Glass-Forming Ability and Soft Magnetic Properties of (Co75Ti25)100−xFex (x; 0–20 at.%) Systems Fabricated by SPS of Mechanically Alloyed Nanopowders
Abstract
:1. Introduction
1.1. Background
1.2. Metallic Glassy Soft Magnetic Materials
1.3. Aim of the Present Work
2. Materials and Methods
2.1. Starting Feedstock Materials
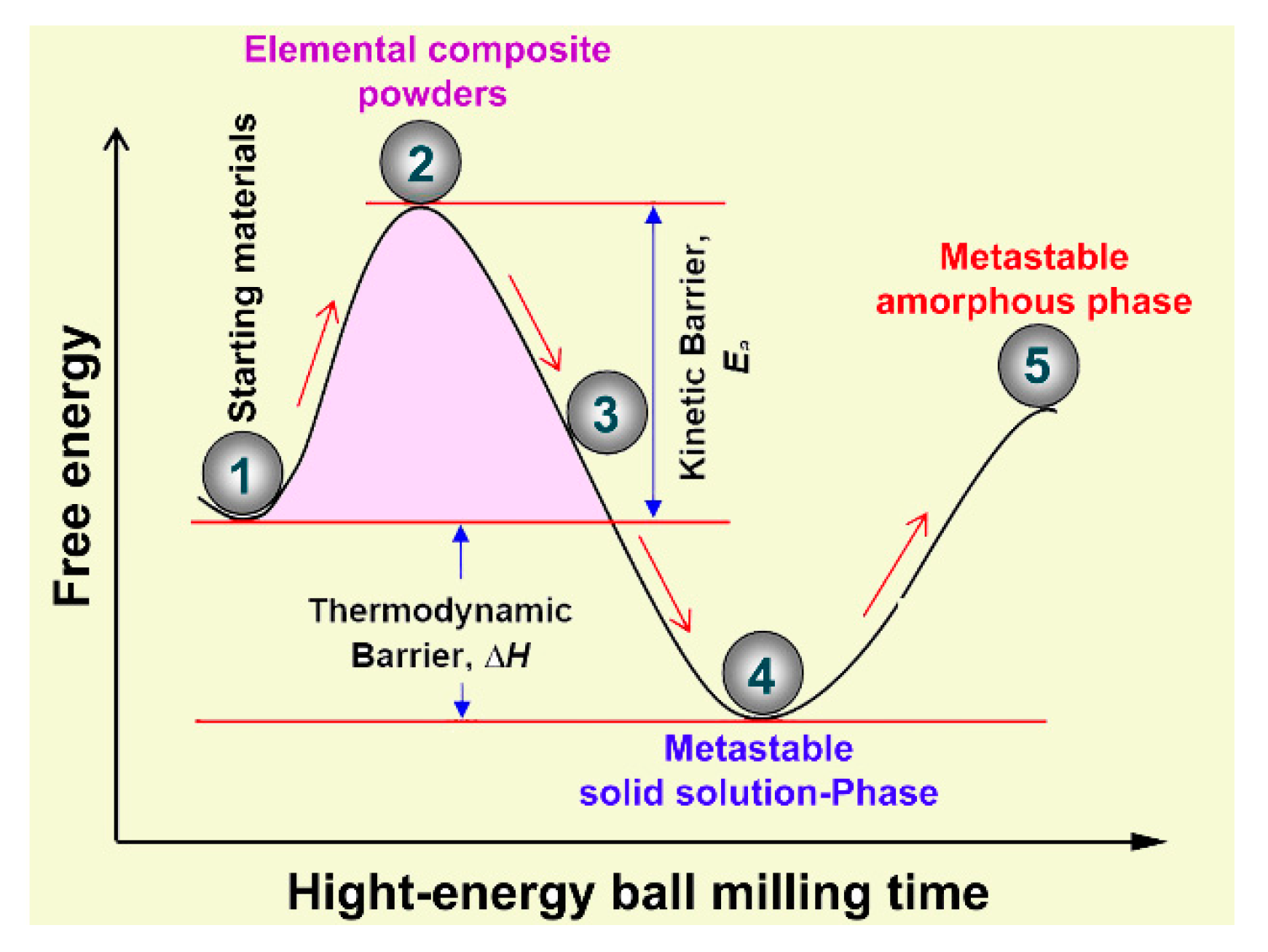
2.2. Preparations of Metallic Glassy Alloy Powders
2.3. Powder Consolidation by Spark Plasma Sintering (SPS)
2.4. Sample Characterizations
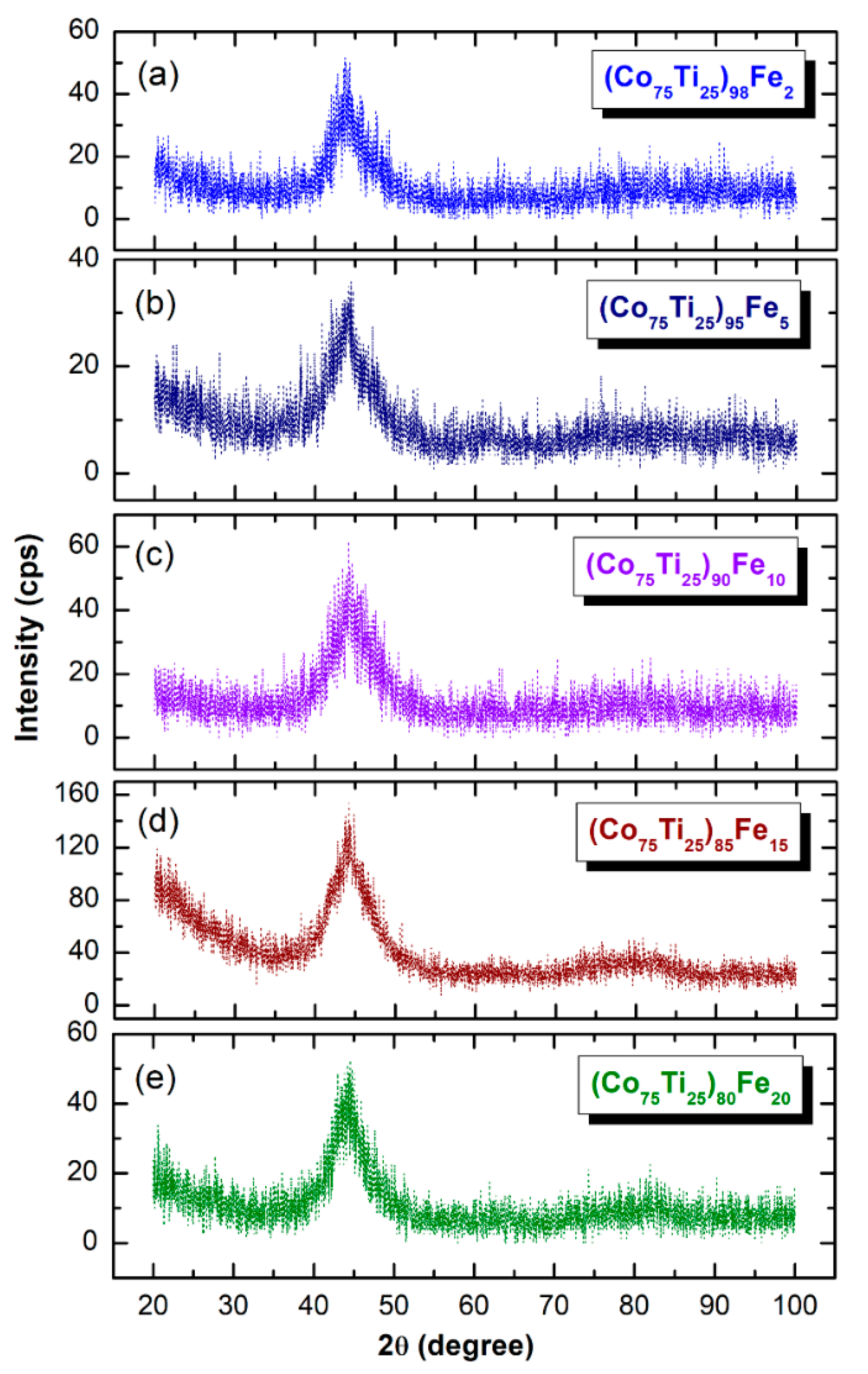
2.4.1. Crystal Structure
2.4.2. Morphology and Elemental Analysis
2.4.3. Thermal Stability
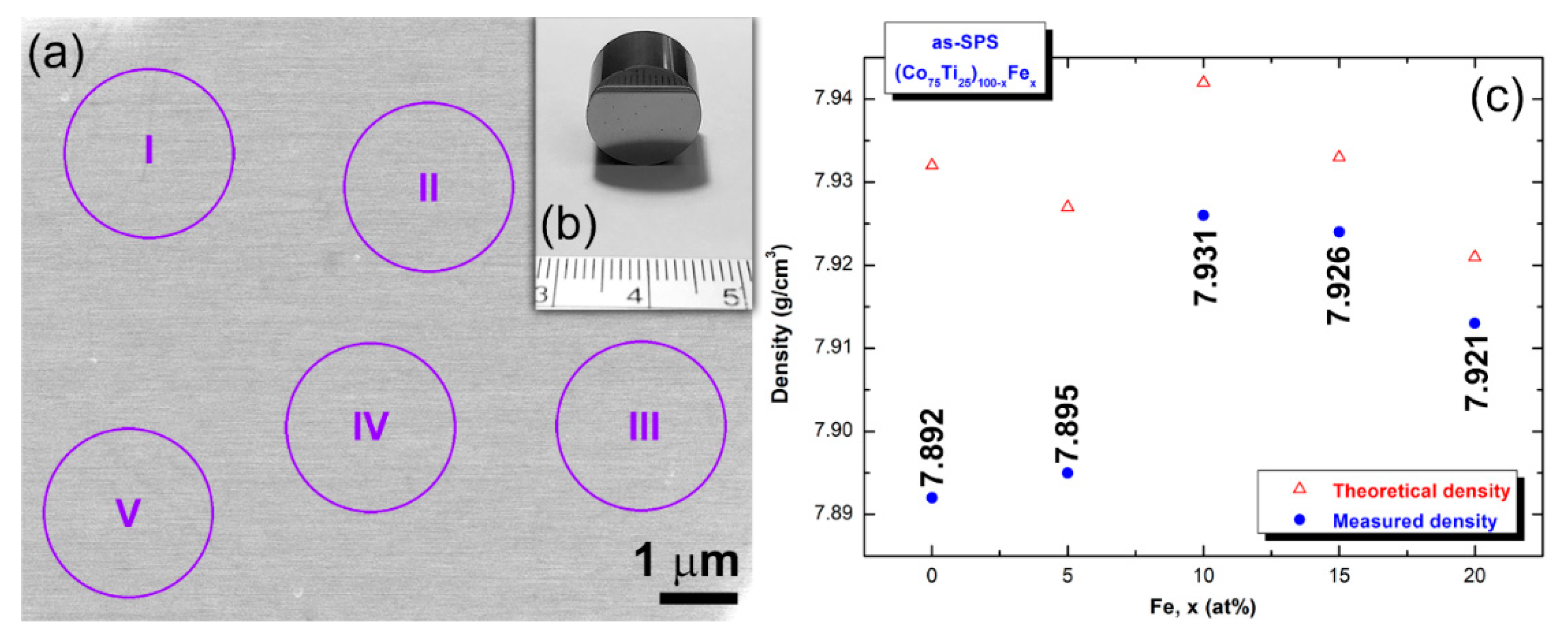
2.4.4. Density Measurements
2.4.5. Magnetization Measurements
3. Results and Discussion
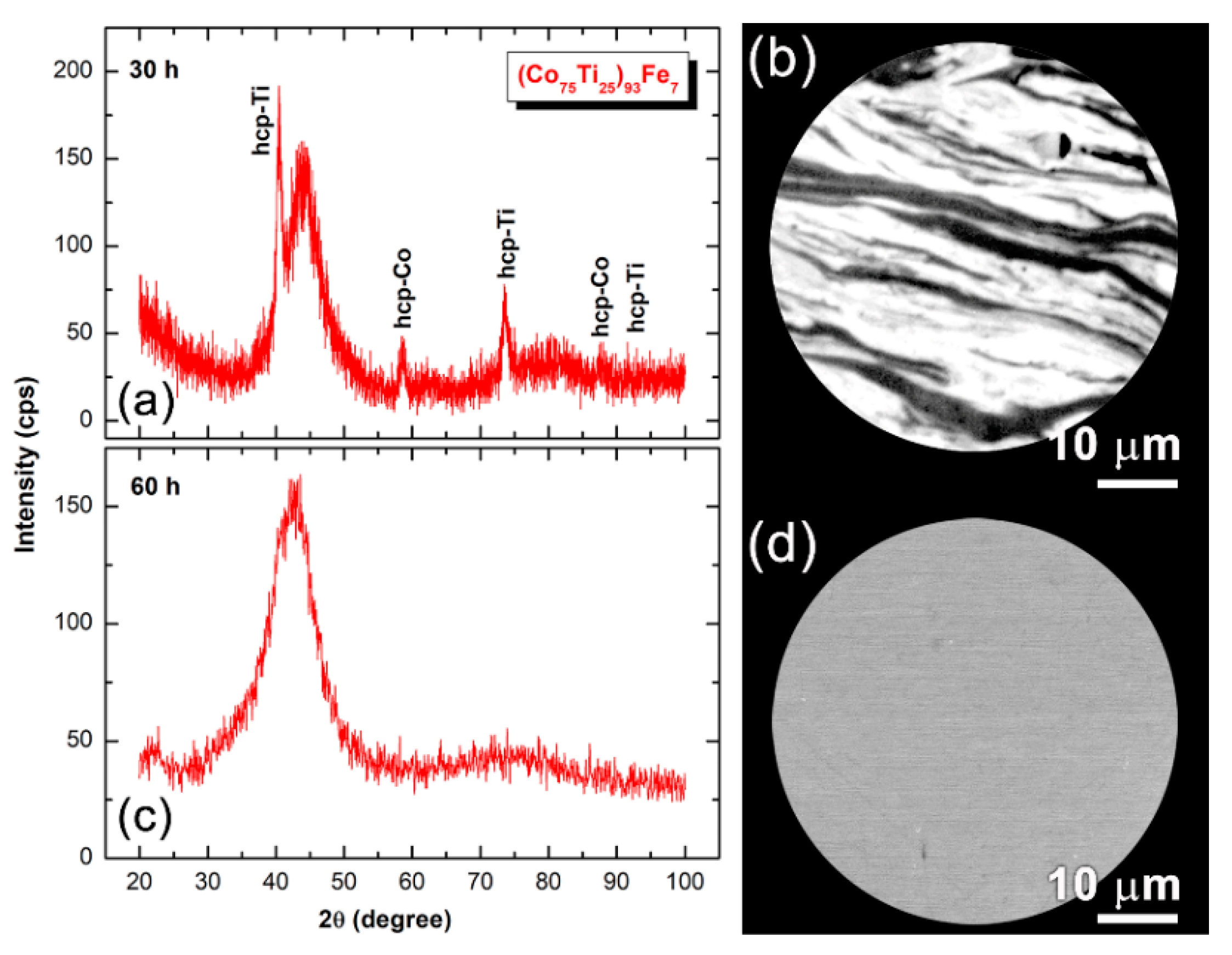
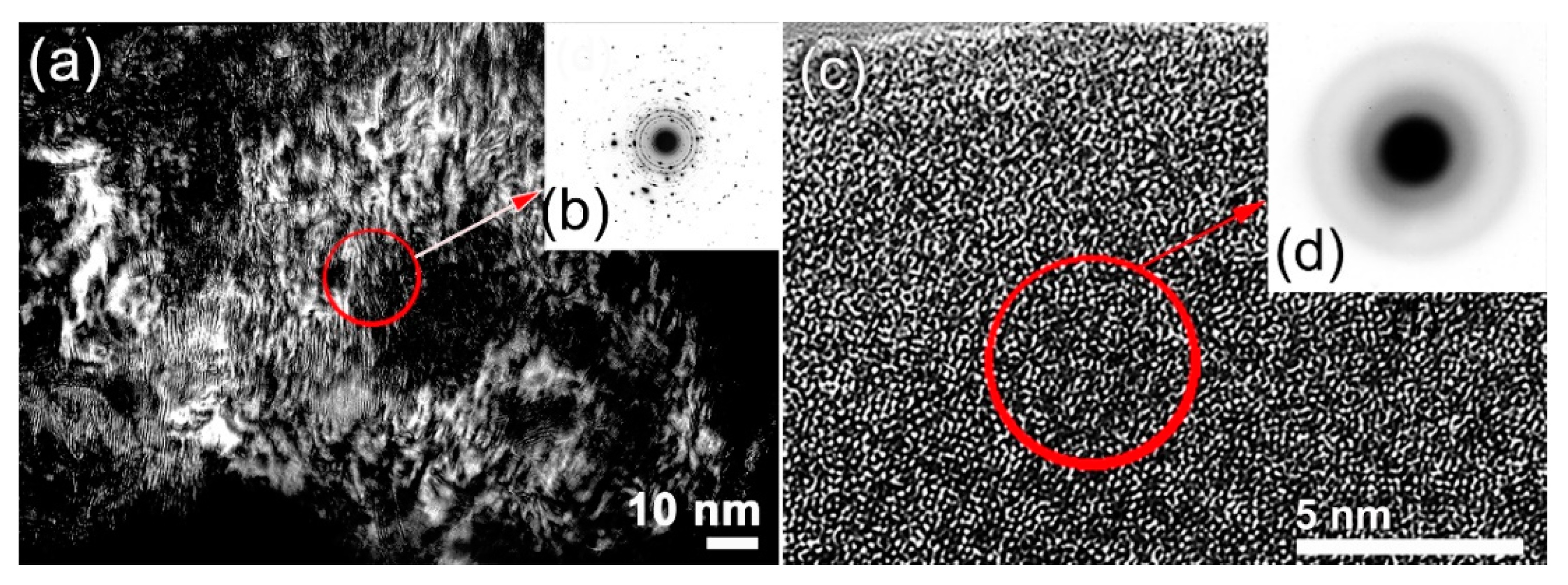
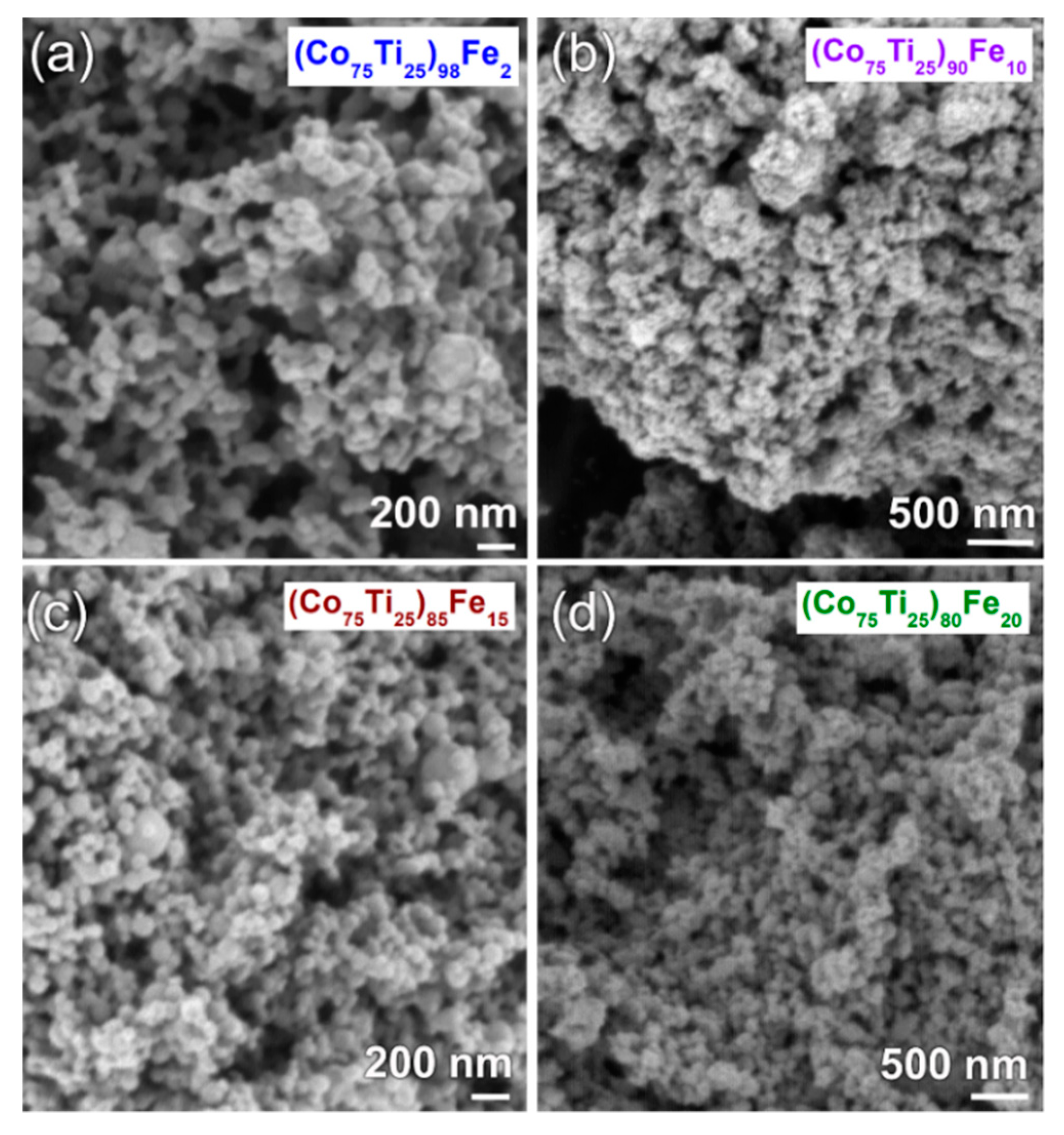
3.1. Ball-Milling Effect on Structure and Morphology of the Mechanically Alloyed Powders
3.2. Consolidation of as-MA Powders into BMG by SPS Technique
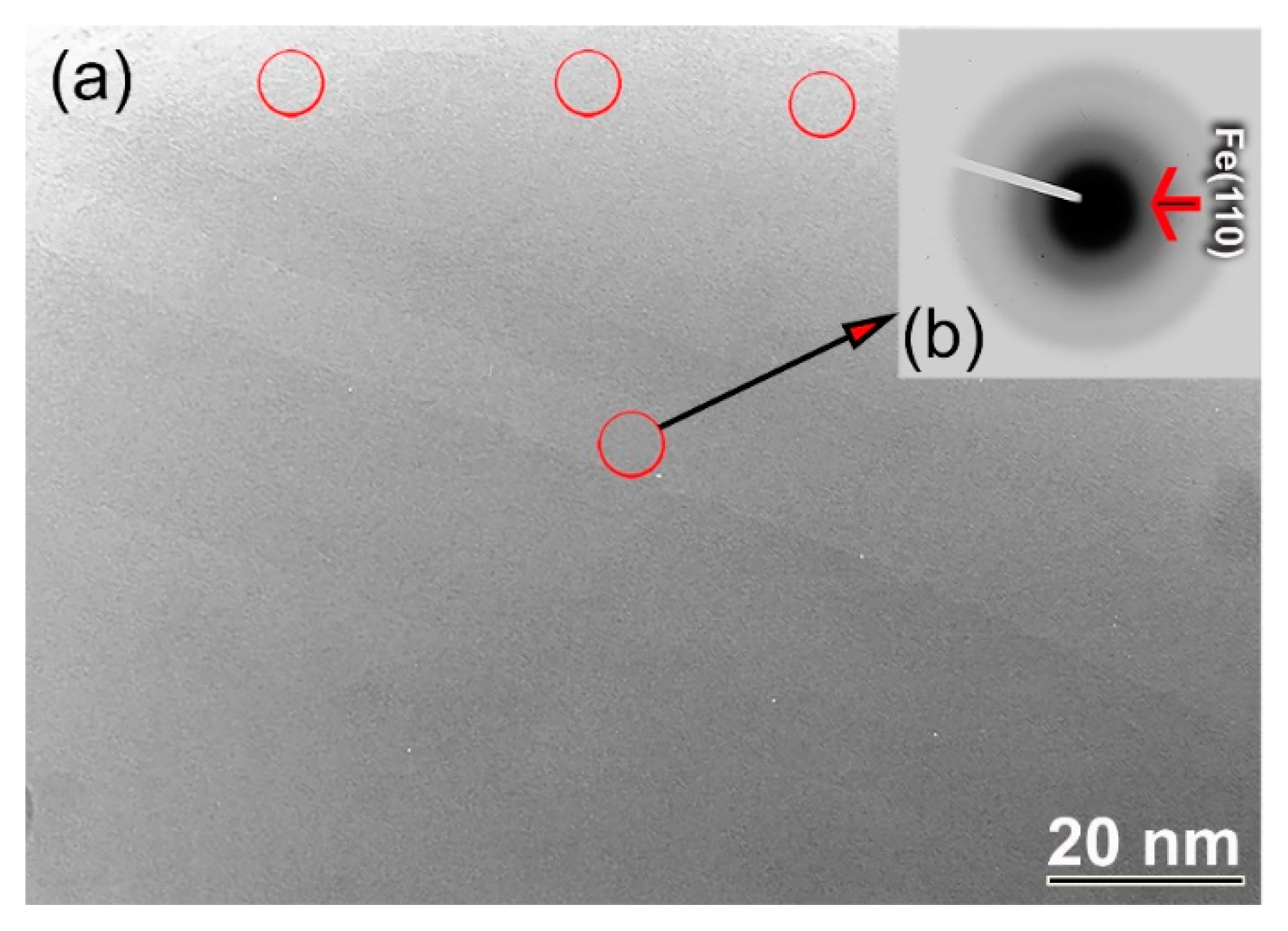
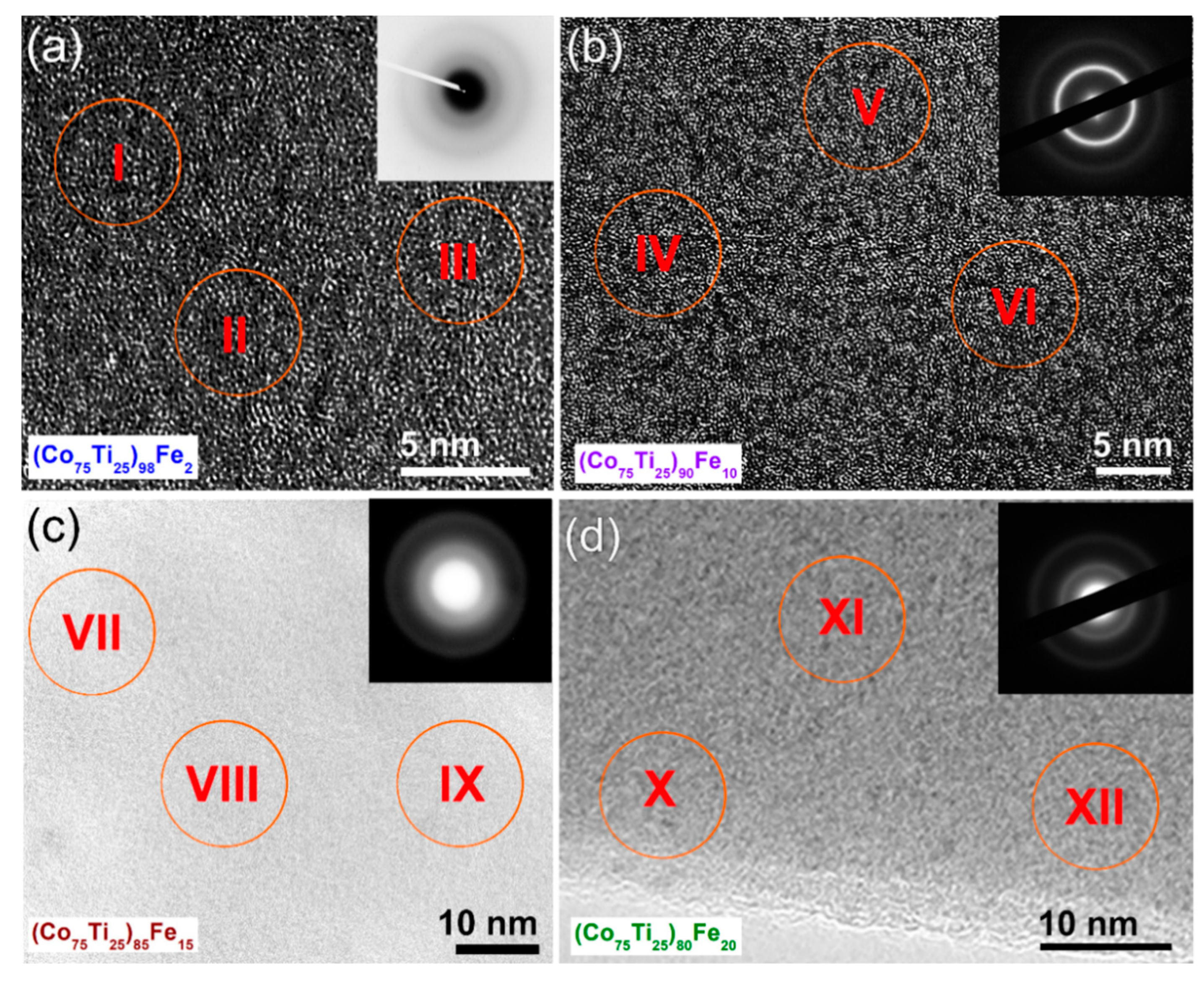
3.2.1. Structural and Morphological Characteristics
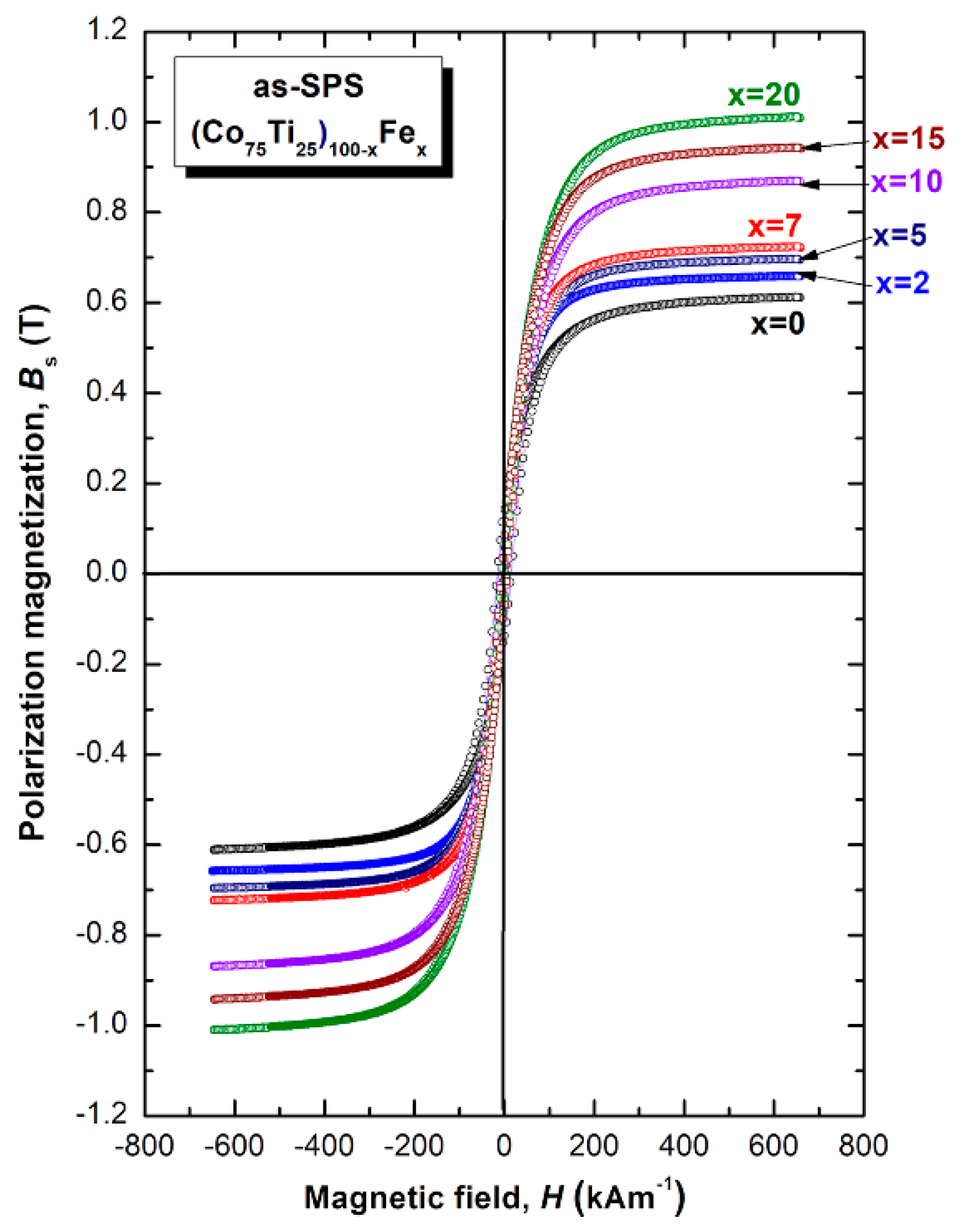
3.2.2. Magnetic Properties
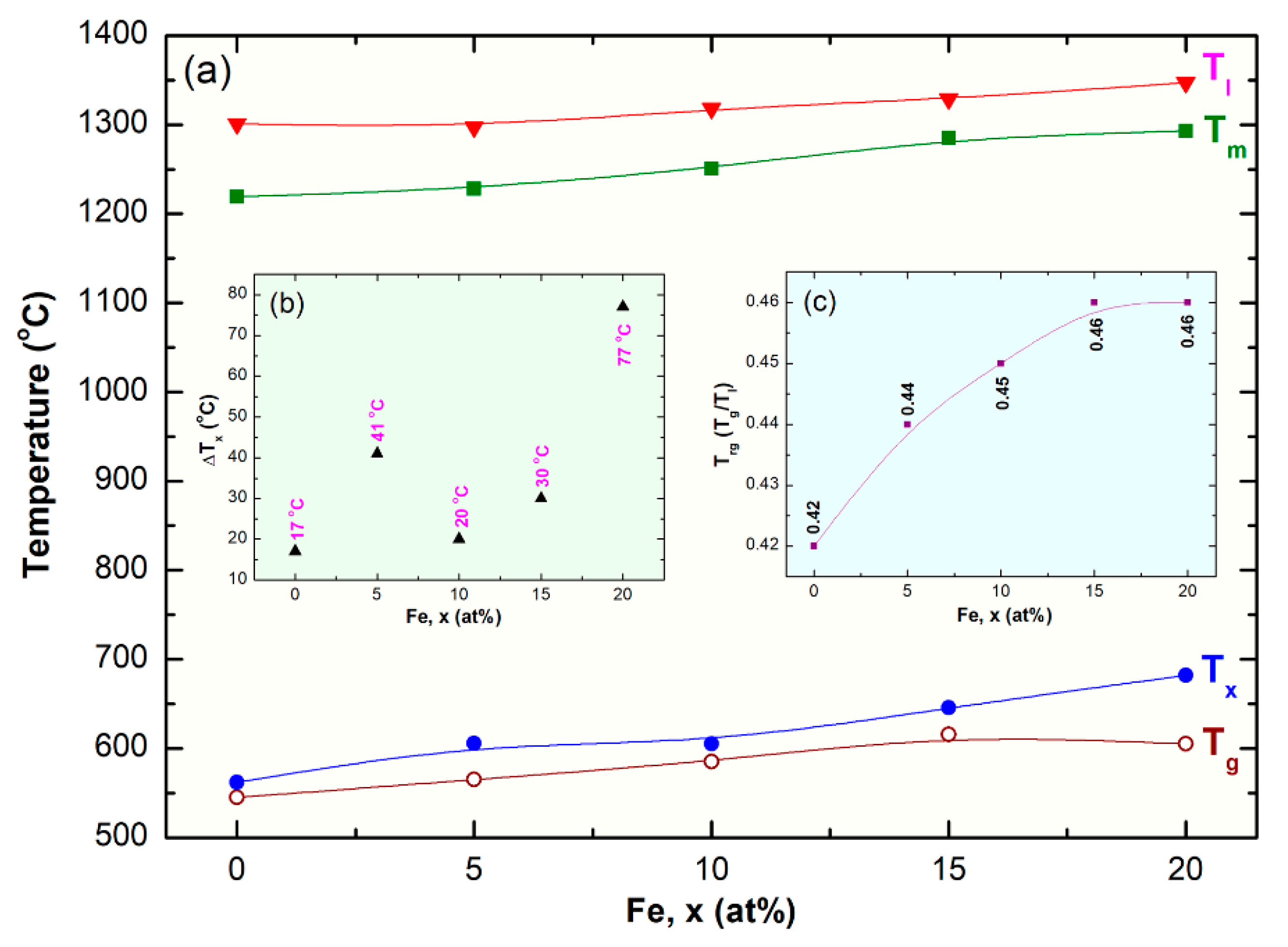
3.2.3. Thermal Analysis
4. Conclusions
- (1)
- The (Co75Ti25)100−xFex system can be prepared successfully in a wide Fe concentration ranging from 0 to 20 at.%.
- (2)
- The end product of the glassy phases obtained after 60 h of milling coexisted with a marginal volume fraction of nanocrystalline Fe powders.
- (3)
- In MA-(Co75Ti25)80Fe20 system, a bcc-FeCoTi solid-solution phase was obtained after BM for 30 h. The powders of this obtained bcc-solid-solution were severely plastically deformed due to the effect of ball–powder–ball collisions, leading to the generation of intensive lattice imperfections composited of dislocations and point and lattice defects.
- (4)
- Increasing the BM time enhanced the mechanically induced imperfections, leading to a solid-solution-to-amorphous phase transformation upon BM for 60 h.
- (5)
- The as-fabricated (Co75Ti25)80Fe20 glassy alloy system revealed excellent GFA and good thermal stability, indicated by their wide ΔTx and high Tx values.
- (6)
- Based on the their wide ΔTx before crystallizations and high Tx, the as-fabricated powders were consolidated into nearly full dense (above 99.95%) bulk buttons, using SPS technique.
- (7)
- The SPS consolidation step maintained the original short-range order structure after consolidation without experience of any partial crystallizations.
- (8)
- The as-prepared metallic glassy (Co75Ti25)100−xFex systems possesses good soft magnetic properties, indicated by high values of saturation magnetization (0.61 to 1.01 T), which increased with increasing Fe concentration.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Axinte, E. Metallic glasses from “alchemy” to pure science: Present and future of design, processing and applications of glassy metals. Mater. Des. 2012, 35, 518–556. [Google Scholar] [CrossRef]
- Karmakar, B. Fundamentals of Glass and Glass Nanocomposites. In Glass Nanocomposites: Synthesis, Properties and Applications; Karmakar, B., Rademann, K., Stepanov, A.L., Eds.; Elsevier: Oxford, UK, 2016; Chapter 1; pp. 3–53. [Google Scholar]
- Sherif El-Eskandarany, M. Mechanical Alloying, Energy Storage, Protective Coatings, and Medical Applications, 3rd ed.; Elsevier: Oxford, UK, (in press, to be appeared on 1st May 2020).
- Klement, W.; Willens, R.H.; Duwez, P. Non-crystalline structure in solidified gold–silicon alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Chen, H.S.; Turnbull, D. Formation, stability and structure of palladium-silicon based alloy glasses. Acta Metall. 1969, 17, 1021–1031. [Google Scholar] [CrossRef]
- Chen, H.S. Thermodynamic considerations on the formation and stability of metallic glasses. Acta Metall. 1974, 22, 1505–1511. [Google Scholar] [CrossRef]
- Turnbull, D. Metastable structures in metallurgy. Metall. Trans. B 1981, 12B, 217–230. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Al–La–Ni amorphous alloys with a wide supercooled liquid region. Mater. Trans. JIM 1989, 30, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Sherif El-Eskandarany, M.; Itoh, F.; Aoki, K.; Suzuki, K. Preparation of AlxTa1-x amorphous alloy powder by mechanical alloying. J. Non-Cryst. Solids 1990, 119, 729–732. [Google Scholar] [CrossRef]
- Kündig, A.A.; Löffler, J.F.; Johnson, W.L.; Uggowitzer, P.J.; Thiyagarajan, P. Influence of decomposition on the thermal stability of undercooled Zr–Ti–Cu–Ni–Al alloys. Scr. Mater. 2001, 44, 1269–1273. [Google Scholar] [CrossRef]
- Sherif El-Eskandarany, M.; Inoue, A. Thermally enhanced and mechanically driven glass formation reactions of multilayered Cu33Zr67 powders. Met. Trans. A 2002, 33A, 2145–2153. [Google Scholar] [CrossRef]
- Sherif El-Eskandarany, M.; Al-Hajji, L.A.; Al-Hazza, A. Fabrication of new metallic glassy Ti40.6Cu15.4Ni8.5Al5.5W30 alloy powders by mechanical alloying and subsequent SPS consolidation. Adv. Powder Technol. 2017, 28, 814–819. [Google Scholar] [CrossRef]
- Sherif El-Eskandarany, M.; Inoue, A. Mechanically induced cyclic metastable phase transformations of Zr2Ni Alloys. Phys. Rev. B 2007, 75, 224109-1–224109-9. [Google Scholar] [CrossRef]
- Masood, A.; Belova, L.; Ström, V. On the correlation between glass forming ability (GFA) and soft magnetism of Ni-substituted Fe-based metallic glassy alloys. J. Magn. Magn. Mater. 2020, 504, 166667. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, Z.; Qin, X.D.; Li, Z.; Zhang, H. Highly efficient and stable CuZr-based metallic glassy catalysts for azo dye degradation. J. Mater. Sci. Technol. 2020, 46, 88–97. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, C.; Yang, B.; Wang, J. Deformation behavior of Al-rich metallic glasses under nanoindentation. J. Mater. Sci. Technol. 2017, 33, 1272–1277. [Google Scholar] [CrossRef]
- Kim, J.T.; Hong, S.H.; Park, J.M.; Eckert, J.; Kim, K.B. New para-magnetic (CoFeNi)(CrMo)50−x(CB)x (x = 20, 25, 30) non-equiatomic high entropy metallic glasses with wide supercooled liquid region and excellent mechanical properties. J. Mater. Sci. Technol. 2020, 43, 135–143. [Google Scholar] [CrossRef]
- Shi, M.; Liu, Z.; Zhang, T. Effects of Metalloid B Addition on the Glass Formation, Magnetic and Mechanical Properties of FePCB Bulk Metallic Glasses. J. Mater. Sci. Technol. 2015, 31, 493–497. [Google Scholar] [CrossRef]
- Lesz, S. A Study of Structure and Magnetic Properties of Low Purity Fe-Co-Based Metallic Glasses. Materials 2017, 10, 625. [Google Scholar] [CrossRef]
- Hossein Taghvaei, A.; Eckert, J. Development and characterization of new Co-Fe-Hf-B bulk metallic glass with high thermal stability and superior soft magnetic performance. J. Alloys Compd. 2020, 823. [Google Scholar]
- Zhou, Z.; Wei, Q.; Li, Q.; Jiang, B.; Chen, Y.; Sun, Y. Development of Co-based bulk metallic glasses as potential biomaterials. Mater. Sci. Eng. C 2016, 69, 46–51. [Google Scholar] [CrossRef]
- Sherif El-Eskandrany, M.; Al-Azmi, A. Potential applications of cold sprayed Cu50Ti20Ni30 metallic glassy alloy powders for antibacterial protective coating in medical and food sectors. J. Mech. Behav. Biomed. Mater. 2016, 56, 183–194. [Google Scholar] [CrossRef]
- Inoue, A.; Kong, F.L.; Han, Y.; Zhu, S.L.; Churyumov, A.; Shalaan, E.; Al-Marzouki, F. Development and application of Fe-based soft magnetic bulk metallic glassy inductors. J. Alloys Compd. 2018, 731, 1303–1309. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, M.S.; Zhang, W.W.; Yang, C.; Xie, G.Q.; Louzguine-Luzgin, D.V. Achieving super-high strength in an aluminum based composite by reinforcing metallic glassy flakes. Mater. Lett. 2020, 262, 127059. [Google Scholar] [CrossRef]
- Sherif El-Eskandarany, M.; Ahmed, S.A.; Shaban, E. Metallic Glassy V45Zr20Ni20Cu10Al3Pd2 Alloy Powders for Superior Hydrogenation/Dehydrogenation Kinetics of MgH2. Mater. Today 2018, 5, 13718–13725. [Google Scholar]
- Sherif El-Eskandarany, M. Metallic glassy Ti2Ni grain-growth inhibitor powder for enhancing the hydrogenation/dehydrogenation kinetics of MgH2. RSC Adv. 2019, 9, 1036. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.-X.; Zhang, W.; Zhang, L.; Wang, W.; Zhang, L.-C. Remediation of industrial contaminated water with arsenic and nitrate by mass-produced Fe-based metallic glass: Toward potential industrial applications. SM&T 2019, 22, e00126. [Google Scholar]
- Chen, C.; Zhang, H.; Zhang, W.; Fan, Y.; Wei, R.; Guan, S.; Wang, T.; Zhang, T.; Li, F. A complex multicomponent bulk metallic glass/ultrafine-nanocrystal composite fabricated under industrial-applicable condition. J. Non-Cryst. Solids 2020, 530, 119827. [Google Scholar] [CrossRef]
- Lai, L.; He, R.; Ding, K.; Liu, T.; Liu, R.; Chen, Y.; Guo, S. Ternary Co-Mo-B bulk metallic glasses with ultrahigh strength and good ductility. J. Non-Cryst. Solids 2019, 524, 119657. [Google Scholar] [CrossRef]
- Inoue, A. Soft Magnetic Co-Based Metallic Glass Alloy. U.S. Patent US 2005/0178476A1, 18 August 2005. [Google Scholar]
- Huang, D.; Li, Y.; Yang, Y.; Zhu, Z.; Zhang, W. Soft magnetic Co-based Co–Fe–B–Si–P bulk metallic glasses with high saturation magnetic flux density of over 1.2 T. J. Alloys Compd. 2020, in press. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, L.; Jiang, S.S.; Wang, Y.G.; Liu, S.N.; Chen, F.G. Role of Ni and Co in tailoring magnetic and mechanical properties of Fe84Si2B13P1 metallic glass. J. Alloys Compd. 2020, 816, 152549. [Google Scholar] [CrossRef]
- Taghvaei, A.H.; Stoica, M.; Prashanth, K.G.; Eckert, J. Fabrication and characterization of bulk glassy Co40Fe22Ta8B30 alloy with high thermal stability and excellent soft magnetic properties. Acta Mater. 2013, 61, 6609–6621. [Google Scholar] [CrossRef]
- Mazaleyrat, F.; Barrué, R. Soft amorphous and nanocrystalline magnetic materials. In Handbook of Advanced Electronic and Photonic Materials and Devices, 2nd ed.; Nalwa, H.S., Ed.; Elsevier: Oxford, UK, 2001; pp. 59–102. [Google Scholar]
- Diouf, S.; Menapace, C.; Molinar, A. Study of effect of particle size on densification of copper during spark plasma sintering. Powder Metall. 2012, 55, 228–234. [Google Scholar] [CrossRef]
- Liang, B.Y.; Jin, S.Z. Effect of alloying time on fabrication of Ti3Si(Al)C2 by spark plasma sintering. Adv. App. Ceram. 2009, 108, 162–166. [Google Scholar] [CrossRef]



| Nominal Composition (at.%) | |||||||
| Fe (x) | 0 | 2 | 5 | 7 | 10 | 15 | 20 |
| Co | 75.00 | 73.5 | 71.25 | 69.75 | 67.50 | 63.75 | 60 |
| Ti | 25.00 | 24.5 | 23.75 | 23.25 | 22.5 | 21.25 | 20 |
| Real Composition, After Consolidation (at.%) | |||||||
| Fe (x) | 0 | 1.96 | 5.15 | 7.01 | 9.98 | 14.99 | 20.14 |
| Co | 74.91 | 73.41 | 71.17 | 69.70 | 67.44 | 63.78 | 60.13 |
| Ti | 25.09 | 24.63 | 23.68 | 23.29 | 22.58 | 21.23 | 19.73 |
| Alloying Elements (wt.%) | |||
|---|---|---|---|
| Zone | Co | Ti | Fe |
| I | 63.14 | 17.08 | 19.78 |
| II | 62.98 | 17.10 | 19.92 |
| III | 62.94 | 16.98 | 20.08 |
| IV | 62.97 | 17.05 | 19.98 |
| V | 63.05 | 17.03 | 19.92 |
| VI | 62.91 | 16.96 | 20.13 |
| VII | 62.96 | 16.97 | 20.07 |
| VIII | 63.04 | 17.03 | 19.93 |
| Alloying Elements (wt.%) | |||
|---|---|---|---|
| Zone | Co | Ti | Fe |
| I | 78.75 | 21.25 | - |
| II | 78.64 | 21.36 | - |
| III | 78.72 | 21.28 | - |
| IV | 71.27 | 18.68 | 10.05 |
| V | 71.31 | 18.68 | 10.10 |
| VI | 71.29 | 18.71 | 10.00 |
| VII | 67.22 | 17.93 | 14.85 |
| VIII | 67.19 | 17.91 | 14.90 |
| IX | 67.21 | 17.96 | 14.83 |
| X | 62.98 | 16.97 | 20.05 |
| XI | 63.08 | 16.99 | 19.93 |
| XII | 63.04 | 17.01 | 19.95 |
| System | Temperature (°C) | Trg | Bs (T) | ||||
|---|---|---|---|---|---|---|---|
| Tg | Tx | ΔTx | Tm | Tl | |||
| Co75Ti25 | 545 | 562 | 17 | 1219 | 1301 | 0.42 | 0.61 |
| (Co75Ti25)98Fe2 | 548 | 571 | 23 | 0.70 | |||
| (Co75Ti25)95Fe5 | 565 | 606 | 41 | 1228 | 1297 | 0.44 | 0.71 |
| (Co75Ti25)93Fe7 | 568 | 609 | 41 | 0.72 | |||
| (Co75Ti25)90Fe10 | 561 | 605 | 44 | 1251 | 1318 | 0.43 | 0.87 |
| (Co75Ti25)85Fe15 | 616 | 646 | 30 | 1285 | 1329 | 0.46 | 0.94 |
| (Co75Ti25)80Fe20 | 605 | 682 | 77 | 1293 | 1347 | 0.45 | 1.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Eskandarany, M.S.; Ali, N.; Saeed, M. Glass-Forming Ability and Soft Magnetic Properties of (Co75Ti25)100−xFex (x; 0–20 at.%) Systems Fabricated by SPS of Mechanically Alloyed Nanopowders. Nanomaterials 2020, 10, 849. https://doi.org/10.3390/nano10050849
El-Eskandarany MS, Ali N, Saeed M. Glass-Forming Ability and Soft Magnetic Properties of (Co75Ti25)100−xFex (x; 0–20 at.%) Systems Fabricated by SPS of Mechanically Alloyed Nanopowders. Nanomaterials. 2020; 10(5):849. https://doi.org/10.3390/nano10050849
Chicago/Turabian StyleEl-Eskandarany, Mohamed Sherif, Naser Ali, and Maryam Saeed. 2020. "Glass-Forming Ability and Soft Magnetic Properties of (Co75Ti25)100−xFex (x; 0–20 at.%) Systems Fabricated by SPS of Mechanically Alloyed Nanopowders" Nanomaterials 10, no. 5: 849. https://doi.org/10.3390/nano10050849
APA StyleEl-Eskandarany, M. S., Ali, N., & Saeed, M. (2020). Glass-Forming Ability and Soft Magnetic Properties of (Co75Ti25)100−xFex (x; 0–20 at.%) Systems Fabricated by SPS of Mechanically Alloyed Nanopowders. Nanomaterials, 10(5), 849. https://doi.org/10.3390/nano10050849







