Bactericidal Silver Nanoparticles by Atmospheric Pressure Solution Plasma Processing
Abstract
:1. Introduction
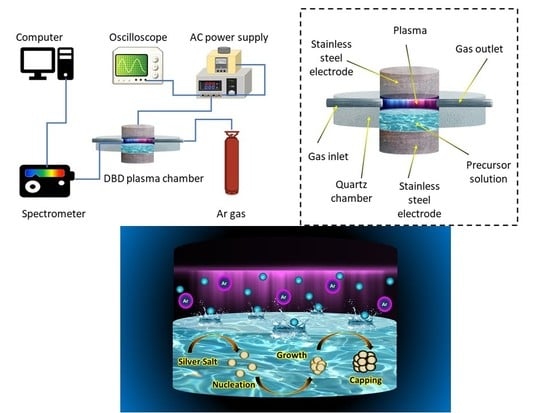
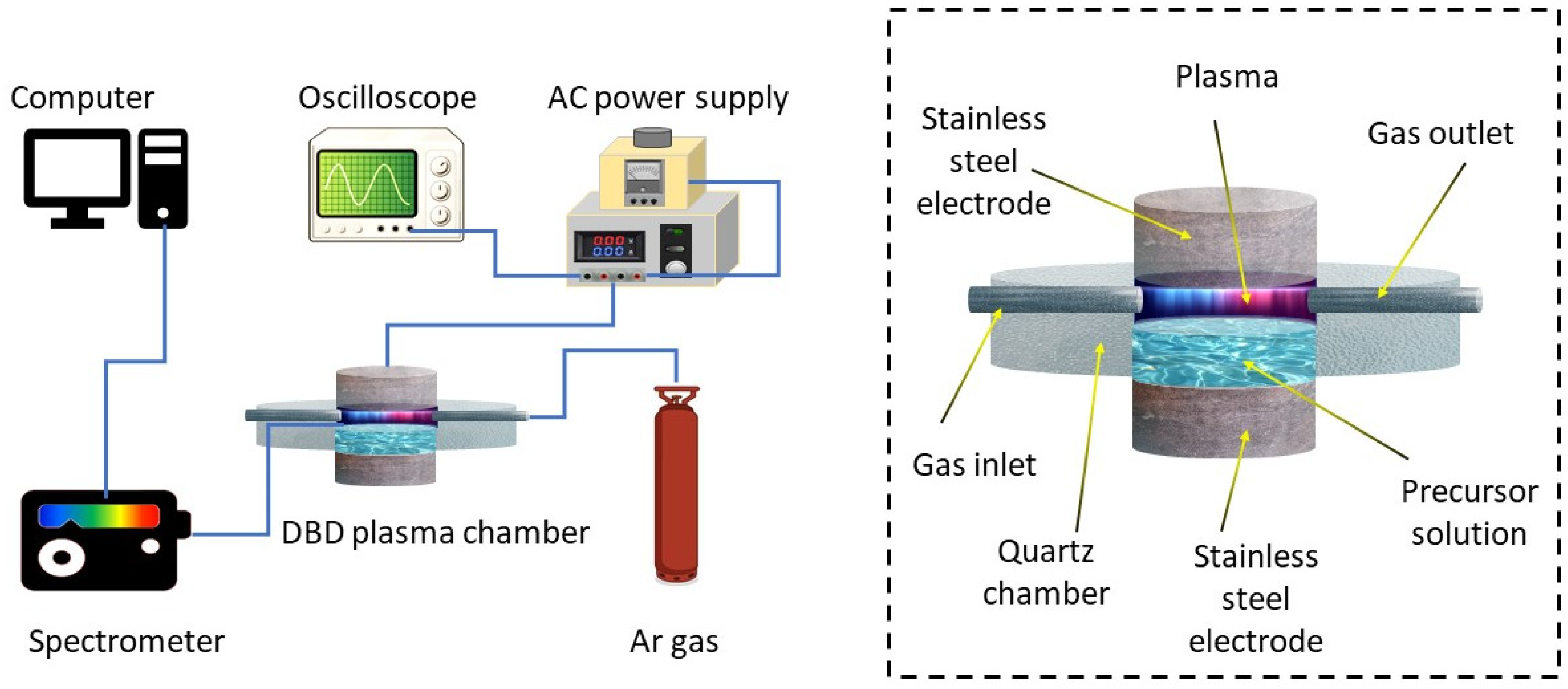
2. Experimental Methods
3. Results and Discussion
3.1. Particle Formation and Characterisation
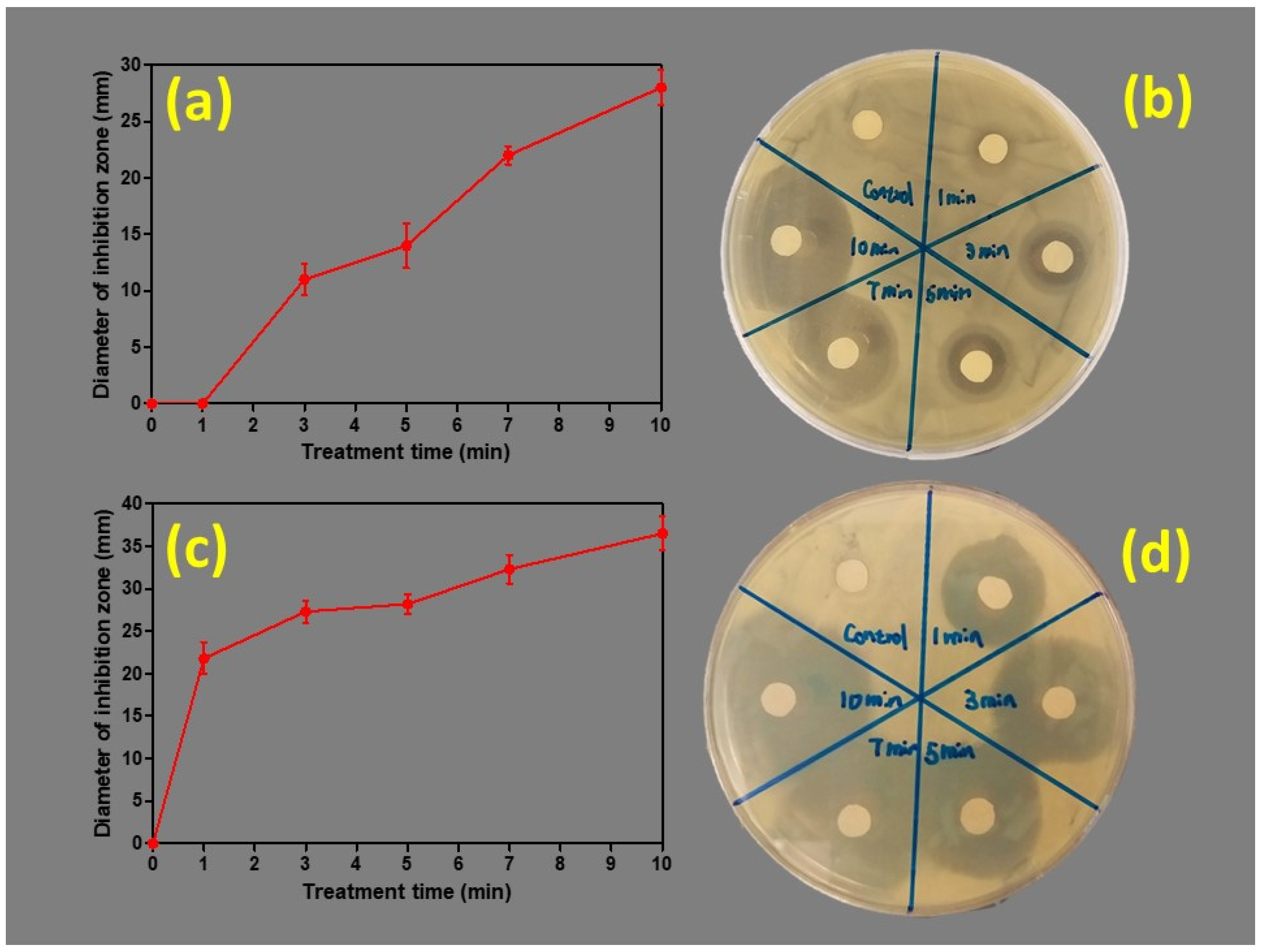
3.2. Antibacterial Properties
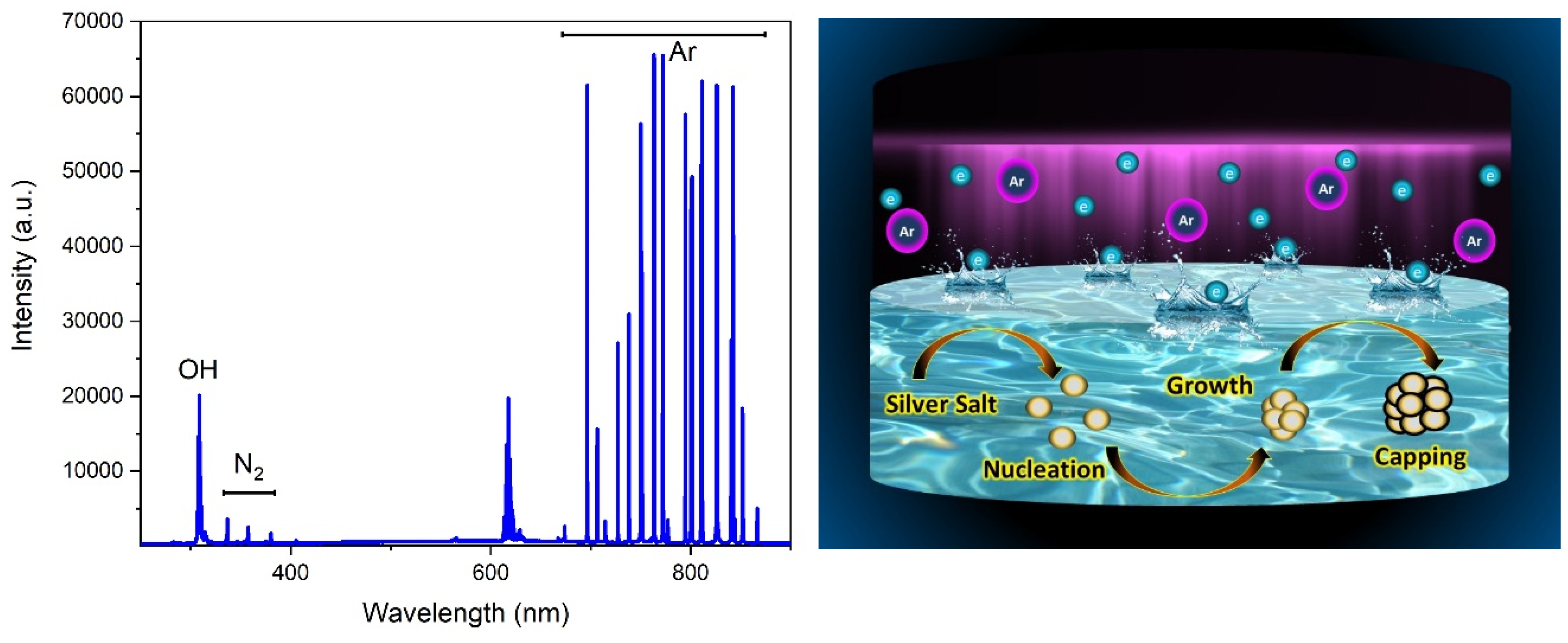
3.3. Mechanism of Nanoparticle Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kondeti, V.S.S.K.; Gangal, U.; Yatom, S.; Bruggeman, P.J. Ag+ reduction and silver nanoparticle synthesis at the plasma–liquid interface by an RF driven atmospheric pressure plasma jet: Mechanisms and the effect of surfactant. J. Vac. Sci. Technol. A 2017, 35, 061302. [Google Scholar] [CrossRef]
- Nishimoto, M.; Abe, S.; Yonezawa, T. Preparation of Ag nanoparticles using hydrogen peroxide as a reducing agent. New J. Chem. 2018, 42, 14493–14501. [Google Scholar] [CrossRef]
- Thai, P.; Abe, S.; Kosugi, K.; Saito, N.; Takahashi, K.; Sasaki, T.; Kikuchi, T. Interaction and transfer of charged particles from an alternating current glow discharge in liquids: Application to silver nanoparticle synthesis. J. Appl. Phys. 2019, 125, 063303. [Google Scholar] [CrossRef]
- Vimala, K.; Sivudu, K.S.; Yallapu, M.M.; Sreedhar, B.; Raju, K.M. Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly(acrylamide) and carbohydrates: A rational methodology for antibacterial application. Carbohydr. Polym. 2009, 75, 463–471. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qiu, L.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, A.J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, F.; Fracassi, F. Atmospheric pressure non-equilibrium plasma jet technology: General features, specificities and applications in surface processing of materials. Surf. Coat. Technol. 2017, 322, 174–201. [Google Scholar] [CrossRef]
- Bardos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Leifert, D.; Studer, A.; O’Connell, D.; Chechik, V. Initiating radical reactions with non-thermal plasmas. Chem. Commun. 2017, 53, 3685–3688. [Google Scholar] [CrossRef] [Green Version]
- Cullen, P.J.; Milosavljevi, V.; Milosavljević, V. Spectroscopic characterization of a radio-frequency argon plasma jet discharge in ambient air. Prog. Theor. Exp. Phys. 2015, 2015, 63. [Google Scholar] [CrossRef] [Green Version]
- Richmonds, C.; Sankaran, R.M. Plasma-liquid electrochemistry: Rapid synthesis of colloidal metal nanoparticles by microplasma reduction of aqueous cations. Appl. Phys. Lett. 2008, 93, 131501. [Google Scholar] [CrossRef]
- Rumbach, P.; Bartels, D.M.; Sankaran, R.M.; Go, D.B. The solvation of electrons by an atmospheric-pressure plasma. Nat. Commun. 2015, 6, 7248. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Akiyama, T. Nanomaterial Synthesis Using Plasma Generation in Liquid. J. Nanomater. 2015, 2015, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.; Nemcova, L.; Maguire, P.D.; Graham, W.; Mariotti, D. Synthesis of surfactant-free electrostatically stabilized gold nanoparticles by plasma-induced liquid chemistry. Nanotechnology 2013, 24, 245604. [Google Scholar] [CrossRef] [Green Version]
- Maugel, T.K.; Goodhew, P.J. Electron Microscopy and Analysis. Trans. Am. Microsc. Soc. 1976, 95, 243. [Google Scholar] [CrossRef]
- Ho, P.-L.; Chow, K.H.; Yuen, K.-Y.; Ng, W.S.; Chau, P.Y. Comparison of a novel, inhibitor-potentiated disc-diffusion test with other methods for the detection of extended-spectrum beta-lactamases in Escherichia coli and Klebsiella pneumoniae. J. Antimicrob. Chemother. 1998, 42, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Skov, R.; Smyth, R.; Larsen, A.R.; Bolmström, A.; Karlsson, A.; Mills, K.; Frimodt-Moller, N.; Kahlmeter, G. Phenotypic Detection of Methicillin Resistance in Staphylococcus aureus by Disk Diffusion Testing and Etest on Mueller-Hinton Agar. J. Clin. Microbiol. 2006, 44, 4395–4399. [Google Scholar] [CrossRef] [Green Version]
- Dong, P.; Cheng, X.; Jin, Z.; Huang, Z.; Nie, X.; Wang, X.; Zhang, X. The green synthesis of Ag-loaded photocatalyst via DBD cold plasma assisted deposition of Ag nanoparticles on N-doped TiO2 nanotubes. J. Photochem. Photobiol. A Chem. 2019, 382, 111971. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cumberland, S.; Lead, J. Particle size distributions of silver nanoparticles at environmentally relevant conditions. J. Chromatogr. A 2009, 1216, 9099–9105. [Google Scholar] [CrossRef]
- Davey, W.P. Precision Measurements of the Lattice Constants of Twelve Common Metals. Phys. Rev. 1925, 25, 753–761. [Google Scholar] [CrossRef]
- Vidhu, V.; Philip, D. Spectroscopic, microscopic and catalytic properties of silver nanoparticles synthesized using Saraca indica flower. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Cheng, Y.H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Boil. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.J.; Shah, S.I. Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Heal. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Quinteros, M.; Aristizábal, V.C.; Dalmasso, P.; Paraje, M.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Xiu, Z.-M.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Mirzajani, F.; Ghassempour, A.; Aliahmadi, A.; Esmaeili, M.A. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res. Microbiol. 2011, 162, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Sihtmäe, M.; Kuzmičiova, J.; Ragelienė, L.; Kahru, A.; Daugelavicius, R. Plasma membrane is the target of rapid antibacterial action of silver nanoparticles in Escherichia coli and Pseudomonas aeruginosa. Int. J. Nanomed. 2018, 13, 6779–6790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirafuji, T.; Nakamura, Y.; Azuma, S.; Sotoda, N.; Isshiki, T. Au-nanoparticle-embedded cross-linked gelatin films synthesized on aqueous solution in contact with dielectric barrier discharge. Jpn. J. Appl. Phys. 2017, 57, 0102BE. [Google Scholar] [CrossRef]
- Xie, P.; Qi, Y.; Wang, R.; Wu, J.; Li, X. Aqueous Gold Nanoparticles Generated by AC and Pulse-Power-Driven Plasma Jet. Nanomaterials 2019, 9, 1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.K.; Jeong, S.; Kim, D.; Moon, J.; Lim, S.; Kim, J.S. Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 2007, 311, 417–424. [Google Scholar] [CrossRef]
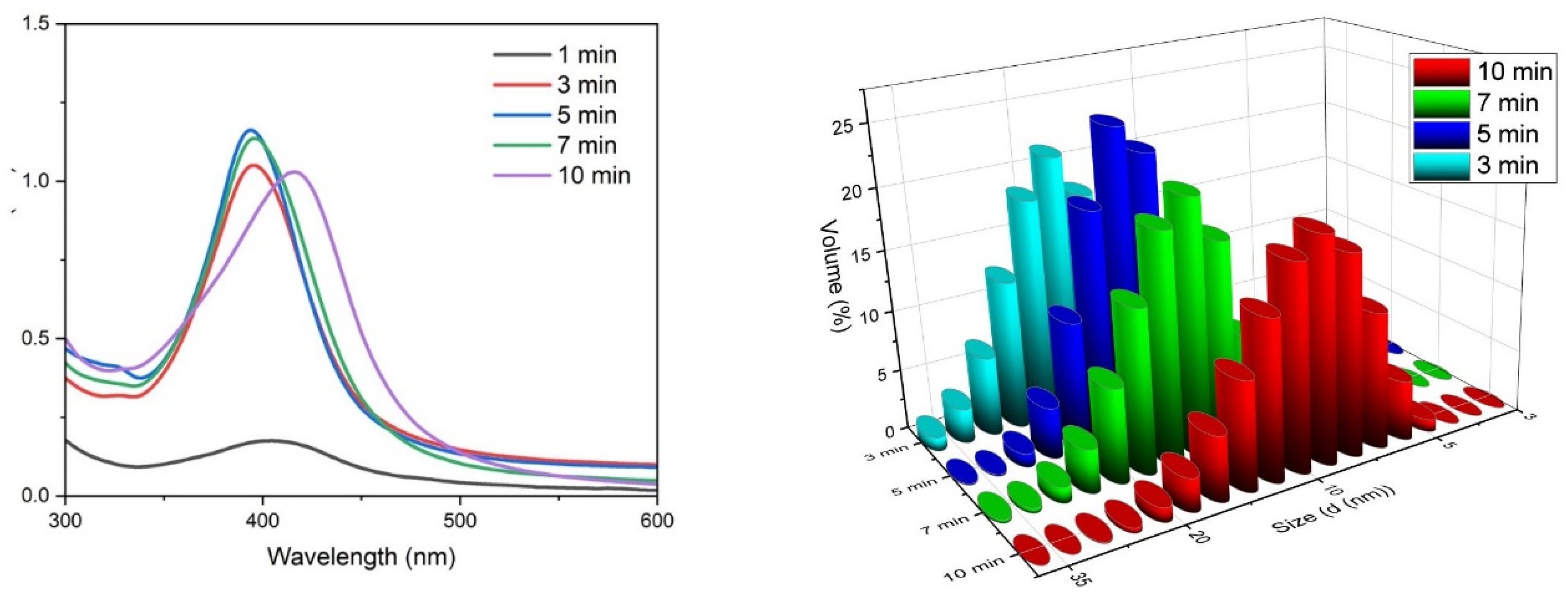
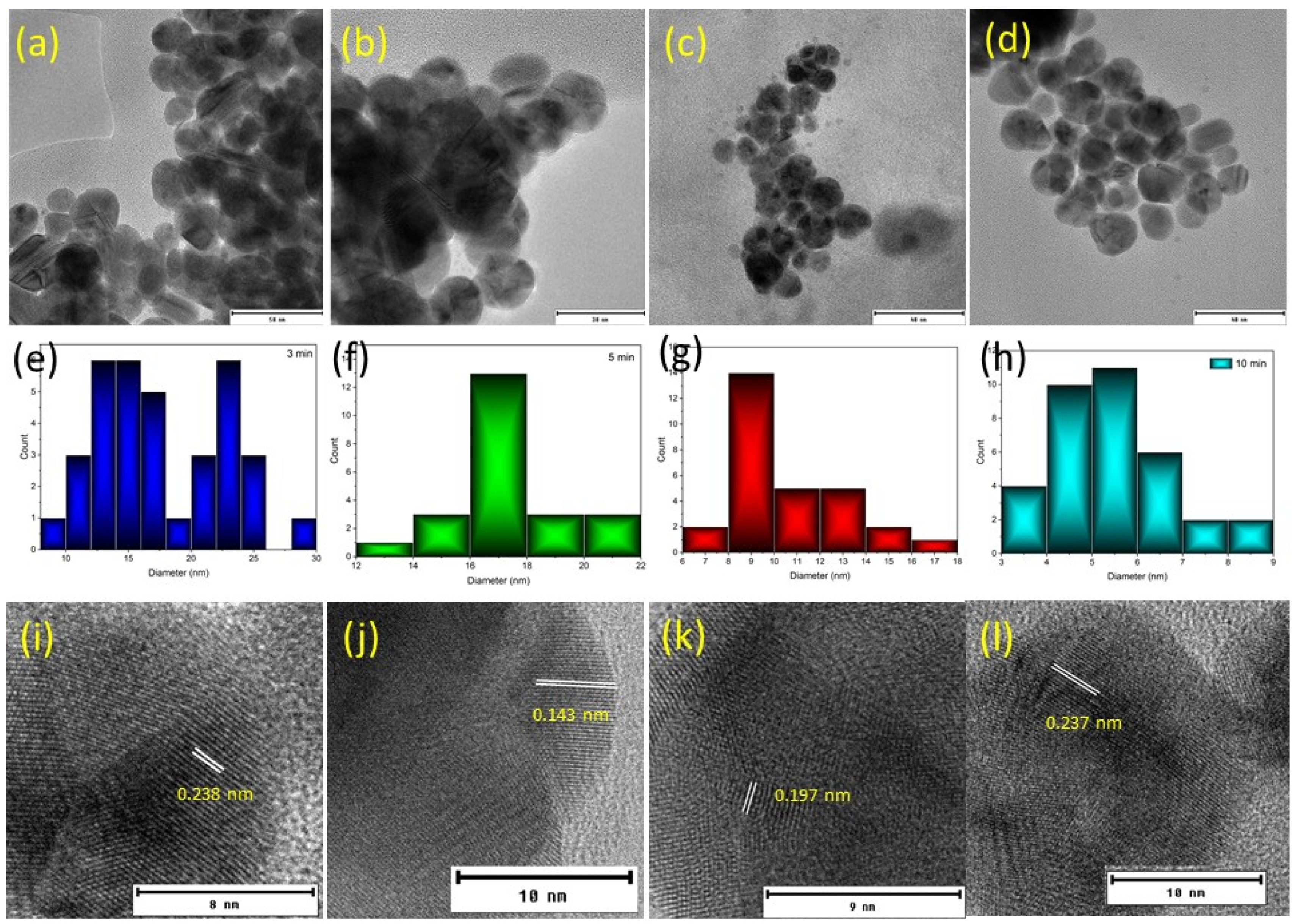
| Plasma Exposure (min) | DLS (nm) | % Volume | Standard Deviation |
|---|---|---|---|
| 3 | 20.06 | 90.9 | 5.543 |
| 5 | 16.16 | 95.9 | 3.746 |
| 7 | 15.27 | 92.2 | 4.322 |
| 10 | 9.99 | 98.4 | 3.405 |
| Wavelength (nm) | Transition | Relative Intensity (a.u) | Ei (eV) | Ef (eV) |
|---|---|---|---|---|
| 696 | 2p2 → 1s5 | 61,371 | 13.33 | 11.55 |
| 707 | 2p3 → 1s5 | 15,587 | 13.30 | 11.55 |
| 715 | 2p5 → 1s5 | 3284 | 13.28 | 11.55 |
| 727 | 2p2 → 1s4 | 26,821 | 13.33 | 11.62 |
| 738 | 2p3 → 1s4 | 30,896 | 13.30 | 11.62 |
| 750 | 2p1 → 1s2 | 55,719 | 13.48 | 11.83 |
| 751 | 2p5 → 1s4 | 36,434 | 13.27 | 11.62 |
| 763 | 2p6 → 1s5 | 65,549 | 13.17 | 11.55 |
| 772 | 2p2 → 1s3 | 65,450 | 13.15 | 11.55 |
| 795 | 2p4 → 1s3 | 57,756 | 13.28 | 11.72 |
| 801 | 2p6 → 1s4 | 48,875 | 13.09 | 11.55 |
| 810 | 2p7 → 1s4 | 50,477 | 13.15 | 11.62 |
| 811 | 2p9 → 1s5 | 62,028 | 13.08 | 11.55 |
| 826 | 2p2 → 1s2 | 61,613 | 13.33 | 11.83 |
| 841 | 2p3 → 1s2 | 27,474 | 13.30 | 11.83 |
| 842 | 2p8 → 1s4 | 61,277 | 13.09 | 11.62 |
| 852 | 2p4 → 1s2 | 18,474 | 13.28 | 11.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerasinghe, J.; Li, W.; Zhou, R.; Zhou, R.; Gissibl, A.; Sonar, P.; Speight, R.; Vasilev, K.; Ostrikov, K. Bactericidal Silver Nanoparticles by Atmospheric Pressure Solution Plasma Processing. Nanomaterials 2020, 10, 874. https://doi.org/10.3390/nano10050874
Weerasinghe J, Li W, Zhou R, Zhou R, Gissibl A, Sonar P, Speight R, Vasilev K, Ostrikov K. Bactericidal Silver Nanoparticles by Atmospheric Pressure Solution Plasma Processing. Nanomaterials. 2020; 10(5):874. https://doi.org/10.3390/nano10050874
Chicago/Turabian StyleWeerasinghe, Janith, Wenshao Li, Rusen Zhou, Renwu Zhou, Alexander Gissibl, Prashant Sonar, Robert Speight, Krasimir Vasilev, and Kostya (Ken) Ostrikov. 2020. "Bactericidal Silver Nanoparticles by Atmospheric Pressure Solution Plasma Processing" Nanomaterials 10, no. 5: 874. https://doi.org/10.3390/nano10050874
APA StyleWeerasinghe, J., Li, W., Zhou, R., Zhou, R., Gissibl, A., Sonar, P., Speight, R., Vasilev, K., & Ostrikov, K. (2020). Bactericidal Silver Nanoparticles by Atmospheric Pressure Solution Plasma Processing. Nanomaterials, 10(5), 874. https://doi.org/10.3390/nano10050874






_Ostrikov.png)