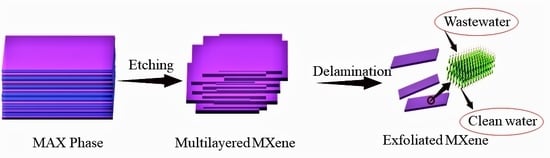
Unveiling Fabrication and Environmental Remediation of MXene-Based Nanoarchitectures in Toxic Metals Removal from Wastewater: Strategy and Mechanism
Abstract
1. Introduction
2. Methods for Toxic Metal Removal from Wastewater
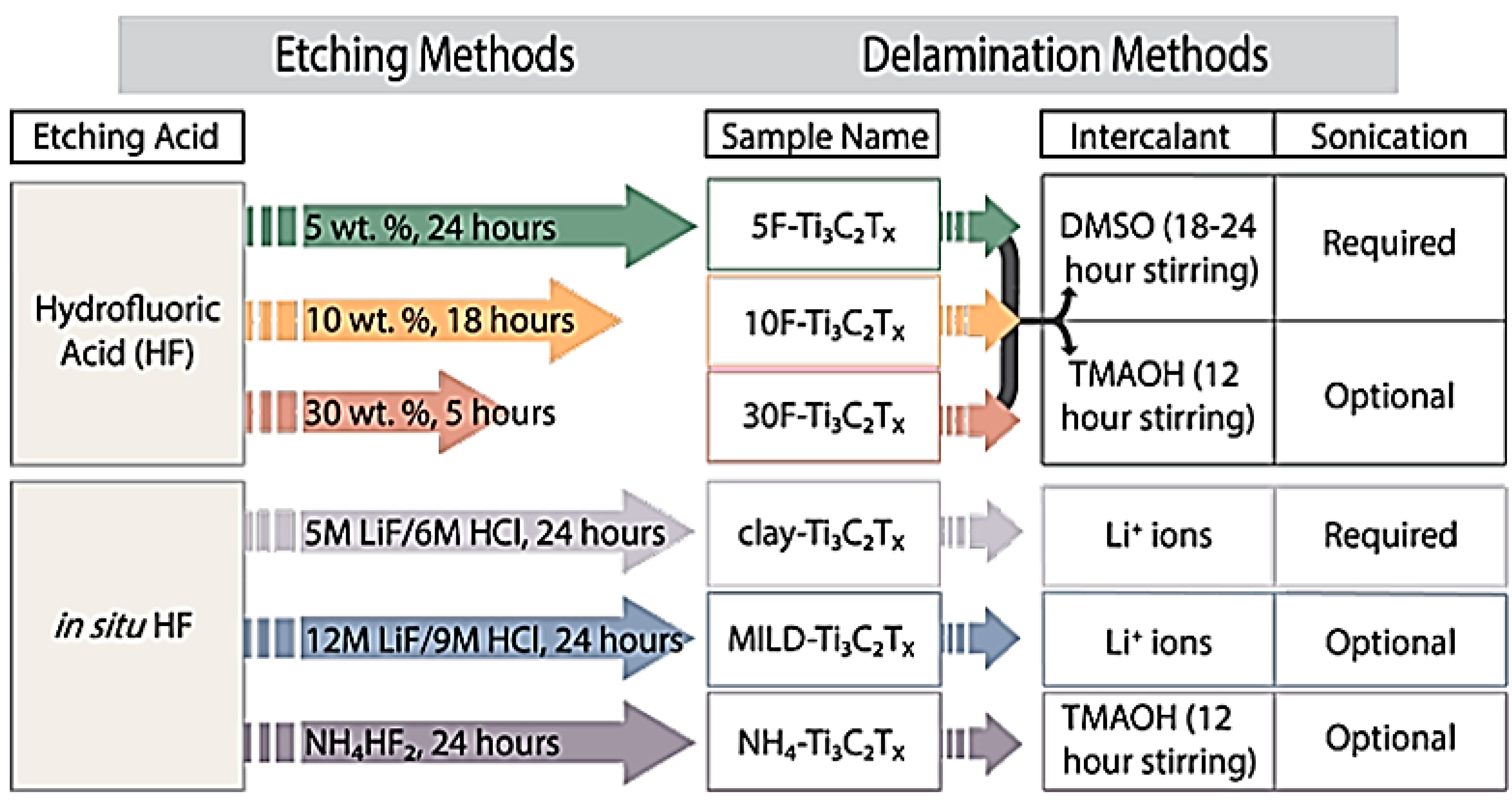
3. Characterization of MXenes and Their Preparation
4. MXenes for Toxic Metal Removal
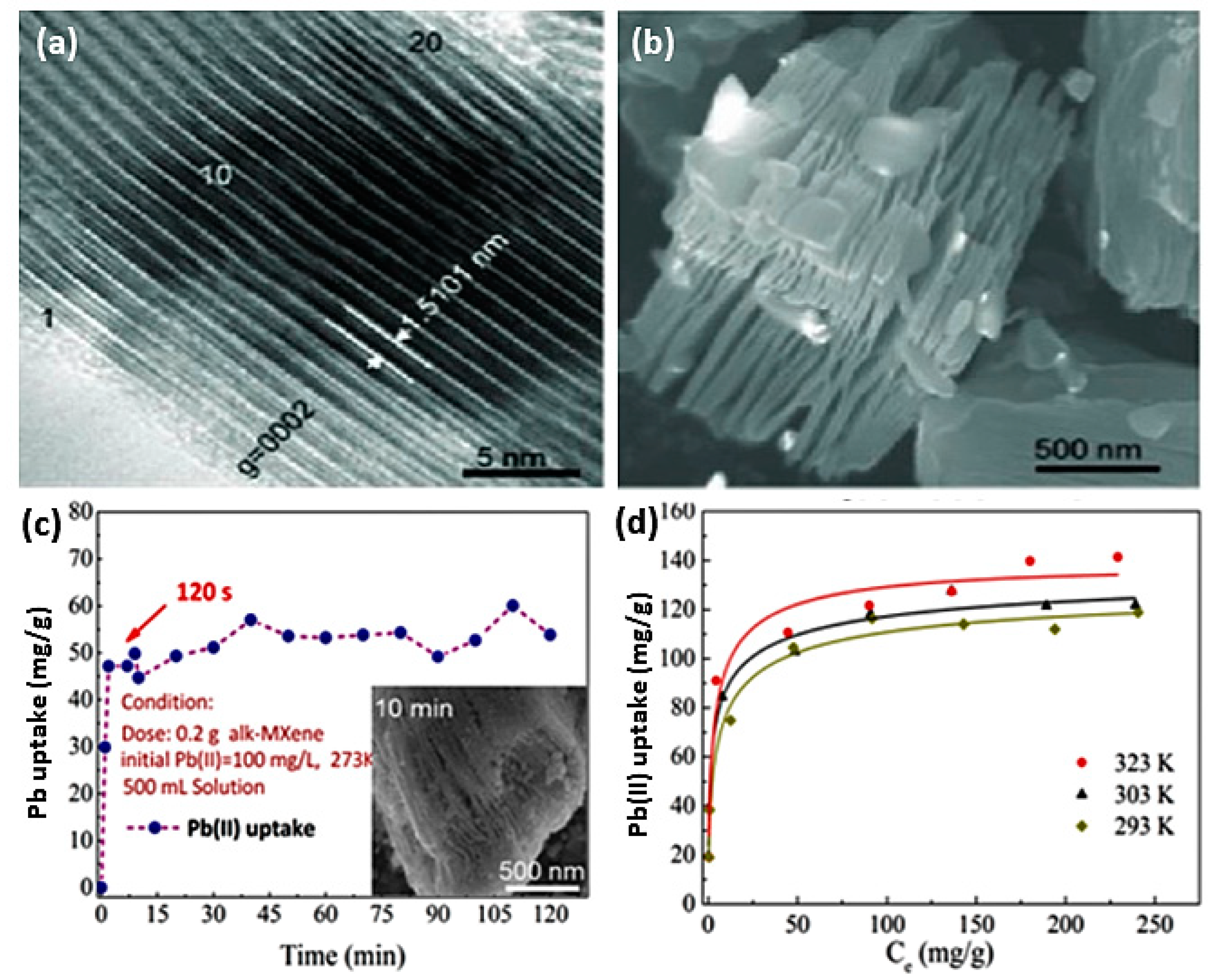
4.1. Sorption of Lead by MXenes
4.2. Removal and Reduction of Chromium Ions by MXenes
4.3. Copper Ion Removal from Wastewater
4.4. Mercury Removal Using MXene Composites
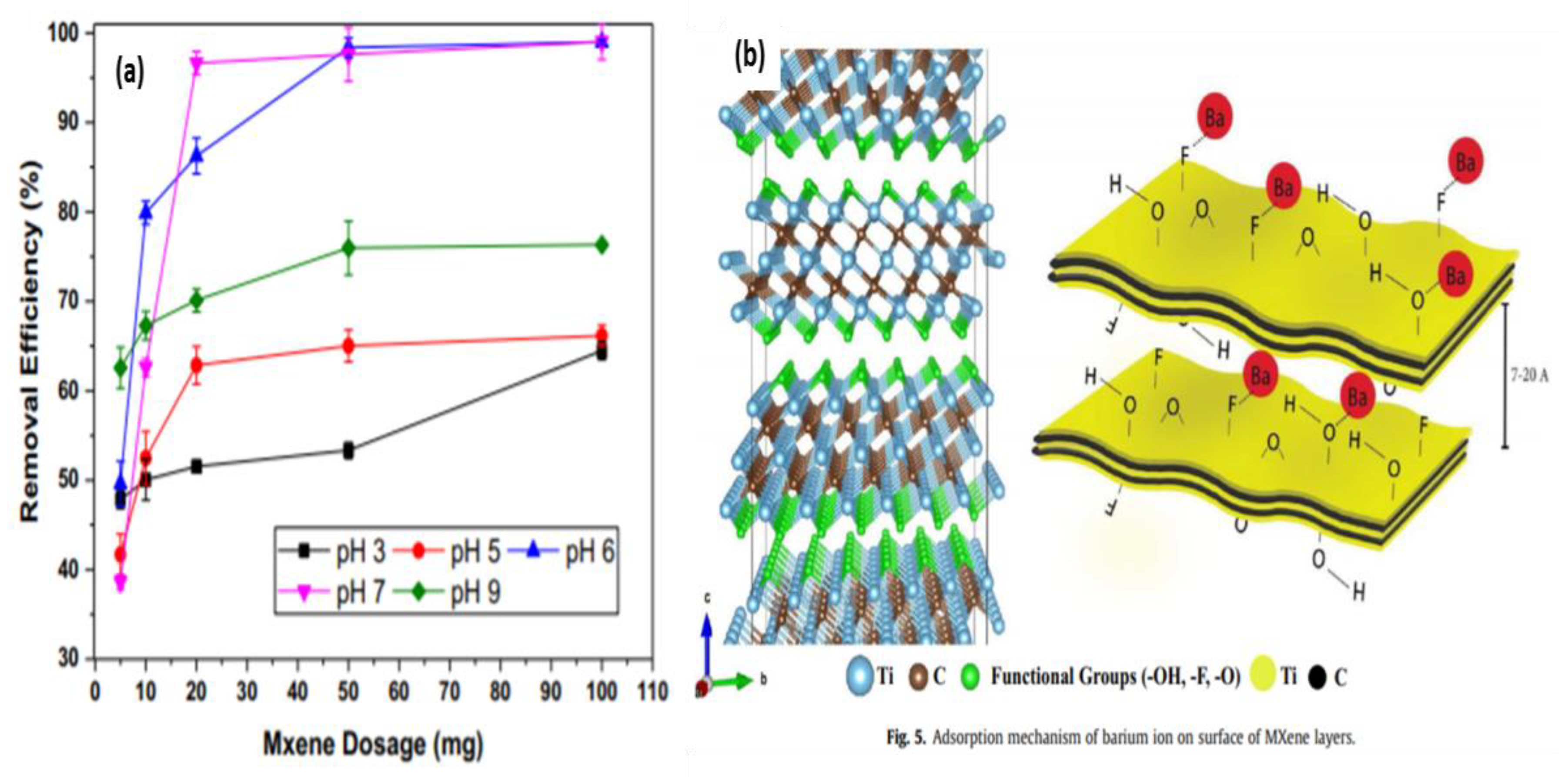
4.5. Barium Extraction Using MXenes
4.6. Phosphate Removal with MXenes
4.7. Limitations of MXenes Regarding Toxic Metal Removal
5. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J. Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.J.N. The nanosized dye adsorbents for water treatment. Nanomaterials 2020, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L.; Qi, P.; Liu, X.; Wei, G.J.N. Synthesis of three-dimensional graphene-based hybrid materials for water purification: A review. Nanomaterials 2019, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, G.; Duan, X.; Liang, X.; Meng, J.; Liang, J. Environmental applications of diatomite minerals in removing heavy metals from water. Ind. Eng. Chem. Res. 2019, 58, 11638–11652. [Google Scholar] [CrossRef]
- Yahaya Pudza, M.; Zainal Abidin, Z.; Abdul Rashid, S.; Md Yasin, F.; Noor, A.; Issa, M.A. Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef]
- Rauwel, P.; Uhl, W.; Rauwel, E. Editorial for the Special Issue on ‘Application and Behavior of Nanomaterials in Water Treatment’. Nanomaterials 2019, 9, 880. [Google Scholar] [CrossRef]
- Chen, R.; Sheehan, T.; Ng, J.L.; Brucks, M.; Su, X. Technology Capacitive deionization and electrosorption for heavy metal removal. Environ. Sci. Water Res. Technol. 2020, 6, 258–282. [Google Scholar] [CrossRef]
- Fu, L.; Yan, Z.; Zhao, Q.; Yang, H. Novel 2D nanosheets with potential applications in heavy metal purification: A review. Adv. Mater. Interfaces 2018, 5, 1801094. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chem. Eng. 2020, 388, 124340. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes); Springer: New York, NY, USA, 2019; ISBN 978-3-030-19025-5. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXene nanomaterials: a review. Rsc. Adv. 2018, 8, 19895–19905. [Google Scholar] [CrossRef]
- Yao, Z.; Sun, H.; Sui, H.; Liu, X. Construction of BPQDs/Ti3C2@ TiO2 Composites with Favorable Charge Transfer Channels for Enhanced Photocatalytic Activity under Visible Light Irradiation. Nanomaterials 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Q.; Huang, H.; Mao, L.; Liu, M.; Zhang, X.; Wei, Y. Recent progress and advances in the environmental applications of MXene related materials. Nanoscale 2020, 12, 3574–3592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Jian, M.; Zhang, Q.; Xu, R.; Liu, R.; Zhang, X.; Liu, H. A new paradigm of ultrathin 2D nanomaterial adsorbents in aqueous media: graphene and GO, MoS 2, MXenes, and 2D MOFs. J. Mater. Chem. A 2019, 7, 16598–16621. [Google Scholar] [CrossRef]
- Burkhard, R.; Deletic, A.; Craig, A. Techniques for water and wastewater management: a review of techniques and their integration in planning. Urban Water 2000, 2, 197–221. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290. [Google Scholar] [CrossRef]
- Bhojwani, S.; Topolski, K.; Mukherjee, R.; Sengupta, D.; El-Halwagi, M.M. Technology review and data analysis for cost assessment of water treatment systems. Sci. Total. Environ. 2019, 651, 2749–2761. [Google Scholar] [CrossRef]
- Yang, H.-L.; Sun, X.-W.; Zhang, Y.-M.; Wang, Z.-H.; Zhu, W.; Fan, Y.-Q.; Wei, T.-B.; Yao, H.; Lin, Q. A bi-component supramolecular gel for selective fluorescence detection and removal of Hg 2+ in water. Soft Matter. 2019, 15, 9547–9552. [Google Scholar] [CrossRef]
- Ijanu, E.M.; Kamaruddin, M.A.; Norashiddin, F.A. Coffee processing wastewater treatment: a critical review on current treatment technologies with a proposed alternative. Appl. Water Sci. 2020, 10, 11. [Google Scholar] [CrossRef]
- Sherlala, A.I.; Raman, A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.W.; Teong, L.; Hanafiah, M.A. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Lim, J.Y.; Mubarak, N.M.; Abdullah, E.C.; Nizamuddin, S.; Khalid, M. Chemistry, E. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. Ind. Eng. Chem. Res. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotecnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the use of metal–organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2016, 4, 3565. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Halfhide, T.; Udom, I.; Gilles, B.; Wolan, J.; Zhang, Q.; Ergas, S. Wastewater use in algae production for generation of renewable resources: A review and preliminary results. Aquat. Biosyst. 2013, 9, 2. [Google Scholar] [CrossRef]
- Ashaghi, K.S.; Ebrahimi, M.; Czermak, P. Ceramic ultra-and nanofiltration membranes for oilfield produced water treatment: A mini review. Open Environ. Sci. 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Skouteris, G.; Saroj, D.; Melidis, P.; Hai, F.I.; Ouki, S. The effect of activated carbon addition on membrane bioreactor processes for wastewater treatment and reclamation–a critical review. Bioresour. Technol. 2015, 185, 399–410. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Melchior, S.A.; Palaniyandy, N.; Sigalas, I.; Iyuke, S.E.; Ozoemena, K.I. Probing the electrochemistry of MXene (Ti2CTx)/electrolytic manganese dioxide (EMD) composites as anode materials for lithium-ion batteries. Electrochim. Acta 2019, 297, 961–973. [Google Scholar] [CrossRef]
- Habib, I.; Ferrer, P.; Ray, S.C.; Ozoemena, K.I. Interrogating the impact of onion-like carbons on the supercapacitive properties of MXene (Ti2CTX). J. Appl. Phys. 2019, 126, 134301. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2T x MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Halim, J.; Kota, S.; Lukatskaya, M.R.; Naguib, M.; Zhao, M.-Q.; Moon, E.J.; Pitock, J.; Nanda, J.; May, S.J.; Gogotsi, Y.; et al. Synthesis and characterization of 2D molybdenum carbide (MXene). Adv. Funct. Mater. 2016, 26, 3118–3127. [Google Scholar] [CrossRef]
- Hong Ng, V.M.; Huang, H.; Zhou, K.; Lee, P.S.; Que, W.; Xu, J.Z.; Kong, L.B. Recent progress in layered transition metal carbides and/or nitrides (MXenes) and their composites: synthesis and applications. R. Soc. Chem. J. Mater. Chem. A 2017, 5, 3039–3068. [Google Scholar] [CrossRef]
- Xiu, L.; Wang, Z.; Yu, M.; Wu, X.; Qiu, J. Aggregation-resistant 3D MXene-based architecture as efficient bifunctional electrocatalyst for overall water splitting. ACS Nano 2018, 12, 8017–8028. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation stability of colloidal two-dimensional titanium carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Seif, A.; Ahmadi, T.S.; Zargar, B.; Rashidi, F.; Rouzbahani, G.M. Thermodynamic and kinetic studies for the adsorption of Hg (II) by nano-TiO2 from aqueous solution. Adv. Powder Technol. 2018, 23, 148–156. [Google Scholar] [CrossRef]
- Cheng, K.; Cai, Z.; Fu, J.; Sun, X.; Sun, W.; Chen, L.; Zhang, D.; Liu, W. Synergistic adsorption of Cu (II) and photocatalytic degradation of phenanthrene by a jaboticaba-like TiO2/titanate nanotube composite: An experimental and theoretical study. Chem. Eng. J. 2019, 358, 1155–1165. [Google Scholar] [CrossRef]
- Almeida, J.C.; Cardoso, C.E.; Tavares, D.S.; Freitas, R.; Trindade, T.; Vale, C.; Pereira, E. Chromium removal from contaminated waters using nanomaterials–a review. Trends Analyt. Chem. 2019, 118, 277–291. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, Y.; Xu, W.; Huang, D.; Wang, Z.; Hong, M. Fabrication and thermal stability of two-dimensional carbide Ti3C2 nanosheets. Ceram. Int. 2016, 42, 8419–8424. [Google Scholar] [CrossRef]
- Wu, Y.; Nie, P.; Wang, J.; Dou, H.; Zhang, X. Few-layer MXenes delaminated via high-energy mechanical milling for enhanced sodium-ion batteries performance. ACS Appl. Mater. Interfaces 2017, 9, 39610–39617. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.; Mathew, K.; Hennig, R.G.; Sinnott, S.B. Predicted surface composition and thermodynamic stability of MXenes in solution. J. Phys. Chem. 2016, 120, 3550–3556. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Q.; Fu, H.; Zou, G.; Zhang, Q. Heavy-metal adsorption behavior of two-dimensional alkalization-intercalated MXene by first-principles calculations. J. Phys. Chem. 2015, 119, 20923–20930. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Jun, B.M.; Her, N.; Park, C.M.; Yoon, Y. Effective removal of Pb (ii) from synthetic wastewater using Ti 3 C 2 T x MXene. Environ. Sci. Water Res. Technol. 2020, 6, 173–180. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Lü, Q.-F.; Zhuang, H. Facile preparation of biosurfactant-functionalized Ti2CTX MXene nanosheets with an enhanced adsorption performance for Pb (II) ions. J. Mol. Liq. 2020, 297, 111810. [Google Scholar] [CrossRef]
- Du, Y.; Yu, B.; Lianqi, W.; Wang, Y.; Zhang, X.; Shufeng, Y. Efficient removal of Pb (II) by Ti 3 C 2 T x powder modified with a silane coupling agent. J. Mater. Sci. 2019, 54, 13283–13297. [Google Scholar] [CrossRef]
- Dong, Y.; Sang, D.; He, C.; Sheng, X.; Lei, L. Mxene/alginate composites for lead and copper ion removal from aqueous solutions. Rsc. Adv. 2019, 9, 29015–29022. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, Y.; Wang, X.; Mao, Y.; Cao, W.; Hu, P.; Peng, X. Two-dimensional titanium carbide for efficiently reductive removal of highly toxic chromium (VI) from water. ACS Appl. Mater. Interfaces 2015, 7, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, C.; Que, W. A novel two-dimensional accordion-like titanium carbide (MXene) for adsorption of Cr (VI) from aqueous solution. J. Adv. Dielectr. 2018, 8. [Google Scholar] [CrossRef]
- Zou, G.; Guo, J.; Peng, Q.; Zhou, A.; Zhang, Q.; Liu, B. Synthesis of urchin-like rutile titania carbon nanocomposites by iron-facilitated phase transformation of MXene for environmental remediation. J. Mater. Chem. A. 2016, 4, 489–499. [Google Scholar] [CrossRef]
- Wang, H.; Cui, H.; Song, X.; Xu, R.; Wei, N.; Tian, J.; Niu, H. Facile synthesis of heterojunction of MXenes/TiO2 nanoparticles towards enhanced hexavalent chromium removal. J. Colloid Interface Sci. 2020, 561, 46–57. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, D.; He, Z.; Yang, X.; Yue, G.; Zhu, J.; Astruc, D.; Zhao, P. Nanoscale zero-valent iron intercalated 2D titanium carbides for removal of Cr (VI) in aqueous solution and the mechanistic aspect. J. Hazard. Mater. 2019, 121761. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Chai, L.; Yang, W.; Wang, H.; Zhang, L. Two-Dimensional Titanium Carbides (Ti3C2Tx) Functionalized by Poly (m-phenylenediamine) for Efficient Adsorption and Reduction of Hexavalent Chromium. Int. J. Environ. Res. Public Health. 2019, 17, 167. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S. Two-Dimensional Ti3C2Tx MXene Nanosheets for Efficient Copper Removal from Water. ACS Sustain. Chem. 2017, 5, 11481–11488. [Google Scholar] [CrossRef]
- Gan, D.; Huang, Q.; Dou, J.; Huang, H.; Chen, J.; Liu, M.; Wen, Y.; Yang, Z.; Zhang, X.; Wei, Y. Bioinspired functionalization of MXenes (Ti3C2TX) with amino acids for efficient removal of heavy metal ions. Appl. Surf. Sci. 2020, 504, 144603. [Google Scholar] [CrossRef]
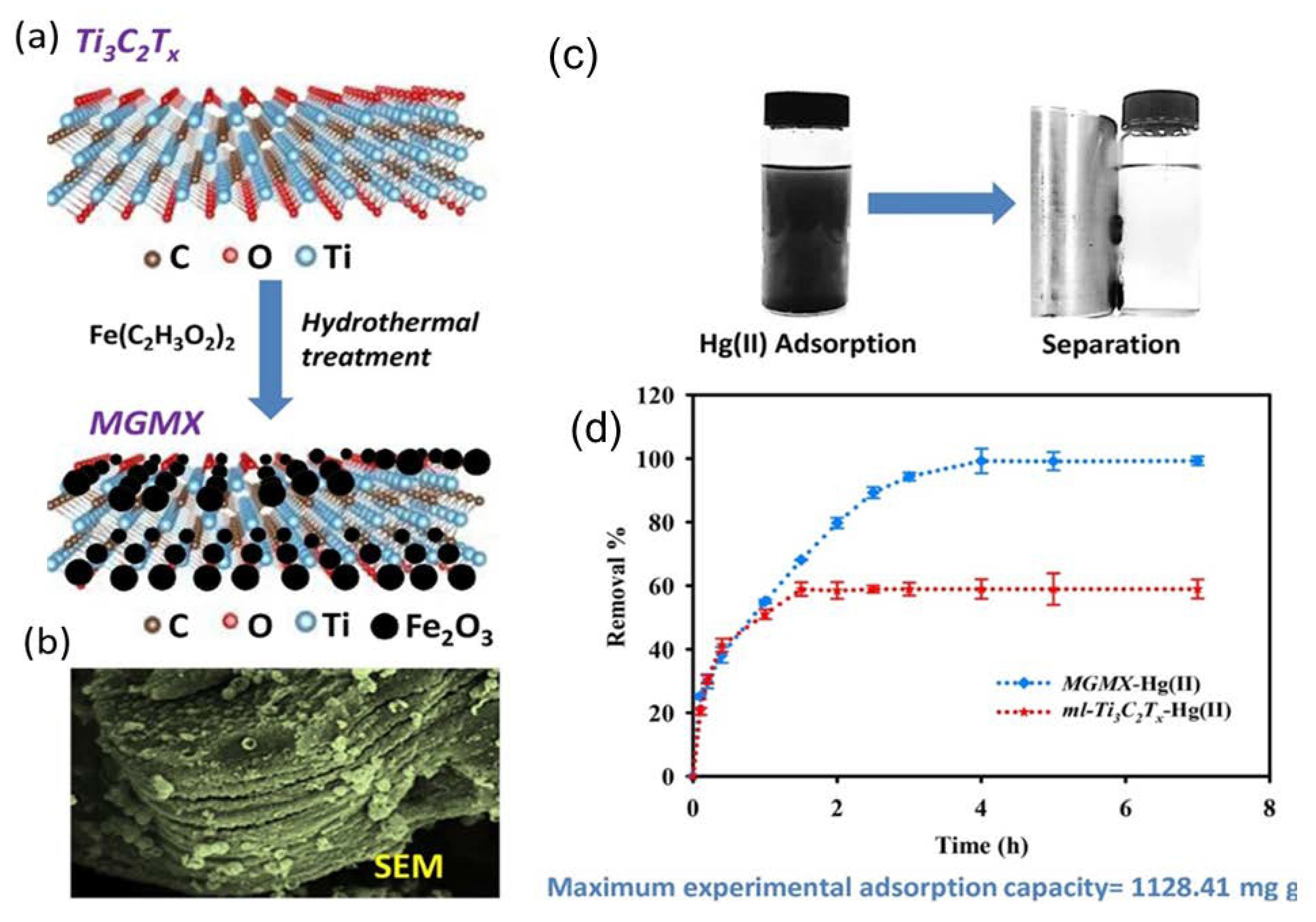
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S. Mercuric ion capturing by recoverable titanium carbide magnetic nanocomposite. J. Hazard. Mater. 2018, 344, 811–818. [Google Scholar] [CrossRef]
- Shahzad, A.; Nawaz, M.; Moztahida, M.; Jang, J.; Tahir, K.; Kim, J.; Lim, Y.; Vassiliadis, V.S.; Woo, S.H.; Lee, D.S. Ti3C2Tx MXene core-shell spheres for ultrahigh removal of mercuric ions. Chem. Eng. 2019, 368, 400–408. [Google Scholar] [CrossRef]
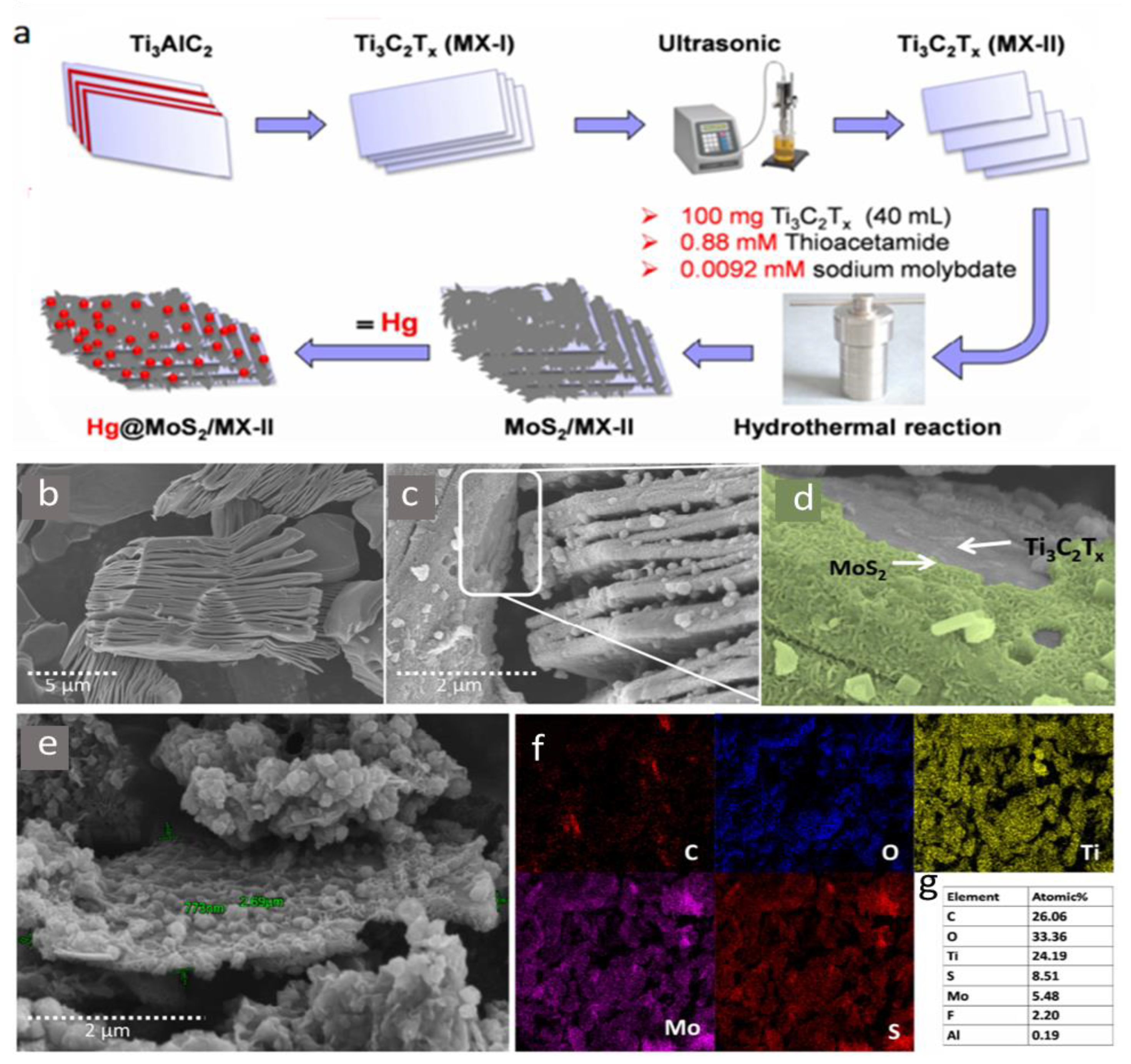
- Shahzad, A.; Jang, J.; Lim, S.R.; Lee, D.S. Unique selectivity and rapid uptake of molybdenum-disulfide-functionalized MXene nanocomposite for mercury adsorption. Environ. Res. 2020, 182. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.K.; McKay, G.; Chamoun, R.; Rhadfi, T.; Preud’Homme, H.; Atieh, M.A. Barium removal from synthetic natural and produced water using MXene as two dimensional (2-D) nanosheet adsorbent. Chem. Eng. J. 2017, 317, 331–342. [Google Scholar] [CrossRef]
- Zhang, Q.; Teng, J.; Zou, G.; Peng, Q.; Du, Q.; Jiao, T.; Xiang, J. Efficient phosphate sequestration for water purification by unique sandwich-like MXene/magnetic iron oxide nanocomposites. Nanoscale 2016, 8, 7085–7093. [Google Scholar] [CrossRef]
- Moore, M.R. Lead in drinking water in soft water areas—health hazards. Sci. Total. Environ. 1977, 7, 109–115. [Google Scholar] [CrossRef]
- Sayre, I.M. International standards for drinking water. J. Am. Water Works Assoc. 1988, 80, 53–60. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.L.; Luo, C.; Wang, X.; Duan, H. Removal of Pb2+ from water environment using a novel magnetic chitosan/graphene oxide imprinted Pb2+. Int. J. Biol. Macromol. 2016, 86, 505–511. [Google Scholar] [CrossRef]
- Zare-Dorabei, R.; Ferdowsi, S.M.; Barzin, A.; Tadjarodi, A. Highly efficient simultaneous ultrasonic-assisted adsorption of Pb (II), Cd (II), Ni (II) and Cu (II) ions from aqueous solutions by graphene oxide modified with 2, 2′-dipyridylamine: central composite design optimization. Ultrason. Sonochem. 2016, 32, 265–276. [Google Scholar] [CrossRef]
- Kurtoglu, M.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W. First principles study of two-dimensional early transition metal carbides. MRS Commun. 2012, 2, 133–137. [Google Scholar] [CrossRef]
- Guo, J.; Fu, H.; Zou, G.; Zhang, Q.; Zhang, Z.; Peng, Q. Theoretical interpretation on lead adsorption behavior of new two-dimensional transition metal carbides and nitrides. J. Alloys Compd. 2016, 184, 504–509. [Google Scholar] [CrossRef]
- Brown, G.E.; Foster, A.L.; Ostergren, J.D. Mineral surfaces and bioavailability of heavy metals: a molecular-scale perspective. Proc. Natl. Acad. Sci. USA 1999, 96, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Giammar, D.E.; Maus, C.J.; Xie, L. Effects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticles. Environ. Eng. Sci. 2007, 24, 85–95. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, S.; Zhang, C.; Wang, X.; Khan, A.; Wen, T.; Hu, B.; Alsaedi, A.; Hayat, T.; Wang, X. Two-dimensional MAX-derived titanate nanostructures for efficient removal of Pb (II). Dalton Trans. 2019, 48, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- GracePavithra, K.; Jaikumar, J.; Kumar, P.; SundarRajan, P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Srivastava, S.; Agrawal, S.B.; Mondal, M.K. Synthesis, characterization and application of Lagerstroemia speciosa embedded magnetic nanoparticle for Cr (VI) adsorption from aqueous solution. Eur. J. Environ. Sci. 2017, 55, 283–293. [Google Scholar] [CrossRef]
- Egodawatte, S.; Greenstein, K.E.; Vance, I.; Rivera, E.; Myung, N.V.; Parkin, G.F.; Cwiertny, D.M.; Larsen, S.C. Electrospun hematite nanofiber/mesoporous silica core/shell nanomaterials as an efficient adsorbent for heavy metals. Rsc. Adv. 2016, 6, 90516–90525. [Google Scholar] [CrossRef]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Becaria, A.; Lahiri, D.K.; Bondy, S.C.; Chen, D.; Hamadeh, A.; Li, H.; Taylor, R.; Campbell, A. Aluminum and copper in drinking water enhance inflammatory or oxidative events specifically in the brain. J. Neuroimmunol. 2006, 176, 16–23. [Google Scholar] [CrossRef]
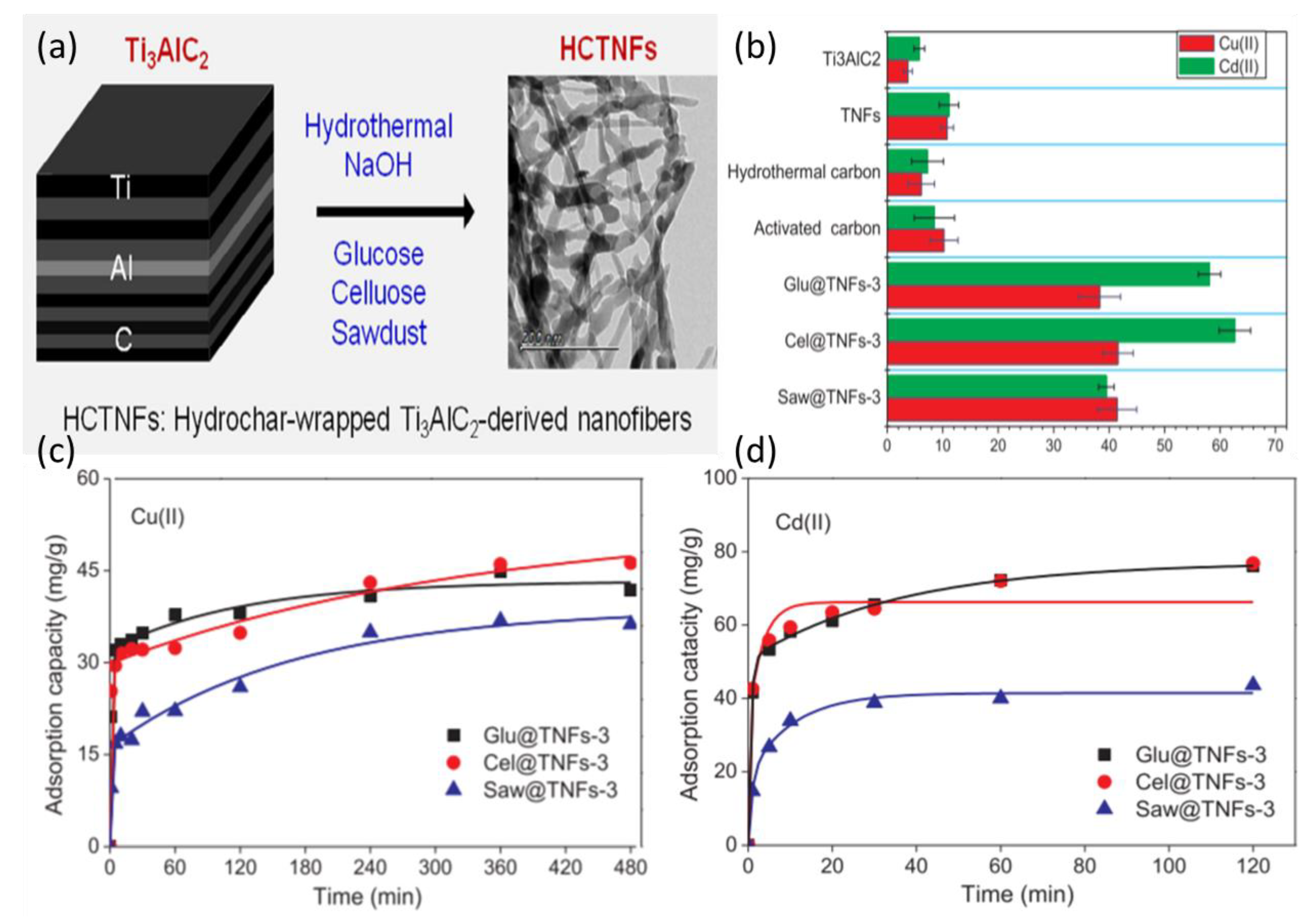
- Dong, X.; Wang, Y.; Jia, M.; Niu, Z.; Cai, J.; Yu, X.; Ke, X.; Yao, J.; Zhang, X. Sustainable and scalable in-situ synthesis of hydrochar-wrapped Ti3AlC2-derived nanofibers as adsorbents to remove heavy metals. Bioresour. Technol. 2019, 282, 222–227. [Google Scholar] [CrossRef]
- Lubick, N.; Malakoff, D. With pact’s completion, the real work begins. Science 2013, 341, 1443–1445. [Google Scholar] [CrossRef]
- Lisha, K.P.; Pradeep, T. Towards a practical solution for removing inorganic mercury from drinking water using gold nanoparticles. Gold Bull. 2009, 42, 144–152. [Google Scholar] [CrossRef]
- Yu, J.G.; Yue, B.Y.; Wu, X.W.; Liu, Q.; Jiao, F.P.; Jiang, X.Y.; Chen, X.Q. Removal of mercury by adsorption: a review. Environ. Sci. Pollut. 2016, 23, 5056–5076. [Google Scholar] [CrossRef] [PubMed]
- Chinh, N.T.; Thai, H.; Tran, T.D.M.; Nguyen, T.T.; Nguyen, O.N.; Tran, M.T.; Nguyen, T.T.T. Technology. ADSORPTION OF MERCURIC ION FROM AQUEOUS SOLUTIONS USING MODIFIED FLY ASH. J. Sci. Technol. 2018, 56, 688. [Google Scholar] [CrossRef]
- Ravi, S.; Ahn, W.S. Materials, M. Facile synthesis of a mesoporous organic polymer grafted with 2-aminoethanethiol for Hg2+ removal. Microporous Mesoporous Mater. 2018, 271, 59–67. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Yang, F. Preparation of polyaniline/reduced graphene oxide nanocomposite and its application in adsorption of aqueous Hg (II). Chem. Eng. J. 2013, 229, 460–468. [Google Scholar] [CrossRef]
- Fontana, K.B.; Chaves, E.S.; Kosera, V.S.; Lenzi, G.G. Barium removal by photocatalytic process: An alternative for water treatment. J. Water Process. Eng. 2018, 22, 163–171. [Google Scholar] [CrossRef]
- Kresse, R.; Baudis, U.; Jäger, P.; Riechers, H.H.; Wagner, H.; Winkler, J.; Wolf, H.U. Barium and barium compounds. Ullmann’s Encycl. Ind. Chem 2007. [Google Scholar] [CrossRef]
- Brenniman, G.R.; Kojola, W.H.; Levy, P.S.; Carnow, B.W.; Namekata, T. High barium levels in public drinking water and its association with elevated blood pressure. Arch. Environ. Health 1981, 36, 28–32. [Google Scholar] [CrossRef]
- Kondash, A.J.; Warner, N.R.; Lahav, O.; Vengosh, A. Radium and barium removal through blending hydraulic fracturing fluids with acid mine drainage. Environ. Sci. Technol. 2014, 48, 1334–1342. [Google Scholar] [CrossRef]
- Kapashi, E.; Kapnisti, M.; Dafnomili, A.; Noli, F. AloeVera as an effective biosorbent for the removal of thorium and barium from aqueous solutions. J. Radioanal. Nucl. Chem. 2019, 321, 217–226. [Google Scholar] [CrossRef]
- Majidnia, Z.; Idris, A.; Majid, M.; Zin, R.; Ponraj, M. Efficiency of barium removal from radioactive waste water using the combination of maghemite and titania nanoparticles in PVA and alginate beads. App. Radiat. Isot. 2015, 105, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Venkiteshwaran, K.; McNamara, P.J.; Mayer, B.K. Meta-analysis of non-reactive phosphorus in water, wastewater, and sludge, and strategies to convert it for enhanced phosphorus removal and recovery. Sci. Total Environ. 2018, 644, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, H.; Rechberger, H.; Egle, L. Phosphorus in water quality and waste management. InTech 2011, 2. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Xu, Y.; Qian, G. Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ. Technol. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Xu, X.; Yang, T.; Zhang, Q.; Xia, W.; Ding, Z.; Eid, K.; Abdullah, A.M.; Hossain, M.S.A.; Zhang, S.; Tang, J. Ultrahigh capacitive deionization performance by 3D interconnected MOF-derived nitrogen-doped carbon tubes. Chem. Eng. J. 2020, 390, 124493. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Al-Kandari, H.; Sharaf, M.A.; Abdullah, A.M. Rational synthesis of porous graphitic-like carbon nitride nanotubes codoped with Au and Pd as an efficient catalyst for carbon monoxide oxidation. Langmuir 2019, 35, 3421–3431. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.; Sliem, M.H.; Abdullah, A.M. Unraveling template-free fabrication of carbon nitride nanorods codoped with Pt and Pd for efficient electrochemical and photoelectrochemical carbon monoxide oxidation at room temperature. Nanoscale 2019, 11, 11755–11764. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Eldesoky, A.S.; Al-Kandari, H.; Abdullah, A.M. Rational synthesis of one-dimensional carbon nitride-based nanofibers atomically doped with Au/Pd for efficient carbon monoxide oxidation. Int. J. Hydrogen Energy 2019, 44, 17943–17953. [Google Scholar] [CrossRef]
- Jlassi, K.; Eid, K.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Rational synthesis, characterization, and application of environmentally friendly (polymer–carbon dot) hybrid composite film for fast and efficient UV-assisted Cd 2+ removal from water. Environ. Sci Eur. 2020, 32, 12. [Google Scholar] [CrossRef]
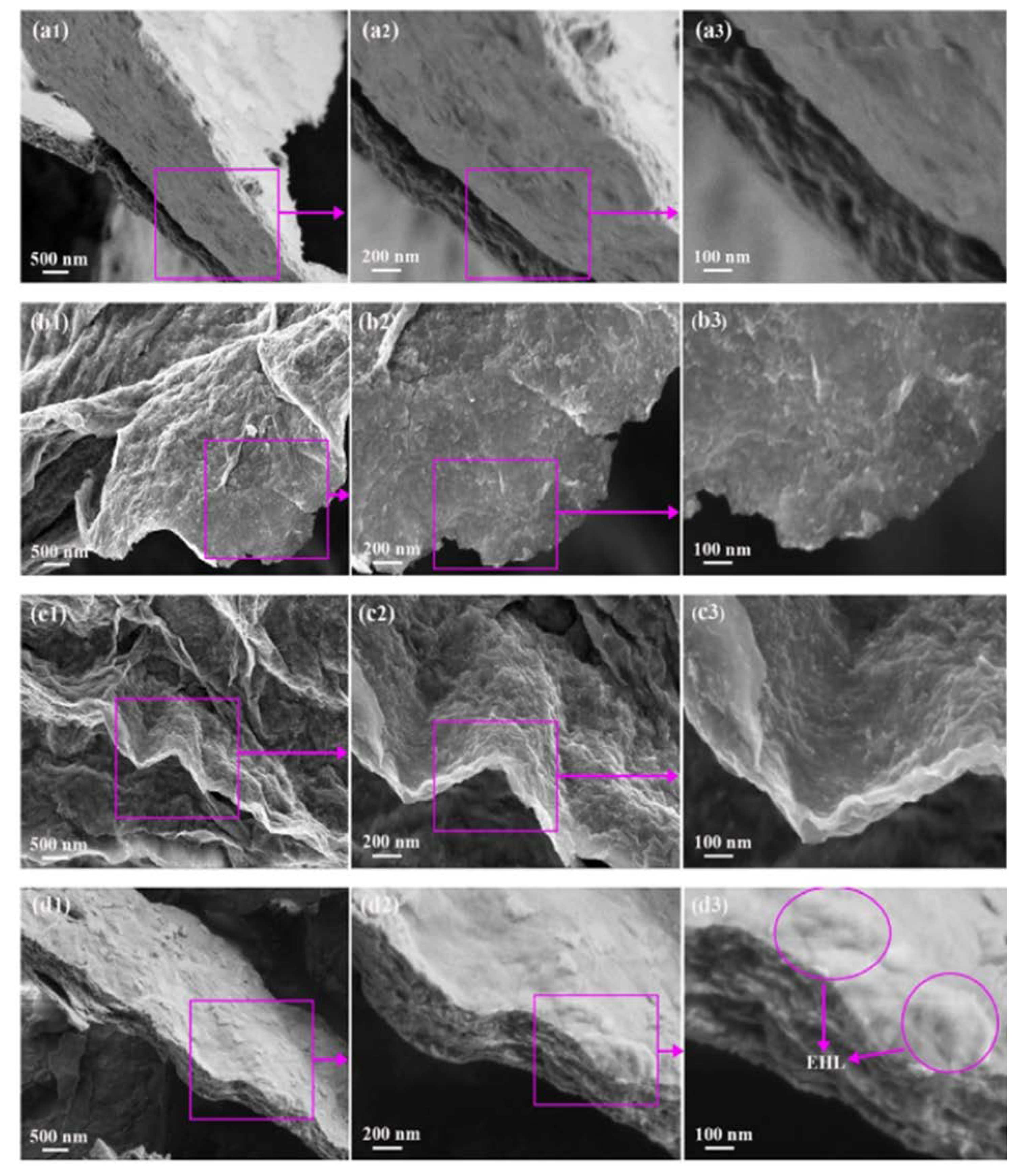
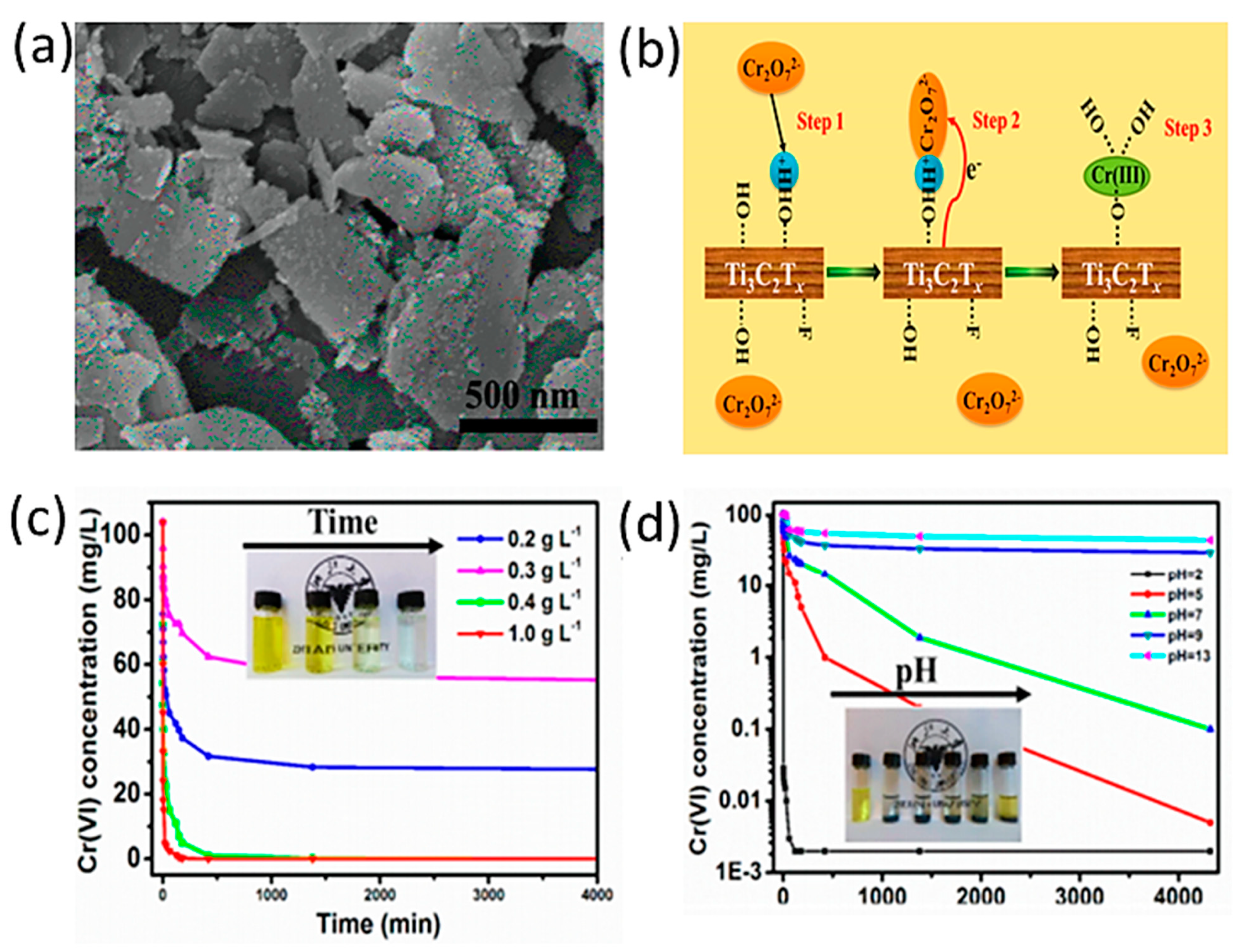
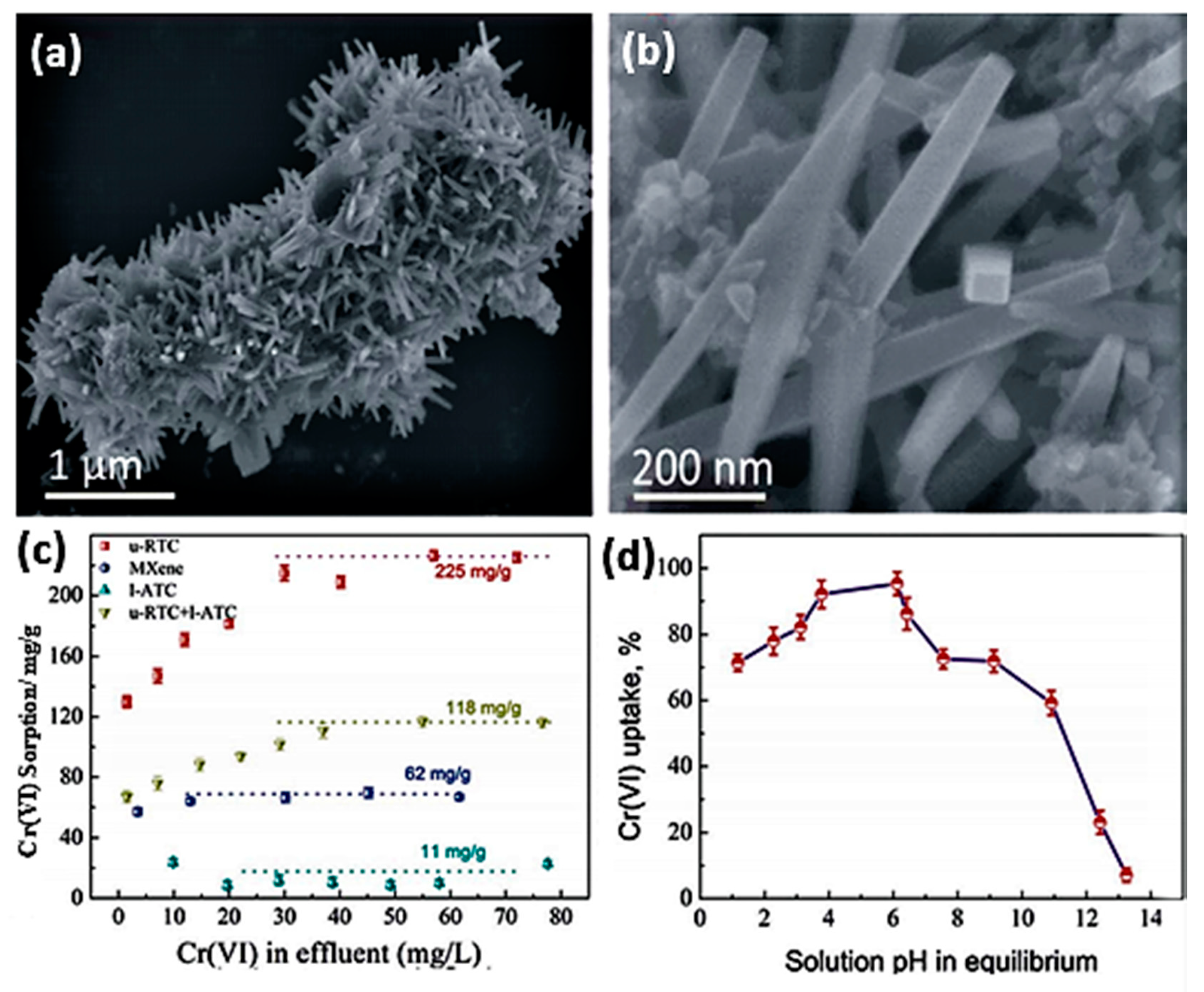
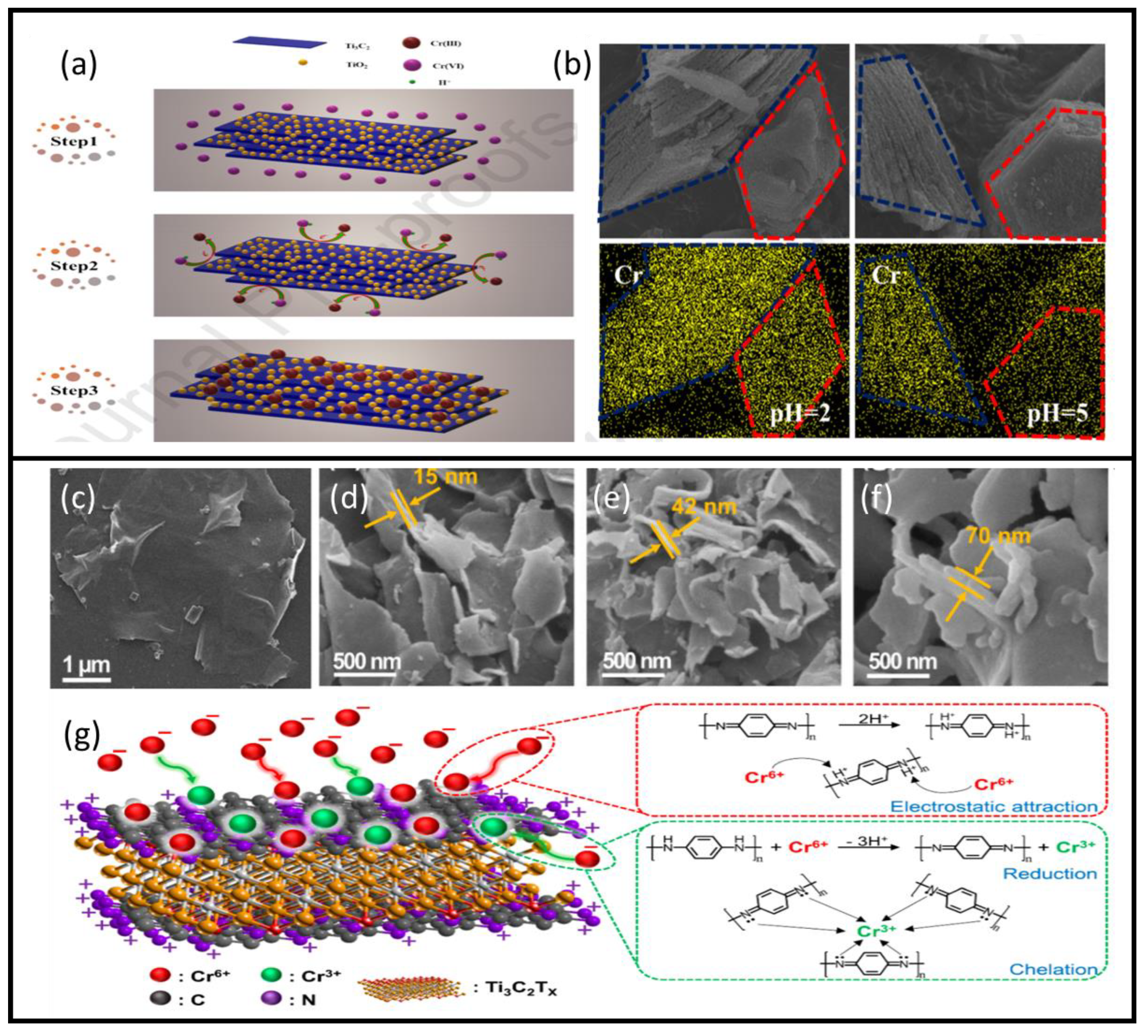
| MXene | Preparation Method | Toxic Metals | Adsorption Conditions | Adsorption Capacity/Removal Efficiency | Mechanism | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| pH | Time | Temp | ||||||
| Ti3C2(OH/ONa)xF2−x | Chemical etching of (Ti3AlC2) by HF followed by alkalization treatment with NaOH. | Pb(II) | 5.8–6.2 | 2 min | 323 K | 140.1 mg/g | Adsorption | [48] |
| Ti3C2Tx | - | Pb(II) | 6 | 2 h | 293 K | 36.6 mg/g | Adsorption | [49] |
| Biosurfactant- functionalized Ti2CTX MXene nanosheets | Chemical etching of (Ti3AlC2) by LiF+HCl solution followed by the addition of either CS, LS, or EHL solutions. | Pb(II) | 5 | 24 h | 30 °C | 232.9 mg/g | Adsorption | [50] |
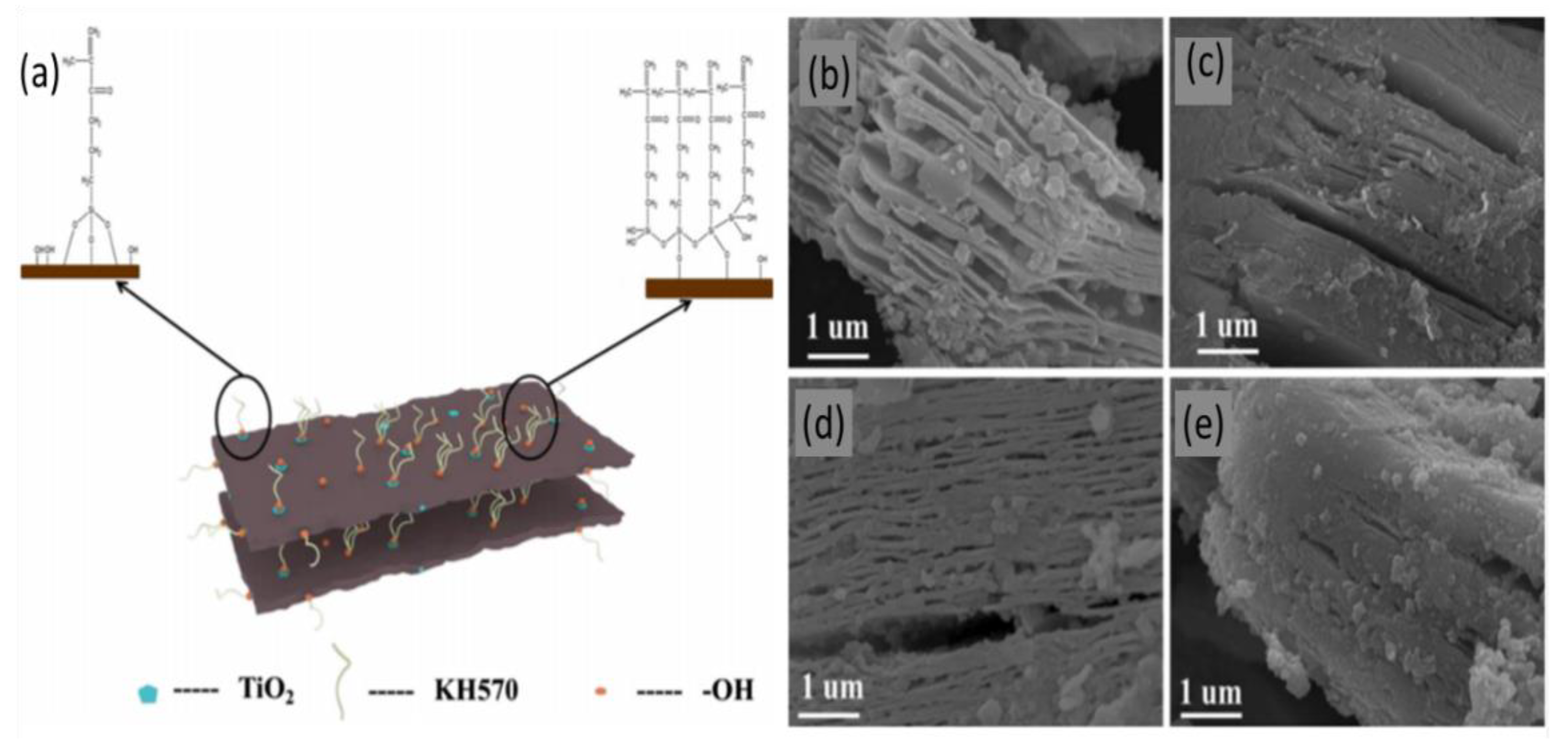
| (Ti3C2Tx- KH570) | Chemical etching of (Ti3AlC2) by 40% HF and then modified by KH570 with mechanical aggigtation to oxidize the MXene into TiO2 particles. | Pb(II) | 1–6 | 2 h | 30 °C | 147.97 mg/g | Adsorption | [51] |
| Mxene/alginate | Adding MXene powder to varying amounts of sodium alginate. | Pb(II) | 5–7 | 15 min | ~50 °C | 382.7 mg/g | Adsorption | [52] |
| Ti3C2Tx | Chemical etching of (Ti3AlC2) by 10% HF followed by intercalation and ultrasonication | Cr(VI) | 5 | 72 h | Room temperature | 250 mg/g | Reduction/adsorption | [53] |
| Ti3C2 | Chemical etching of (Ti3AlC2) by 40% HF followed by intercalation and sonication. | Cr(VI) | - | 14 h | 298 K | 80 mg/g | Adsorption | [54] |
| TiO2-C (u-RTC) | Chemical etching of (Ti3AlC2) by LiF+HCl solution followed by in situ decomposition of the MXene in a mixed solution of ethylene glycol (EG), FeCl3, and isopropyl alcohol (IPA). | Cr(VI) | 3–6 | 120 min | - | ~225 mg/g | Adsorption | [55] |
| Ti3C2/TiO2 | Chemical etching of (Ti3AlC2) by HF followed by hydrothermal heating (160 °C) to obtain Ti3C2/TiO2 particles. | Cr(VI) | acidic | 12 min | - | Reduction Efficiency 99.35% | Reduction/ adsorption | [56] |
| nZVI-alk-Ti3C2 | Chemical etching of (Ti3AlC2) by HF followed by alkalization treatment with KOH and then adding NaBH4 to reduce Fe2+ into nZVI. | Cr(VI) | 2 | ~1500 min | - | 194.87 mg/g | Adsorption | [57] |
| Ti3C2Tx/PmPD-5/1 | Intercalation of poly(m-phenylenediamine) (PmPD) into regular MXene sheets. | Cr(VI) | 2 | ~700 min | - | 540.47 mg/g | Reduction/ adsorption | [58] |
| Ti3C2Tx | Chemical etching of (Ti3AlC2) by HF followed by intercalation and ultrasonication | Cu(II) | 5 | 3 min | 298 K | 78.45 mg/g | Reduction/ adsorption | [59] |
| Amino acids modified MXenes (Ti3C2TX-PDOPA) | Prepared through Single-step via self-polymerization of DOPA then adding it to the MXene. | Cu(II) | 7 | 1 h | 298 K | 18.36 mg/g | Adsorption | [60] |
| Magnetic Ti3C2TX nanocomposite | Magnetizing MXene flakes through the deposition of Fe2O3 and Fe3O4 nanoparticles on its surface. | Hg(II) | 6 | 24 h | 298K | 1128.41 mg/g | Adsorption | [61] |
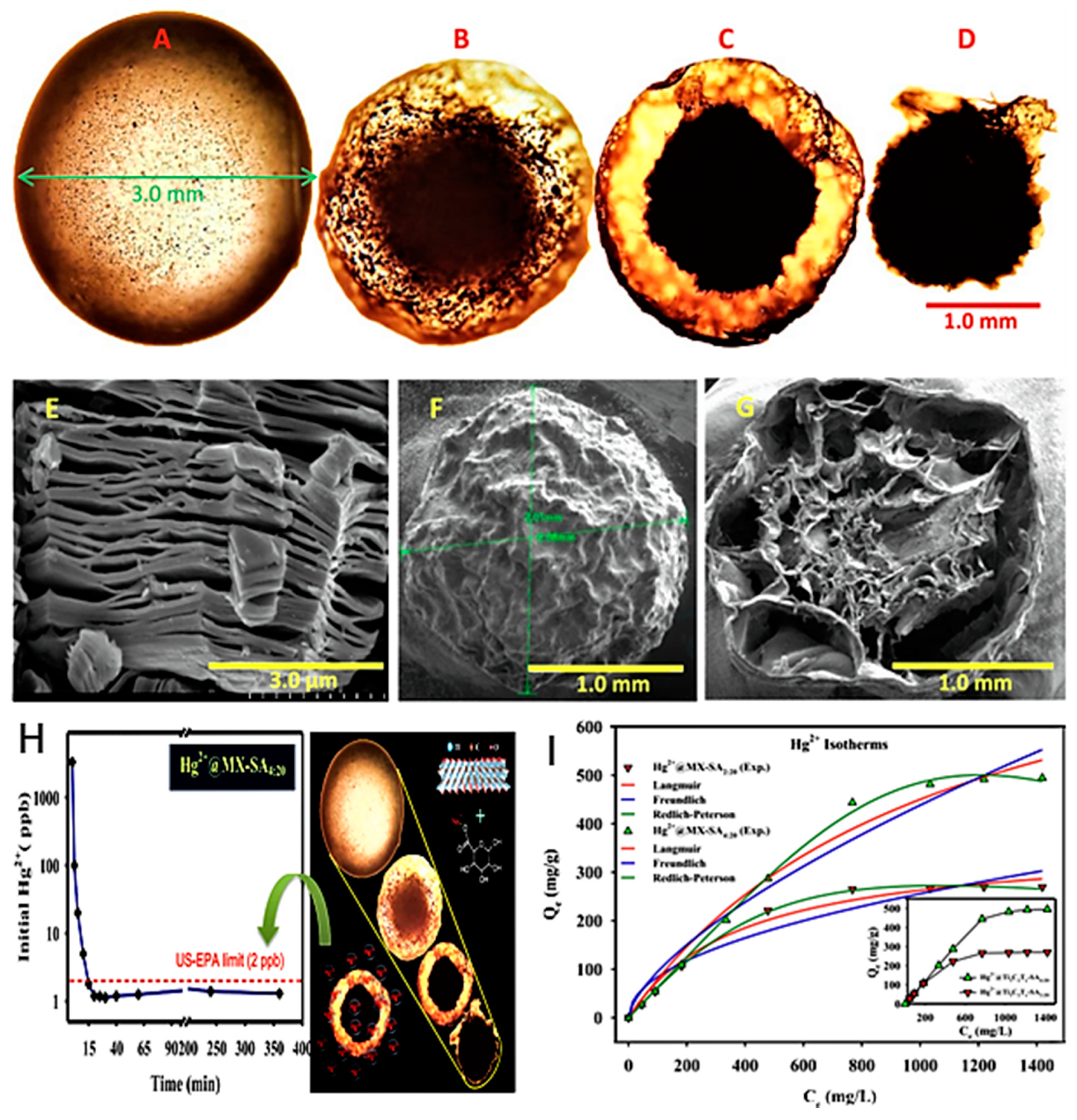
| Ti3C2TX core-shell spheres containing sodium alginate | Chemical etching of (Ti3AlC2) by NH4F followed by ultrasonication under Ar gas with sodium alginate (SA) powder. | Hg (II) | 4.5 | 24 h | 298 K | 932.84 mg/g | Adsorption | [62] |
| Molybdenum-disulfidefunctionalized MXenes (MoS2/MX) | Synthesized by a facile hydrothermal treatment method. | Hg (II) | 2–11 | 2 min | - | 1435.2 mg/g removal efficiency of 98.5% | Adsorption | [63] |
| Ti3C2Tx | Chemical etching of (Ti3AlC2) by HF followed by intercalation and ultrasonication | Ba(II) | 7 | 2 h | 25 °C | 9.3 | Adsorption | [64] |
| Ti3C2(OH)xF1−x | Chemical etching of (Ti3AlC2) by followed by magnetic ferric oxide intercalation. | PO43− | 2.5–6 | ~250 min | - | 2400 mg/g | Adsorption | [65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, Y.; Kassab, A.; Eid, K.; M. Abdullah, A.; Ozoemena, K.I.; Elzatahry, A. Unveiling Fabrication and Environmental Remediation of MXene-Based Nanoarchitectures in Toxic Metals Removal from Wastewater: Strategy and Mechanism. Nanomaterials 2020, 10, 885. https://doi.org/10.3390/nano10050885
Ibrahim Y, Kassab A, Eid K, M. Abdullah A, Ozoemena KI, Elzatahry A. Unveiling Fabrication and Environmental Remediation of MXene-Based Nanoarchitectures in Toxic Metals Removal from Wastewater: Strategy and Mechanism. Nanomaterials. 2020; 10(5):885. https://doi.org/10.3390/nano10050885
Chicago/Turabian StyleIbrahim, Yassmin, Amal Kassab, Kamel Eid, Aboubakr M. Abdullah, Kenneth I. Ozoemena, and Ahmed Elzatahry. 2020. "Unveiling Fabrication and Environmental Remediation of MXene-Based Nanoarchitectures in Toxic Metals Removal from Wastewater: Strategy and Mechanism" Nanomaterials 10, no. 5: 885. https://doi.org/10.3390/nano10050885
APA StyleIbrahim, Y., Kassab, A., Eid, K., M. Abdullah, A., Ozoemena, K. I., & Elzatahry, A. (2020). Unveiling Fabrication and Environmental Remediation of MXene-Based Nanoarchitectures in Toxic Metals Removal from Wastewater: Strategy and Mechanism. Nanomaterials, 10(5), 885. https://doi.org/10.3390/nano10050885