Stearic Acid Coated MgO Nanoplate Arrays as Effective Hydrophobic Films for Improving Corrosion Resistance of Mg-Based Metallic Glasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Glassy Ribbons
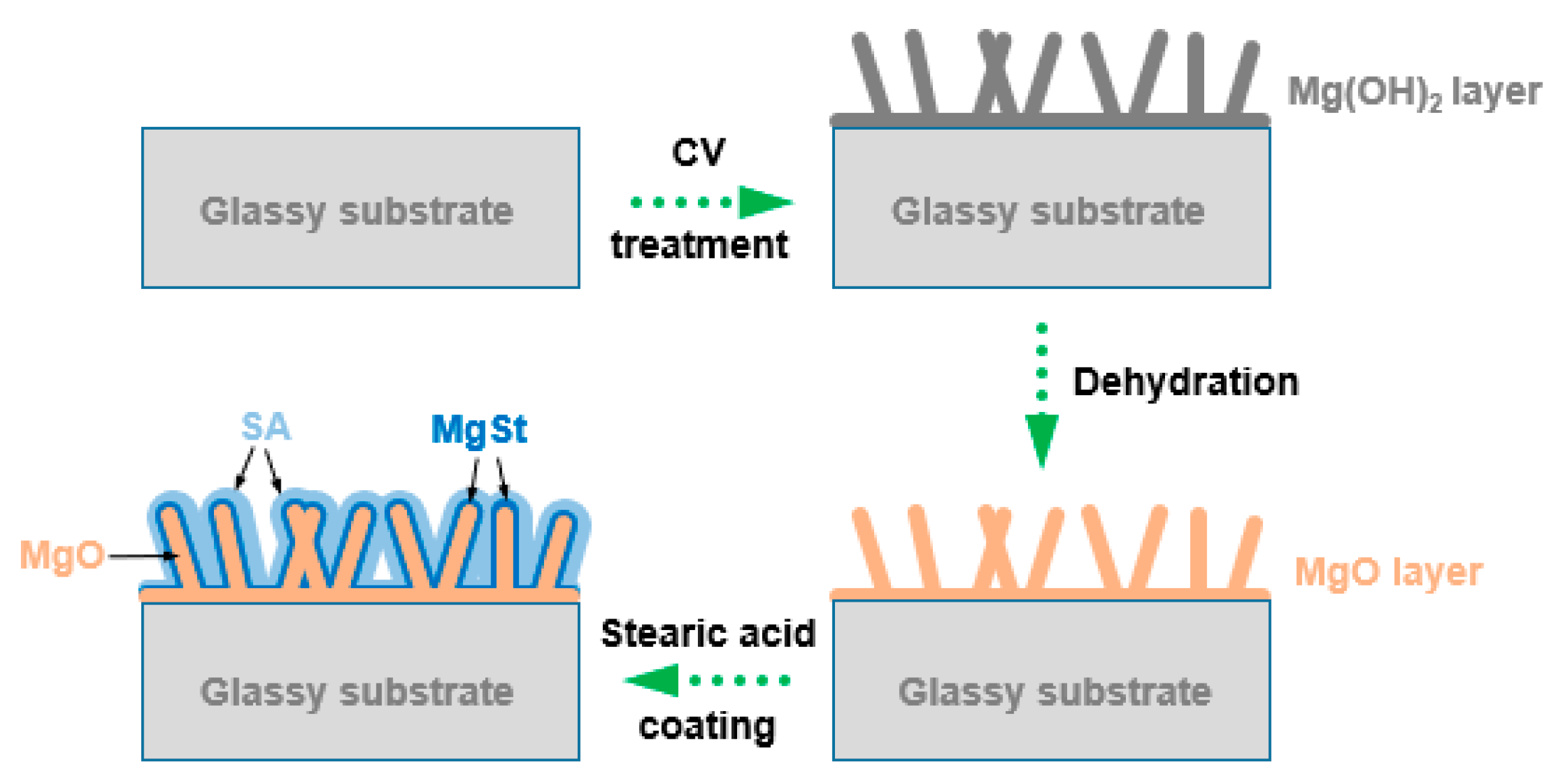
2.2. Preparation of Hydrophobic Surface
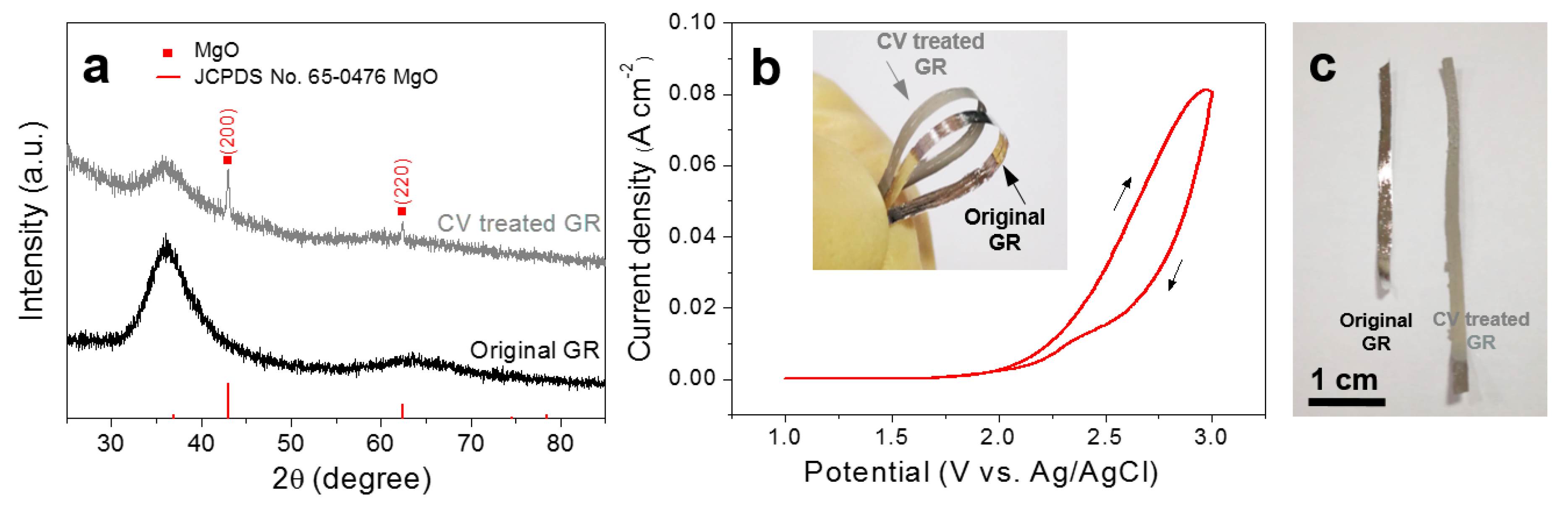
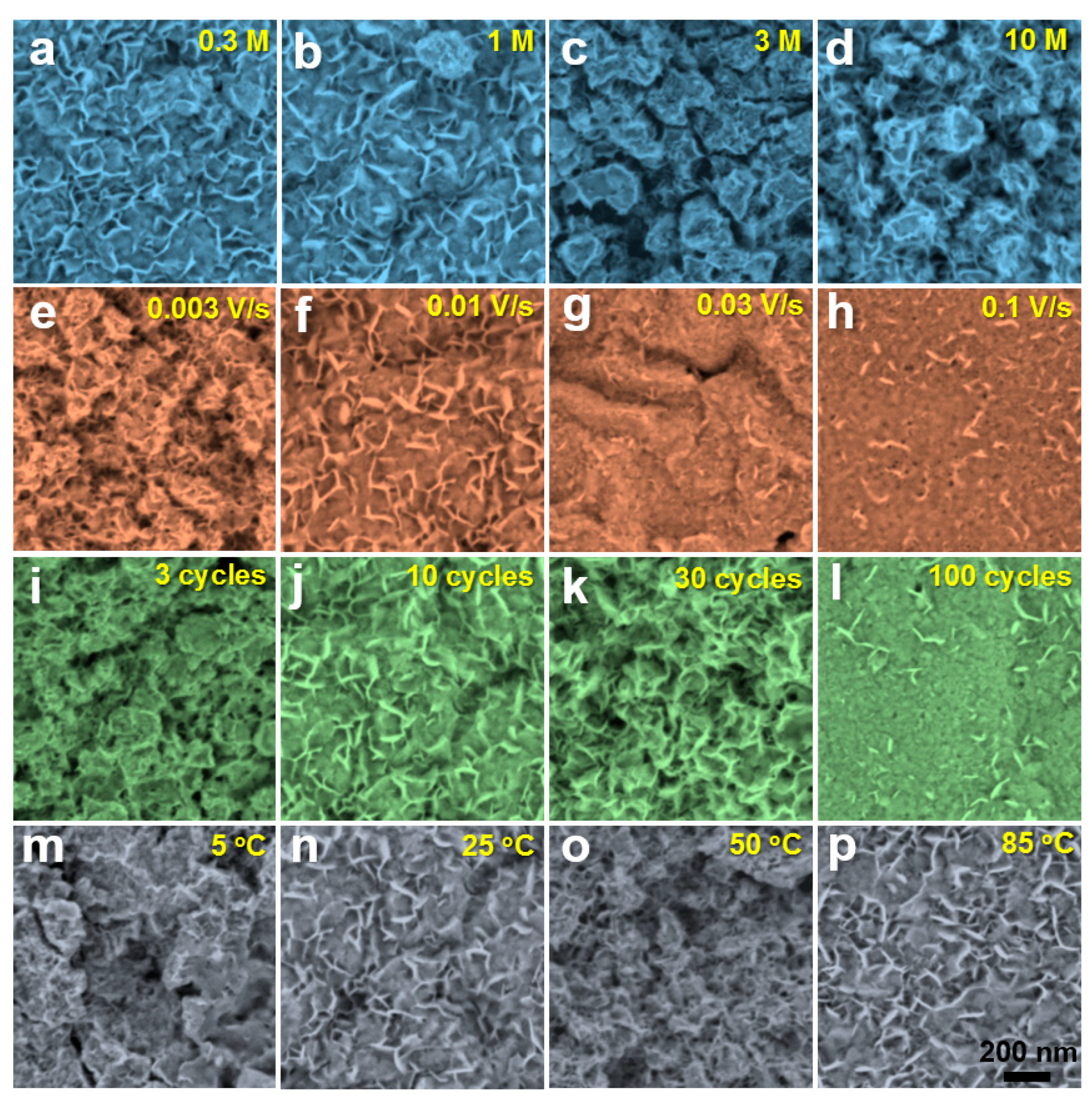
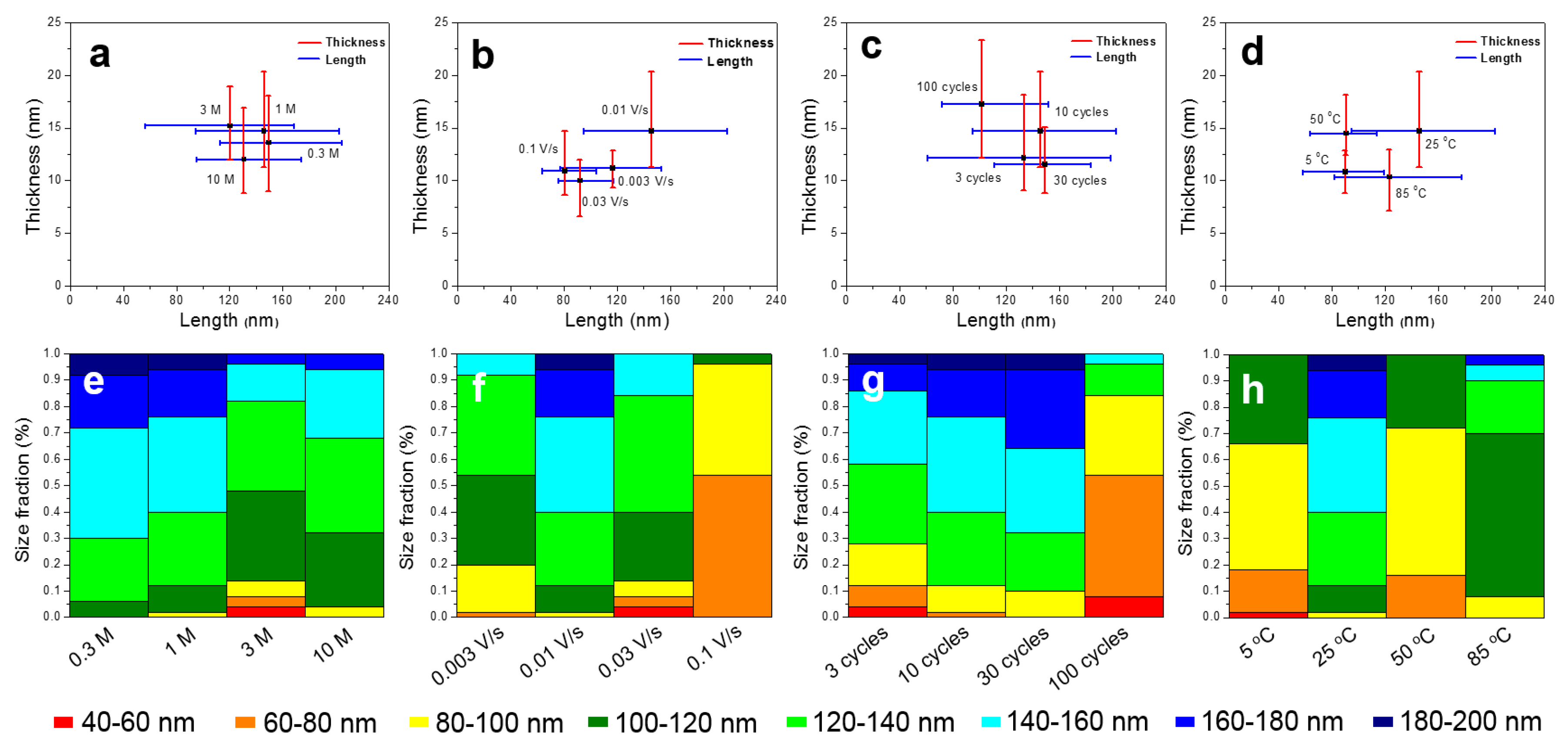
2.3. Characterization
2.4. Corrosion Resistance and Immersing Test
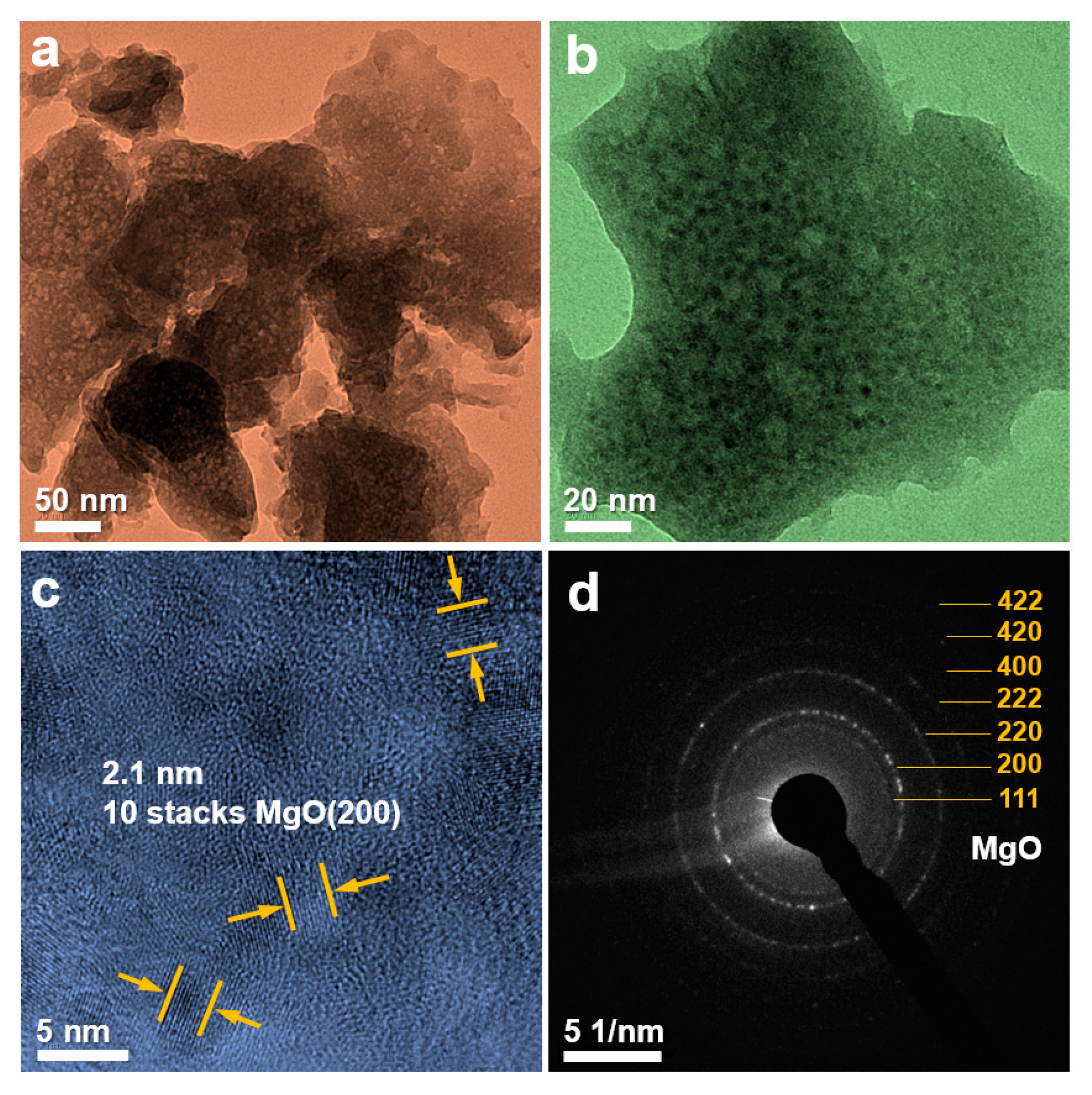
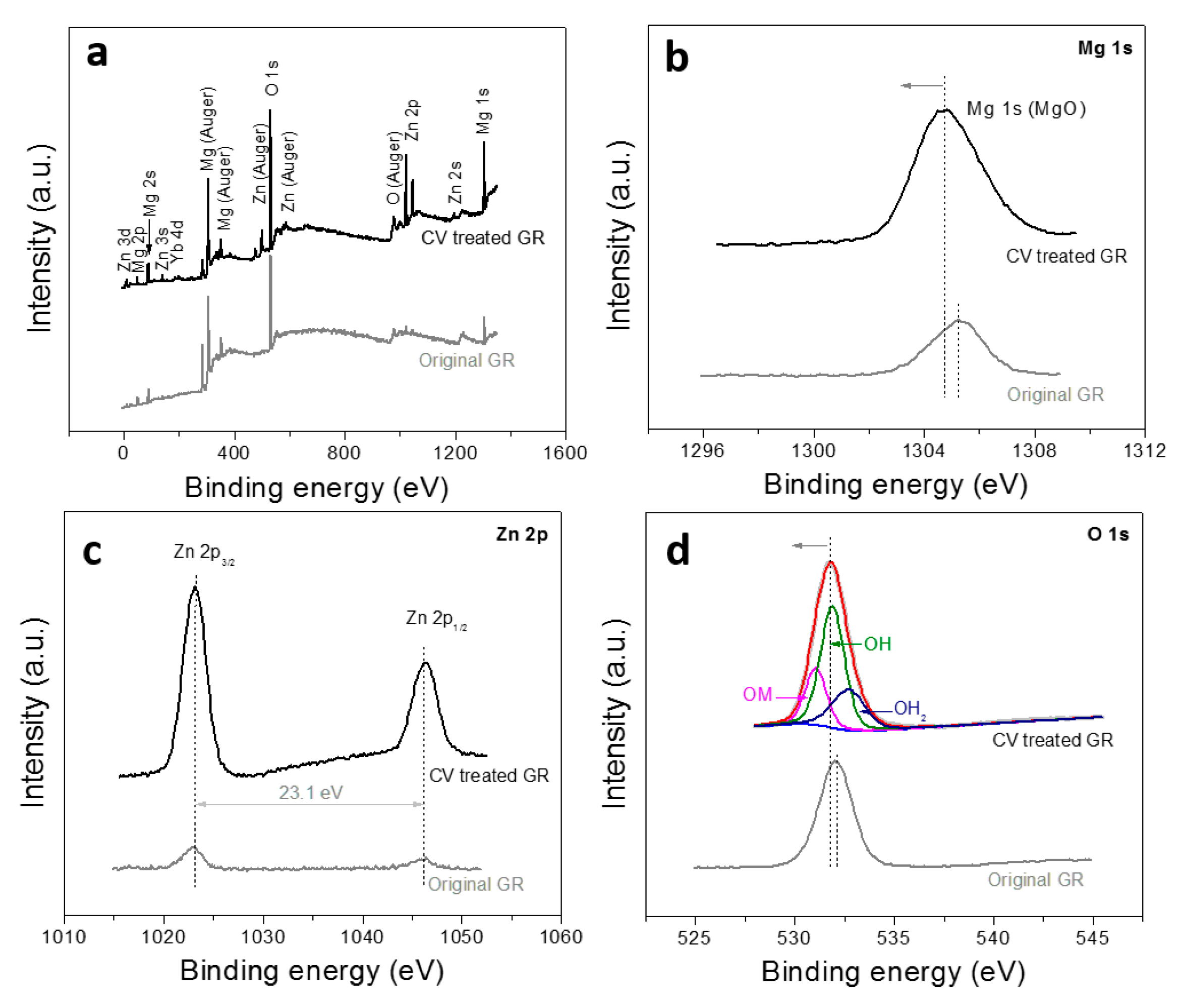
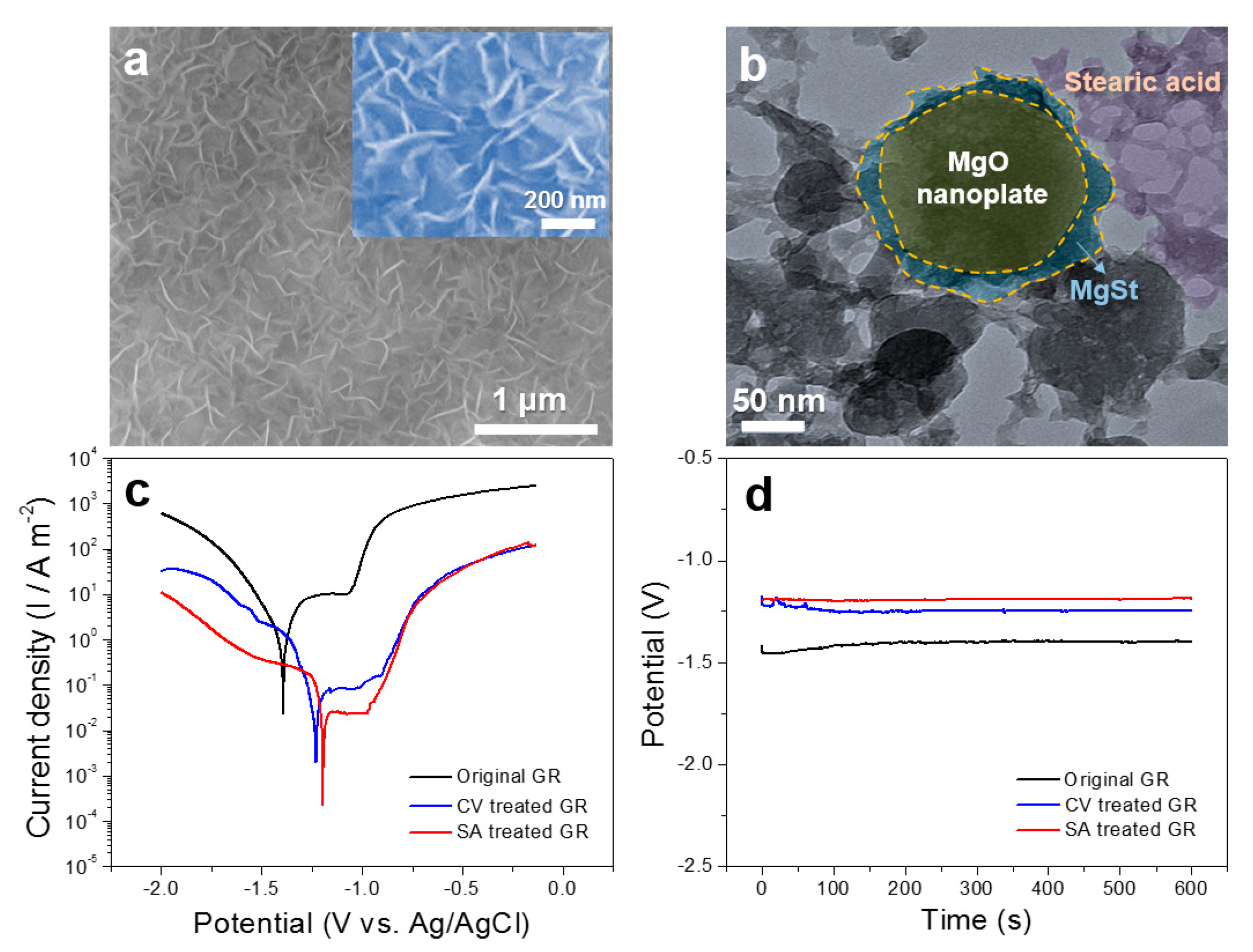
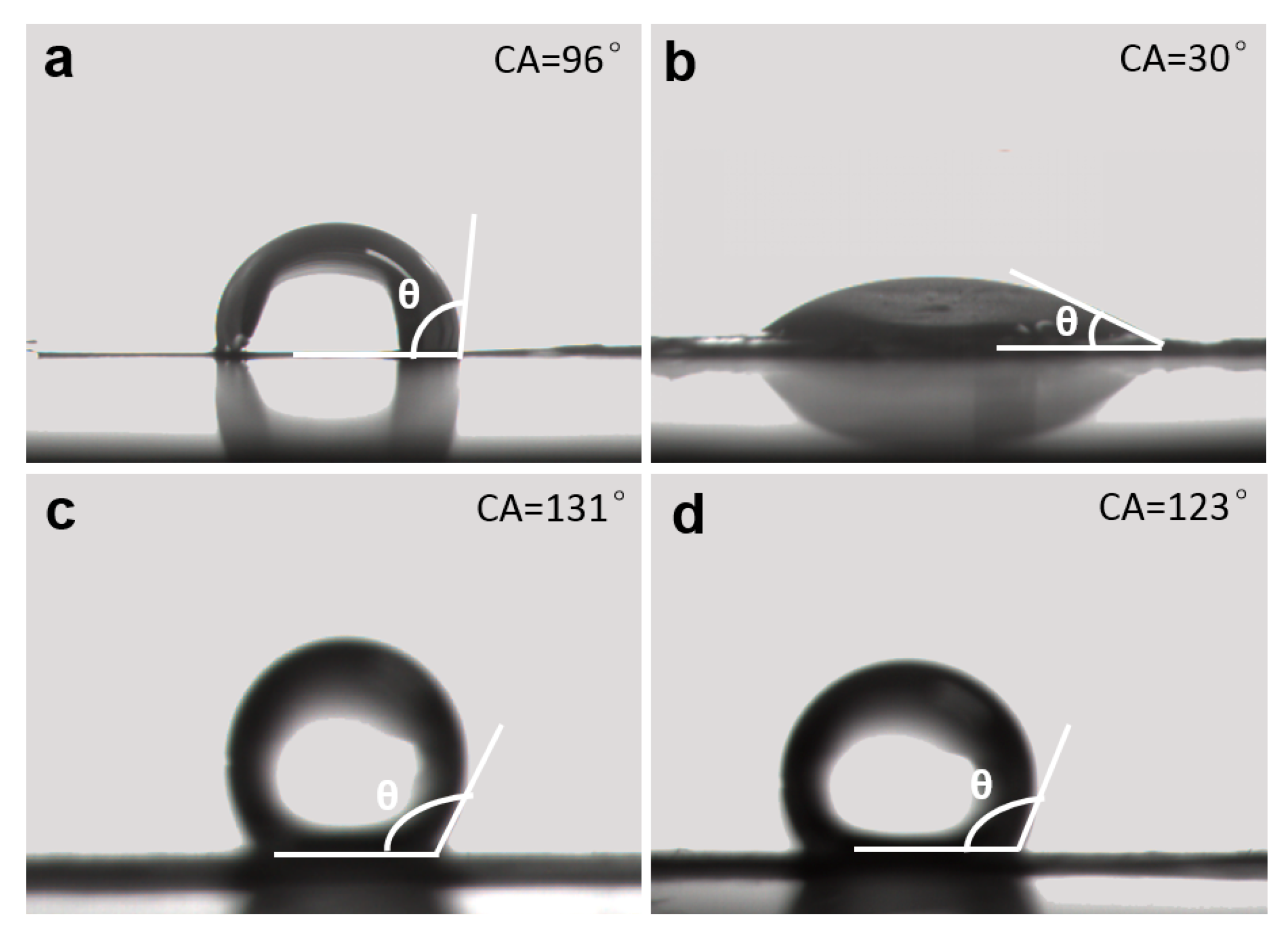
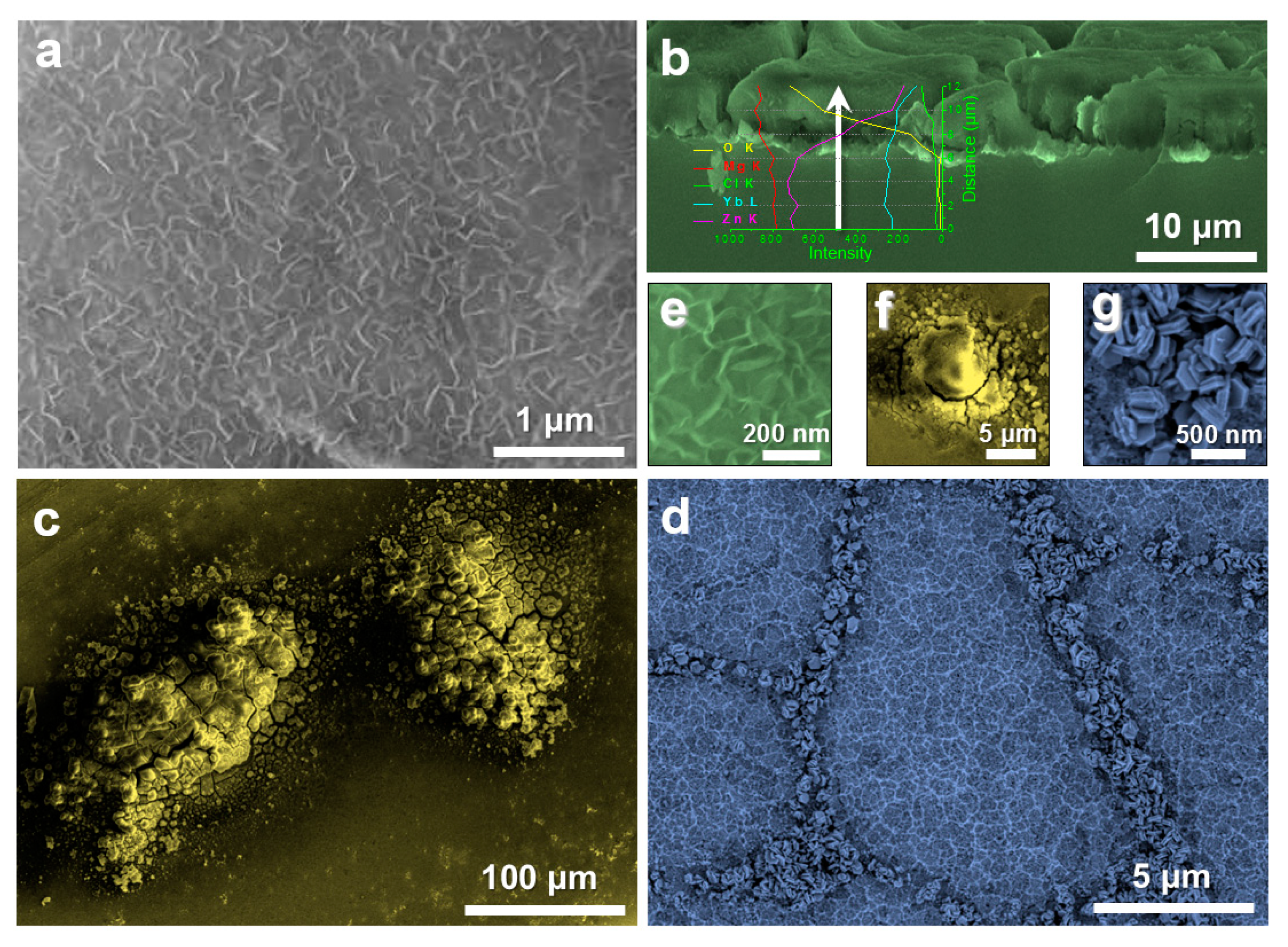
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wei, J.F.; Li, B.C.; Jing, L.Y.; Tian, N.; Zhao, X.; Zhang, J.P. Efficient protection of Mg alloy enabled by combination of a conventional anti-corrosion coating and a superamphiphobic coating. Chem. Eng. J. 2020, 390, 124562. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.X.; Wen, C.E.; Wright, P.F.A.; Li, Y.C. Mechanical, corrosion, and biocompatibility properties of Mg-Zr-Sr-Sc alloys for biodegradable implant applications. Acta Biomater. 2020, 102, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Zhao, W.M.; Li, H.P.; Ding, J.; Li, Y.Y.; Liang, C.Y. Effect of titanium, antimony, cerium and carbon nanotubes on the morphology and microhardness of Mg-based icosahedral quasicrystal phase. J. Mater. Sci. Technol. 2010, 26, 27–32. [Google Scholar] [CrossRef]
- Wu, Y.P.; Wang, Z.F.; Liu, Y.; Li, G.F.; Xie, S.H.; Yu, H.; Xiong, H.Q. AZ61 and AZ61-La alloys as anodes for Mg-air battery. J. Mater. Eng. Perform. 2019, 28, 2006–2016. [Google Scholar] [CrossRef]
- Xiong, H.Q.; Liang, Z.F.; Wang, Z.F.; Qin, C.L.; Zhao, W.M.; Yu, H. Mechanical properties and degradation behavior of Mg(100-7x) Zn6xYx (x = 0.2, 0.4, 0.6, 0.8) alloys. Metals 2018, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.M.; Cheng, W.L.; Gu, X.J.; Yu, H.; Wang, Z.F.; Wang, H.X.; Wang, L.F. Discharge properties of low-alloyed Mg-Bi-Ca alloys as anode materials for Mg-air batteries: Influence of Ca alloying. J. Alloys Compd. 2020, 823, 153779. [Google Scholar] [CrossRef]
- Wang, X.J.; Chen, Z.N.; Ren, J.; Kang, H.J.; Guo, E.Y.; Li, J.H.; Wang, T.M. Corrosion behavior of as-cast Mg–5Sn based alloys with in additions in 3.5 wt.% NaCl solution. Corros. Sci. 2020, 164, 108318. [Google Scholar] [CrossRef]
- Xu, Y.K.; Ma, H.; Xu, J.; Ma, E. Mg-based bulk metallic glass composites with plasticity and gigapascal strength. Acta. Mater. 2005, 53, 1857–1866. [Google Scholar] [CrossRef]
- Eckert, J.; Das, J. Mechanical properties of bulk metallic glasses and composites. J. Mater. Res. 2007, 22, 285–301. [Google Scholar] [CrossRef]
- Gao, R.; Hui, X.; Fang, H.Z.; Liu, X.J.; Chen, G.L.; Liu, Z.K. Structural characterization of Mg65Cu25Y10 metallic glass from ab initio molecular dynamics. Comput. Master. Sci. 2008, 44, 802–806. [Google Scholar] [CrossRef]
- Men, H.; Hu, Z.Q.; Xu, J. Bulk metallic glass formation in the Mg–Cu–Zn–Y system. Scr. Mater. 2002, 46, 699–703. [Google Scholar] [CrossRef]
- Yao, H.B.; Li, Y.; Wee, A.T.S. Corrosion behavior of melt-spun Mg65Ni20Nd15 and Mg65Cu25Y10 metallic glasses. Electrochim. Acta 2003, 48, 2641–2650. [Google Scholar] [CrossRef]
- Babilas, R.; Bajorek, A.; Simka, W.; Babilas, D. Study on corrosion behavior of Mg-based bulk metallic glasses in NaCl solution. Electrochim. Acta 2016, 209, 632–642. [Google Scholar] [CrossRef]
- Guo, S.F.; Chan, K.C.; Jiang, X.Q.; Zhang, H.J.; Zhang, D.F.; Wang, J.F.; Jiang, B.; Pan, F.S. Atmospheric RE-free Mg-based bulk metallic glass with high bio-corrosion resistance. J. Non-Cryst. Solids. 2013, 379, 107–111. [Google Scholar] [CrossRef]
- Babilas, R.; Bajorek, A.; Sakiewicz, P.; Kania, A.; Szyba, D. Corrosion resistance of resorbable Ca-Mg-Zn-Yb metallic glasses in Ringer’s solution. J. Non-Cryst. Solids 2018, 488, 69–78. [Google Scholar] [CrossRef]
- Zhang, X.L.; Chen, G.; Bauer, T. Mg-based bulk metallic glass composite with high bio-corrosion resistance and excellent mechanical properties. Intermetallics 2012, 29, 56–60. [Google Scholar] [CrossRef]
- Wang, J.F.; Huang, S.; Wei, Y.Y.; Guo, S.F.; Pan, F.S. Enhanced mechanical properties and corrosion resistance of a Mg-Zn-Ca bulk metallic glass composite by Fe particle addition. Mater. Lett. 2013, 91, 311–314. [Google Scholar] [CrossRef]
- Chen, S.S.; Tu, J.X.; Hu, Q.; Xiong, X.B.; Wu, J.J.; Zou, J.Z.; Zeng, X.R. Corrosion resistance and in vitro bioactivity of Si-containing coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by micro-arc oxidation. J. Non-Cryst. Solids 2017, 456, 125–131. [Google Scholar] [CrossRef]
- Yang, J.X.; Cui, F.Z.; Lee, I.S.; Wang, X.M. Plasma surface modification of magnesium alloy for biomedical application. Surf. Coat. Technol. 2010, 205, S182–S187. [Google Scholar] [CrossRef]
- Wu, G.S.; Ibrahim, J.M.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Xie, J.; Hu, J.; Fang, L.; Liao, X.L.; Du, R.L.; Wu, F.; Wu, L. Facile fabrication and biological properties of super-hydrophobic coating on magnesium alloy used as potential implant materials. Surf. Coat. Technol. 2020, 384, 125223. [Google Scholar] [CrossRef]
- Zhou, J.; Li, K.; Wang, B.; Ai, F.R. Nano-hydroxyapatite/ZnO coating prepared on a biodegradable Mg–Zn–Ca bulk metallic glass by one-step hydrothermal method in acid situation. Ceram. Int. 2020, 46, 6958–6964. [Google Scholar] [CrossRef]
- Bhushan, B.; Katiyar, P.K.; Murty, B.S.; Mondal, K. Synthesis of hydrophobic Ni-VN alloy powder by ball milling. Adv. Powder. Technol. 2019, 30, 1600–1610. [Google Scholar] [CrossRef]
- Wang, K.; Pang, J.B.; Li, L.W.; Zhou, S.Z.; Li, Y.H.; Zhang, T.Z. Synthesis of hydrophobic carbon nanotubes/reduced graphene oxide composite films by flash light irradiation. Front. Chem. Sci. Eng. 2018, 12, 376–382. [Google Scholar] [CrossRef]
- Du, X.Y.; Gao, B.; Li, Y.H.; Song, Z.X. Super-robust and anti-corrosive NiCrN hydrophobic coating fabricated by multi-arc ion plating. Appl. Surf. Sci. 2020, 511, 145653. [Google Scholar] [CrossRef]
- Fang, L.; Hou, L.X.; Zhang, Y.H.; Wang, Y.K.; Yan, G.H. Synthesis of highly hydrophobic rutile titania-silica nanocomposites by an improved hydrolysis co-precipitation method. Ceram. Int. 2017, 43, 5592–5598. [Google Scholar] [CrossRef]
- Cui, X.J.; Lin, X.Z.; Lin, C.H.; Yang, R.S.; Zheng, X.W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Hu, R.G.; Zhang, S.; Bu, J.F.; Lin, C.J.; Song, G.L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Dai, X.J.; Xiong, J.W. Study on the preparation and corrosion resistance of hydroxyapatite/stearic acid superhydrophobic composite coating on magnesium alloy surface. AIP Adv. 2019, 9, 045314. [Google Scholar] [CrossRef] [Green Version]
- Khalifeh, S.; Burleigh, T.D. Super-hydrophobic stearic acid layer formed on anodized high purified magnesium for improving corrosion resistance of bioabsorbable implants. J. Magnes. Alloy. 2018, 6, 327–336. [Google Scholar] [CrossRef]
- Qiao, L.Y.; Wang, Y.; Wang, W.L.; Mohedano, M.; Gong, C.H.; Gao, J.C. The preparation and corrosion performance of self-assembled monolayers of stearic acid and MgO layer on pure magnesium. Mater. Trans. 2014, 55, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.F.; Zhang, X.M.; Liu, X.L.; Wang, Y.C.; Zhang, Y.G.; Li, Y.Y.; Zhao, W.M.; Qin, C.L.; Mukanova, A.; Bakenov, Z. Bimodal nanoporous NiO@Ni–Si network prepared by dealloying method for stable Li-ion storage. J. Power Sources 2020, 449, 227550. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Liu, X.L.; Zhang, W.Q.; Zhang, Y.G.; Li, Y.Y.; Qin, C.L.; Zhao, W.M.; Bakenov, Z. Dual-network nanoporous NiFe2O4/NiO composites for high performance Li-ion battery anodes. Chem. Eng. J. 2020, 388, 124207. [Google Scholar] [CrossRef]
- Qin, C.L.; Zheng, D.H.; Hu, Q.F.; Zhang, X.M.; Wang, Z.F.; Li, Y.Y.; Zhu, J.S.; Ou, J.Z.; Yang, C.H.; Wang, Y.C. Flexible integrated metallic glass-based sandwich electrodes for high-performance wearable all-solid-state supercapacitors. Appl. Mater. Today 2020, 19, 100539. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liang, Z.F.; Yang, L.Z.; Zhao, W.M.; Wang, Y.L.; Yu, H.; Qin, C.L.; Wang, Z.F. Surface morphologies and mechanical properties of Mg-Zn-Ca amorphous alloys under chemistry-mechanics interactive environments. Metals 2019, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.F.; Liu, J.Y.; Qin, C.L.; Yu, H.; Xia, X.C.; Wang, C.Y.; Zhang, Y.S.; Hu, Q.F.; Zhao, W.M. Dealloying of Cu-based metallic glasses in acidic solutions: Products and energy storage applications. Nanomaterials 2015, 5, 697–721. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Wang, Y.S.; Fan, H.L.; Falco, G.; Yang, S.; Shangguan, J.; Bandosz, T.J. Bifunctional ZnO-MgO/activated carbon adsorbents boost H2S room temperature adsorption and catalytic oxidation. Appl. Catal. B-Environ. 2020, 266, 118674. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Zheng, X.K.; Wang, C.; Ma, J.W.; Yan, B.B.; Du, Z.N.; Li, M.Y.; Wang, W.J.; Fan, H.Q. 3D porous flower-like ZnO microstructures loaded by large-size Ag and their ultrahigh sensitivity to ethanol. J. Alloys Compd. 2020, 829, 154453. [Google Scholar] [CrossRef]
- Wang, Z.F.; Fei, P.Y.; Xiong, H.Q.; Qin, C.L.; Zhao, W.M.; Liu, X.Z. CoFe2O4 nanoplates synthesized by dealloying method as high performance Li-ion battery anodes. Electrochim. Acta 2017, 252, 295–305. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Wang, Z.F.; Qin, C.L.; Zhang, M.M.; Li, Y.Y. Porous CuxO/Ag2O (x = 1, 2) nanowires anodized on nanoporous Cu-Ag bimetal network as a self-supported flexible electrode for glucose sensing. Appl. Surf. Sci. 2020, 515, 146062. [Google Scholar] [CrossRef]
- Liu, A.H.; Xu, J.L. Preparation and corrosion resistance of superhydrophobic coatings on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 2287–2293. [Google Scholar] [CrossRef]
- Wu, L.; Wu, J.H.; Zhang, Z.Y.; Zhang, C.; Zhang, Y.H.; Tang, A.T.; Li, L.J.; Zhang, G.; Zheng, Z.C.; Atrens, A.; et al. Corrosion resistance of fatty acid and fluoroalkylsilane-modified hydrophobic Mg-Al LDH films on anodized magnesium alloy. Appl. Surf. Sci. 2019, 487, 569–580. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Liu, X.; Xiong, H.; Zhou, J.; Yu, H.; Qin, C.; Wang, Z. Stearic Acid Coated MgO Nanoplate Arrays as Effective Hydrophobic Films for Improving Corrosion Resistance of Mg-Based Metallic Glasses. Nanomaterials 2020, 10, 947. https://doi.org/10.3390/nano10050947
Yan Y, Liu X, Xiong H, Zhou J, Yu H, Qin C, Wang Z. Stearic Acid Coated MgO Nanoplate Arrays as Effective Hydrophobic Films for Improving Corrosion Resistance of Mg-Based Metallic Glasses. Nanomaterials. 2020; 10(5):947. https://doi.org/10.3390/nano10050947
Chicago/Turabian StyleYan, Yonghui, Xiaoli Liu, Hanqing Xiong, Jun Zhou, Hui Yu, Chunling Qin, and Zhifeng Wang. 2020. "Stearic Acid Coated MgO Nanoplate Arrays as Effective Hydrophobic Films for Improving Corrosion Resistance of Mg-Based Metallic Glasses" Nanomaterials 10, no. 5: 947. https://doi.org/10.3390/nano10050947
APA StyleYan, Y., Liu, X., Xiong, H., Zhou, J., Yu, H., Qin, C., & Wang, Z. (2020). Stearic Acid Coated MgO Nanoplate Arrays as Effective Hydrophobic Films for Improving Corrosion Resistance of Mg-Based Metallic Glasses. Nanomaterials, 10(5), 947. https://doi.org/10.3390/nano10050947





