Recent Advances in Functionalized Mesoporous Silica Frameworks for Efficient Desulfurization of Fuels
Abstract
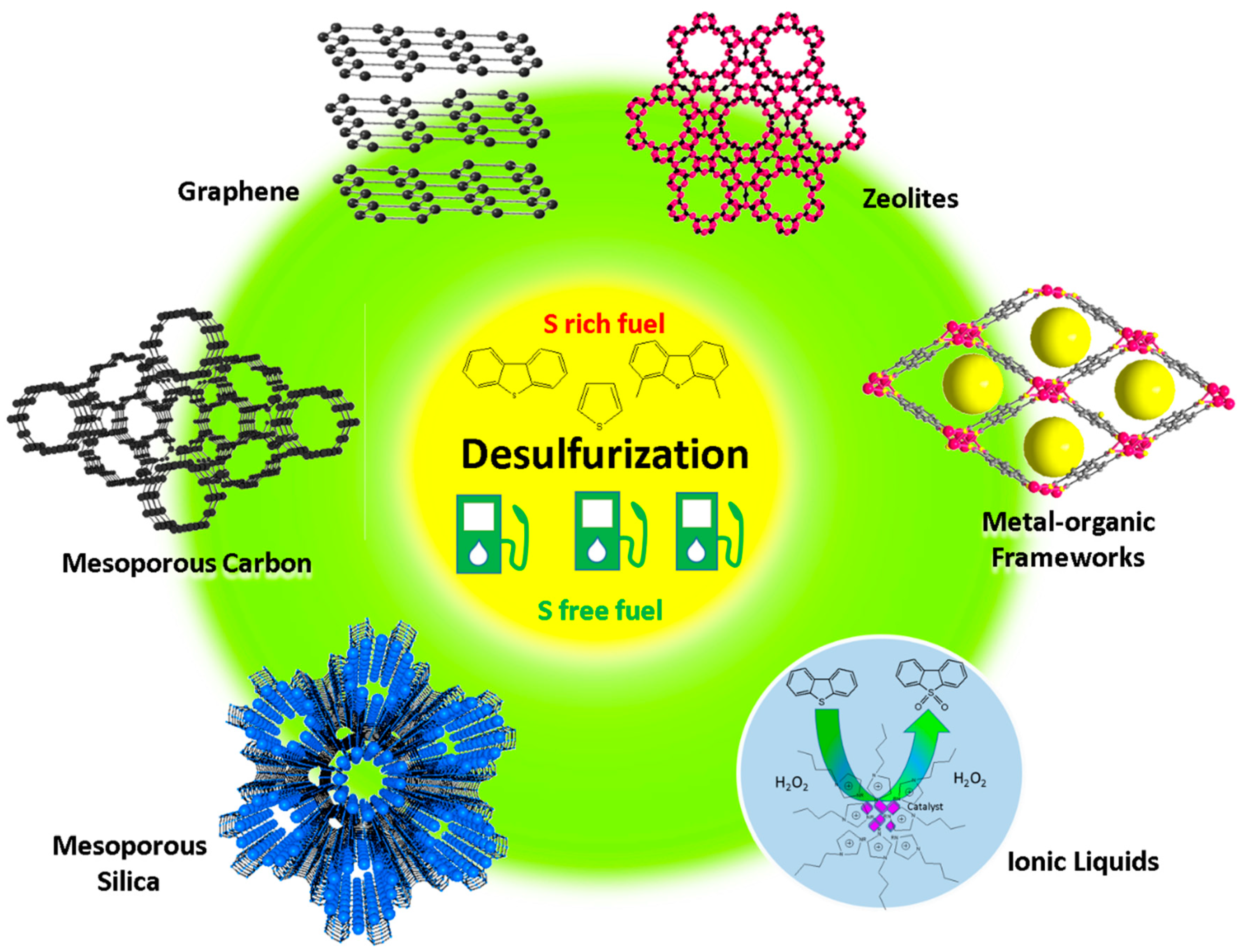
1. Introduction
2. Strategies to Synthesize Mesoporous Silica Catalytic Host Materials
2.1. Templating Strategies
2.1.1. Nanocellulose Template
2.1.2. Triblock Copolymer (P123) as a Template
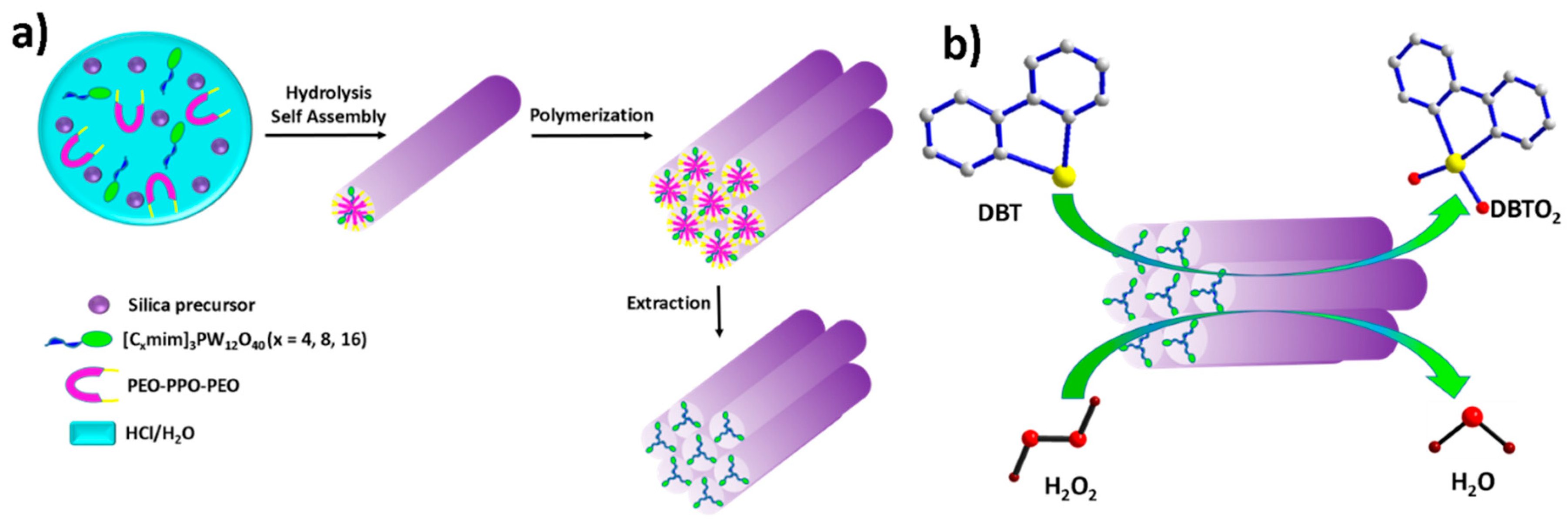
2.1.3. Task-Specific Ionic Liquid Strategy
2.1.4. Polystyrene Colloidal Crystal Template
2.2. Molecular Imprinting Polymer Technology
2.3. 1D Silica Fibers and Nanowires
3. Strategies to Desulfurize Fuel
3.1. Hydrodesulfurization (HDS)
3.2. Oxidative Desulfurization (ODS)
3.3. Adsorptive Desulfurization (ADS)
3.4. Biodesulfurization (BDS)
3.5. Other Forms of Desulfurization
4. Mesoporous Silica and Mesoporous Silica Nanoparticles in Desulfurization
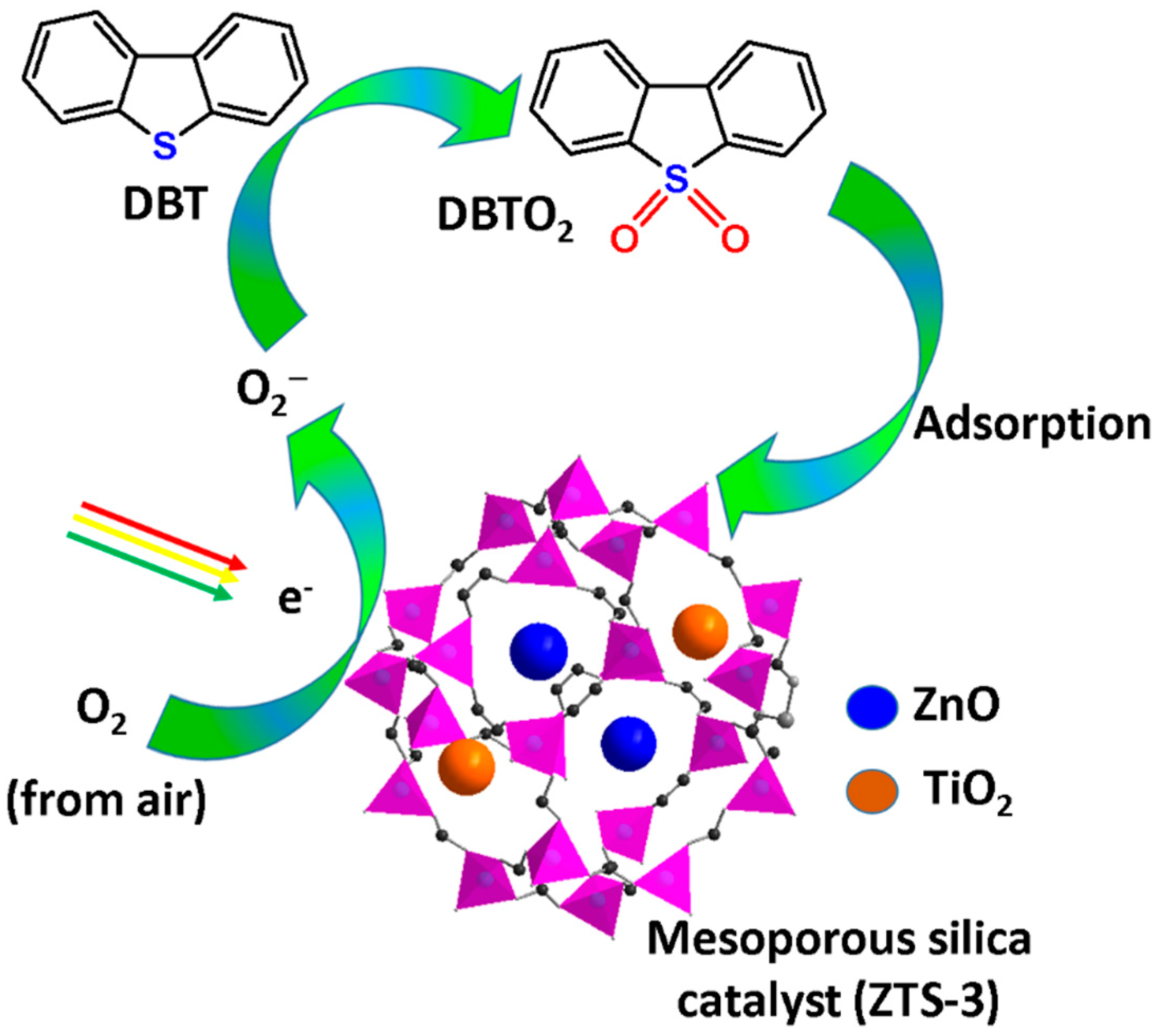
4.1. Photocatalytic MSs
4.2. Redox Active MSNs
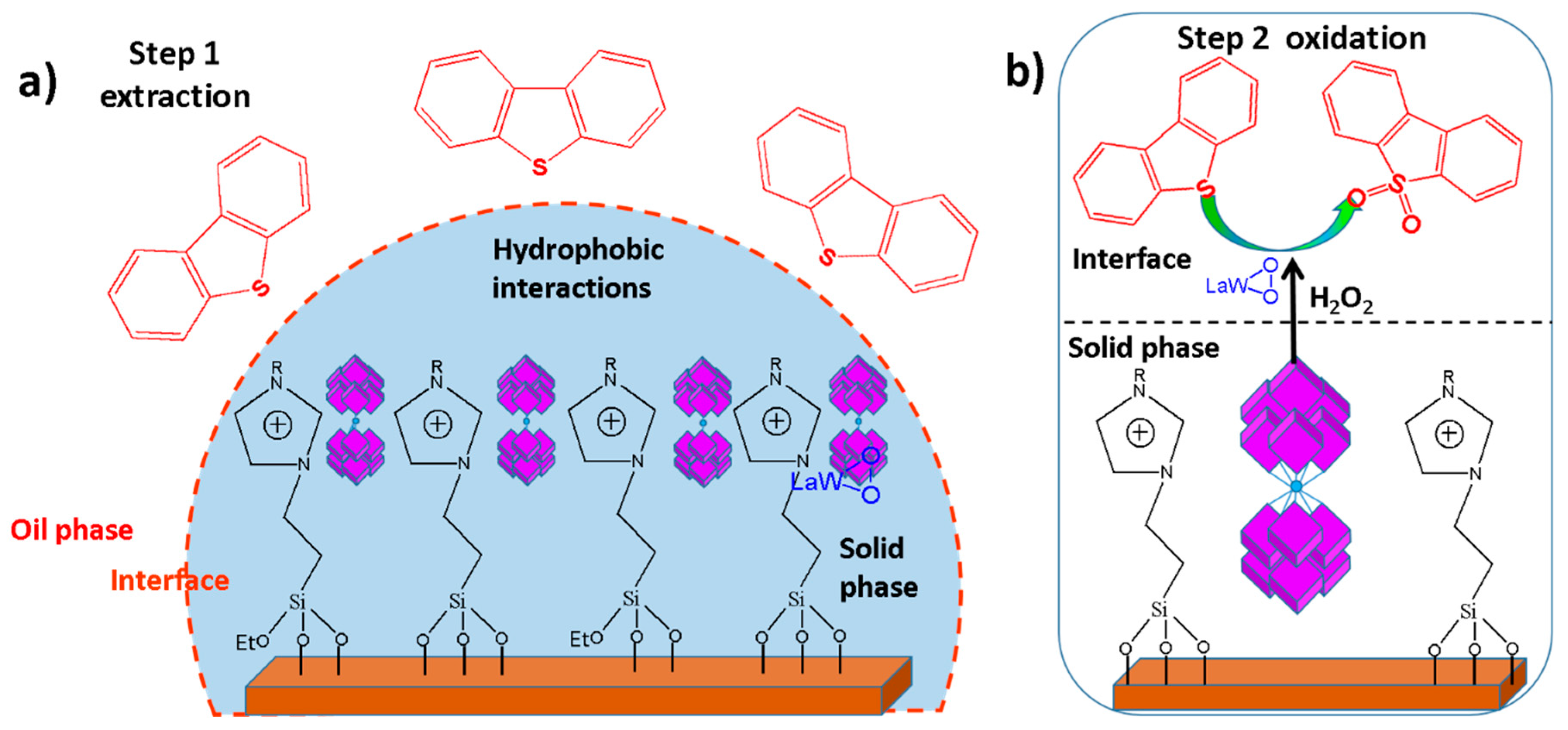
4.3. Ionic Liquid MSN Catalysts
4.4. Magnetic MS Catalysts
4.5. Spinel Embedded Silicoaluminophosphate Catalysts
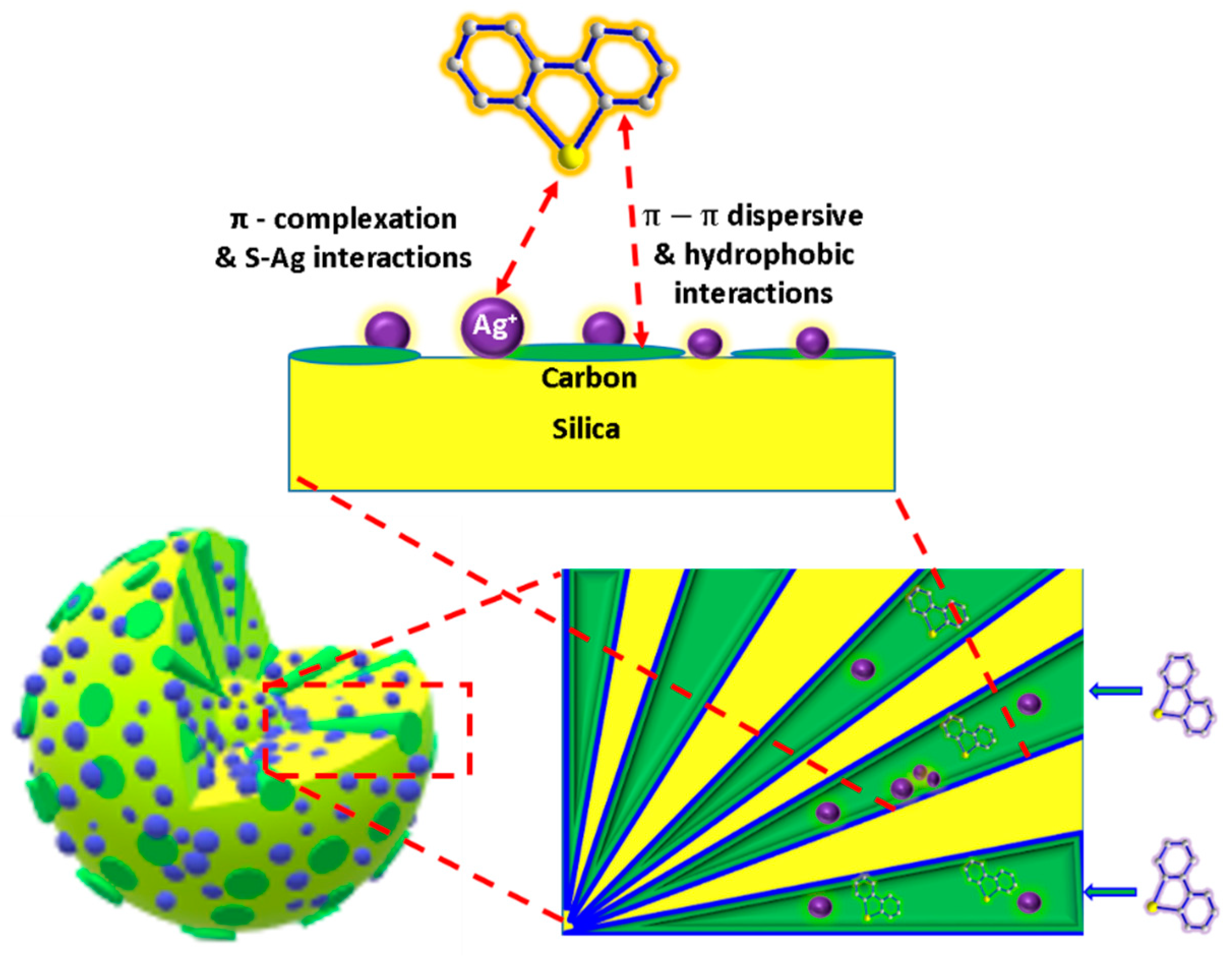
4.6. MSN-Carbon Composites
4.7. Silica Gels
5. MS Frameworks and Nanomaterials in Desulfurization of JP-8 and JP-5 Aviation Fuel
6. Summary
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/gho/publications/world_health_statistics/2018/en/ (accessed on 4 June 2020).
- Available online: https://www.unece.org/info/about-unece/mission/unece-and-the-sdgs.html (accessed on 4 June 2020).
- Available online: https://ccacoalition.org/en/resources/global-strategy-introduce%C2%A0low-sulfur-fuels-and-cleaner-diesel-vehicles (accessed on 4 June 2020).
- Li, H.; Su, S.; Peng, Y.; Wu, L.; Xu, K.; Liu, L.; Qing, M.; Hu, S.; Wang, Y.; Xiang, J. Effect of La-Modified Supporter on H2S Removal Performance of Mn/La/Al2O3 Sorbent in a Reducing Atmosphere. Ind. Eng. Chem. Res. 2019, 58, 8260–8270. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, W.; Wu, J. CeO2–MnOx/ZSM-5 sorbents for H2S removal at high temperature. Chem. Eng. J. 2016, 284, 862–871. [Google Scholar] [CrossRef]
- Guo, L.F.; Pan, K.L.; Lee, H.M.; Chang, M.B. High-temperature gaseous H2S removal by Zn–Mn-based sorbent. Ind. Eng. Chem. Res. 2015, 54, 11040–11047. [Google Scholar] [CrossRef]
- Wu, M.; Chang, B.; Lim, T.-T.; Oh, W.-D.; Lei, J.; Mi, J. High-sulfur capacity and regenerable Zn-based sorbents derived from layered double hydroxide for hot coal gas desulfurization. J. Hazard. Mater. 2018, 360, 391–401. [Google Scholar] [CrossRef]
- Zeng, B.; Yue, H.; Liu, C.; Huang, T.; Li, J.; Zhao, B.; Zhang, M.; Liang, B. Desulfurization behavior of Fe–Mn-based regenerable sorbents for high-temperature H2S removal. Energy Fuels 2015, 29, 1860–1867. [Google Scholar] [CrossRef]
- Xia, H.; Liu, B.; Li, Q.; Huang, Z.; Cheung, A.S.-C. High capacity Mn-Fe-Mo/FSM-16 sorbents in hot coal gas desulfurization and mechanism of elemental sulfur formation. Appl. Catal. B Environ. 2017, 200, 552–565. [Google Scholar] [CrossRef]
- Chen, C.-S.; Budi, C.S.; Wu, H.-C.; Saikia, D.; Kao, H.-M. Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous silica as highly active catalysts for CO2 hydrogenation. ACS Catal. 2017, 7, 8367–8381. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2019, 30, 1902634. [Google Scholar] [CrossRef]
- Mendiratta, S.; Hussein, M.; Nasser, H.A.; Ali, A.A.A. Multidisciplinary Role of Mesoporous Silica Nanoparticles in Brain Regeneration and Cancers: From Crossing the Blood–Brain Barrier to Treatment. Part. Part. Syst. Charact. 2019, 36, 1900195. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-impregnated mesoporous silica nanotube as an emerging nanocomposite for CO2 capture. ACS Appl. Mater. Interfaces 2016, 8, 17312–17320. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Han, Y.H.; Na, J.; Lee, C.H.; Sun, Z.; Wang, S.B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.Z. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 1907035. [Google Scholar] [CrossRef] [PubMed]
- Shieh, F.-K.; Wang, S.-C.; Yen, C.-I.; Wu, C.-C.; Dutta, S.; Chou, L.-Y.; Morabito, J.V.; Hu, P.; Hsu, M.-H.; Wu, K.C.-W. Imparting functionality to biocatalysts via embedding enzymes into nanoporous materials by a de novo approach: Size-selective sheltering of catalase in metal–organic framework microcrystals. J. Am.Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Sue, Y.-C.; Wu, J.-W.; Chung, S.-E.; Kang, C.-H.; Tung, K.-L.; Wu, K.C.-W.; Shieh, F.-K. Synthesis of hierarchical micro/mesoporous structures via solid–aqueous interface growth: Zeolitic imidazolate framework-8 on siliceous mesocellular foams for enhanced pervaporation of water/ethanol mixtures. ACS Appl. Mater. Interfaces 2014, 6, 5192–5198. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Ding, B.; Chang, Z.; Zhang, X.; Yamauchi, Y.; Wu, K.C.W. Confined Self-Assembly in Two-Dimensional Interlayer Space: Monolayered Mesoporous Carbon Nanosheets with In-Plane Orderly Arranged Mesopores and a Highly Graphitized Framework. Angew. Chem. Int. Ed. 2018, 57, 2894–2898. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Matsagar, B.M.; Wu, K.C.-W. Metal–organic framework (MOF)-derived effective solid catalysts for valorization of lignocellulosic biomass. ACS Sustain. Chem. Eng. 2018, 6, 13628–13643. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Dutta, S.; Hossain, M.S.; Shiddiky, M.J.; Tung, K.L.; Shieh, F.K.; Tsung, C.K.; Wu, K.C.W.; Yamauchi, Y. Strategies for improving the functionality of zeolitic imidazolate frameworks: Tailoring nanoarchitectures for functional applications. Adv. Mater. 2017, 29, 1700213. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Shen, D.; Dai, Y.; Han, J.; Gan, L.; Liu, J.; Long, M. A nanocellulose template strategy for the controllable synthesis of tungsten-containing mesoporous silica for ultra-deep oxidative desulfurization. Chem. Eng. J. 2018, 332, 563–571. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Cheng, S. Acid-free synthesis of mesoporous silica using triblock copolymer as template with the aid of salt and alcohol. Chem. Mater. 2007, 19, 3041–3051. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am.Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Chen, D.; Li, Z.; Yu, C.; Shi, Y.; Zhang, Z.; Tu, B.; Zhao, D. Nonionic block copolymer and anionic mixed surfactants directed synthesis of highly ordered mesoporous silica with bicontinuous cubic structure. Chem. Mater. 2005, 17, 3228–3234. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kleitz, F.; Paul, B.; Ryoo, R. MCM-48-like large mesoporous silicas with tailored pore structure: Facile synthesis domain in a ternary triblock copolymer− butanol− water system. J. Am. Chem. Soc. 2005, 127, 7601–7610. [Google Scholar] [CrossRef] [PubMed]
- Flodström, K.; Alfredsson, V.; Källrot, N. Formation of a New Ia3-d Cubic Meso-Structured Silica via Triblock Copolymer-Assisted Synthesis. J. Am. Chem. Soc. 2003, 125, 4402–4403. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Motozuka, S.; Tagaya, M. Synthesis of nanostructured silica/hydroxyapatite hybrid particles containing amphiphilic triblock copolymer for effectively controlling hydration layer structures with cytocompatibility. J. Mater. Chem. B 2020, 8, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.; Raut, D.; Darvatkar, N.; Salunkhe, M. Recent developments of task-specific ionic liquids in organic synthesis. Green Chem. Lett. Rev. 2011, 4, 41–54. [Google Scholar] [CrossRef]
- Ward, A.J.; Pujari, A.A.; Costanzo, L.; Masters, A.F.; Maschmeyer, T. Ionic liquid-templated preparation of mesoporous silica embedded with nanocrystalline sulfated zirconia. Nanoscale Res. Lett. 2011, 6, 192. [Google Scholar] [CrossRef]
- Pujari, A.A.; Chadbourne, J.J.; Ward, A.J.; Costanzo, L.; Masters, A.F.; Maschmeyer, T. The use of acidic task-specific ionic liquids in the formation of high surface area mesoporous silica. New J. Chem. 2009, 33, 1997–2000. [Google Scholar] [CrossRef]
- Wang, T.; Kaper, H.; Antonietti, M.; Smarsly, B. Templating behavior of a long-chain ionic liquid in the hydrothermal synthesis of mesoporous silica. Langmuir 2007, 23, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Mori, S.; Shimojima, A.; Wada, H.; Kuroda, K. Fabrication of colloidal crystals composed of pore-expanded mesoporous silica nanoparticles prepared by a controlled growth method. Nanoscale 2017, 9, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qi, K.; Rong, J.; Wang, L.; Liu, Z.; Yang, Y. Template synthesis of 3-D bimodal ordered porous silica. Chin. Sci. Bull. 2001, 46, 1785–1789. [Google Scholar] [CrossRef]
- Villaescusa, L.A.; Mihi, A.; Rodríguez, I.; García-Bennett, A.E.; Míguez, H. Growth of mesoporous materials within colloidal crystal films by spin-coating. J. Phys. Chem. B 2005, 109, 19643–19649. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhang, Z.; Rong, M.; Zhang, M. Core-Shell Structure Design of Hollow Mesoporous Silica Nanospheres Based on Thermo-Sensitive PNIPAM and pH-Responsive Catechol-Fe3+ Complex. Polymers 2019, 11, 1832. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.-R.; Moon, J.H.; Yang, S.-M. Ordered macroporous particles by colloidal templating. Chem. Mater. 2001, 13, 2613–2618. [Google Scholar] [CrossRef]
- Qin, L.; Shi, W.; Liu, W.; Yang, Y.; Liu, X.; Xu, B. Surface molecularly imprinted polymers grafted on ordered mesoporous carbon nanospheres for fuel desulfurization. RSC Adv. 2016, 6, 12504–12513. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Xu, L.; Zhang, J. Bisphenol A sensing based on surface molecularly imprinted, ordered mesoporous silica. Electrochim. Acta 2011, 56, 2105–2109. [Google Scholar] [CrossRef]
- Pan, S.-D.; Shen, H.-Y.; Zhou, L.-X.; Chen, X.-H.; Zhao, Y.-G.; Cai, M.-Q.; Jin, M.-C. Controlled synthesis of pentachlorophenol-imprinted polymers on the surface of magnetic graphene oxide for highly selective adsorption. J. Mater. Chem. A 2014, 2, 15345–15356. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Fu, L.; Liu, D.; Chen, L. Magnetic molecularly imprinted microsensor for selective recognition and transport of fluorescent phycocyanin in seawater. J. Mater. Chem. A 2015, 3, 7437–7444. [Google Scholar] [CrossRef]
- Chandra Srivastava, V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Austin, G.T. Shreve’s Chemical Process Industries; McGraw-Hill Companies: New York, NY, USA, 1984. [Google Scholar]
- Bej, S.K.; Maity, S.K.; Turaga, U.T. Search for an efficient 4, 6-DMDBT hydrodesulfurization catalyst: A review of recent studies. Energy Fuels 2004, 18, 1227–1237. [Google Scholar] [CrossRef]
- Egorova, M.; Prins, R. Competitive hydrodesulfurization of 4, 6-dimethyldibenzothiophene, hydrodenitrogenation of 2-methylpyridine, and hydrogenation of naphthalene over sulfided NiMo/γ-Al2O3. J. Catal. 2004, 224, 278–287. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Egorova, M.; Prins, R. Kinetics of the HDS of 4, 6-dimethyldibenzothiophene and its hydrogenated intermediates over sulfided Mo and NiMo on γ-Al2O3. J. Catal. 2007, 250, 283–293. [Google Scholar] [CrossRef]
- Layan Savithra, G.H.; Bowker, R.H.; Carrillo, B.A.; Bussell, M.E.; Brock, S.L. Mesoporous matrix encapsulation for the synthesis of monodisperse Pd5P2 nanoparticle hydrodesulfurization catalysts. ACS Appl. Mater. Interfaces 2013, 5, 5403–5407. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Nava, R.; Peza-Ledesma, C.L.; Lara-Romero, J.; Alonso-Núez, G.; Pawelec, B.; Rivera-Muñoz, E.M. SBA-15 mesoporous silica as catalytic support for hydrodesulfurization catalysts. Materials 2013, 6, 4139–4167. [Google Scholar] [CrossRef]
- Marafi, M.; Furimsky, E. Hydroprocessing catalysts containing noble metals: Deactivation, regeneration, metals reclamation, and environment and safety. Energy Fuels 2017, 31, 5711–5750. [Google Scholar] [CrossRef]
- Kumaran, G.M.; Garg, S.; Soni, K.; Kumar, M.; Sharma, L.; Rama Rao, K.; Dhar, G.M. Effect of Al-SBA-15 support on catalytic functionalities of hydrotreating catalysts. II. Effect of variation of molybdenum and promoter contents on catalytic functionalities. Ind. Eng. Chem. Res. 2007, 46, 4747–4754. [Google Scholar] [CrossRef]
- Soni, K.; Rana, B.; Sinha, A.; Bhaumik, A.; Nandi, M.; Kumar, M.; Dhar, G. 3-D ordered mesoporous KIT-6 support for effective hydrodesulfurization catalysts. Appl. Catal. B Environ. 2009, 90, 55–63. [Google Scholar] [CrossRef]
- Sorensen, A.C.; Fuller, B.L.; Eklund, A.G.; Landry, C.C. Mo-Doped mesoporous silica for thiophene hydrodesulfurization: Comparison of materials and methods. Chem. Mater. 2004, 16, 2157–2164. [Google Scholar] [CrossRef]
- Danforth, S.J.; Liyanage, D.R.; Hitihami-Mudiyanselage, A.; Ilic, B.; Brock, S.L.; Bussell, M.E. Probing hydrodesulfurization over bimetallic phosphides using monodisperse Ni2-xMxP nanoparticles encapsulated in mesoporous silica. Surf. Sci. 2016, 648, 126–135. [Google Scholar] [CrossRef]
- Chiranjeevi, T.; Kumar, P.; Maity, S.; Rana, M.; Dhar, G.M.; Rao, T.P. Characterization and hydrodesulfurization catalysis on WS2 supported on mesoporous Al–HMS material. Microporous Mesoporous Mater. 2001, 44, 547–556. [Google Scholar] [CrossRef]
- Campos-Martin, J.; Capel-Sanchez, M.; Fierro, J. Highly efficient deep desulfurization of fuels by chemical oxidation. Green Chem. 2004, 6, 557–562. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Wei, Y.; Li, H.; Liu, J.; Zhang, Q.; Zhu, W.; Li, H. Ionic liquid-supported 3DOM silica for efficient heterogeneous oxidative desulfurization. Inorg. Chem. Front. 2018, 5, 2478–2485. [Google Scholar] [CrossRef]
- Yuzbashi, S.; Mousazadeh, M.; Ramezani, N.; Sid Kalal, H.; Sabour, B. Mesoporous zirconium–silica nanocomposite modified with heteropoly tungstophosphoric acid catalyst for ultra-deep oxidative desulfurization. Appl. Organomet. Chem. 2020, 34, e5326. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, G.; Tian, M.; Liu, B.; Chen, W. Oxidative desulfurization of dibenzothiophene by central metal ions of chlorophthalocyanines-tetracarboxyl complexes. Inorg. Chim. Acta 2019, 485, 58–63. [Google Scholar] [CrossRef]
- Javadli, R.; De Klerk, A. Desulfurization of heavy oil. Appl. Petrochem. Res. 2012, 1, 3–19. [Google Scholar] [CrossRef]
- Zhu, W.; Li, H.; Jiang, X.; Yan, Y.; Lu, J.; He, L.; Xia, J. Commercially available molybdic compound-catalyzed ultra-deep desulfurization of fuels in ionic liquids. Green Chem. 2008, 10, 641–646. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.-Y.; Fan, H.-X.; Yang, Z.; Feng, J.; Li, W. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Hossain, M.N.; Park, H.C.; Choi, H.S. A comprehensive review on catalytic oxidative desulfurization of liquid fuel oil. Catalysts 2019, 9, 229. [Google Scholar] [CrossRef]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative desulfurization of heavy oils with high sulfur content: A review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, S.; Jin, Q.; Zhao, J. Review on oxidative desulfurization of fuel by supported heteropolyacid catalysts. J. Ind. Eng. Chem. 2020, 82, 1–16. [Google Scholar] [CrossRef]
- Ja’fari, M.; Ebrahimi, S.L.; Khosravi-Nikou, M.R. Ultrasound-assisted oxidative desulfurization and denitrogenation of liquid hydrocarbon fuels: A critical review. Ultrason. Sonochem. 2018, 40, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.; Ebert, S.; Foght, J.; Fedorak, P.; Gray, M. Bacterial biodegradation of aliphatic sulfides under aerobic carbon-or sulfur-limited growth conditions. J. Appl. Microbiol. 2005, 99, 1444–1454. [Google Scholar] [CrossRef]
- Kirkwood, K.M.; Foght, J.M.; Gray, M.R. Selectivity among organic sulfur compounds in one-and two-liquid-phase cultures of Rhodococcus sp. strain JVH1. Biodegradation 2007, 18, 473–480. [Google Scholar] [CrossRef]
- Kirkwood, K.M.; Andersson, J.T.; Fedorak, P.M.; Foght, J.M.; Gray, M.R. Sulfur from benzothiophene and alkylbenzothiophenes supports growth of Rhodococcus sp. strain JVH1. Biodegradation 2007, 18, 541–549. [Google Scholar] [CrossRef]
- McFarland, B.L.; Boron, D.J.; Deever, W.; Meyer, J.; Johnson, A.R.; Atlas, R.M. Biocatalytic sulfur removal from fuels: Applicability for producing low sulfur gasoline. Crit. Rev. Microbiol. 1998, 24, 99–147. [Google Scholar] [CrossRef]
- Gupta, N.; Roychoudhury, P.; Deb, J. Biotechnology of desulfurization of diesel: Prospects and challenges. Appl. Microbiol. Biotechnol. 2005, 66, 356–366. [Google Scholar] [CrossRef]
- Sadare, O.O.; Obazu, F.; Daramola, M.O. Biodesulfurization of petroleum distillates—Current status, opportunities and future challenges. Environments 2017, 4, 85. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y. Alkylation desulfurization of the C9 fraction over Amberlyst 36 resin. RSC Adv. 2015, 5, 2908–2913. [Google Scholar] [CrossRef]
- Han, D.Y.; Chen, C.J.; Zhang, F.J. Deep Desulfurization of Light Oil Using the Imitating Silver Salt ILs Method. Petrol. Sci. Technol. 2011, 29, 2487–2493. [Google Scholar] [CrossRef]
- He, J.; Wu, P.; Wu, Y.; Li, H.; Jiang, W.; Xun, S.; Zhang, M.; Zhu, W.; Li, H. Taming interfacial oxygen vacancies of amphiphilic tungsten oxide for enhanced catalysis in oxidative desulfurization. ACS Sustain. Chem. Eng. 2017, 5, 8930–8938. [Google Scholar] [CrossRef]
- Wang, X.-S.; Huang, Y.-B.; Lin, Z.-J.; Cao, R. Phosphotungstic acid encapsulated in the mesocages of amine-functionalized metal–organic frameworks for catalytic oxidative desulfurization. Dalton Trans. 2014, 43, 11950–11958. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Ding, Y.; Zhang, L.; Wu, H.; Guo, J. Synthesis of mesoporous ZnO/TiO 2–SiO2 composite material and its application in photocatalytic adsorption desulfurization without the addition of an extra oxidant. Dalton Trans. 2020, 49, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, T.; He, H.; Fu, Y.; Zhang, X.; Sun, J.; Xing, L.; Liu, B.; Zhang, Y.; Xue, X. Enhanced H2 production of TiO2/ZnO nanowires Co-using solar and mechanical energy through piezo-photocatalytic effect. ACS Sustain. Chem. Eng. 2018, 6, 10162–10172. [Google Scholar] [CrossRef]
- Qin, B.; Shen, Y.; Xu, B.; Zhu, S.; Li, P.; Liu, Y. Mesoporous TiO2–SiO2 adsorbent for ultra-deep desulfurization of organic-S at room temperature and atmospheric pressure. RSC Adv. 2018, 8, 7579–7587. [Google Scholar] [CrossRef]
- Meizhen, X.; Lina, Y.; Jian, L. Photocatalytic oxidative desulfurization of dibenzothiophene on TiO2 modified bimodal mesoporous silica. China Pet. Process. Petrochem.Technol. 2017, 19, 59–67. [Google Scholar]
- Zaccariello, G.; Moretti, E.; Storaro, L.; Riello, P.; Canton, P.; Gombac, V.; Montini, T.; Rodriguez-Castellon, E.; Benedetti, A. TiO2–mesoporous silica nanocomposites: Cooperative effect in the photocatalytic degradation of dyes and drugs. RSC Adv. 2014, 4, 37826–37837. [Google Scholar] [CrossRef]
- Julião, D.; Gomes, A.C.; Pillinger, M.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Gonçalves, I.S.; Cunha Silva, L.; Balula, S.S. Zinc-Substituted Polyoxotungstate@ amino-MIL-101 (Al)–An Efficient Catalyst for the Sustainable Desulfurization of Model and Real Diesels. Eur. J. Inorg. Chem. 2016, 2016, 5114–5122. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate modified pine cone biochar carbon for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3939–3947. [Google Scholar] [CrossRef]
- Ghahramaninezhad, M.; Soleimani, B.; Shahrak, M.N. A simple and novel protocol for Li-trapping with a POM/MOF nano-composite as a new adsorbent for CO2 uptake. New J. Chem. 2018, 42, 4639–4645. [Google Scholar] [CrossRef]
- Xun, S.; Zhu, W.; Chang, Y.; Li, H.; Zhang, M.; Jiang, W.; Zheng, D.; Qin, Y.; Li, H. Synthesis of supported SiW12O40-based ionic liquid catalyst induced solvent-free oxidative deep-desulfurization of fuels. Chem. Eng. J. 2016, 288, 608–617. [Google Scholar] [CrossRef]
- Li, S.-W.; Li, J.-R.; Gao, Y.; Liang, L.-L.; Zhang, R.-L.; Zhao, J.-S. Metal modified heteropolyacid incorporated into porous materials for a highly oxidative desulfurization of DBT under molecular oxygen. Fuel 2017, 197, 551–561. [Google Scholar] [CrossRef]
- Shen, D.; Yang, J.; Li, X.; Zhou, L.; Zhang, R.; Li, W.; Chen, L.; Wang, R.; Zhang, F.; Zhao, D. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett. 2014, 14, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Chen, L.; Yang, J.; Zhang, R.; Wei, Y.; Li, X.; Li, W.; Sun, Z.; Zhu, H.; Abdullah, A.M. Ultradispersed palladium nanoparticles in three-dimensional dendritic mesoporous silica nanospheres: Toward active and stable heterogeneous catalysts. ACS Appl. Mater. Interfaces 2015, 7, 17450–17459. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Yang, J.; Chen, X.; Wang, M.; Li, H.; Zhu, W.; Li, H. Molybdenum-containing dendritic mesoporous silica spheres for fast oxidative desulfurization in fuel. Inorg. Chem. Front. 2019, 6, 451–458. [Google Scholar] [CrossRef]
- Casuscelli, S.; Crivello, M.; Perez, C.; Ghione, G.; Herrero, E.; Pizzio, L.; Vázquez, P.; Cáceres, C.; Blanco, M. Effect of reaction conditions on limonene epoxidation with H2O2 catalyzed by supported Keggin heteropolycompounds. Appl. Catal. A Gen. 2004, 274, 115–122. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.; Silva, P.; Paz, F.A.A.; Saini, V.K.; Pires, J.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Monovacant polyoxometalates incorporated into MIL-101 (Cr): Novel heterogeneous catalysts for liquid phase oxidation. Appl. Catal. A Gen. 2013, 453, 316–326. [Google Scholar] [CrossRef]
- Ribeiro, S.; Barbosa, A.D.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; Cunha-Silva, L.; Balula, S.S. Catalytic oxidative desulfurization systems based on Keggin phosphotungstate and metal-organic framework MIL-101. Fuel Process. Technol. 2013, 116, 350–357. [Google Scholar] [CrossRef]
- Singh, S.; Patel, A.; Prakashan, P. One pot oxidative esterification of aldehyde over recyclable cesium salt of nickel substituted phosphotungstate. Appl. Catal. A Gen. 2015, 505, 131–140. [Google Scholar] [CrossRef]
- Coronel, N.C.; da Silva, M.J. Lacunar keggin heteropolyacid salts: Soluble, solid and solid-supported catalysts. J. Clust. Sci. 2018, 29, 195–205. [Google Scholar] [CrossRef]
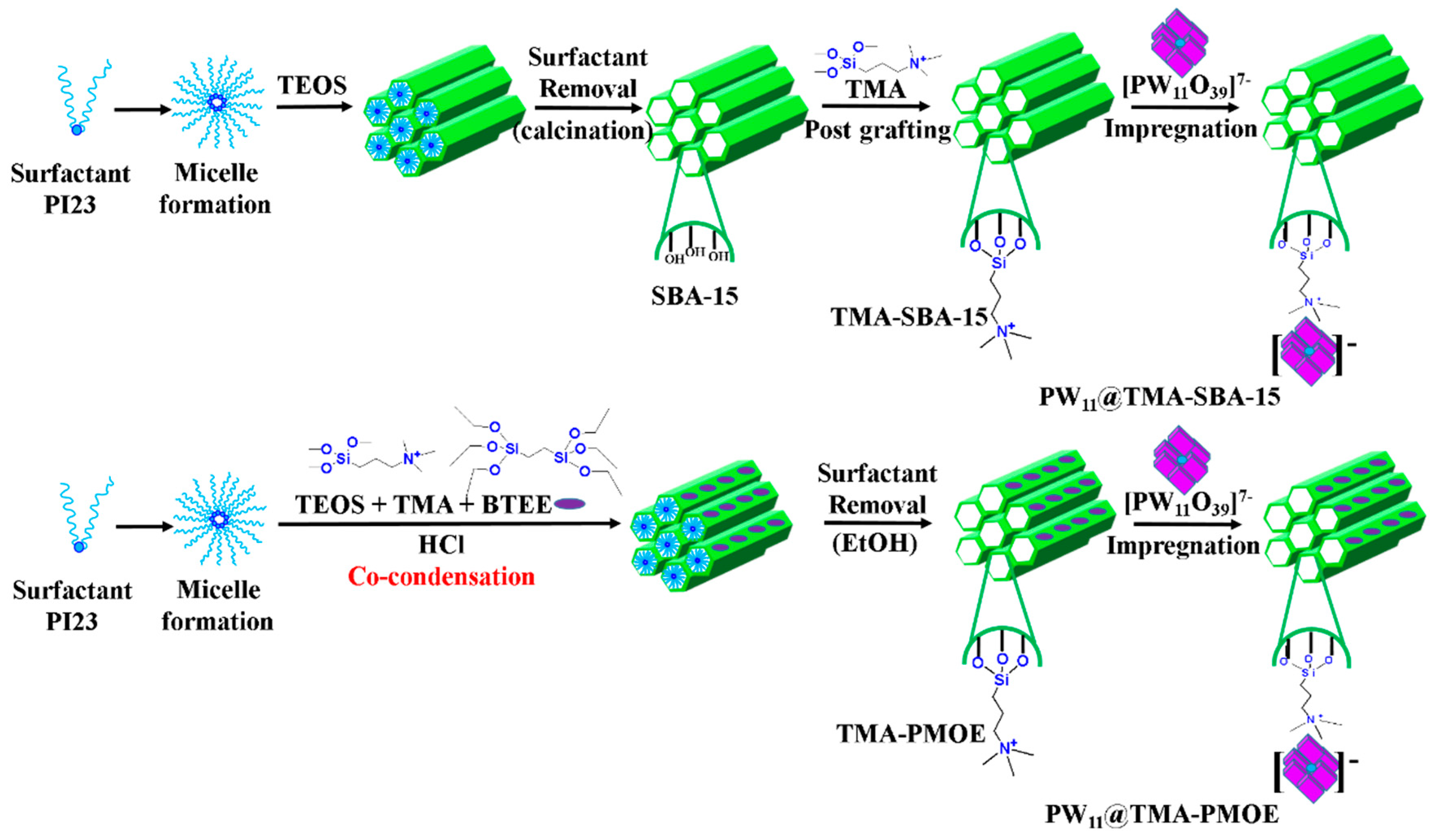
- Abdalla, Z.E.A.; Li, B. Preparation of MCM-41 supported (Bu4N)4H3(PW11O39) catalyst and its performance in oxidative desulfurization. Chem. Eng. J. 2012, 200, 113–121. [Google Scholar] [CrossRef]
- Abdalla, Z.E.A.; Li, B.; Tufail, A. Direct synthesis of mesoporous (C19H42N)4H3(PW11O39)/SiO2 and its catalytic performance in oxidative desulfurization. Colloids Surf. A Physicochem. Eng. Asp. 2009, 341, 86–92. [Google Scholar] [CrossRef]
- Wu, N.; Li, B.; Liu, Z.; Han, C. Synthesis of Keggin-type lacunary 11-tungstophosphates encapsulated into mesoporous silica pillared in clay interlayer galleries and their catalytic performance in oxidative desulfurization. Catal. Commun. 2014, 46, 156–160. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Granadeiro, C.M.; Almeida, P.L.; Pires, J.; Capel-Sanchez, M.C.; Campos-Martin, J.M.; Gago, S.; De Castro, B.; Balula, S.S. Oxidative desulfurization strategies using Keggin-type polyoxometalate catalysts: Biphasic versus solvent-free systems. Catal. Today 2019, 333, 226–236. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Granadeiro, C.M.; Corvo, M.C.; Pires, J.; Campos-Martin, J.M.; de Castro, B.; Balula, S.S. Mesoporous Silica vs. Organosilica Composites to Desulfurize Diesel. Front. Chem. 2019, 7, 756. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.O.; Almeida, P.; Pires, J.; de Castro, B.; Balula, S.S. Polyoxometalate@ Periodic mesoporous organosilicas as active materials for oxidative desulfurization of diesels. Microporous Mesoporous Mater. 2020, 110193. [Google Scholar] [CrossRef]
- Ni, B.; Wang, X. Chemistry and properties at a sub-nanometer scale. Chem. Sci. 2016, 7, 3978–3991. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef]
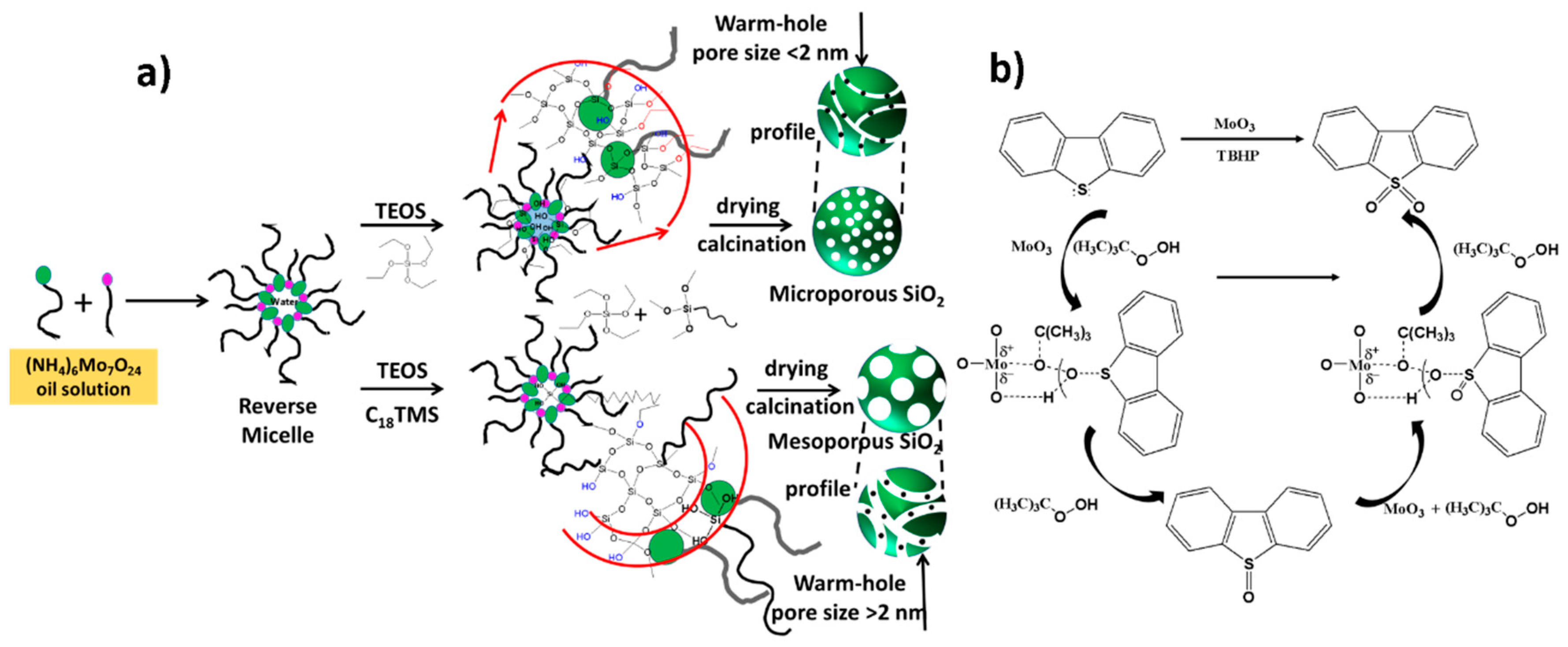
- Wang, J.; Li, X.; Zhang, S.; Lu, R. Facile synthesis of ultrasmall monodisperse “raisin–bun”-type MoO 3/SiO2 nanocomposites with enhanced catalytic properties. Nanoscale 2013, 5, 4823–4828. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, W.; Ye, H.; Zhao, Y.; Wang, W.-H.; Bao, M. MoO3 subnanoclusters on ultrasmall mesoporous silica nanoparticles: An efficient catalyst for oxidative desulfurization. RSC Adv. 2017, 7, 44827–44833. [Google Scholar] [CrossRef]
- Dou, J.; Zeng, H.C. Integrated Networks of Mesoporous Silica Nanowires and Their Bifunctional Catalysis–Sorption Application for Oxidative Desulfurization. ACS Catal. 2014, 4, 566–576. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of inorganic materials using ionic liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, J.; Zhu, D.; Ren, X.; Ge, H.; Shen, L. Heteropolyanion-based ionic liquids: Reaction-induced self-separation catalysts for esterification. Angew. Chem. Int. Ed. 2009, 48, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qing, S.; Wang, L.; Islam, N.; Guan, S.; Gao, Z.; Mamat, X.; Li, H.; Eli, W.; Wang, T. Production of 5-hydroxymethylfurfural from fructose by a thermo-regulated and recyclable Brønsted acidic ionic liquid catalyst. RSC Adv. 2015, 5, 47377–47383. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W.; Chang, Y.; Chao, Y.; Yin, S.; Liu, H.; Zhu, F.; Li, H. Ionic liquid extraction and catalytic oxidative desulfurization of fuels using dialkylpiperidinium tetrachloroferrates catalysts. Chem. Eng. J. 2014, 250, 48–54. [Google Scholar] [CrossRef]
- Lü, H.; Wang, S.; Deng, C.; Ren, W.; Guo, B. Oxidative desulfurization of model diesel via dual activation by a protic ionic liquid. J. Hazard. Mater. 2014, 279, 220–225. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, B.; Gupta, N.; Su, D.S. Heterogenization of homogenous reaction system on carbon surface with ionic liquid as mediator. Green Chem. 2015, 17, 1107–1112. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, G.; Zhao, C.; Li, X.; Li, M.; Na, H. MOFs synthesized by the ionothermal method addressing the leaching problem of IL–polymer composite membranes. Chem. Commun. 2014, 50, 14121–14124. [Google Scholar] [CrossRef]
- Wan, H.; Chen, C.; Wu, Z.; Que, Y.; Feng, Y.; Wang, W.; Wang, L.; Guan, G.; Liu, X. Encapsulation of heteropolyanion-based ionic liquid within the metal–organic framework MIL-100 (Fe) for biodiesel production. ChemCatChem 2015, 7, 441–449. [Google Scholar] [CrossRef]
- Patil, J.D.; Patil, S.A.; Pore, D.M. A polymer supported ascorbate functionalized task specific ionic liquid: An efficient reusable catalyst for 1, 3-dipolar cycloaddition. RSC Adv. 2015, 5, 21396–21404. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, M. Silica functionalized sulphonic acid coated with ionic liquid: An efficient and recyclable heterogeneous catalyst for the one-pot synthesis of 1, 4-dihydropyridines under solvent-free conditions. Green Chem. 2015, 17, 1100–1106. [Google Scholar] [CrossRef]
- Sidhpuria, K.B.; Daniel-da-Silva, A.L.; Trindade, T.; Coutinho, J.A. Supported ionic liquid silica nanoparticles (SILnPs) as an efficient and recyclable heterogeneous catalyst for the dehydration of fructose to 5-hydroxymethylfurfural. Green Chem. 2011, 13, 340–349. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Li, X.; Ma, X. Oxidative desulfurization of dibenzothiophene and diesel over [Bmim] 3PMo12O40. J. Catal. 2011, 279, 269–275. [Google Scholar] [CrossRef]
- Virtanen, P.; Mikkola, J.-P.; Toukoniitty, E.; Karhu, H.; Kordas, K.; Eränen, K.; Wärnå, J.; Salmi, T. Supported ionic liquid catalysts—From batch to continuous operation in preparation of fine chemicals. Catal. Today 2009, 147, S144–S148. [Google Scholar] [CrossRef]
- Bordoloi, A.; Sahoo, S.; Lefebvre, F.; Halligudi, S. Heteropoly acid-based supported ionic liquid-phase catalyst for the selective oxidation of alcohols. J. Catal. 2008, 259, 232–239. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.M. A heteropolyacid-based ionic liquid immobilized onto fibrous nano-silica as an efficient catalyst for the synthesis of cyclic carbonate from carbon dioxide and epoxides. Green Chem. 2015, 17, 3059–3066. [Google Scholar] [CrossRef]
- Eguizábal, A.; Lemus, J.; Urbiztondo, M.; Moschovi, A.; Ntais, S.; Soler, J.; Pina, M. Ammonium based ionic liquids immobilized in large pore zeolites: Encapsulation procedures and proton conduction performance. J. Power Sources 2011, 196, 4314–4323. [Google Scholar] [CrossRef]
- Gu, Q.; Zhu, W.; Xun, S.; Chang, Y.; Xiong, J.; Zhang, M.; Jiang, W.; Zhu, F.; Li, H. Preparation of highly dispersed tungsten species within mesoporous silica by ionic liquid and their enhanced catalytic activity for oxidative desulfurization. Fuel 2014, 117, 667–673. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Chen, Q.; Zhu, W.; Li, H.; Yin, S.; Li, Y.; Li, H. One-pot synthesis of ordered mesoporous silica encapsulated polyoxometalate-based ionic liquids induced efficient desulfurization of organosulfur in fuel. RSC Adv. 2015, 5, 76048–76056. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Chen, W.; Wang, M.; Song, Y.F. Highly efficient extraction and oxidative desulfurization system using Na7H2LaW10O36⋅32H2O in [bmim] BF4 at room temperature. Chem. Eur. J. 2012, 18, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, S.; Ji, Y.; Song, Y.F. Deep Desulfurization by Amphiphilic Lanthanide-Containing Polyoxometalates in Ionic-Liquid Emulsion Systems under Mild Conditions. Chem. Eur. J. 2013, 19, 709–715. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.F. Immobilization of LaW10 onto Ionic-Liquid-Modified Mesoporous Silica: Deep Desulfurization with Zero-Order Reaction Kinetics. ChemPlusChem 2014, 79, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, X.; Han, D.; Gu, F. Structural and electronic engineering of 3DOM WO3 by alkali metal doping for improved NO2 sensing performance. Nanoscale 2016, 8, 10622–10631. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Zheng, Z.; Gao, Y.; Ma, K.; Ye, J.; Yang, Y. Enhanced thermal decomposition properties of ammonium perchlorate through addition of 3DOM core-shell Fe2O3/Co3O4 composite. J. Alloys Compd. 2017, 724, 720–727. [Google Scholar] [CrossRef]
- Yue, D.; Lei, J.; Lina, Z.; Zhenran, G.; Du, X.; Li, J. Oxidation desulfurization of fuels by using amphiphilic hierarchically meso/macroporous phosphotungstic acid/SiO2 catalysts. Catal. Letters 2018, 148, 1100–1109. [Google Scholar] [CrossRef]
- Du, Y.; Yang, P.; Zhou, S.; Li, J.; Du, X.; Lei, J. Direct synthesis of ordered meso/macrostructured phosphotungstic acid/SiO2 by EISA method and its catalytic performance of fuel oil. Mater. Res. Bull. 2018, 97, 42–48. [Google Scholar] [CrossRef]
- Mirante, F.; Gomes, N.; Branco, L.C.; Cunha-Silva, L.; Almeida, P.L.; Pillinger, M.; Gago, S.; Granadeiro, C.M.; Balula, S.S. Mesoporous nanosilica-supported polyoxomolybdate as catalysts for sustainable desulfurization. Microporous Mesoporous Mater. 2019, 275, 163–171. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysts for fine chemical synthesis. In Catalysis by Polyoxometalates; John Wiley & Sons Inc.: New York, NY, USA, 2002; Volume 2. [Google Scholar]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic chemistry of heteropoly compounds. In Advances in Catalysis; Academic Press; Elsevier: Amsterdam, The Netherlands, 1996; Volume 41, pp. 113–252. [Google Scholar]
- Xiong, J.; Zhu, W.; Ding, W.; Yang, L.; Zhang, M.; Jiang, W.; Zhao, Z.; Li, H. Hydrophobic mesoporous silica-supported heteropolyacid induced by ionic liquid as a high efficiency catalyst for the oxidative desulfurization of fuel. RSC Adv. 2015, 5, 16847–16855. [Google Scholar] [CrossRef]
- Huang, W.; Xu, P.; Yang, W.; Xu, W. Thermosensitive molecularly imprinted polymers based on magnetic nanoparticles for the recognition of sulfamethazine. RSC Adv. 2016, 6, 74734–74741. [Google Scholar] [CrossRef]
- Gao, R.; Mu, X.; Hao, Y.; Zhang, L.; Zhang, J.; Tang, Y. Combination of surface imprinting and immobilized template techniques for preparation of core–shell molecularly imprinted polymers based on directly amino-modified Fe3O4 nanoparticles for specific recognition of bovine hemoglobin. J. Mater. Chem. B 2014, 2, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Chu, J.; Dong, C.; Qi, J.; Yuan, Y. Synthesis of core-shell magnetic molecular imprinted polymer by the surface RAFT polymerization for the fast and selective removal of endocrine disrupting chemicals from aqueous solutions. Environ. Pollut. 2010, 158, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Men, H.-F.; Liu, H.-Q.; Zhang, Z.-L.; Huang, J.; Zhang, J.; Zhai, Y.-Y.; Li, L. Synthesis, properties and application research of atrazine Fe3O4@ SiO2 magnetic molecularly imprinted polymer. Environ. Sci. Pollut. Res. 2012, 19, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, J. Selective and simultaneous removal of dibenzothiophene and 4-methyldibenzothiophene using double-template molecularly imprinted polymers on the surface of magnetic mesoporous silica. J. Mater. Chem. A 2017, 5, 4651–4659. [Google Scholar] [CrossRef]
- Yue, Q.; Zhang, Y.; Jiang, Y.; Li, J.; Zhang, H.; Yu, C.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.; Zhao, D. Nanoengineering of core–shell magnetic mesoporous microspheres with tunable surface roughness. J. Am.Chem. Soc. 2017, 139, 4954–4961. [Google Scholar] [CrossRef]
- Jiang, W.; Jia, H.; Fan, X.; Dong, L.; Guo, T.; Zhu, L.; Zhu, W.; Li, H. Ionic liquid immobilized on magnetic mesoporous microspheres with rough surface: Application as recyclable amphiphilic catalysts for oxidative desulfurization. Appl. Surf. Sci. 2019, 484, 1027–1034. [Google Scholar] [CrossRef]
- Bian, L.; Li, Y.-J.; Li, J.; Nie, J.-N.; Dong, F.-Q.; Song, M.-X.; Wang, L.-S.; Dong, H.-L.; Li, H.-L.; Nie, X.-Q. Photovoltage response of (XZn) Fe2O4-BiFeO3 (X= Mg, Mn or Ni) interfaces for highly selective Cr3+, Cd2+, Co2+ and Pb2+ ions detection. J. Hazard. Mater. 2017, 336, 174–187. [Google Scholar] [CrossRef]
- Ko, T.-H.; Wang, S.; Chang, F.-H.; Chu, C.-Y. Performance of ZnMn2O4/SiO2 sorbent for high temperature H2S removal from hot coal gas. RSC Adv. 2017, 7, 35795–35804. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Z.; Liu, B.; Xia, H. Rare earth oxide doping and synthesis of spinel ZnMn2O4/KIT-1 with double gyroidal mesopores for desulfurization nature of hot coal gas. Appl. Catal. B Environ. 2018, 237, 855–865. [Google Scholar] [CrossRef]
- Babaahamdi-Milani, M.; Nezamzadeh-Ejhieh, A. A comprehensive study on photocatalytic activity of supported Ni/Pb sulfide and oxide systems onto natural zeolite nanoparticles. J. Hazard. Mater. 2016, 318, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Ejhieh, A.N.; Khorsandi, M. Photodecolorization of Eriochrome Black T using NiS–P zeolite as a heterogeneous catalyst. J. Hazard. Mater. 2010, 176, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohseni, M.H.; Nezamzadeh-Ejhieh, A. Modification of carbon paste electrode with Ni-clinoptilolite nanoparticles for electrocatalytic oxidation of methanol. Electrochim. Acta 2014, 147, 572–581. [Google Scholar] [CrossRef]
- Tamiji, T.; Nezamzadeh-Ejhieh, A. A comprehensive study on the kinetic aspects and experimental design for the voltammetric response of a Sn (IV)-clinoptilolite carbon paste electrode towards Hg (II). J. Electroanal. Chem. 2018, 829, 95–105. [Google Scholar] [CrossRef]
- Radko, M.; Rutkowska, M.; Kowalczyk, A.; Mikrut, P.; Święs, A.; Díaz, U.; Palomares, A.E.; Macyk, W.; Chmielarz, L. Catalytic oxidation of organic sulfides by H2O2 in the presence of titanosilicate zeolites. Microporous Mesoporous Mater. 2020, 302, 110219. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Liu, Q.; Xu, R.; Xia, H. Lattice substitution and desulfurization kinetic analysis of Zn-based spinel sorbents loading onto porous silicoaluminophosphate zeolites. J. Hazard. Mater. 2020, 383, 121151. [Google Scholar] [CrossRef]
- Chao, Y.; Ju, H.; Luo, J.; Jin, Y.; Wang, C.; Xiong, J.; Wu, P.; Ji, H.; Zhu, W. Synthesis of porous carbon via a waste tire leavening strategy for adsorptive desulfurization. RSC Adv. 2019, 9, 30575–30580. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Wang, L.; Liu, G. MOF-derived porous carbon for adsorptive desulfurization. AIChE J. 2014, 60, 2747–2751. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, P.; Duan, A.; Mei, J.; Zheng, P.; Meng, Q.; Cai, A.; Cheng, T.; Gong, Y. Monodispersed dendritic mesoporous silica/carbon nanospheres with enhanced active site accessibility for selective adsorptive desulfurization. J. Mater. Sci. 2019, 54, 8148–8162. [Google Scholar] [CrossRef]
- Ma, X.; Sun, L.; Song, C. A new approach to deep desulfurization of gasoline, diesel fuel and jet fuel by selective adsorption for ultra-clean fuels and for fuel cell applications. Catal. Today 2002, 77, 107–116. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Du, L.; Li, S.; Cai, H.; Liu, W. Nickel-heteropolyacids supported on silica gel for ultra-deep desulfurization assisted by Ultrasound and Ultraviolet. Fuel 2013, 105, 353–357. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Z.; Chen, X.; Zhao, Q.; Wang, X.; Jiang, Z. A micro reaction-controlled phase-transfer catalyst for oxidative desulfurization based on polyoxometalate modified silica. Appl. Catal. A Gen. 2013, 467, 26–32. [Google Scholar] [CrossRef]
- Xun, S.; Zhu, W.; Zheng, D.; Zhang, L.; Liu, H.; Yin, S.; Zhang, M.; Li, H. Synthesis of metal-based ionic liquid supported catalyst and its application in catalytic oxidative desulfurization of fuels. Fuel 2014, 136, 358–365. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Jiang, H.; Hu, Y. Desulfurization performance of ether-functionalized imidazolium-based ionic liquids supported on porous silica gel. Energy Fuels 2015, 29, 1941–1945. [Google Scholar] [CrossRef]
- Safa, M.; Mokhtarani, B.; Mortaheb, H.R.; Tabar Heidar, K.; Sharifi, A.; Mirzaei, M. Oxidative desulfurization of diesel fuel using a Brønsted acidic ionic liquid supported on silica gel. Energy Fuels 2017, 31, 10196–10205. [Google Scholar] [CrossRef]
- Samokhvalov, A.; Tatarchuk, B.J. Review of experimental characterization of active sites and determination of molecular mechanisms of adsorption, desorption and regeneration of the deep and ultradeep desulfurization sorbents for liquid fuels. Catal. Rev. 2010, 52, 381–410. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Yang, R.T. Desulfurization of transportation fuels by adsorption. Catal. Rev. 2004, 46, 111–150. [Google Scholar] [CrossRef]
- Yang, R.T.; Hernández-Maldonado, A.J.; Yang, F.H. Desulfurization of transportation fuels with zeolites under ambient conditions. Science 2003, 301, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Palomino, J.M.; Oliver, S.R. Desulfurization of JP-8 jet fuel: Challenges and adsorptive materials. RSC Adv. 2018, 8, 7301–7314. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.T.; Heinzel, J.M. Desulfurization of jet fuel by π-complexation adsorption with metal halides supported on MCM-41 and SBA-15 mesoporous materials. Chem. Eng. Sci. 2008, 63, 356–365. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.T.; Heinzel, J.M. Desulfurization of jet fuel JP-5 light fraction by MCM-41 and SBA-15 supported cuprous oxide for fuel cell applications. Ind. Eng. Chem. Res. 2009, 48, 142–147. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Yang, F.H.; Yang, R.T. Desulfurization of high-sulfur jet fuel by mesoporous π-complexation adsorbents. Chem. Eng. Sci. 2009, 64, 5240–5246. [Google Scholar] [CrossRef]
- Palomino, J.M.; Tran, D.T.; Hauser, J.L.; Dong, H.; Oliver, S.R. Mesoporous silica nanoparticles for high capacity adsorptive desulfurization. J. Mater. Chem. A 2014, 2, 14890–14895. [Google Scholar] [CrossRef]
- Gonzalez, J.; Wang, J.; Chen, L.; Manriquez, M.; Dominguez, J. Structural defects, Lewis acidity, and catalysis properties of mesostructured WO3/SBA-15 nanocatalysts. J. Phys. Chem. C 2017, 121, 23988–23999. [Google Scholar] [CrossRef]
- González, J.; Wang, J.A.; Chen, L.; Manríquez, M.; Salmones, J.; Limas, R.; Arellano, U. Quantitative determination of oxygen defects, surface lewis acidity, and catalytic properties of mesoporous MoO3/SBA-15 catalysts. J. Solid State Chem. 2018, 263, 100–114. [Google Scholar] [CrossRef]




| Catalyst | Sulfur Species | Conversion % (time, min) | qm (mg-S per g-Cat) | Ref. |
|---|---|---|---|---|
| ZnO-Mesoporous TiO2-SiO2 | DBT | 97% | 46.1 | [78] |
| Mesoporous TiO2-SiO2-40 | DBT | 99% | 13.7 | [81] |
| Mo/10SiO2-550 | 4,6-DMDBT | 100% (40 m) | - | [91] |
| WO3/SBA-15 | 4,6-DMDBT | 100% (60 m) | - | [170] |
| 15%MoO3/SBA-15 | 4,6-DMDBT | 100% (60 m) | - | [171] |
| PW11@aptes-SBA-15 | 1-BT, DBT, 4-MDBT, 4,6-DMDBT | 100% (60 m) | - | [100] |
| PW11@TMA-SBA-15 | DBT | 100% (30 m) | - | [101] |
| PW11@TMA-SBA-15 | 4-MDBT | 100% (30 m) | - | [101] |
| PW11@TMA-SBA-15 | 4,6-DMDBT | 100% (30 m) | - | [101] |
| PW11@TMA-SBA-15 | 1-BT | 93.9% (30 m) | - | [101] |
| PW11@TMA-PMOE | DBT | 98.2% (60 m) | - | [101] |
| PW11@TMA-PMOE | 4-MDBT | 99.0% (60 m) | - | [101] |
| PW11@TMA-PMOE | 4,6-DMDBT | 99.3% (60 m) | - | [101] |
| PW11@TMA-PMOE | 1-BT | 92.8% (60 m) | - | [101] |
| 20%HPW/Zr-HMS | DBT | 95.0% (30 m) | - | [59] |
| Mesoporous W-SiO2 | BT, DBT, 4,6-MDBT | 100% (60 m) | - | [24] |
| subnano-MoO3/UMSN | DBT | 100% (15 m) | - | [106] |
| 10% Mo/mSiO2 nanowire | DBT | 100% (30 m) | - | [107] |
| W-mSiO2-450 | DBT | 99.6% (30 m) | - | [124] |
| [C4mim]3PW12O40@OMS | DBT | 99.5% (60 m) | - | [125] |
| LaW10/IL-SiO2 | DBT | 100% (1 m, small batch)100% (25 m, large scale) | - | [128] |
| IL-3DOM SiO2 | BT, DBT, 3-MBT, 4-MDBT, 4,6-DMDBT | 100% (60 m) | - | [58] |
| PMo12@TBA-MSN | BT, DBT, 3-MBT, 4-MDBT, 4,6-DMDBT | 98% (70 m) | - | [133] |
| PMo12@TMA-MSN | 92% (70 m) | - | [133] | |
| 0.05HPMo-IL/SBA-15 | DBT | 90% (90 m) | - | [136] |
| 0.05HPMo-IL/SBA-15 | BT | 71% (90 m) | - | [136] |
| 0.05HPMo-IL/SBA-15 | 4,6-DMDBT | 69.8% (90 m) | - | [136] |
| Fe3O4@mSiO2@DT-MIP | DBT | - | 104.2 | [141] |
| Fe3O4@mSiO2@DT-MIP | 4-MDBT | - | 113.6 | [141] |
| Fe3O4@SiO2-IL/RS--MMS | 4,6-DMDBT | 98.3% (50 m) | - | [143] |
| Fe3O4@SiO2-IL/RS--MMS | 4-MDBT | 99.5% (50 m) | - | [143] |
| Fe3O4@SiO2-IL/RS--MMS | DBT | 99.7% (50 m) | - | [143] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendiratta, S.; Ali, A.A.A. Recent Advances in Functionalized Mesoporous Silica Frameworks for Efficient Desulfurization of Fuels. Nanomaterials 2020, 10, 1116. https://doi.org/10.3390/nano10061116
Mendiratta S, Ali AAA. Recent Advances in Functionalized Mesoporous Silica Frameworks for Efficient Desulfurization of Fuels. Nanomaterials. 2020; 10(6):1116. https://doi.org/10.3390/nano10061116
Chicago/Turabian StyleMendiratta, Shruti, and Ahmed Atef Ahmed Ali. 2020. "Recent Advances in Functionalized Mesoporous Silica Frameworks for Efficient Desulfurization of Fuels" Nanomaterials 10, no. 6: 1116. https://doi.org/10.3390/nano10061116
APA StyleMendiratta, S., & Ali, A. A. A. (2020). Recent Advances in Functionalized Mesoporous Silica Frameworks for Efficient Desulfurization of Fuels. Nanomaterials, 10(6), 1116. https://doi.org/10.3390/nano10061116





