Deriving Optimized PID Parameters of Nano-Ag Colloid Prepared by Electrical Spark Discharge Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Logic Circuit Design
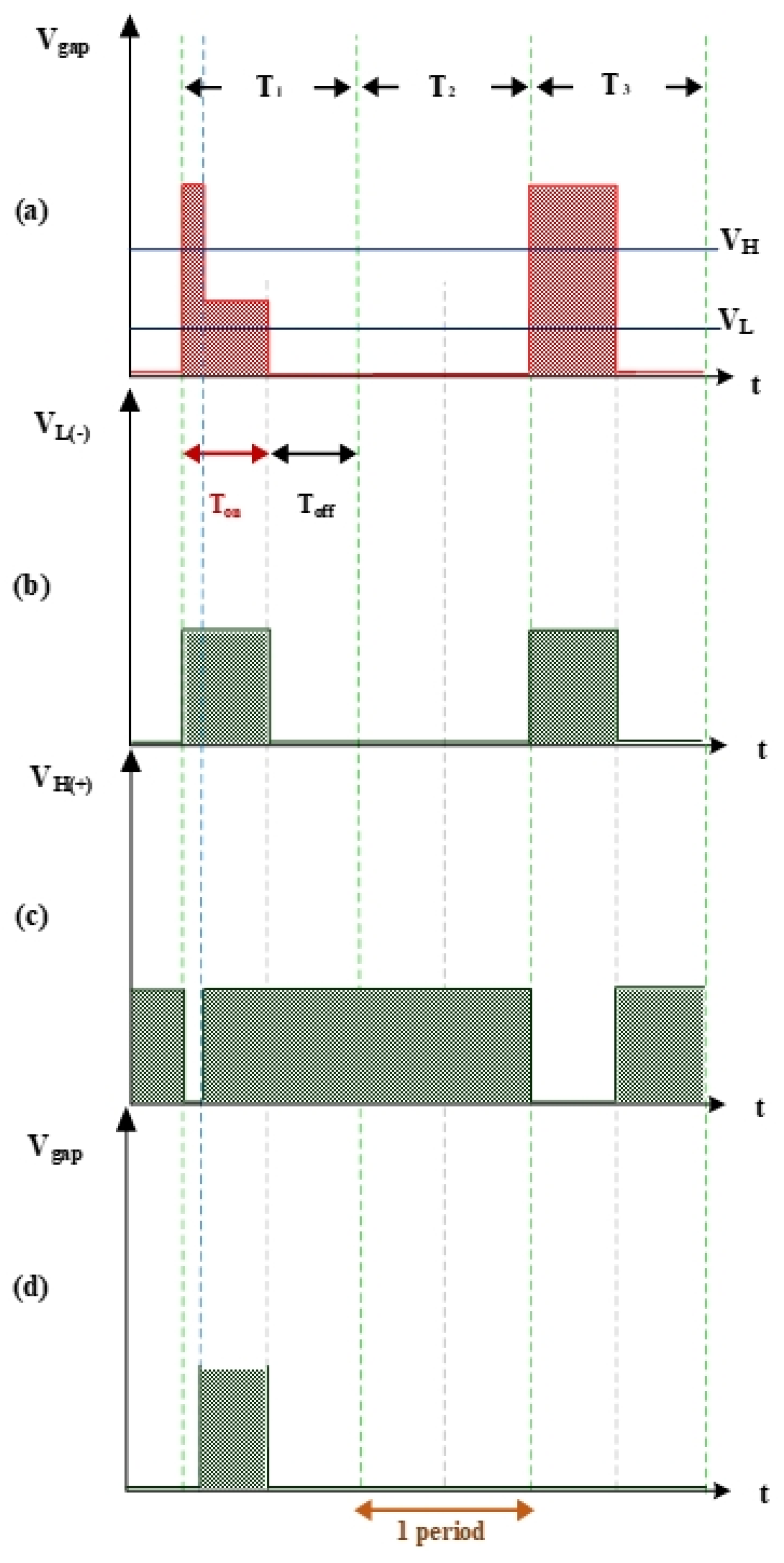
2.1.1. Vgap High/Low Levels and Igap Simulation
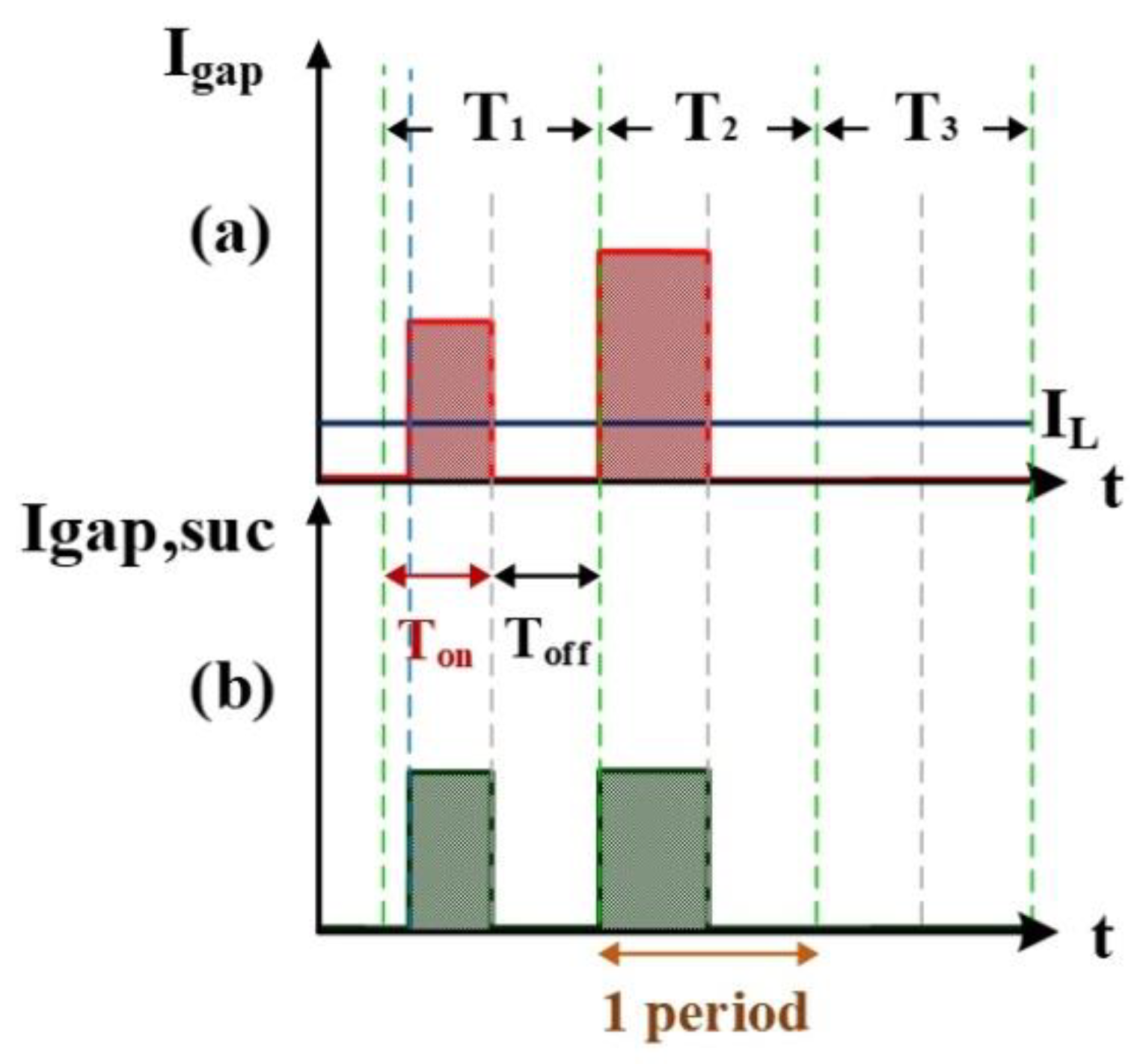
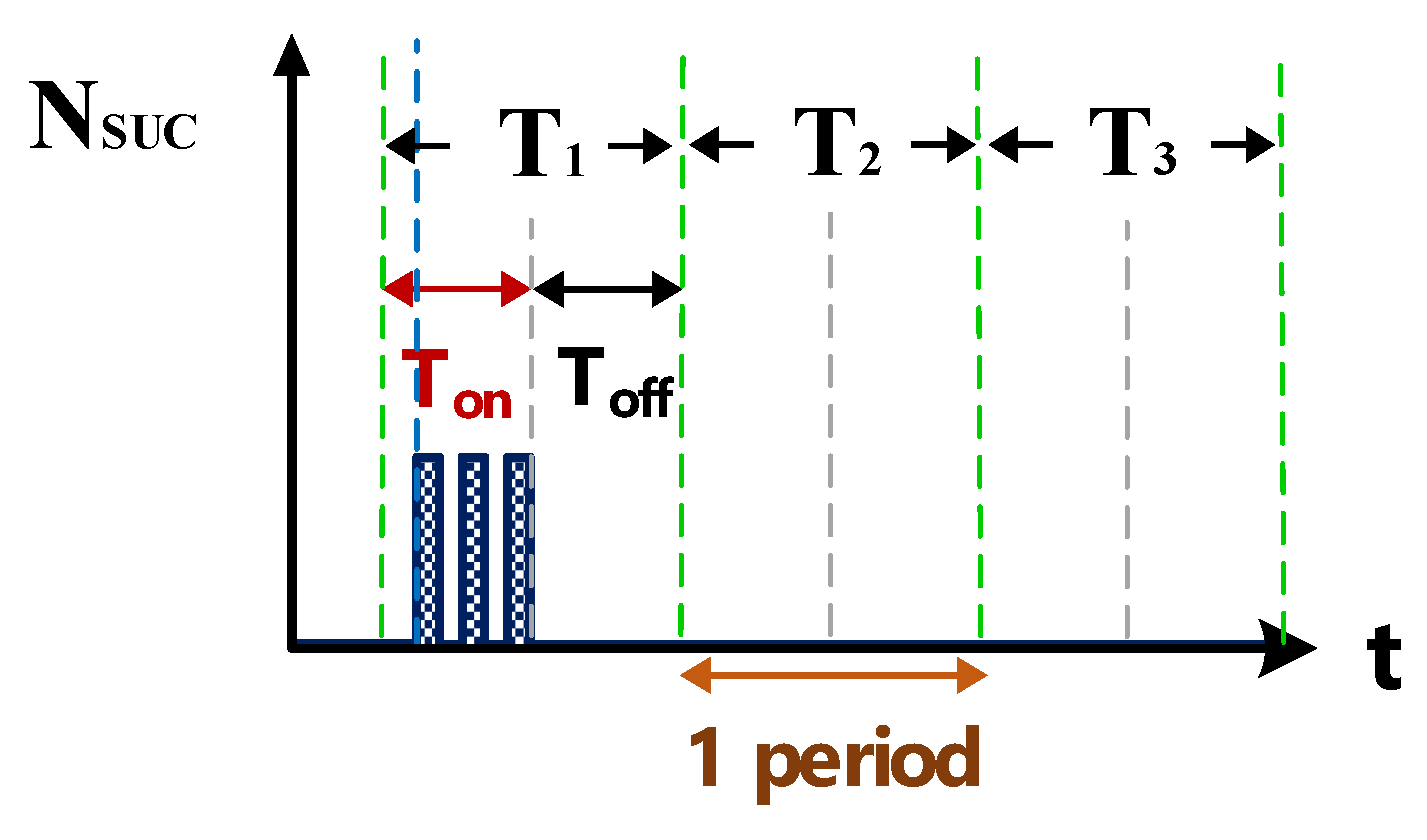
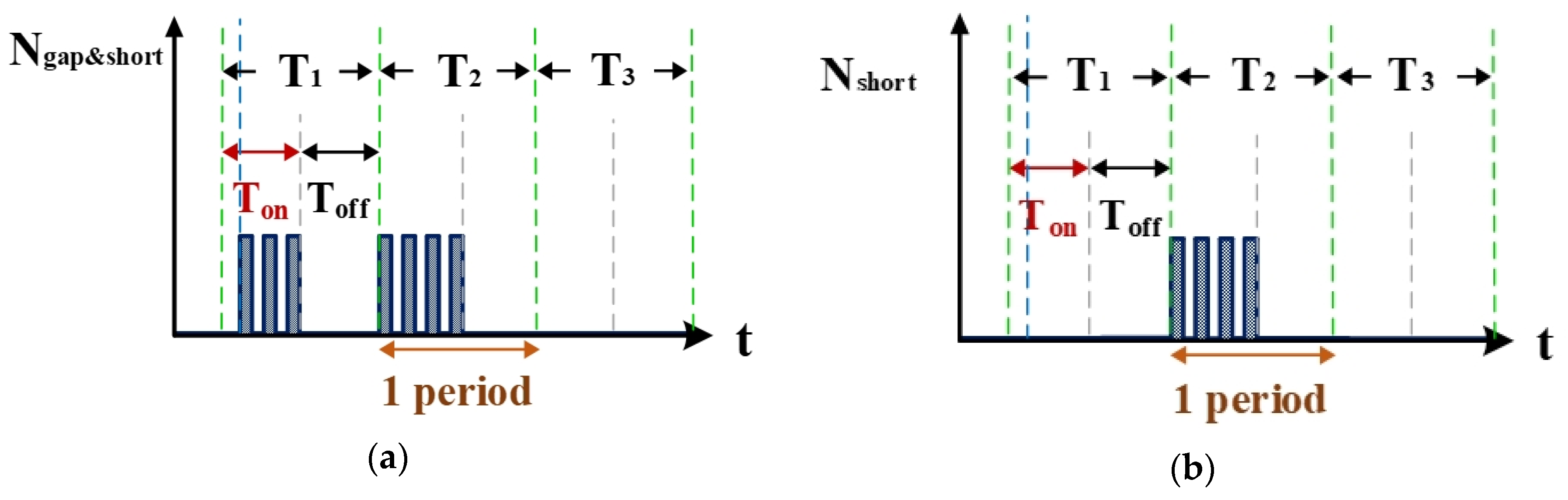
2.1.2. Computation Simulation of Electrical Discharge Success Rate
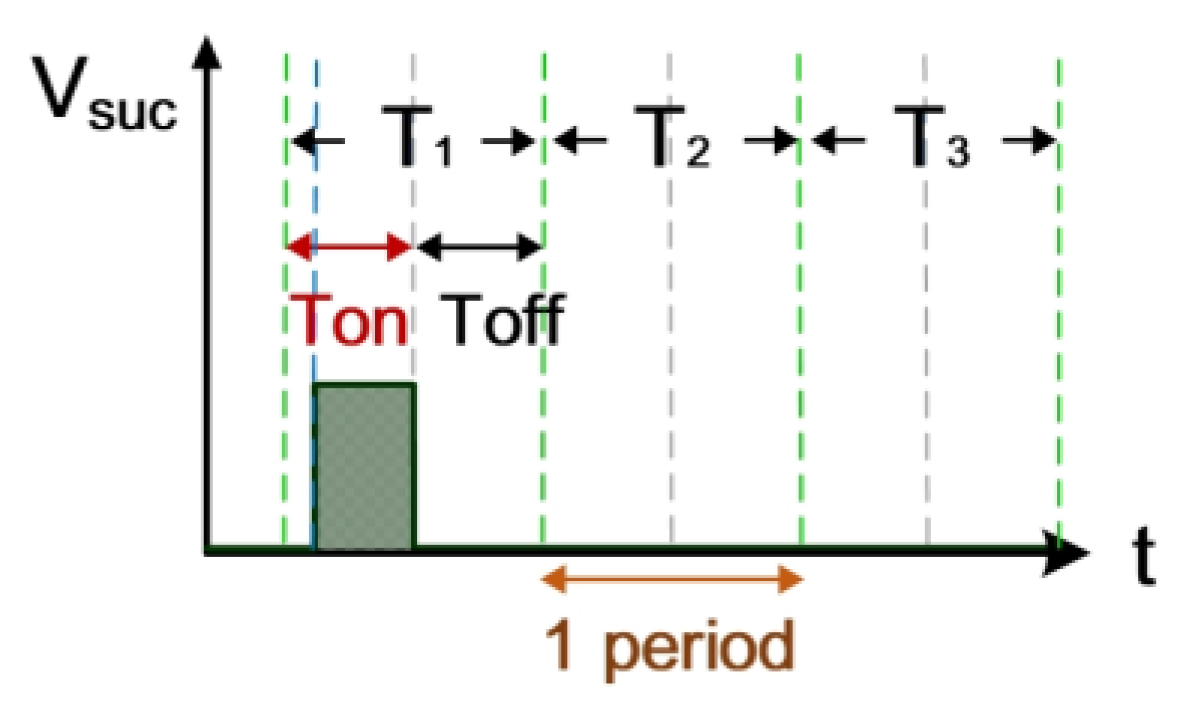
2.1.3. Computation Simulation of Short-Circuit Rate
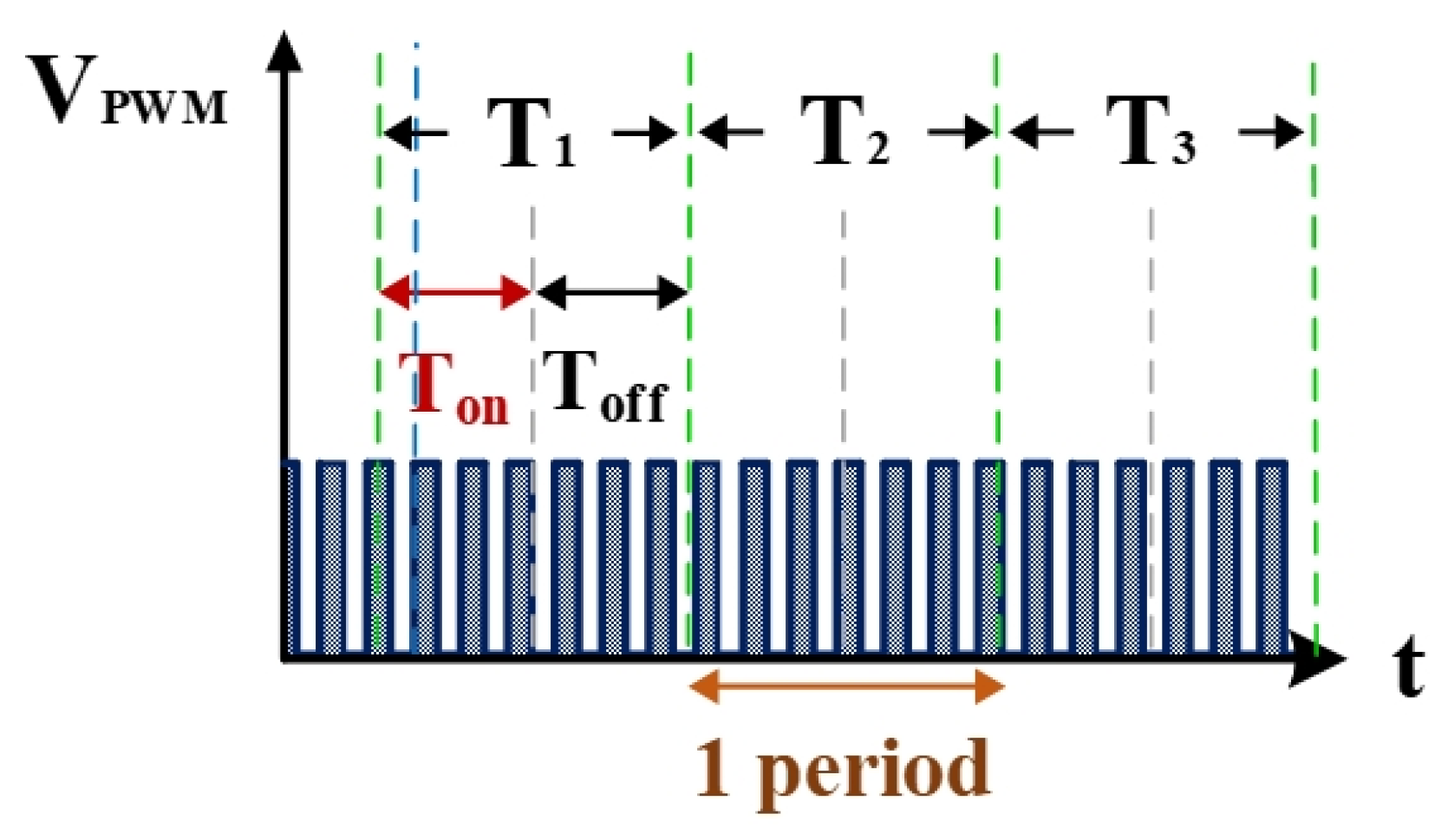
2.2. Electrical Discharge Process Optimization
3. Results
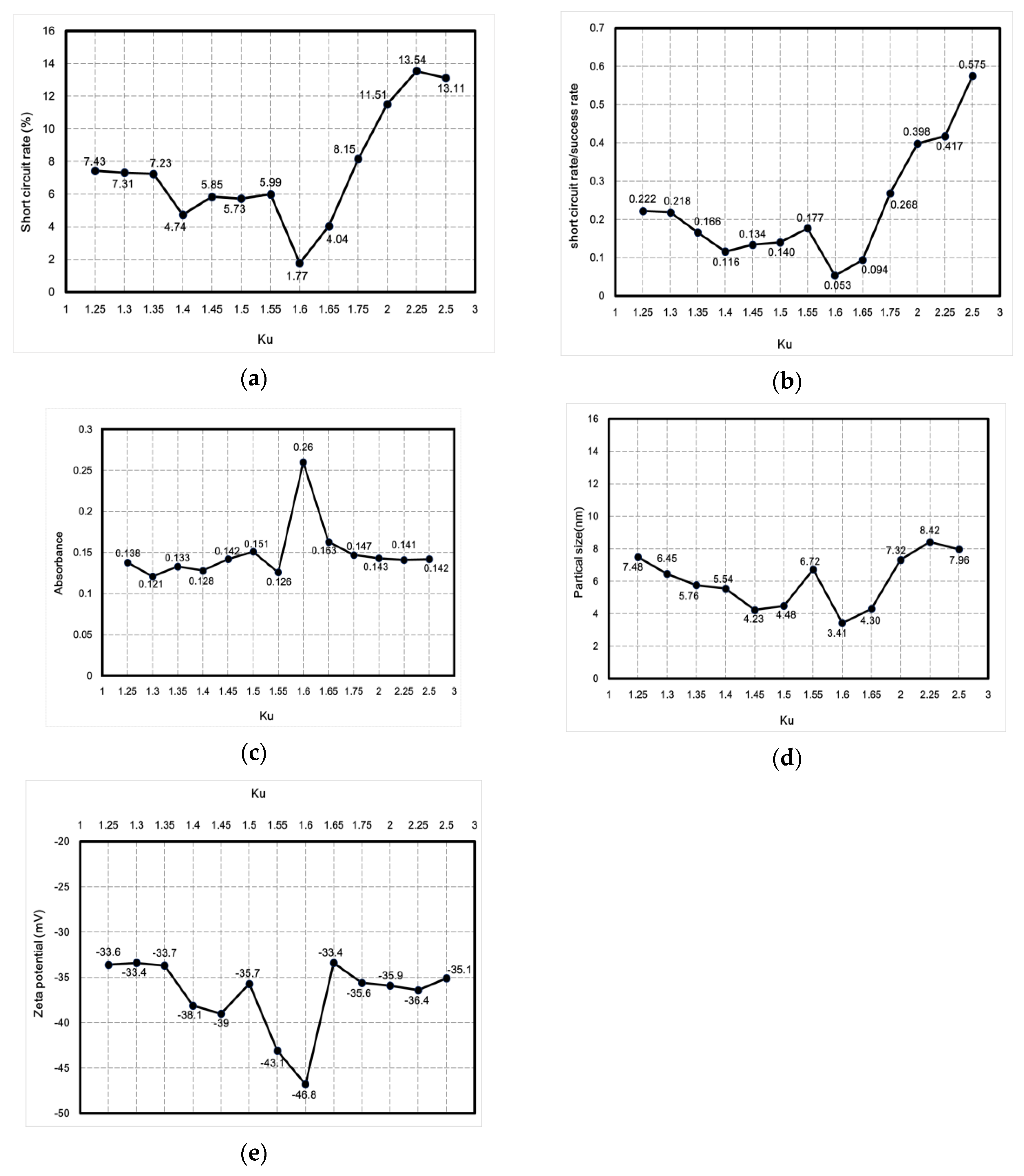
3.1. PID Fine-Tuning Optimization
3.2. Nano-Ag Colloid Characteristics Analysis
3.2.1. UV-Vis and Zeta Potential Analysis
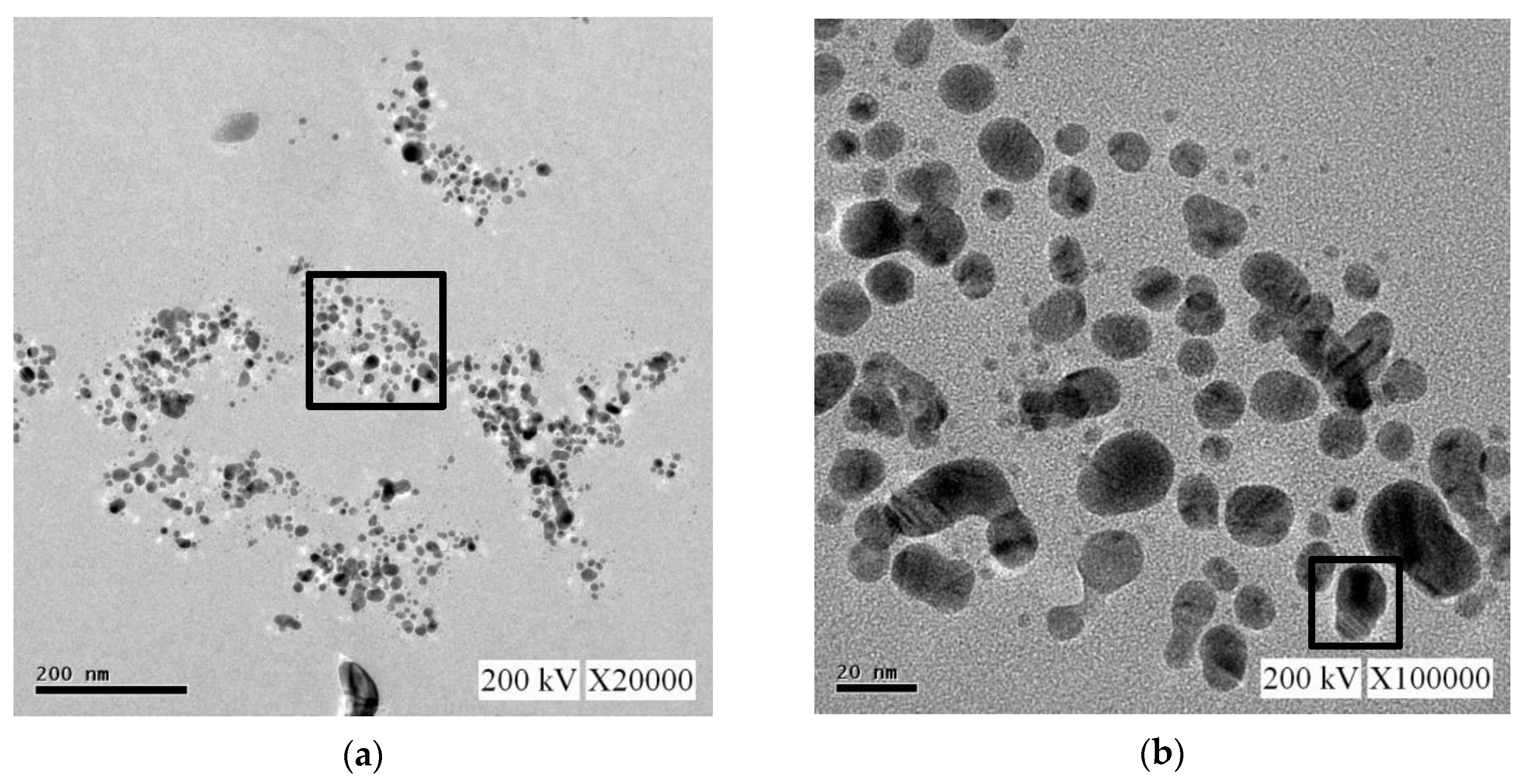
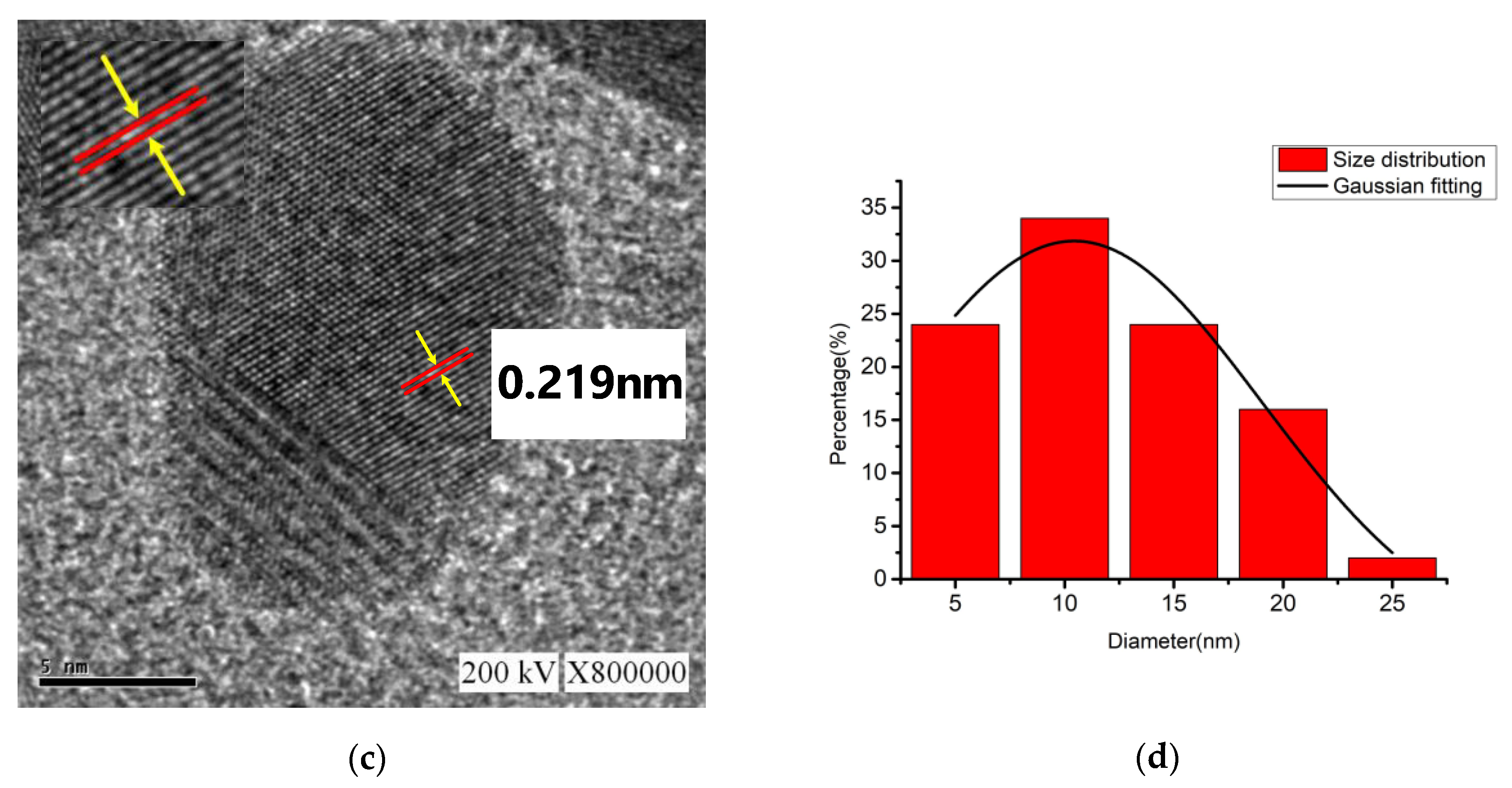
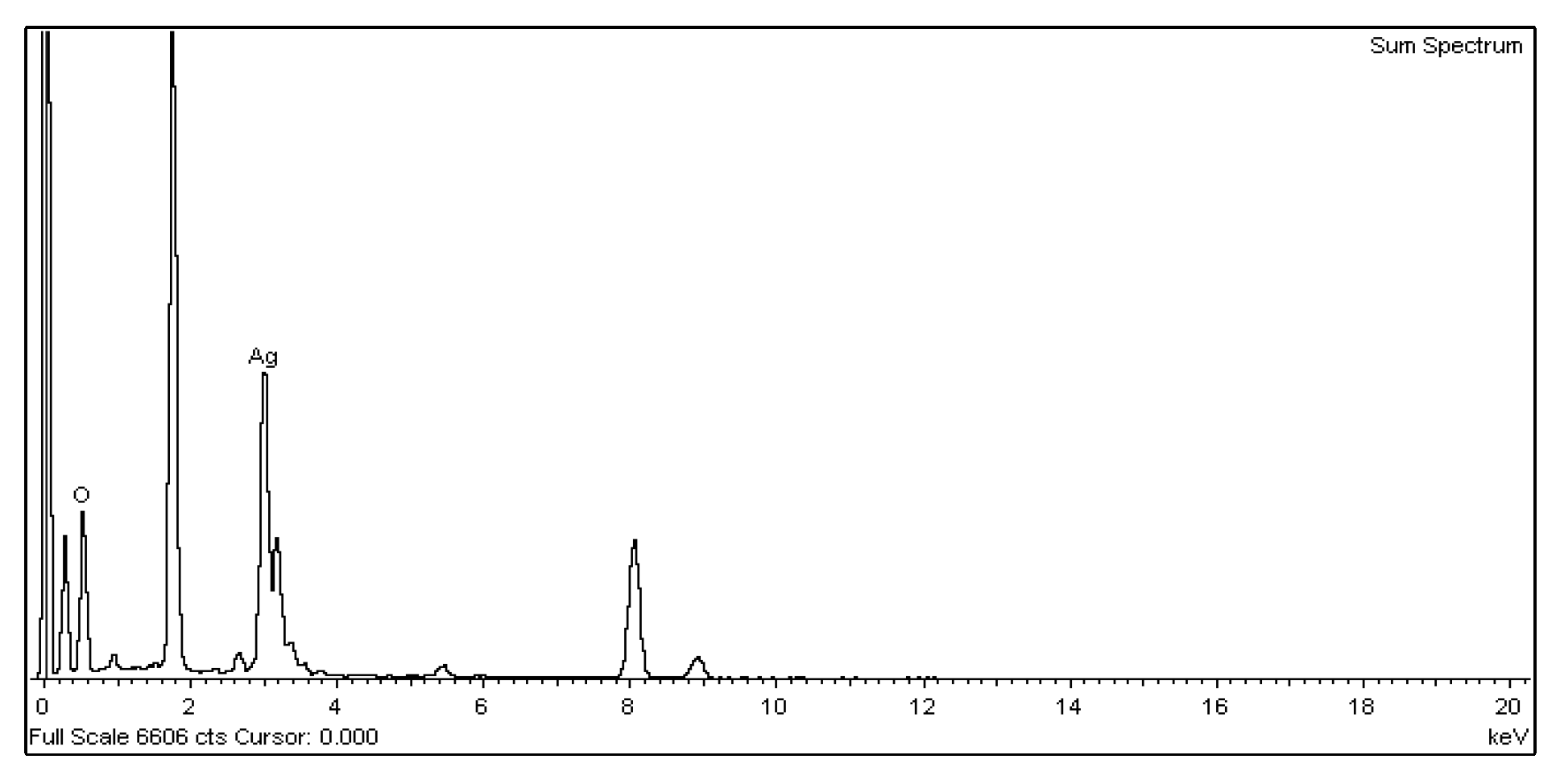
3.2.2. Transmission Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
4. Conclusions
- In this study, a nano-Ag colloid was prepared using the electric spark discharge method (ESDM) with electrode material (Ag with 99.9% purity) in 150 mL of deionized water. The diameter of the anode electrode was 1 mm, and the diameter of the cathode electrode was 2 mm. With the electrical discharge voltage = 100 V, duty cycle (Ton-Toff) = 10-10 μs, and a process time of 120 s, silver nanoparticles with an absorbance of 0.26 could be prepared.
- The study used self-developed micro-EDM and ESDM to prepare a nano-Ag colloid. This method requires no additional surfactant or other chemical materials and can be used at the ambient temperature and pressure. Moreover, this novel physical method for preparing nano-Ag colloids results in no chemical pollution.
- When using ESDM to prepare a nano-Ag colloid, the electrical discharge conditions were observed to comprise gap electrical discharge, short circuits, and open circuits. The short-circuit phenomenon was explored in depth in this study, and a self-developed logic judgment circuit set was applied to identify short circuits. Signals were then sent to computer software for computation of the rate. Short circuits may have exerted an adverse effect on the equipment.
- This study showed that PID parameters such that Kp was 0.96, Ki was 5.760576, and Kd was 0.039996 (and, as a result, Ku was 1.6) produced optimal values for absorbance (0.26), surface plasmon resonance (390 nm), zeta potential (−46.8 mV), particle size (3.41 nm), short-circuit rate (1.77%), and the ratio between the short-circuit rate and the discharge success rate (0.053). Therefore, this study demonstrated that the lower the short-circuit rate is, the more the nanocharacteristics are optimized.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roll, R. A Possible Explanation of the Small Firm Effect. J. Financ. 1981, 36, 879–888. [Google Scholar] [CrossRef]
- Bødker, F.; Mørup, S.; Linderoth, S. Surface effects in metallic iron nanoparticles. Phys. Rev. Lett. 1994, 72, 282. [Google Scholar] [CrossRef] [PubMed]
- Chamarro, M.; Gourdon, C.; Lavallard, P.; Lublinskaya, O.; Ekimov, A.I. Enhancement of electron-hole exchange interaction in CdSe nanocrystals: A quantum confinement effect. Phys. Rev. B 1996, 53, 1336. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.C. Synthesis of nanostructured materials by mechanical milling: Problems and opportunities. Nanostruct. Mater. 1997, 9, 13–22. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Kumar, R.R.; Selvan, V.T.; Sathyanarayanan, M.; Rao, K.N. Growth of ZnSe nano and microstructures at high vacuum by thermal evaporation. Appl. Nanosci. 2014, 4, 469–475. [Google Scholar] [CrossRef][Green Version]
- Lo, C.H.; Tsung, T.T.; Lin, H.M. Preparation of silver nanofluid by the submerged arc nanoparticle synthesis system (SANSS). J. Alloys Compd. 2007, 434, 659–662. [Google Scholar] [CrossRef]
- Rajurkar, K.P.; Levy, G.; Malshe, A.; Sundaram, M.M.; McGeough, J.; Hu, X.; DeSilva, A. Micro and nano machining by electro-physical and chemical processes. CIRP Ann. 2006, 55, 643–666. [Google Scholar] [CrossRef]
- Tseng, K.H.; Chen, Y.C.; Shyue, J.J. Continuous Synthesis of Colloidal Silver Nanoparticles by Electrochemical Discharge in Aqueous Solutions. J. Nanopart. Res. 2011, 13, 1865–1872. [Google Scholar] [CrossRef]
- Yeo, S.H.; Tan, P.C.; Kurnia, W. Effects of powder additives suspended in dielectric on crater characteristics for micro electrical discharge machining. J. Micromech. Microeng. 2007, 17, N91. [Google Scholar] [CrossRef]
- Lo, C.H.; Tsung, T.T.; Chen, L.C. Shape-controlled synthesis of Cu-based nanofluid using submerged arc nanoparticle synthesis system (SANSS). J. Cryst. Growth 2005, 277, 636–642. [Google Scholar] [CrossRef]
- Lung, J.K.; Huang, J.C.; Tien, D.C.; Liao, C.Y.; Tseng, K.H.; Tsung, T.T. Preparation of gold nanoparticles by arc discharge in water. J. Alloys Compd. 2007, 434–435, 655–658. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Chang, C.-Y.; Chung, M.-Y.; Cheng, T.-S. Fabricating TiO2 Nanocolloids by Electric Spark Discharge Method at Normal Temperature and Pressure. Nanotechnology 2017, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-Y.; Tseng, K.-H.; Lin, H.-S. Preparation of Metallic Aluminum Compound Particles by Submerged Arc Discharge Method in Aqueous Media. Metall. Mater. Trans. B 2013, 44, 91–97. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Ku, H.-C.; Tien, D.-C.; Stobinski, L. Parameter control and concentration analysis of graphene colloids prepared by electric spark discharge method. Nanotechnol. Rev. 2019, 8, 201–209. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Chung, M.-Y.; Chang, C.-Y. Parameters for Fabricating Nano-Au Colloids through the Electric Spark Discharge Method with Micro-Electrical Discharge Machining. Nanomaterials 2017, 7, 133. [Google Scholar] [CrossRef]
- Song, K.C.; Lee, S.M.; Park, T.S.; Lee, B.S. Preparation of colloidal silver nanoparticles by chemical reduction method. Korean J. Chem. Eng. 2009, 26, 153–155. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Browning, N.D.; Chisholm, M.F.; Pennycook, S.J. Atomic-resolution chemical analysis using a scanning transmission electron microscope. Nature 1993, 366, 143–146. [Google Scholar] [CrossRef]
- Khanna, P.K.; Singh, N.; Kulkarni, D.; Deshmukh, S.; Charan, S.; Adhyapak, P.V. Water based simple synthesis of re-dispersible silver nano-particles. Mater. Lett. 2007, 61, 3366–3370. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Chang, C.-Y.; Chen, M.-J.; Tseng, Y.-K. Novel electrical discharge machining system with real-time control and monitoring for preparing nanoiron Colloid. Adv. Mech. Eng. 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Kao, Y.-S.; Chang, C.-Y. Development and Implementation of a Micro-electric Discharge Machine: Real-Time Monitoring System of Fabrication of Nanosilver Colloid. J. Clust. Sci. 2016, 27, 763–773. [Google Scholar] [CrossRef]
- Foley, M.W.; Julien, R.H.; Copeland, B.R. A comparison of PID controller tuning methods. Can. J. Chem. Eng. 2005, 83, 712–722. [Google Scholar] [CrossRef]
- Ang, K.H.; Chong, G.; Li, Y. PID control system analysis, design, and technology. IEEE Trans. Control Syst. Technol. 2005, 13, 559–576. [Google Scholar]
- Darwish, N.M. Design of robust PID controllers for first-order plus time delay systems based on frequency domain specifications. J. Eng. Sci. Assiut Univ. Fac. Eng. 2015, 43, 472–489. [Google Scholar]




| Kp | Ti | Td | |
|---|---|---|---|
| Ziegler–Nichols method | 0.6 × Ku | Tu/2 | Tu/8 |
| Ku = critical gain, Tu = period of oscillation, Ki = Kp/Ti, Kd = Kp × Td | |||
| Title | Parameters | Title | Parameters |
|---|---|---|---|
| Ambient temperature | 25 °C | Atmospheric pressure | 1 atm |
| Electrical Discharge voltage | 100 V | Electrical discharge current | 4 A |
| Duty cycle | Ton-Toff 10-10 (μs) | Electrode material (purity) | Ag (99.99%) |
| Process time | 120 s | Dielectric fluid | Deionized water |
| Electrode diameter | Anode 1 mm Cathode 2 mm | Preparation capacity | 150 mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, K.-H.; Lin, Y.-S.; Lin, Y.-C.; Tien, D.-C.; Stobinski, L. Deriving Optimized PID Parameters of Nano-Ag Colloid Prepared by Electrical Spark Discharge Method. Nanomaterials 2020, 10, 1091. https://doi.org/10.3390/nano10061091
Tseng K-H, Lin Y-S, Lin Y-C, Tien D-C, Stobinski L. Deriving Optimized PID Parameters of Nano-Ag Colloid Prepared by Electrical Spark Discharge Method. Nanomaterials. 2020; 10(6):1091. https://doi.org/10.3390/nano10061091
Chicago/Turabian StyleTseng, Kuo-Hsiung, Yur-Shan Lin, Yun-Chung Lin, Der-Chi Tien, and Leszek Stobinski. 2020. "Deriving Optimized PID Parameters of Nano-Ag Colloid Prepared by Electrical Spark Discharge Method" Nanomaterials 10, no. 6: 1091. https://doi.org/10.3390/nano10061091
APA StyleTseng, K.-H., Lin, Y.-S., Lin, Y.-C., Tien, D.-C., & Stobinski, L. (2020). Deriving Optimized PID Parameters of Nano-Ag Colloid Prepared by Electrical Spark Discharge Method. Nanomaterials, 10(6), 1091. https://doi.org/10.3390/nano10061091





