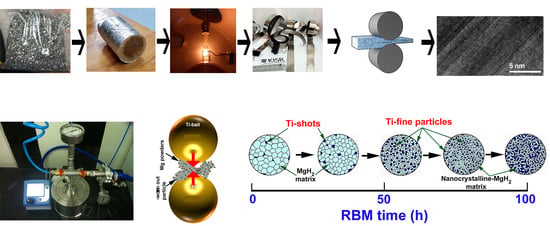
Solid-State Conversion of Magnesium Waste to Advanced Hydrogen-Storage Nanopowder Particles
Abstract
1. Introduction
1.1. Background
1.1.1. Solid-Waste Management
1.1.2. Nano-Mg as a Solid-State Hydrogen Storage Materials
1.2. Aim of the Present Study
2. Materials and Methods
2.1. The Feedstock Materials
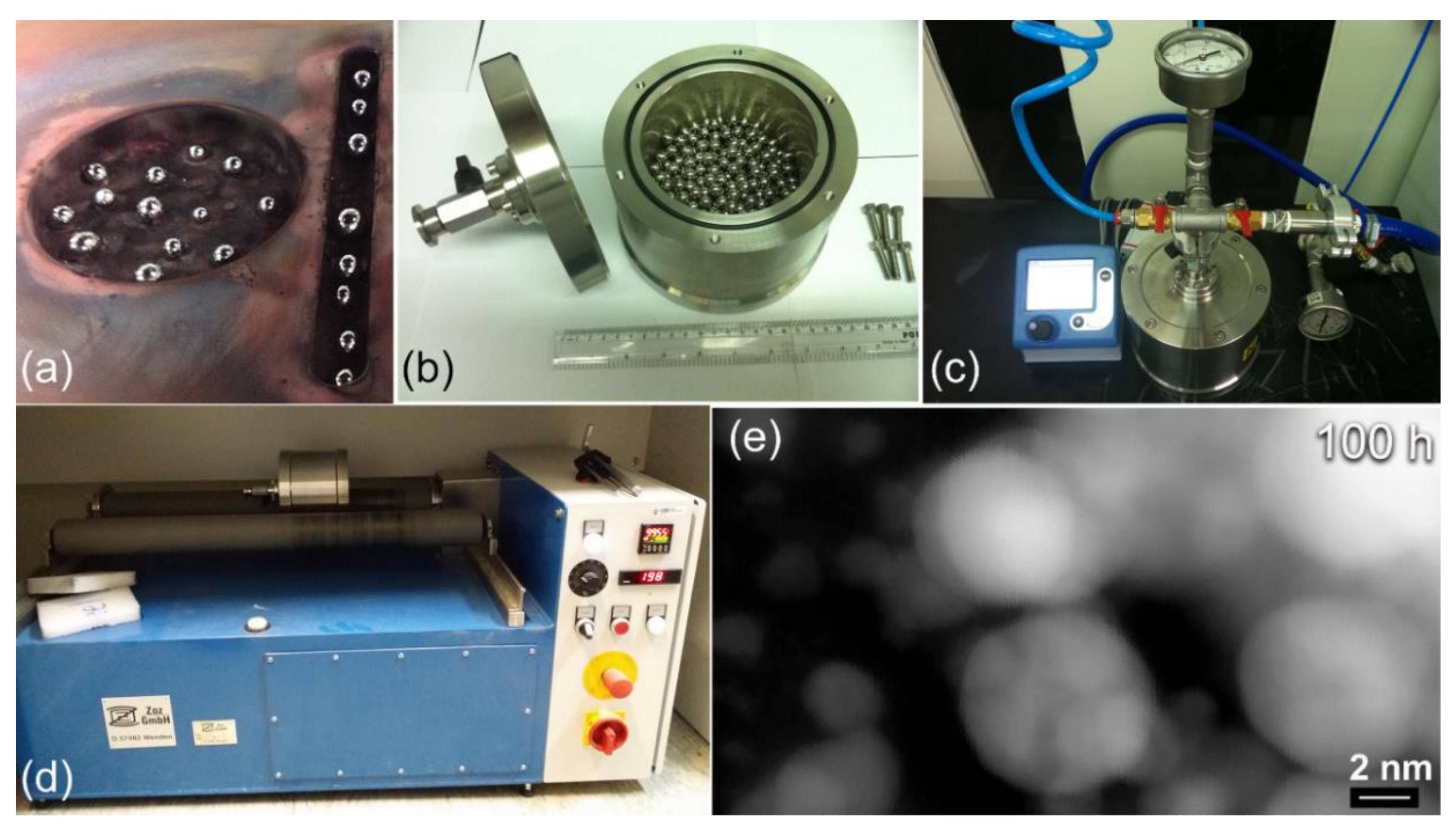
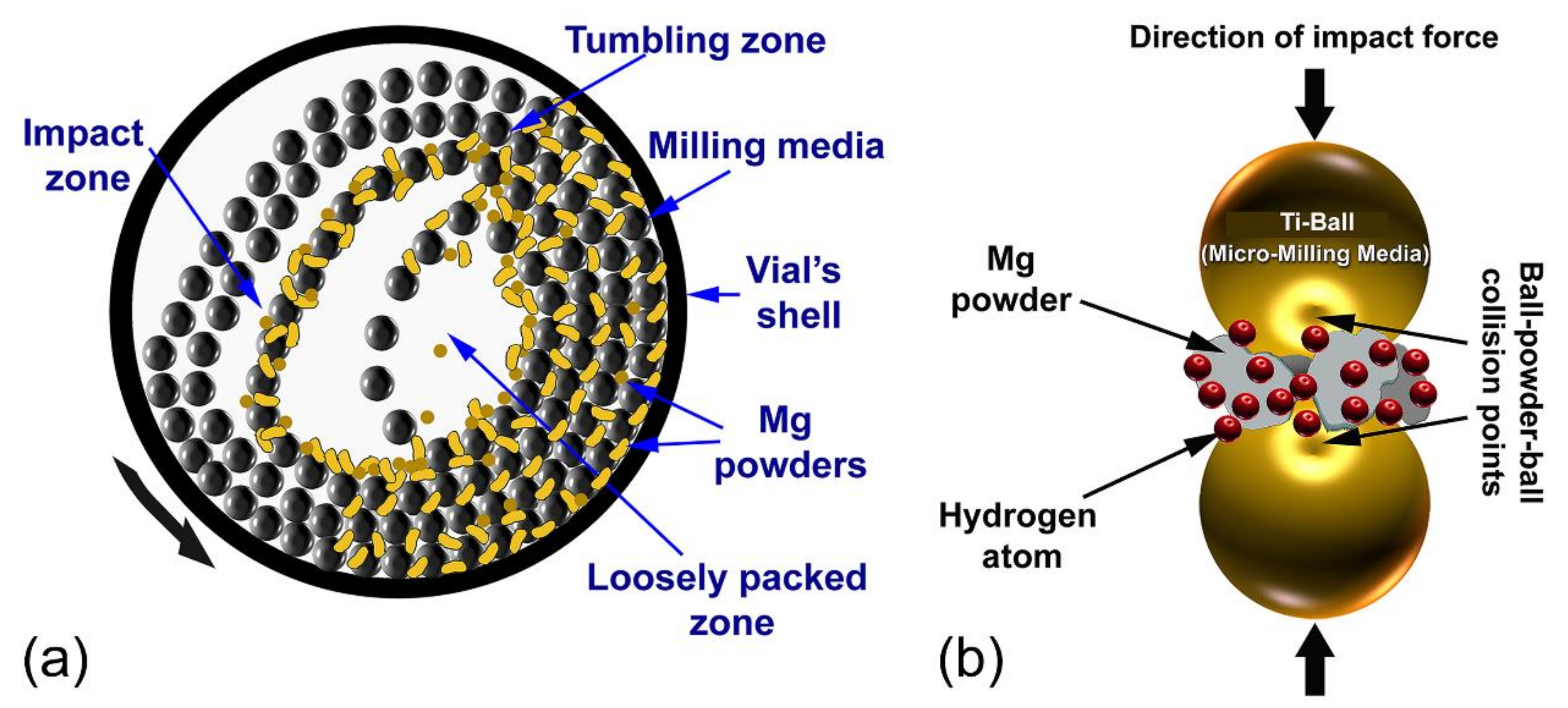
2.2. Preparation of MgH2 Nanopowder Particles
2.3. Sample Characterizations
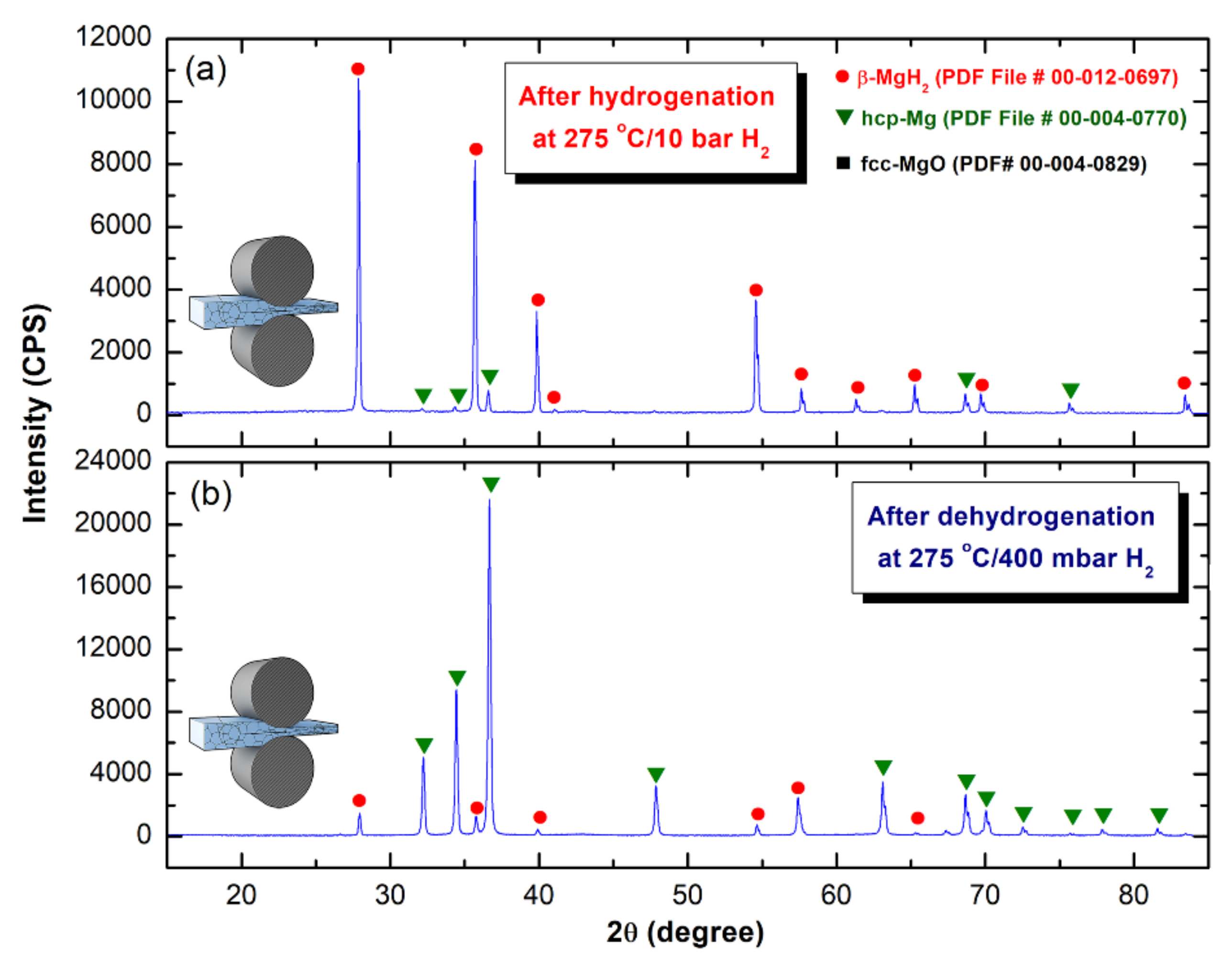
2.3.1. Crystal Structure
2.3.2. Morphology and Elemental Analysis
2.3.3. Thermal Stability
2.3.4. Hydrogenation/Dehydrogenation Kinetics Behavior
3. Results and Discussion
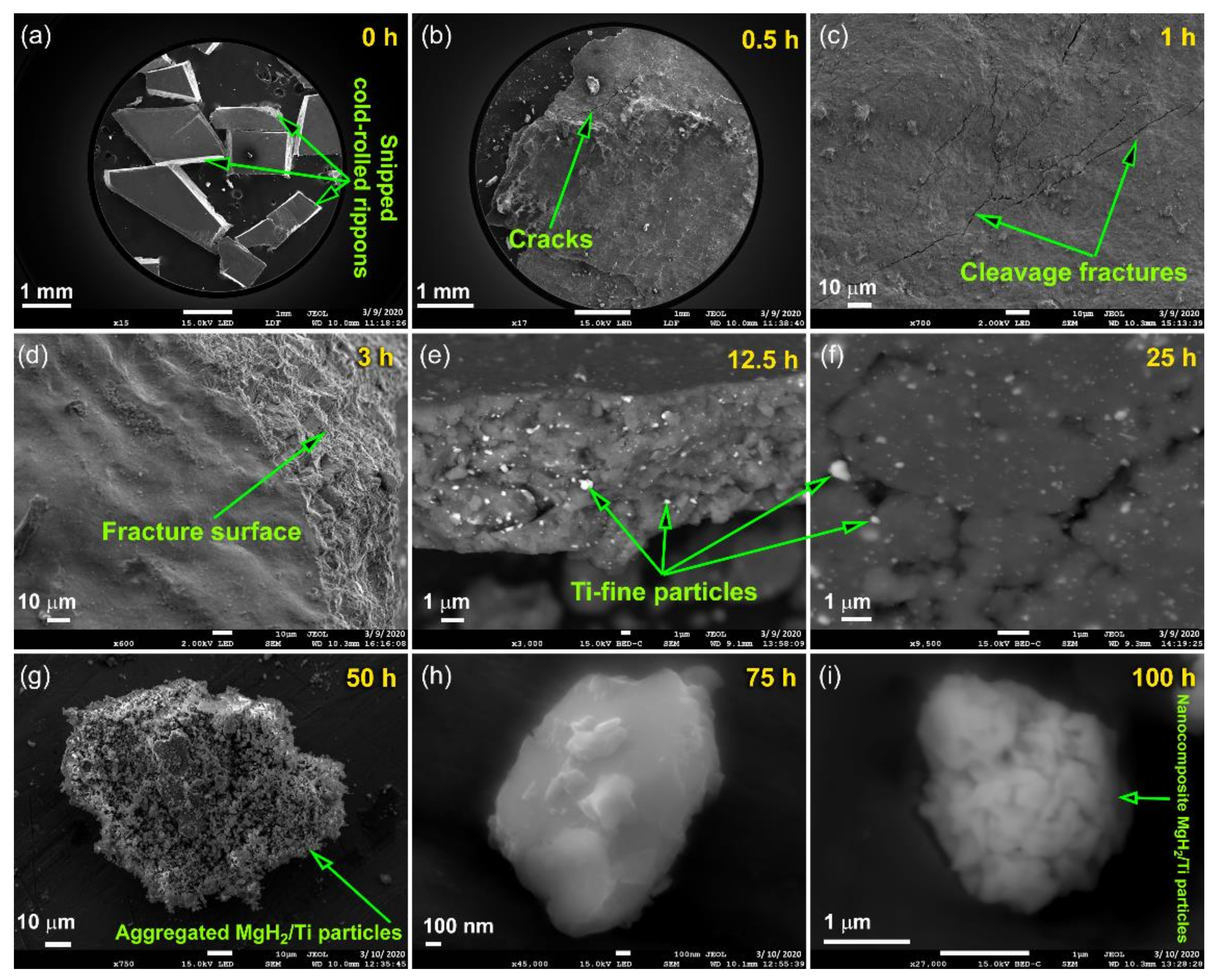
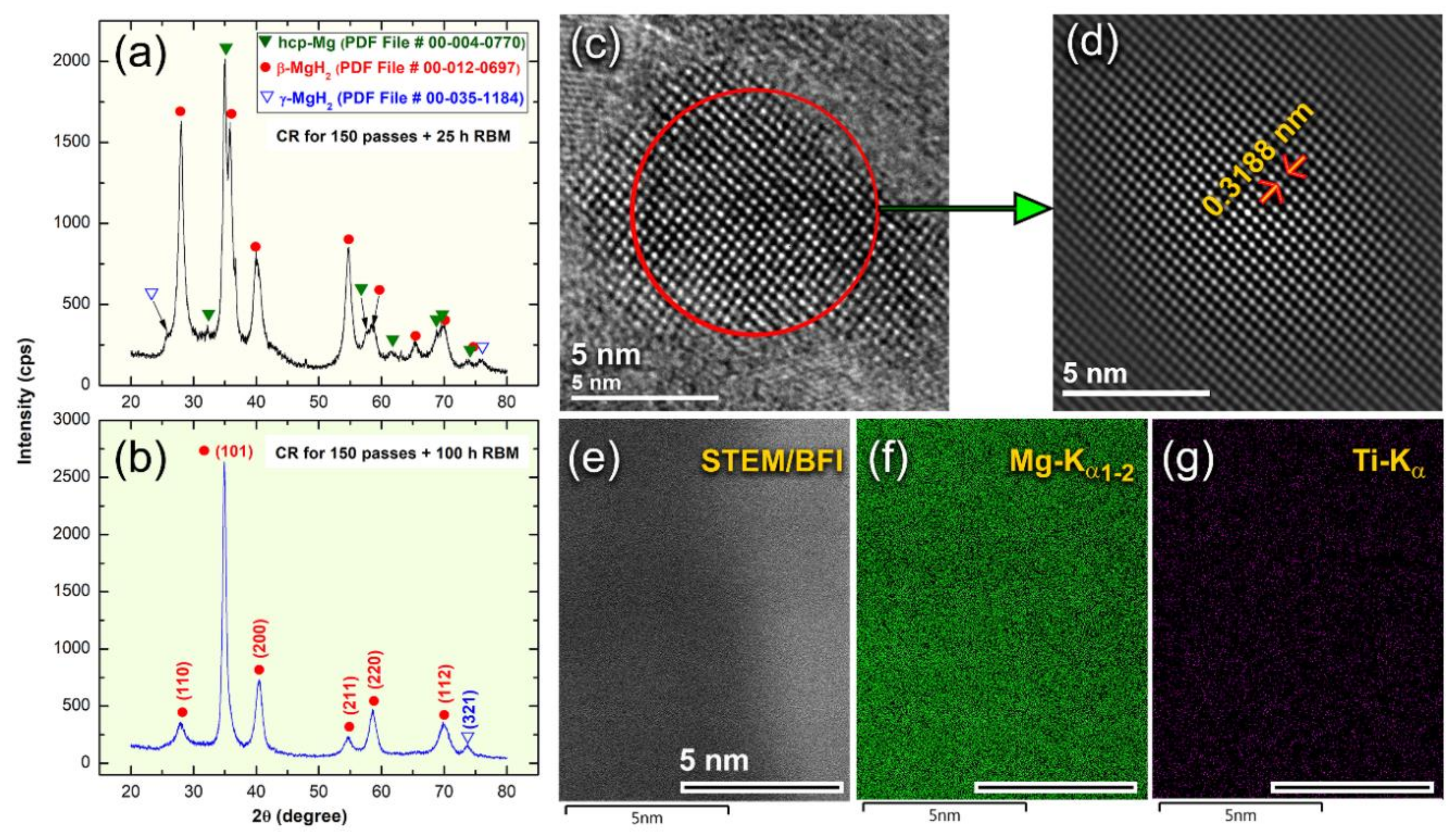
3.1. Morphology and Crystal Structure
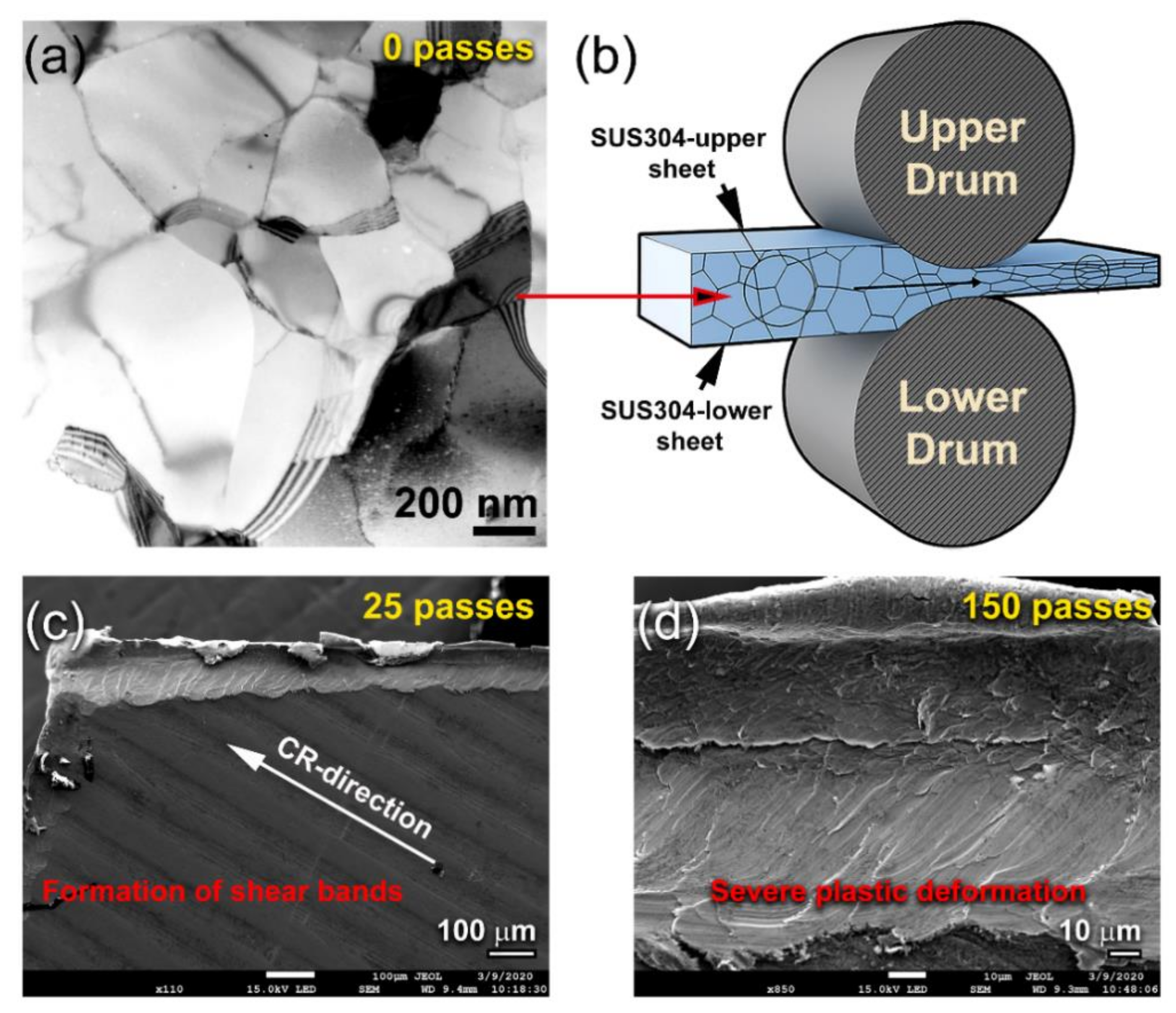
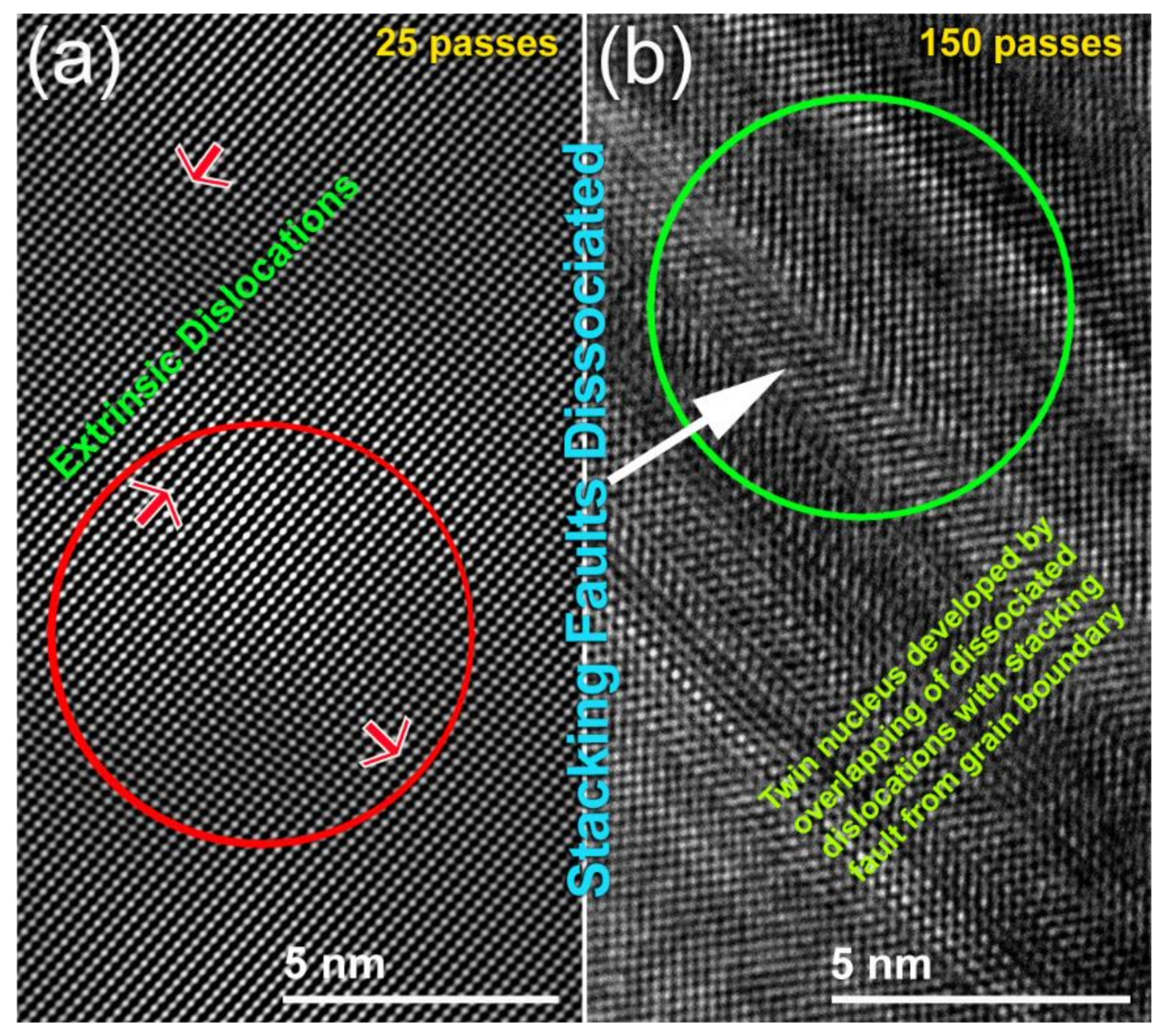
3.1.1. CR Mg-Ribbons
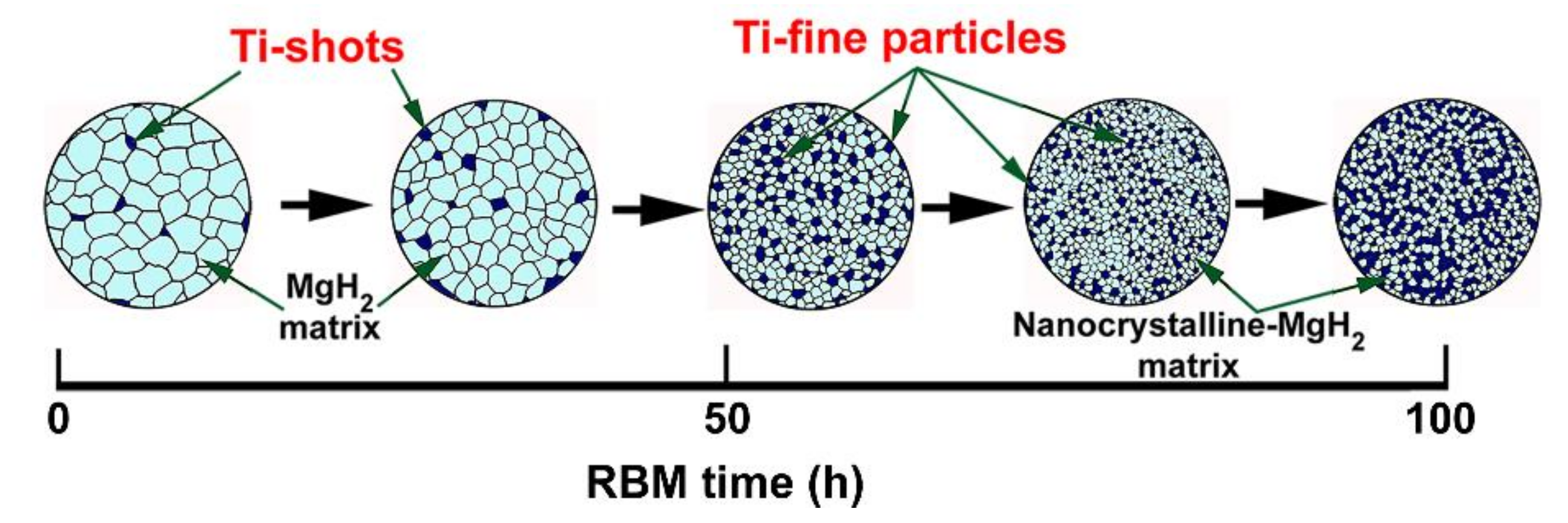
3.1.2. Reactive Ball Milling of Mg Powders
3.1.3. Color Changes with Changing the RBM Time
3.2. Thermal Analysis
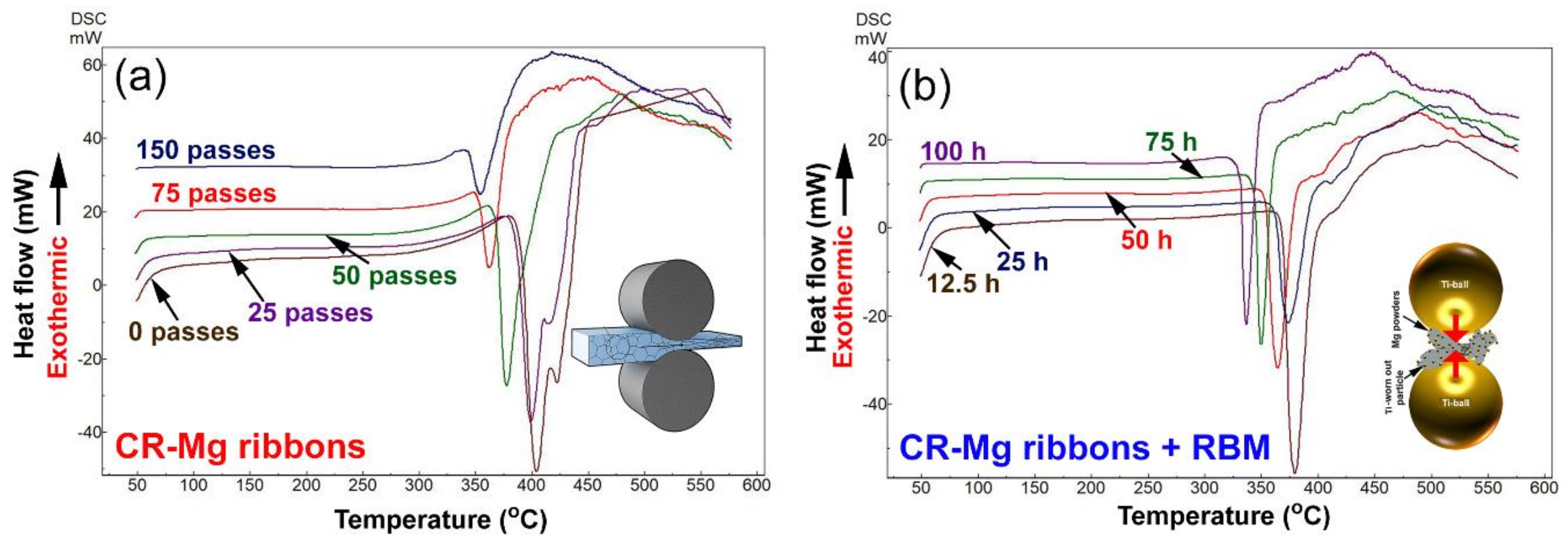
3.2.1. Differential Scanning Calorimetry Measurements
3.2.2. Correlations between Processing Approaches, Grain Size and Decomposition Temperatures
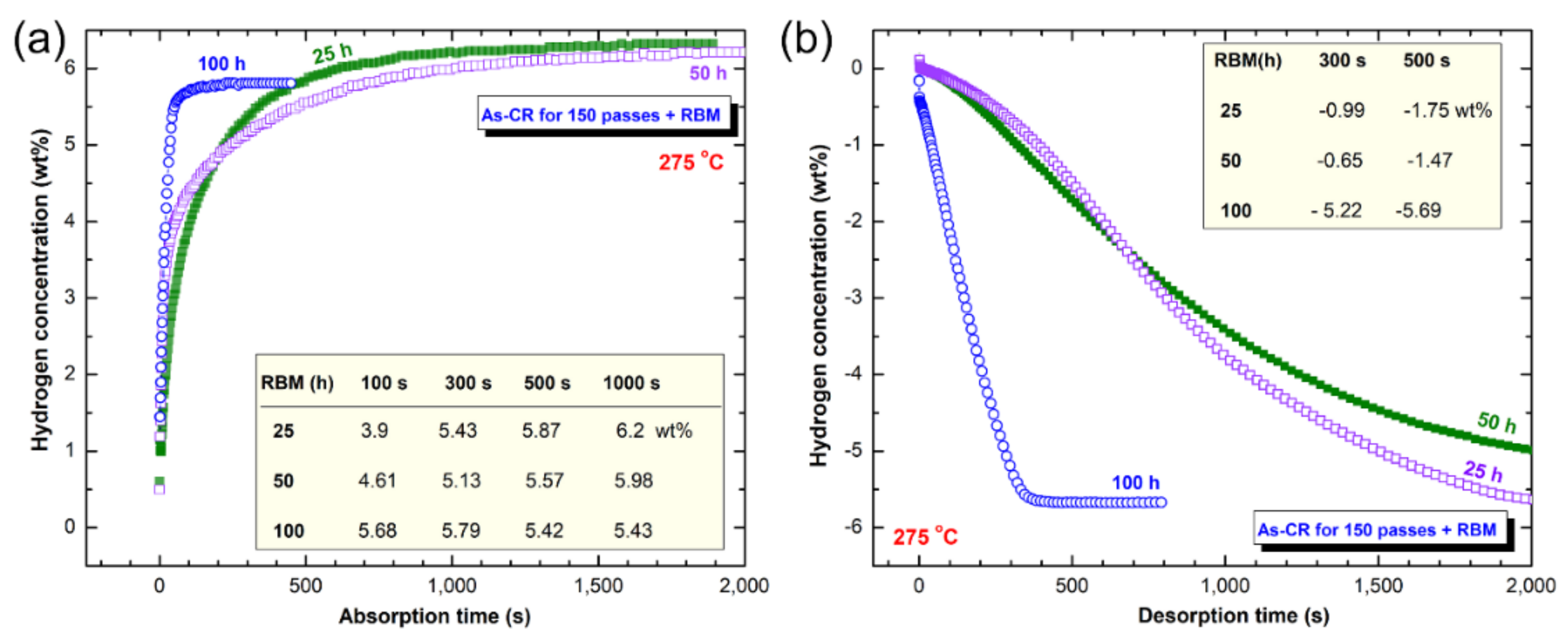
3.3. Hydrogenation/Dehydrogenation Kinetics
3.3.1. CR-Mg Ribbons
3.3.2. RBM Mg Powders
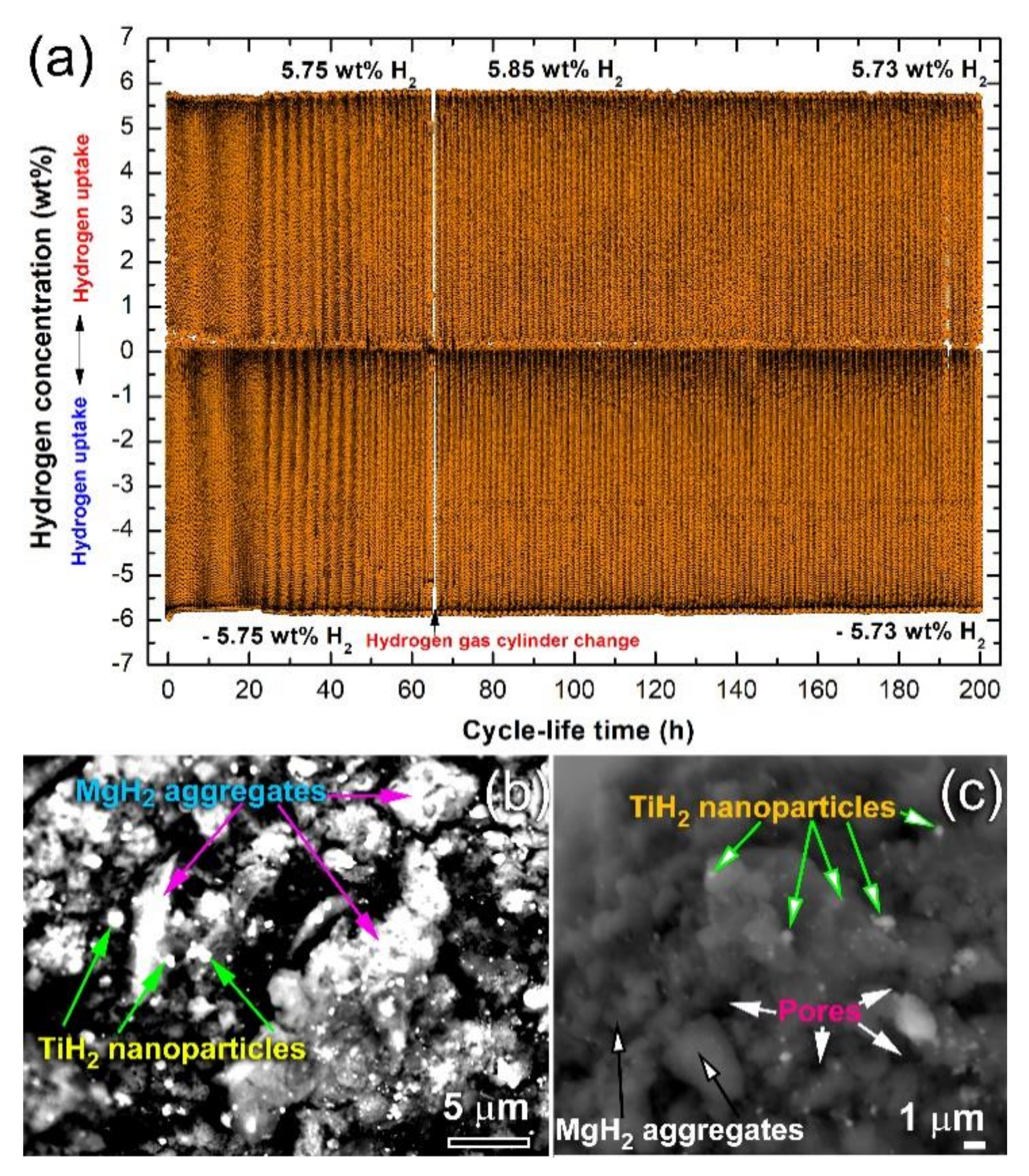
3.3.3. Cycle Lifetime Test
4. Conclusions
- MS technique is an ideal technique used to produce thin Mg-ribbons.
- CR process is a vital step used to introduce severe plastic deformation in Mg-ribbons, resulting in the formation of fine crystallites.
- Increasing the number of CR passes led to a significant reduction in the Mg crystallites. Accordingly, this led to an increase in the hydrogen storage capacity of metallic Mg and enhance the hydrogenation/dehydrogenation kinetics.
- Further increase in the hydrogen storage capacity and superior improvement in the gas uptake/release kinetics can be attained upon ball milling the as-CR Mg ribbons under high H2 pressure.
- Using Ti-balls milling media had obvious benefits for improving the hydrogenation/dehydrogenation processes that were taken place very fast when compared with MgH2 powders milled with tool steel-balls.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, F. Amirkhanian, Recent applications of waste solid materials in pavement engineering. J. Waste Manag. 2020, 108, 78–105. [Google Scholar] [CrossRef] [PubMed]
- Yue, K. Comparative Analysis of scrap car recycling management policies. Procedia Environ. Sci. 2012, 16, 44–50. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Tam, V.; Xue, C. Advanced progress in recycling municipal and construction solid wastes for manufacturing sustainable construction materials. Resour. Conserv. Recycl. X 2020. [Google Scholar] [CrossRef]
- Cui, F.; Wang, G.; Yu, D.; Gan, X.; Tian, Q.; Guo, X. Towards “zero waste” extraction of nickel from scrap nickel-based superalloy using magnesium. J. Clean. Prod. 2020, 262. [Google Scholar] [CrossRef]
- Wu, S.; Ji, Z.; Ming, H.; Hu, M.; Huang, Z.; Tian, C.; Wu, M. Microstructure and mechanical properties of AZ31B magnesium alloy prepared by solid state recycling. Rare Metal Mater. Eng. 2018, 47, 736–741. [Google Scholar] [CrossRef]
- Lucci, R.; López Padilla, R.; Cantero, S.; Bariles, R.; Oldani, C. Refining of AZ91 magnesium alloy obtained in machining chips recycling. Procedia Mater. Sci. 2015, 8, 886–893. [Google Scholar] [CrossRef]
- Paraskevas, D.; Dadbakhsh, S.; Vleugels, J.; Vanmeensel, K.; Dewulf, W.; Duflou, J. Solid state recycling of pure Mg and AZ31 Mg machining chips via spark plasma sintering. Mater. Des. 2016, 109, 520–529. [Google Scholar] [CrossRef]
- Ali, Y.; Qiu, D.; Jiang, B.; Pan, F.; Zhang, M.-X. Current research progress in grain refinement of cast magnesium alloys: A review article. J. Alloys Compd. 2015, 619, 639–651. [Google Scholar] [CrossRef]
- Shao, H.; Xin, G.; Zheng, J.; Li, X.; Akiba, E. Nanotechnology in Mg-based materials for hydrogen storage. Nano Energy 2012, 1, 590–601. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrog. Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Metallic glassy Zr70Ni20Pd10 powders for improving the hydrogenation/dehydrogenation behavior of MgH2. Nature. Sci. Rep. 2016, 6, 26936. Available online: www.nature.com/scientificreports (accessed on 8 May 2020). [CrossRef] [PubMed]
- Luo, Q.; Li, J.; Li, B.; Liu, B.; Shao, H.; Li, Q. Kinetics in Mg-based hydrogen storage materials: Enhancement and mechanism. J. Magnes. Alloy. 2019, 7, 58–71. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Banyan, M.; Al-Ajmi, F. Environmentally friendly nanocrystalline magnesium hydride decorated with metallic glassy zirconium palladium nanopowders for fuel cell applications. RSC Adv. 2019, 9, 27987. [Google Scholar] [CrossRef]
- Colbe, B.; Ares, J.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrog. Energy 2019, 27, 7780. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Banyan, M.; Al-Ajmi, F. Cold-rolled magnesium hydride strips decorated with cold-sprayed Ni powders for solid-state hydrogen storage. Int. J. Hydrog. Energy 2019, 44, 16852–16861. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, C.; Li, Y.; Zhang, Q. Crystallite growth characteristics of Mg during hydrogen desorption of MgH2. Prog. Nat. Sci. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Recent developments in the fabrication, characterization and implementation of MgH2-based solid-hydrogen materials in the Kuwait Institute for Scientific Research. RSC Adv. 2019, 9, 9907–9930. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Francois, K.; Zinsou, A. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.; Baricco, M.; Bellosta von Colbed, J.; Blanchard, D.; Bowman, R. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J. Alloys Compd. 2020, 827. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Chen, J.; Xia, G.; Guo, Z.; Huang, Z.; Liu, H.; Yu, X. Porous Ni Nanofibers with Enhanced Catalytic Effect on the Hydrogen Storage Performance of MgH2. J. Mater. Chem. A 2015, 3, 15843–15848. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.F.; Ding, B.Z.; Hu, Z.Q. Structural and hydriding properties of composite Mg–ZrFe1.4Cr0.6. Acta Mater. 2001, 49, 921–926. [Google Scholar] [CrossRef]
- Yu, X.B.; Yang, Z.X.; Liu, H.K.; Grant, D.M.; Walker, G.S. The effect of a Ti-V-based BCC alloy as a catalyst on the hydrogen storage properties of MgH2. Int. J. Hydrog. Energy 2010, 35, 6338–6344. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti intermetallic catalysts on hydrogen storage properties of magnesium hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Matrouk, H.; Shaban, E.; Al-Duweesh, A. Effect of mechanically-induced solid-state doping time on the morphology and hydrogenation cyclability of MgH2/7 Mn3.6Ti2.4 nanocomposite powders. Int. J. Hydrog. Energy 2015, 40, 10139–10149. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Matrouk, H.; Behbehani, M.; Alkandary, A.; Aldakheel, F.; Ali, N.; Ahmed, S.A. Structure, morphology and hydrogen storage kinetics of nanocomposite MgH2/10 wt% ZrNi5 powders. Mater. Today Energy 2017, 3, 60–71. [Google Scholar] [CrossRef]
- Kumar, S.; Kojima, Y.G.; Dey, K. Morphological effects of Nb2O5 on Mg–MgH2 system for thermal energy storage application. Int. J. Hydrog. Energy 2018, 43, 809–816. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Nasrallah, E.; Banyan, M.; Al-Ajmi, F. Bulk nanocomposite MgH2/10 wt% (8 Nb2O5/2 Ni) solid-hydrogen storage system for fuel cell applications. Int. J. Hydrog. Energy 2018, 43, 23382–23396. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Varin, R.; Plocinski, T.; Pisarek, M. The effect of chromium (III) oxide (Cr2O3) nanopowder on the microstructure and cyclic hydrogen storage behavior of magnesium hydride (MgH2). J. Alloys Compd. 2011, 509, 2386–2391. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Zhu, Y.; Zhao, Y.; Lin, H.; Zhang, Y.; Li, H.; Zhang, J.; Liu, Y.; Gao, W.; et al. Crystal-facet-dependent catalysis of anatase TiO2 on hydrogen storage of MgH2. J. Alloys Compd. 2020, 822. [Google Scholar] [CrossRef]
- Ranjbara, A.; Guoa, Z.P.; Yua, X.B.; Wexler, D.; Calka, A.; Kimd, C.J.; Liu, H.K. Hydrogen storage properties of MgH2–SiC composites. Mater. Chem. Phys. 2009, 114, 168–172. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Alsairafi, A. Synergistic dosing effect of TiC/FeCr nanocatalysts on the hydrogenation/dehydrogenation kinetics of nanocrystalline MgH2 powders. Energy 2016, 104, 158–170. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Alkandary, A.; Aldakheel, F.; Al-Saidi, M.; Al-Ajmi, F.; Banyan, M. Performance and fuel cell applications of reacted ball-milled MgH2/5.3 wt% TiH2 nanocomposite powders. RSC Adv. 2018, 8, 38175–38185. [Google Scholar] [CrossRef]
- Song, J.-Z.; Zhao, Z.-Y.; Zhao, X.; Fu, R.-D.; Han, S.-M. Hydrogen storage properties of MgH2 co-catalyzed by LaH3 and NbH. Int. J. Miner. Metall. Mater. 2017, 24, 1183–1191. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Xiao, X.; Chen, M.; Sun, M.; Yao, Z.; Hu, Z.; Chen, L. Novel 1D carbon nanotubes uniformly wrapped nanoscale MgH2 for efficient hydrogen storage cycling performances with extreme high gravimetric and volumetric capacities. Nano Energy 2019, 61, 540–549. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9. [Google Scholar] [CrossRef]
- Amira, S.; Huot, J. Effect of cold rolling on hydrogen sorption properties of die-cast and as-cast magnesium alloys. J. Alloys Compd. 2012, 520, 287–294. [Google Scholar] [CrossRef]
- Jorge, A.M.; de Lima, G.F.; Triques, M.R.M.; Botta, W.J.; Langdon, T.G. Correlation between hydrogen storage properties and textures induced in magnesium through ECAP and cold rolling. Int. J. Hydrog. Energy 2014, 39, 3810–3821. [Google Scholar] [CrossRef]
- Kumar, G.; Lodh, A.; Jaiveer, S.; Singh, R.K.; Srivastava, D.; Dey, G.K.; Samajdar, I. Experimental characterization and finite element modeling of through thickness deformation gradient in a cold rolled zirconium sheet. CIRP J. Manuf. Sci. Technol. 2017, 19, 176–190. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying, Energy Storage, Protective Coatings, and Medical Applications, 3rd ed.; Elsevier: Oxford, UK, 2020. [Google Scholar]
- El-Eskandarany, M.S. Metallic glassy Ti2Ni grain-growth inhibitor powder for enhancing the hydrogenation/dehydrogenation kinetics of MgH2. RSC Adv. 2019, 9, 1036. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Ajmi, F.; Banyan, M. Mechanically-induced catalyzation of MgH2 powders with Zr2Ni-ball milling media. Catalysts 2019, 9, 382. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Halaili, B. Nanocrystalline β-γ-β cyclic phase transformation in reacted ball milled MgH2 powders. Int. J. Hydrog. Energy 2014, 39, 12727–12740. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Shemmiri, A. Integrated Ni/Nb2O5 nanocatalytic agent dose for improving the hydrogenation/dehydrogenation kinetics of reacted ball milled MgH2 powders. J. Hydrog. Energy 2014, 39, 21097–21106. [Google Scholar] [CrossRef]
- Huot, J.; Tousignant, M. Effect of cold rolling on metal hydrides. Mater. Trans. 2019, 60, 1571–1576. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Eskandarany, M.S.; Ali, N.; Al-Salem, S.M. Solid-State Conversion of Magnesium Waste to Advanced Hydrogen-Storage Nanopowder Particles. Nanomaterials 2020, 10, 1037. https://doi.org/10.3390/nano10061037
El-Eskandarany MS, Ali N, Al-Salem SM. Solid-State Conversion of Magnesium Waste to Advanced Hydrogen-Storage Nanopowder Particles. Nanomaterials. 2020; 10(6):1037. https://doi.org/10.3390/nano10061037
Chicago/Turabian StyleEl-Eskandarany, Mohamed Sherif, Naser Ali, and Sultan Majed Al-Salem. 2020. "Solid-State Conversion of Magnesium Waste to Advanced Hydrogen-Storage Nanopowder Particles" Nanomaterials 10, no. 6: 1037. https://doi.org/10.3390/nano10061037
APA StyleEl-Eskandarany, M. S., Ali, N., & Al-Salem, S. M. (2020). Solid-State Conversion of Magnesium Waste to Advanced Hydrogen-Storage Nanopowder Particles. Nanomaterials, 10(6), 1037. https://doi.org/10.3390/nano10061037