Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Milk-Derived Exosomes
2.2. Protein Content
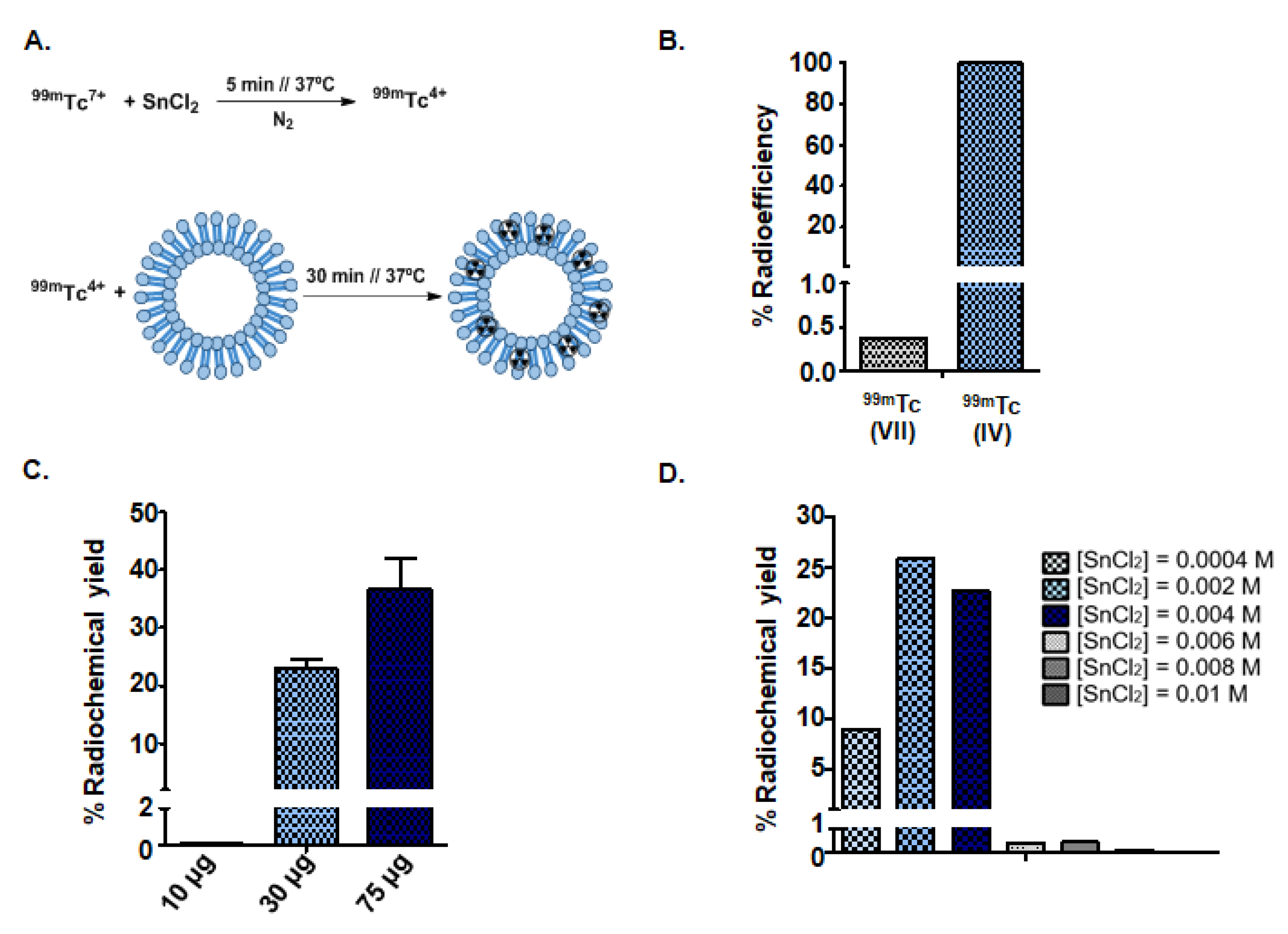
2.3. Radiolabeling of Goat Milk Exosomes with Reduced Technetium (99mTc (IV))
2.4. Radiolabeling of Milk Exosomes with Commercial Pertechnetate (99mTc (VII))
2.5. High-Performance Liquid Chromatography (HPLC)
2.6. Transmission Electron Microscopy (TEM)
2.7. Dynamic Light Scattering (DLS) Analysis
2.8. In Vitro Stability Studies
2.9. Ethics Statement
2.10. Blood Half-Life
2.11. In Vivo SPECT/CT Imaging
2.12. Ex Vivo Biodistribution Studies
2.13. Autoradiography
2.14. Data Analysis
3. Results
3.1. Protein Content
3.2. Synthesis of Radioactive Exosomes ([99mTc]-Exo) and Optimization
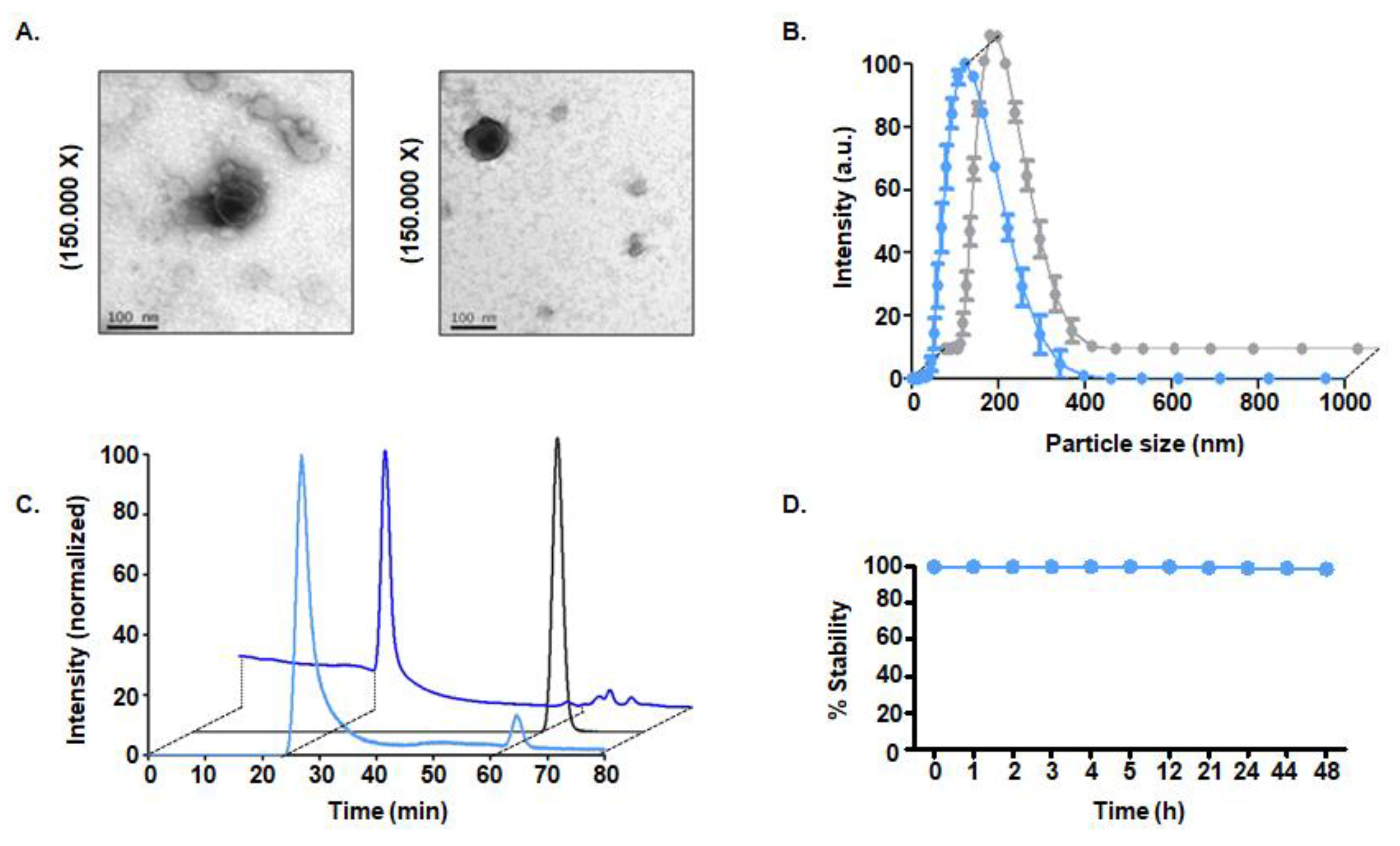
3.3. Physicochemical Characterization
3.3.1. Size and Morphology
3.3.2. Radiopurity of [99mTc]-Exo Tracer
3.3.3. In Vitro Stability Studies
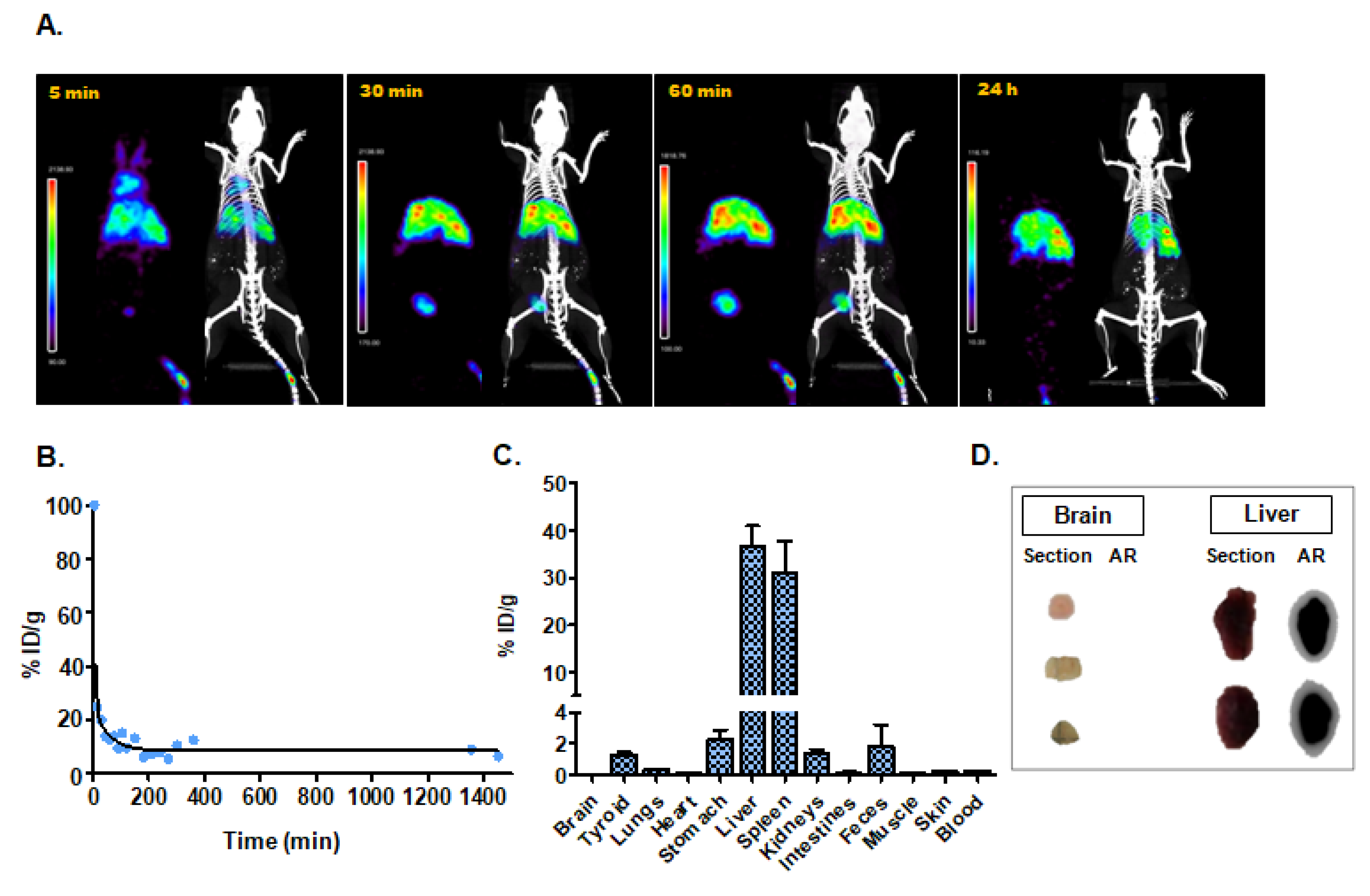
3.4. Pharmacokinetic Study and In Vivo Tracking
3.4.1. Intravenous Administration
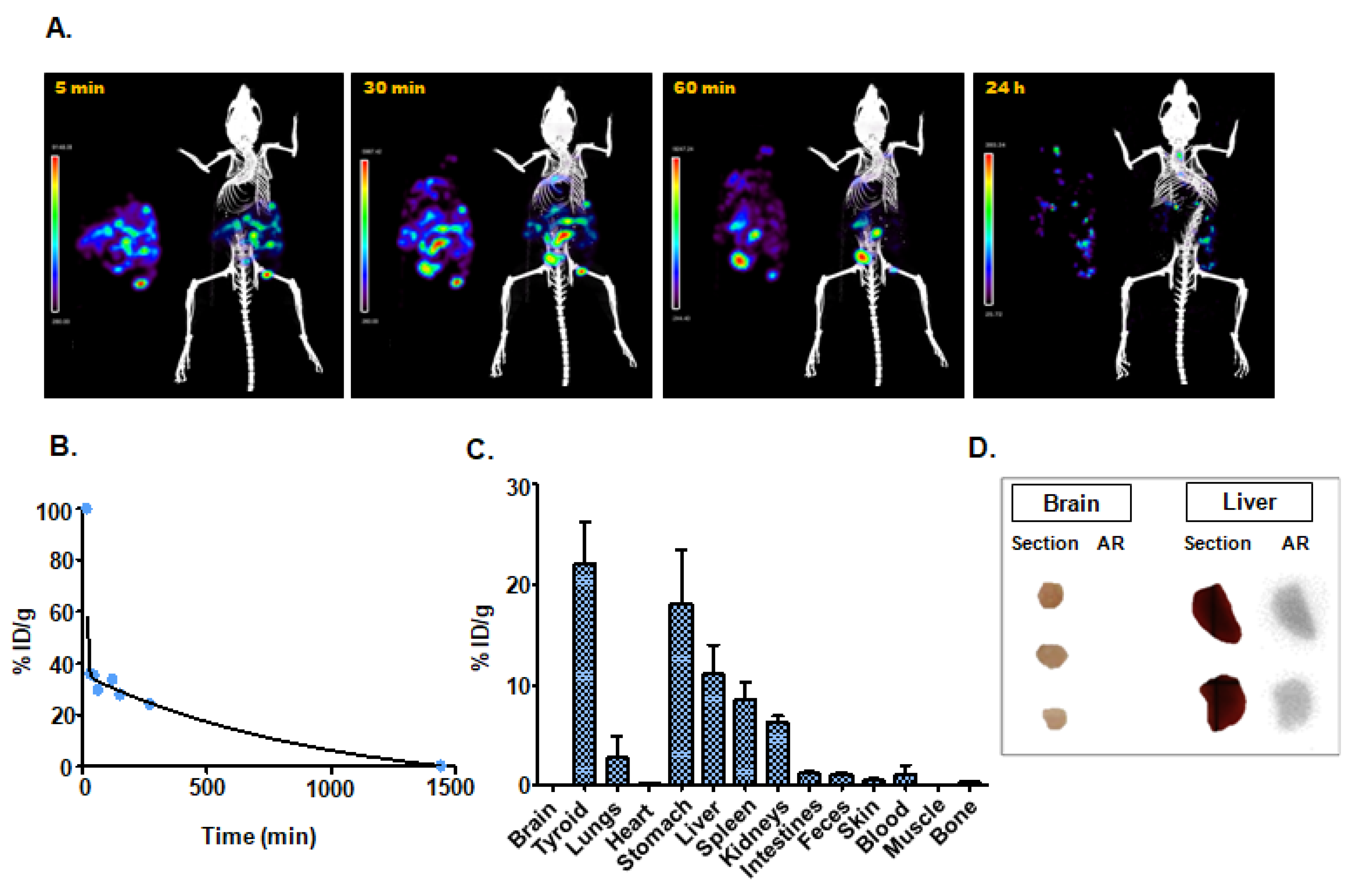
3.4.2. Intraperitoneal Administration
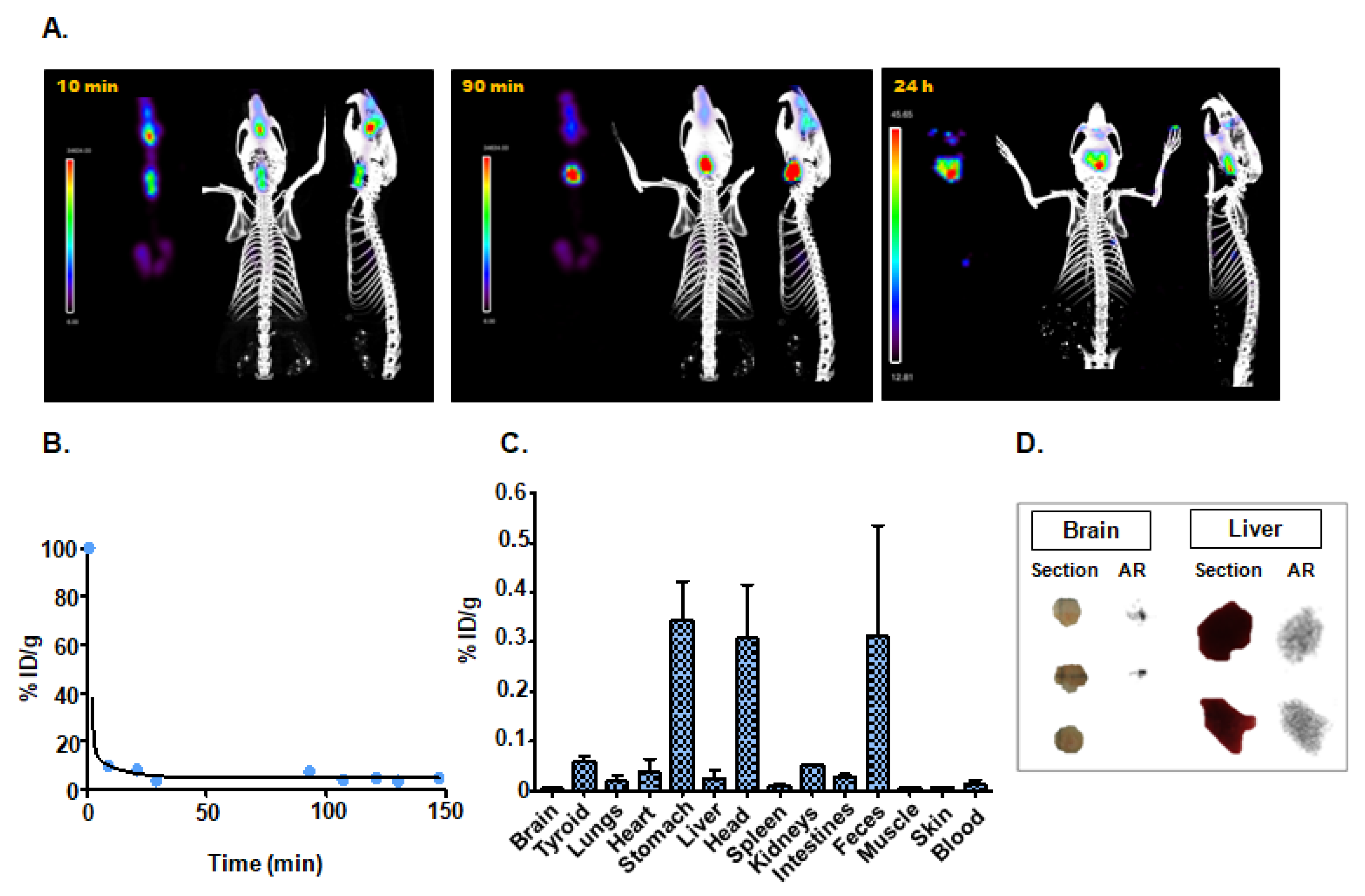
3.4.3. Intranasal Instillation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncology 2010, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; De Hollanda, L.M.; Santini, A.; Reis, L.V.; Souto, S.B.; Souto, E.B.; Silva, A.M. Advances in nanobiomaterials for oncology nanomedicine. In Nanobiomaterials in Cancer Therapy; William Andrew Publishing: New York, NY, USA, 2016; pp. 91–115. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, E. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular vesicles: Unique intercellular delivery vehicles. Trends. Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef] [PubMed]
- Andaloussi, S.E.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Van der Meel, R.; Fens, M.H.; Vader, P.; Van Solinge, W.W.; Eniola-Adefeso, O.; Schiffelers, R.M. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J. Control. Release 2014, 195, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Perez, J.R.; Ybarra, N.; Chagnon, F.; Serban, M.; Lee, S.; Seuntjens, J.; Lesur, O.; El Naqa, I. Tracking of mesenchymal stem cells with fluorescence endomicroscopy imaging in radiotherapy-induced lung injury. Sci. Rep. 2017, 7, 40748. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, S.T.; Kwon, J.; Lee, J.; Chun, Y.O.; Han, J.S.; Han, H.S. Ultrasensitive fluorescence monitoring and in vivo live imaging of circulating tumor cell-derived miRNAs using molecular beacon system. ACS Sens. 2018, 3, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Hong, C.M.; Ahn, B.C. Current perspectives on in vivo noninvasive tracking of extracellular vesicles with molecular imaging. BioMed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.A.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; DePuy, J.; Klimash, J. Strategies to minimize background autofluorescence in live mice during noninvasive fluorescence optical imaging. Lab. Anim. 2007, 36, 40. [Google Scholar] [CrossRef]
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 2005, 11, 227–256. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Van Leeuwen, F.W.B.; de Jong, M. Molecular imaging: The emerging role of optical imaging in nuclear medicine. Eur. J. Nucl Med. Mol Imaging 2014, 41, 2150–2153. [Google Scholar] [CrossRef][Green Version]
- Pascau, J.; Vaquero, J.J.; Abella, M.; Cacho, R.; Lage, E.; Desco, M. Multimodality workstation for small animal image visualization and analysis. Mol. Imaging Biol. 2006, 8, 97–98. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Owunwanne, A.; Church, L.B.; Blau, M. Effect of oxygen on the reduction of pertechnetate by stannous ion. J. Nucl. Med. 1977, 18, 822–826. [Google Scholar]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Therapy 2008, 16, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Hwang, D.W.; Choi, H.; Jang, S.C.; Yoo, M.Y.; Park, J.Y.; Choi, N.E.; Oh, H.J.; Ha, S.; Lee, Y.-S.; Jeong, J.M.; et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using 99mTc-HMPAO. Sci. Rep. 2015, 5, 15636. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Gyurkó, I.; Pálóczi, K.; Buzás, E.I.; Horváth, I.; Hegedűs, N.; Máthé, d.; Szigeti, K. Radiolabeling of Extracellular Vesicles with 99mTc for Quantitative In Vivo Imaging Studies. Cancer Biother. Radiopharm. 2016, 31, 168–173. [Google Scholar] [CrossRef]
- Seldin, D.W.; Simchon, S.; Jan, K.M.; Chien, S.; Alderson, P.O. Dependence of Technetium-99m Red Blood Cell Labeling Efficiency on Red Cell Surface Charge. J. Nucl. Med. 1988, 29, 1710–1713. [Google Scholar]
- Jensen, A.T.I.; Rasmussen, P.; Andresen, T.L. Radiolabeling of Liposomes and Polymeric Micelles with PET-Isotopes; DTU Nutech (Center for Nuclear Technologies): Roskilde, Denmark, 2012. [Google Scholar]
- Love, W.G.; Amos, N.; Williams, B.D.; Kellaway, I.W. Effect of liposome surface charge on the stability of technetium (99mTc) radiolabelled liposomes. J. Microencapsul. 1989, 6, 105–113. [Google Scholar] [CrossRef]
- Richardson, V.J.; Ryman, B.E.; Jewkes, R.F.; Jeyasingh, K.; Tattersall, M.N.; Newlands, E.S.; Kaye, S.B. Tissue distribution and tumour localization of 99m-technetium-labelled liposomes in cancer patients. Br. J. Cancer 1979, 40, 35. [Google Scholar] [CrossRef]
- Man, F.; Gawne, P.J.; de Rosales, R.T. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Deliconstantinos, G.; Ramantanis, G.; Todorou, D.K. Interaction of 99mTc-labeled liposomes with Walker tumour cells in vitro. Liposome-mediated introduction of thaliblastine into resistant Walker tumour cells. Gen. Pharmac. 1983, 14, 407–411. [Google Scholar] [CrossRef]
- Cheng, S.H.; Yu, D.; Tsai, H.M.; Morshed, R.A.; Kanojia, D.; Lo, L.W.; Leoni, L.; Govind, Y.; Zhang, L.; Aboody, K.S.; et al. Dynamic in vivo SPECT imaging of neural stem cells functionalized with radiolabeled nanoparticles for tracking of glioblastoma. J. Nucl. Med. 2016, 57, 279–284. [Google Scholar] [CrossRef]
- Rokka, J.; Snellman, A.; Kaasalainen, M.; Salonen, J.; Zona, C.; La Ferla, B.; Nicotra, F.; Re, F.; Masserini, M.; Forsback, S.; et al. 18F-labeling syntheses and preclinical evaluation of functionalized nanoliposomes for Alzheimer’s disease. Eur. J. Pharm. Sci. 2016, 88, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Pharmacokinetics of exosomes an important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. J. Pharm. Sci. 2017, 106, 2265–2269. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, N.; Babaei, M.H.; Vali, A.M.; Dadashzadeh, S. Effect of liposome size on peritoneal retention and organ distribution after intraperitoneal injection in mice. Int. J. Pharm. 2010, 383, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Campaña, C.; Gómez-Vallejo, V.; Puigivila, M.; Martín, A.; Calvo-Fernández, T.; Moya, S.E.; Ziolo, R.F.; Reese, T.; Llop, J. Biodistribution of different sized nanoparticles assessed by positron emission tomography: A general strategy for direct activation of metal oxide particles. ACS Nano 2013, 7, 3498–3505. [Google Scholar] [CrossRef]
- Ramos, C.D.; Wittmann, D.E.Z.; Etchebehere, E.C.S.D.C.; Tambascia, M.A.; Silva, C.A.M.; Camargo, E.E. Thyroid uptake and scintigraphy using 99mTc pertechnetate: Standardization in normal individuals. Sao Paulo Med. J. 2002, 120, 45–48. [Google Scholar] [CrossRef]
- Anjos, D.A.; Etchebehere, E.C.; Santos, A.O.; Lima, M.C.; Ramos, C.D.; Paula, R.B.; Camargo, E.E. Normal values of [99mTc] pertechnetate uptake and excretion fraction by major salivary glands. Nucl. Med. Commun. 2006, 27, 395–403. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
| Administration Route | T1/2 (min) | Ex Vivo Biodistribution (Main Organs; % ID/g) |
|---|---|---|
| Intravenous injection | 3.84 | Liver (36.6 ± 7.5); Spleen (31.1 ± 11.6); Stomach (2.1 ± 0.9) |
| Intraperitoneal injection | 15.97 | Thyroid (22.0 ± 7.2); Stomach (18.1 ± 9.3); Liver (11.1 ± 5.0) |
| Intranasal instillation | 0.77 | Stomach (0.34 ± 0.11); Head (0.31 ± 0.15); Feces (0.31 ± 0.39) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, M.I.; Martín-Duque, P.; Desco, M.; Salinas, B. Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging. Nanomaterials 2020, 10, 1062. https://doi.org/10.3390/nano10061062
González MI, Martín-Duque P, Desco M, Salinas B. Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging. Nanomaterials. 2020; 10(6):1062. https://doi.org/10.3390/nano10061062
Chicago/Turabian StyleGonzález, María Isabel, Pilar Martín-Duque, Manuel Desco, and Beatriz Salinas. 2020. "Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging" Nanomaterials 10, no. 6: 1062. https://doi.org/10.3390/nano10061062
APA StyleGonzález, M. I., Martín-Duque, P., Desco, M., & Salinas, B. (2020). Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging. Nanomaterials, 10(6), 1062. https://doi.org/10.3390/nano10061062






