Analogous Diamondene Nanotube Structure Prediction Based on Molecular Dynamics and First-Principle Calculations
Abstract
1. Introduction
2. Models and Methods
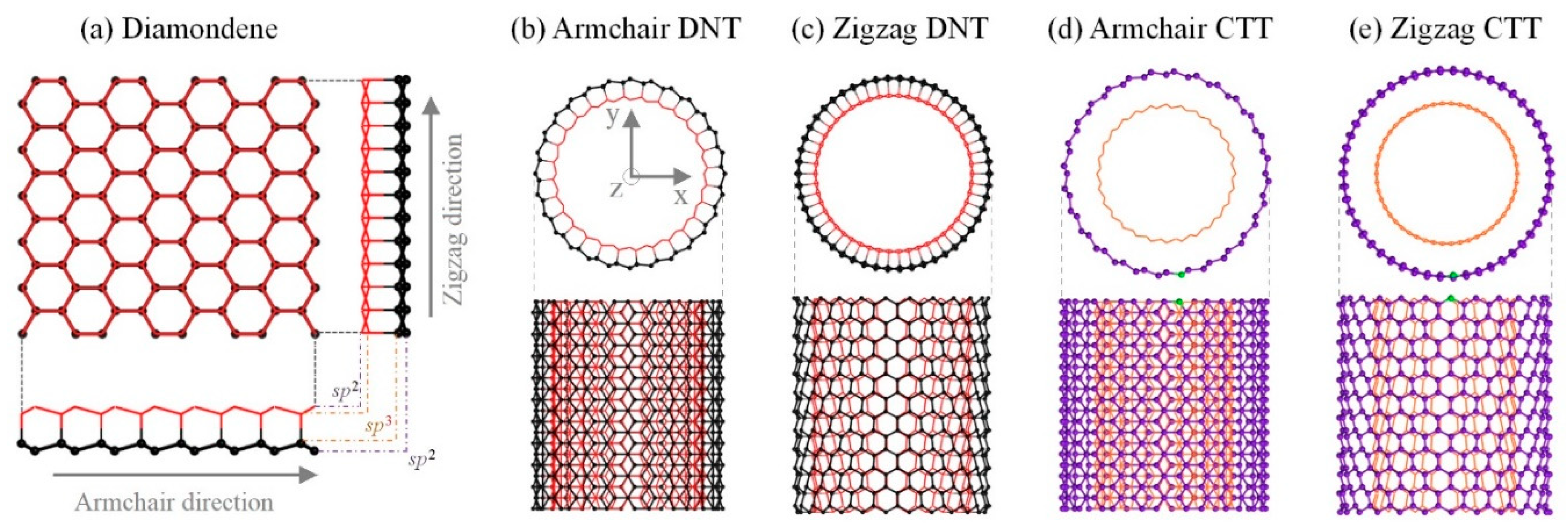
2.1. Models
2.2. Methodology
2.3. Parameter Definition
3. Results and Discussion
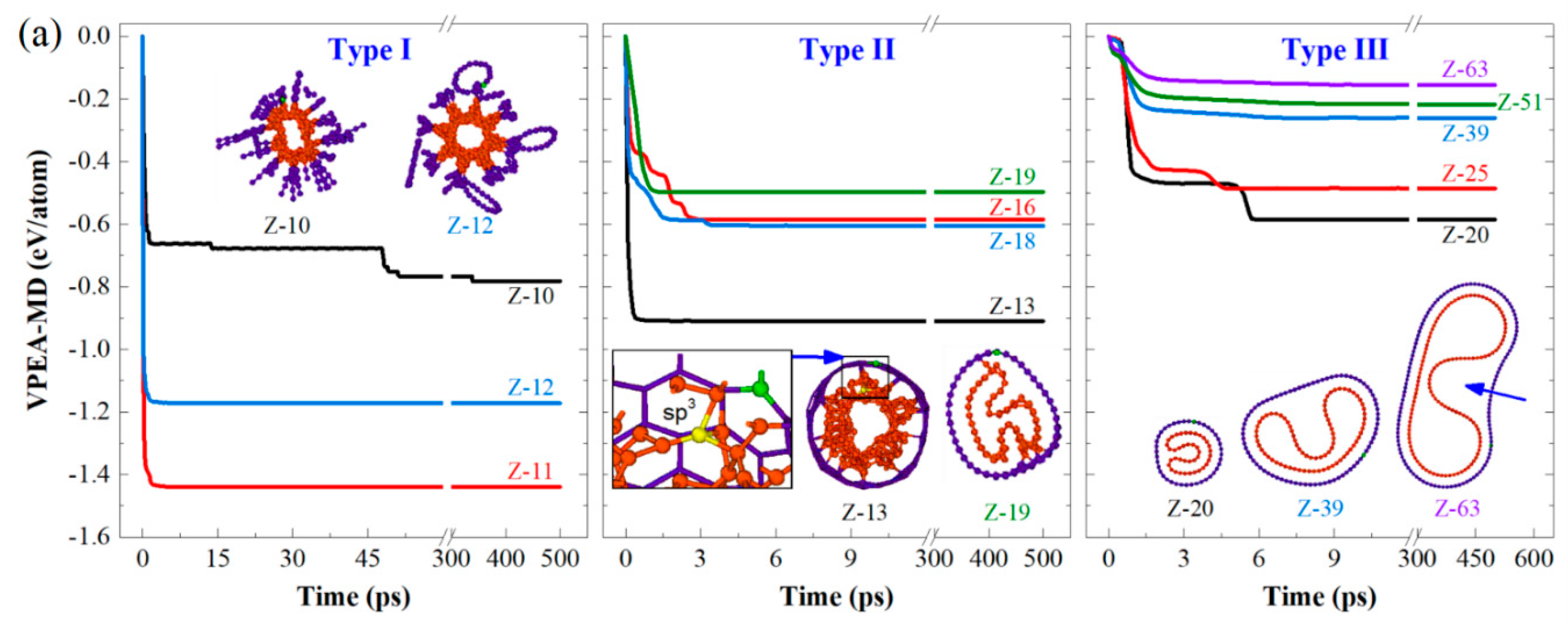
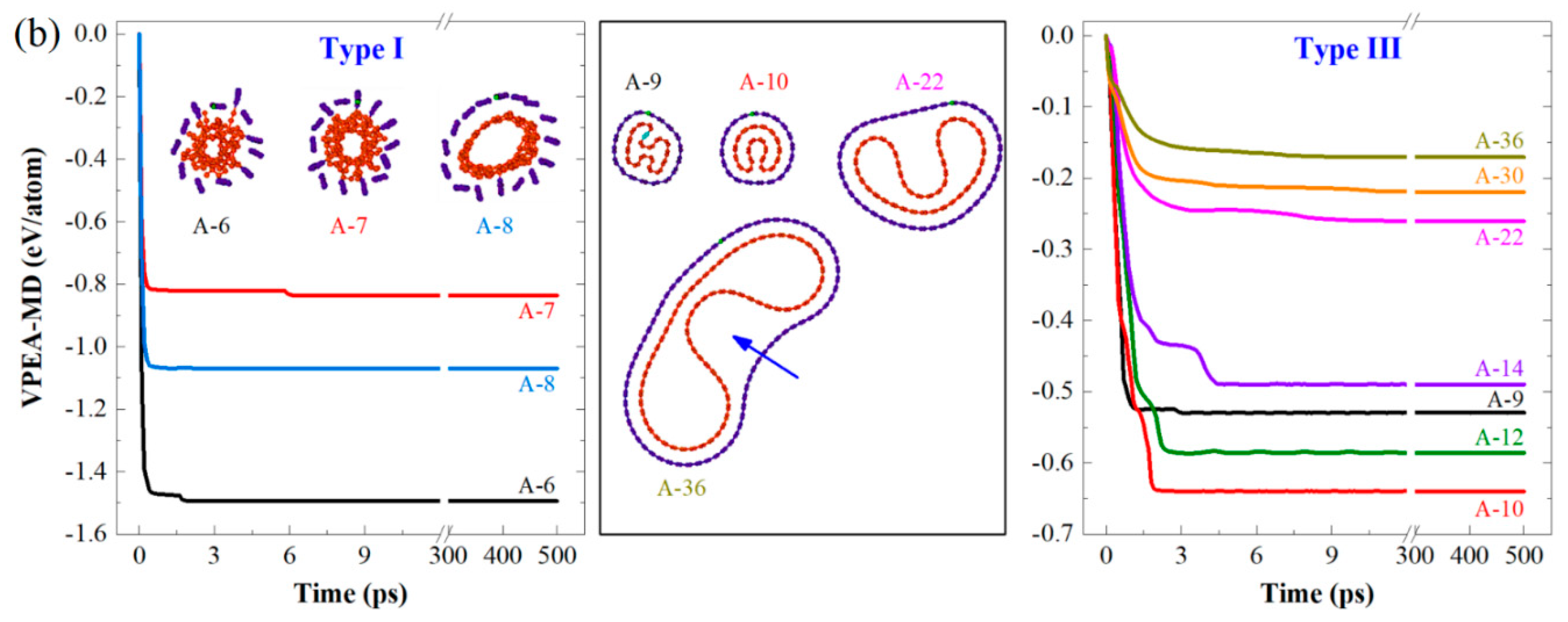
3.1. Stable Configurations of CTTs at 8 K
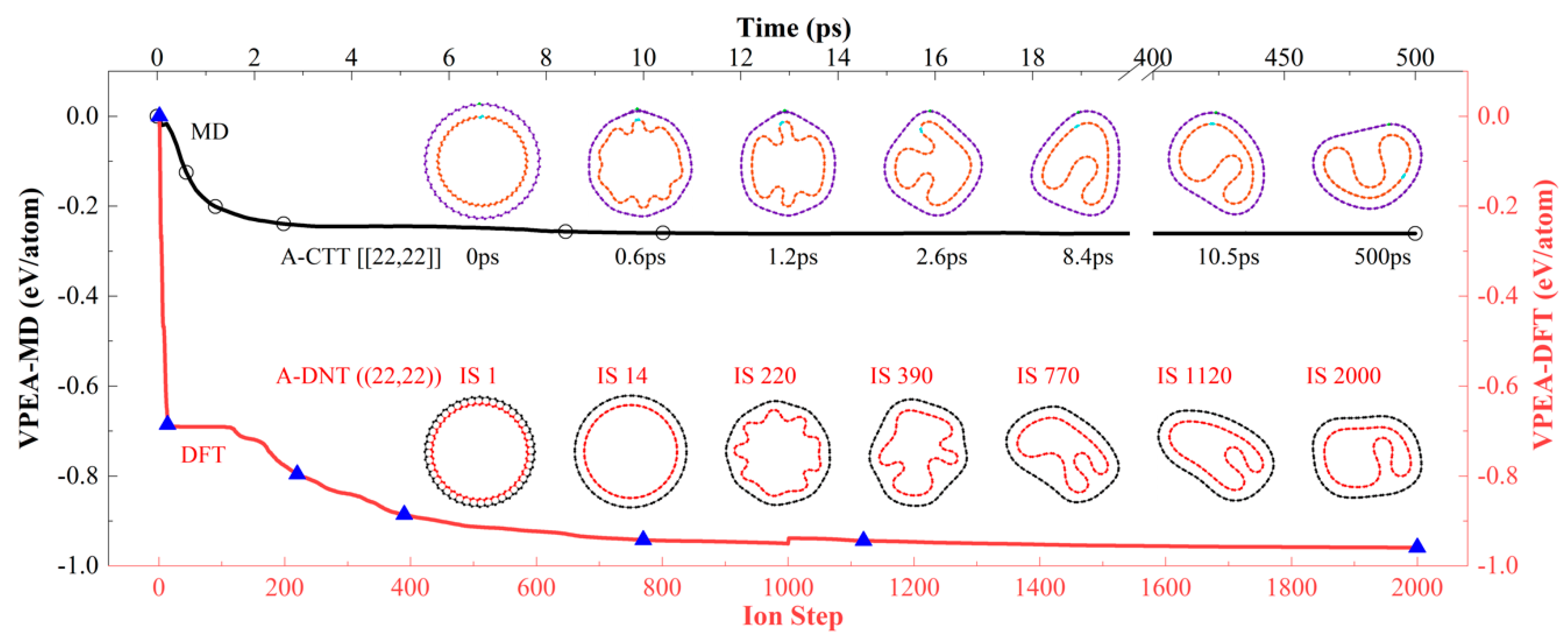
3.2. Comparison of MD and DFT Results
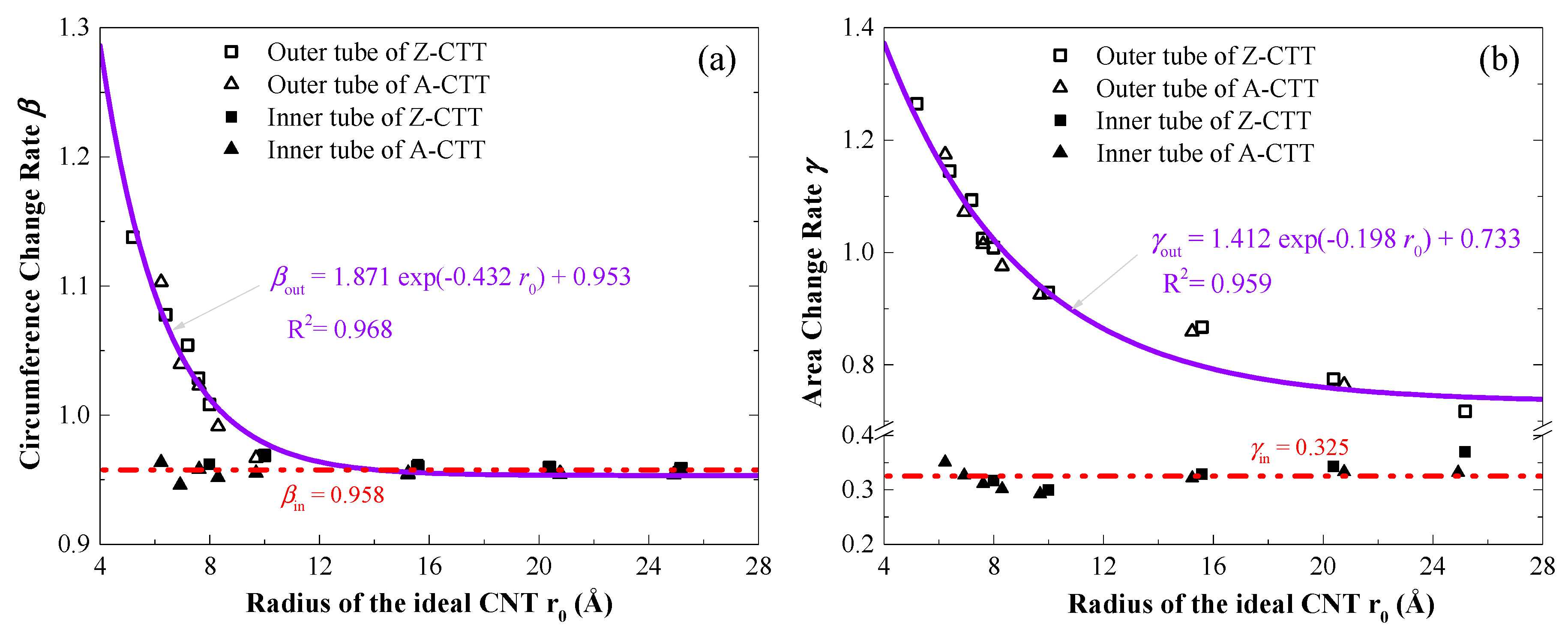
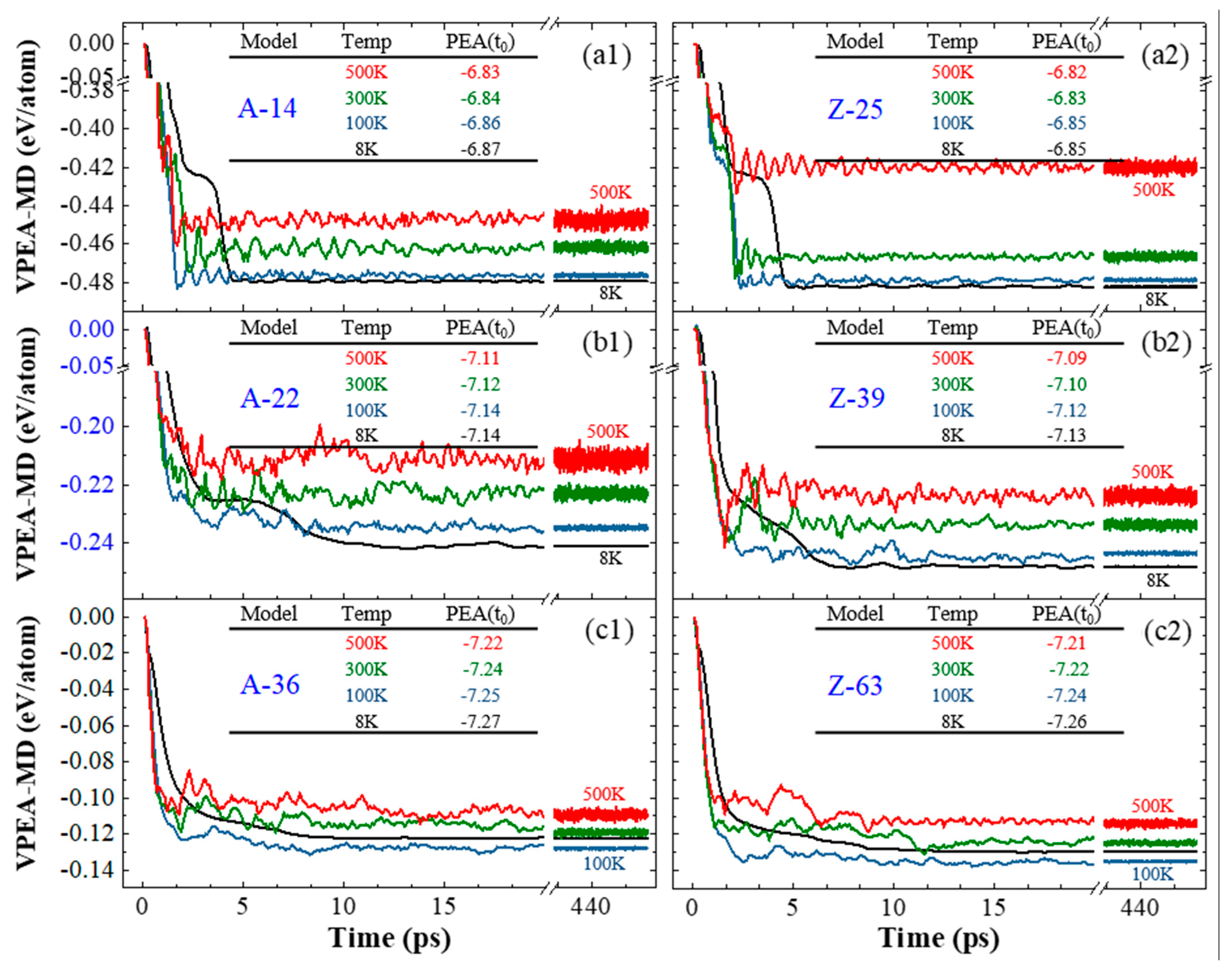
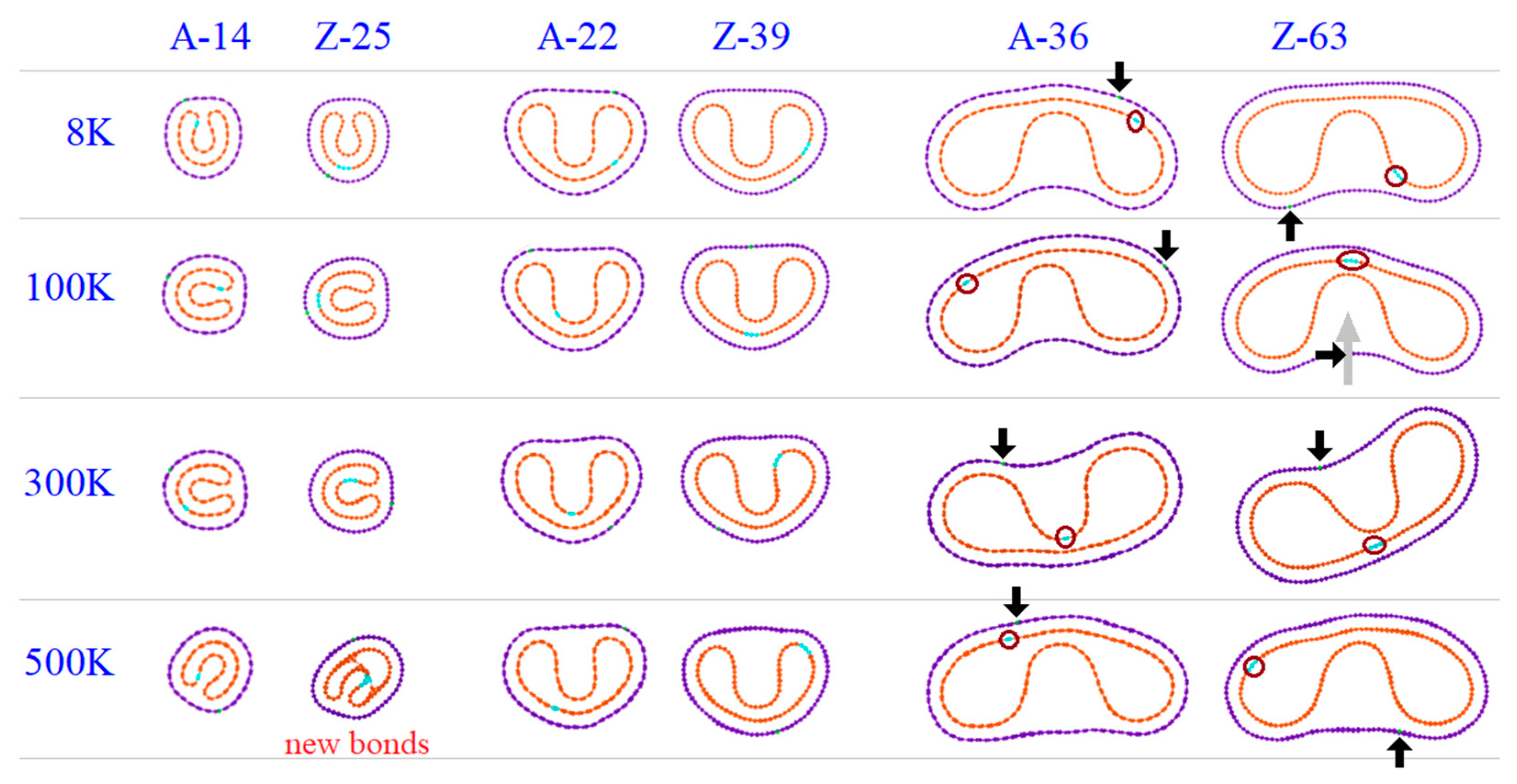
3.3. Geometry and Temperature Effect
3.4. Maximal Radii of A-/Z-CTTs with Convex Outer Tubes at 8 K
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Osawa, E. Superaromaticity. Kagaku 1970, 25, 854–863. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Sofo, J.O.; Chaudhari, A.S.; Barber, G.D. Graphane: A two-dimensional hydrocarbon. Phys. Rev. B 2007, 75, 153401. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, S.H.; Lou, J.Z.; Wang, J.H.; Yang, Q.J.; Liu, C.R.; Huang, D.P.; Zhu, T.H. Graphene’s cousin: The present and future of graphane. Nanoscale Res. Lett. 2014, 9, 26. [Google Scholar] [CrossRef]
- Martins, L.G.P.; Matos, M.J.S.; Paschoal, A.R.; Freire, P.T.C.; Andrade, N.F.; Aguiar, A.L.; Kong, J.; Neves, B.R.A.; de Oliveira, A.B.; Mazzoni, M.S.C.; et al. Raman evidence for pressure-induced formation of diamondene. Nat. Commun. 2017, 8, 96. [Google Scholar] [CrossRef]
- Shi, J.; Cai, K.; Xie, Y.M. Thermal and tensile properties of diamondene at finite temperature: A molecular dynamics study. Mater. Des. 2018, 156, 125–134. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.R.; Shi, J.; Cai, K. Vibration behavior of diamondene nano-ribbon passivated by hydrogen. Sci. Rep. 2019, 9, 15783. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Sorokin, P.B.; Kvashnin, A.G.; Kvashnin, D.G. Diamond-like C2H nanolayer, diamane: Simulation of the structure and properties. Jetp Lett. 2009, 90, 134–138. [Google Scholar] [CrossRef]
- Paul, S.; Momeni, K. Mechanochemistry of stable diamane and atomically thin diamond films synthesis from bi- and multilayer graphene: A computational study. J. Phys. Chem. C 2019, 123, 15751–15760. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Li, W.; Ding, F. Giant thermal conductivity in diamane and the influence of horizontal reflection symmetry on phonon scattering. Nanoscale 2019, 11, 4248–4257. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Sorokin, P.B.; Kuzubov, A.A.; Sorokin, B.P.; Kvashnin, A.G.; Kvashnin, D.G.; Avramov, P.V.; Yakobson, B.I. Influence of size effect on the electronic and elastic properties of diamond films with nanometer thickness. J. Phys. Chem. C 2011, 115, 132–136. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Mavrin, B.N.; Sorokin, P.B. Determination of ultrathin diamond films by Raman spectroscopy. Phys. Status Solidi B 2012, 249, 1550–1554. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Chernozatonskii, L.A.; Yakobson, B.I.; Sorokin, P.B. Phase diagram of quasi-two-dimensional carbon, from graphene to diamond. Nano Lett. 2014, 14, 676–681. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Sorokin, P.B. Lonsdaleite films with nanometer thickness. J. Phys. Chem. Lett. 2014, 5, 541–548. [Google Scholar] [CrossRef]
- Antipina, L.Y.; Sorokin, P.B. Converting chemically functionalized few-layer graphene to diamond films: A computational study. J. Phys. Chem. C 2015, 119, 2828–2836. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Seol, J.H.; Jo, I.; Moore, A.L.; Lindsay, L.; Aitken, Z.H.; Pettes, M.T.; Li, X.S.; Yao, Z.; Huang, R.; Broido, D.; et al. Two-dimensional phonon transport in supported graphene. Science 2010, 328, 213–216. [Google Scholar] [CrossRef]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Savini, G.; Dappe, Y.J.; Oberg, S.; Charlier, J.C.; Katsnelson, M.I.; Fasolino, A. Bending modes, elastic constants and mechanical stability of graphitic systems. Carbon 2011, 49, 62–69. [Google Scholar] [CrossRef]
- Scarpa, F.; Chowdhury, R.; Adhikari, S. Thickness and in-plane elasticity of graphane. Phys. Lett. A 2011, 375, 2071–2074. [Google Scholar] [CrossRef]
- Ansari, R.; Mirnezhad, M.; Rouhi, H. Mechanical properties of fully hydrogenated graphene sheets. Solid State Commun. 2015, 201, 1–4. [Google Scholar] [CrossRef]
- Neek-Amal, M.; Peeters, F.M. Lattice thermal properties of graphane: Thermal contraction, roughness, and heat capacity. Phys. Rev. B 2011, 83, 235437. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.J.; Li, Q.X.; Yang, J.L. Linear band-gap modulation of graphane nanoribbons under uniaxial elastic strain: A density functional theory study. J. Phys. Chem. C 2012, 116, 9356–9359. [Google Scholar] [CrossRef]
- Barboza, A.P.M.; Guimaraes, M.H.D.; Massote, D.V.P.; Campos, L.C.; Neto, N.M.B.; Cancado, L.G.; Lacerda, R.G.; Chacham, H.; Mazzoni, M.S.C.; Neves, B.R.A. Room-temperature compression-induced diamondization of few-layer graphene. Adv. Mater. 2011, 23, 3014–3017. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, T.F.; Cellini, F.; Berger, C.; de Heer, W.A.; Tosatti, E.; Riedo, E.; Bongiorno, A. Ultrahard carbon film from epitaxial two-layer graphene. Nat. Nanotechnol. 2018, 13, 133–138. [Google Scholar] [CrossRef]
- Wen, X.D.; Yang, T.; Hoffmann, R.; Ashcroft, N.W.; Martin, R.L.; Rudin, S.P.; Zhu, J.X. Graphane nanotubes. Acs Nano 2012, 6, 7142–7150. [Google Scholar] [CrossRef]
- Cai, K.; Wang, L.; Xie, Y.M. Buckling behavior of nanotubes from diamondene. Mater. Des. 2018, 149, 34–42. [Google Scholar] [CrossRef]
- Wang, L.; Cai, K.; Wei, S.; Xie, Y.M. Softening to hardening of stretched diamondene nanotubes. Phys. Chem. Chem. Phys. 2018, 20, 21136–21143. [Google Scholar] [CrossRef]
- Wang, L.; Cai, K.; Xie, Y.M.; Qin, Q.H. Thermal shrinkage and stability of diamondene nanotubes. Nanotechnology 2019, 30, 075702. [Google Scholar] [CrossRef]
- Zhang, H.W.; Wang, L.; Wang, J.B. Computer simulation of buckling behavior of double-walled carbon nanotubes with abnormal interlayer distances. Comp. Mater. Sci. 2007, 39, 664–672. [Google Scholar] [CrossRef]
- Xu, Z.P.; Wang, L.F.; Zheng, Q.S. Enhanced mechanical properties of prestressed multi-walled carbon nanotubes. Small 2008, 4, 733–737. [Google Scholar] [CrossRef]
- Ma, M.D.; Liu, J.Z.; Wang, L.F.; Shen, L.M.; Zheng, Q.S. Effects of vacancies on interwall spacings of multi-walled carbon nanotubes. J. Zhejiang Univ. Sci. A 2010, 11, 714–721. [Google Scholar] [CrossRef]
- Shi, J.; Cai, H.F.; Cai, K.; Qin, Q.H. Dynamic behavior of a black phosphorus and carbon nanotube composite system. J. Phys. D Appl. Phys. 2017, 50, 025304. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular-dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- O′Connor, T.C.; Andzelm, J.; Robbins, M.O. AIREBO-M: A reactive model for hydrocarbons at extreme pressures. J. Chem. Phys. 2015, 142, 024903. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Klimes, J.; Bowler, D.R.; Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Phys. Condes. Matter 2010, 22, 022201. [Google Scholar] [CrossRef]
- Klimes, J.; Bowler, D.R.; Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef]
- Cai, K.; Cai, H.; Yin, H.; Qin, Q.H. Dynamic behavior of curved double-wall carbon nanotubes with rotating inner tube. RSC Adv. 2015, 5, 29908–29913. [Google Scholar] [CrossRef]
- Banhart, F.; Ajayan, P.M. Carbon onions as nanoscopic pressure cells for diamond formation. Nature 1996, 382, 433–435. [Google Scholar] [CrossRef]
- Banhart, F.; Ajayan, P.M. Self-compression and diamond formation in carbon onions. Adv. Mater. 1997, 9, 261–263. [Google Scholar] [CrossRef]
- Sun, L.; Banhart, F.; Krasheninnikov, A.V.; Rodriguez-Manzo, J.A.; Terrones, M.; Ajayan, P.M. Carbon nanotubes as high-pressure cylinders and nanoextruders. Science 2006, 312, 1199–1202. [Google Scholar] [CrossRef]
- Cai, K.; Gao, D.Y.; Qin, Q.H. Post-buckling solutions of hyper-elastic beam by canonical dual finite element method. Math. Mech. Solids 2014, 19, 659–671. [Google Scholar] [CrossRef]
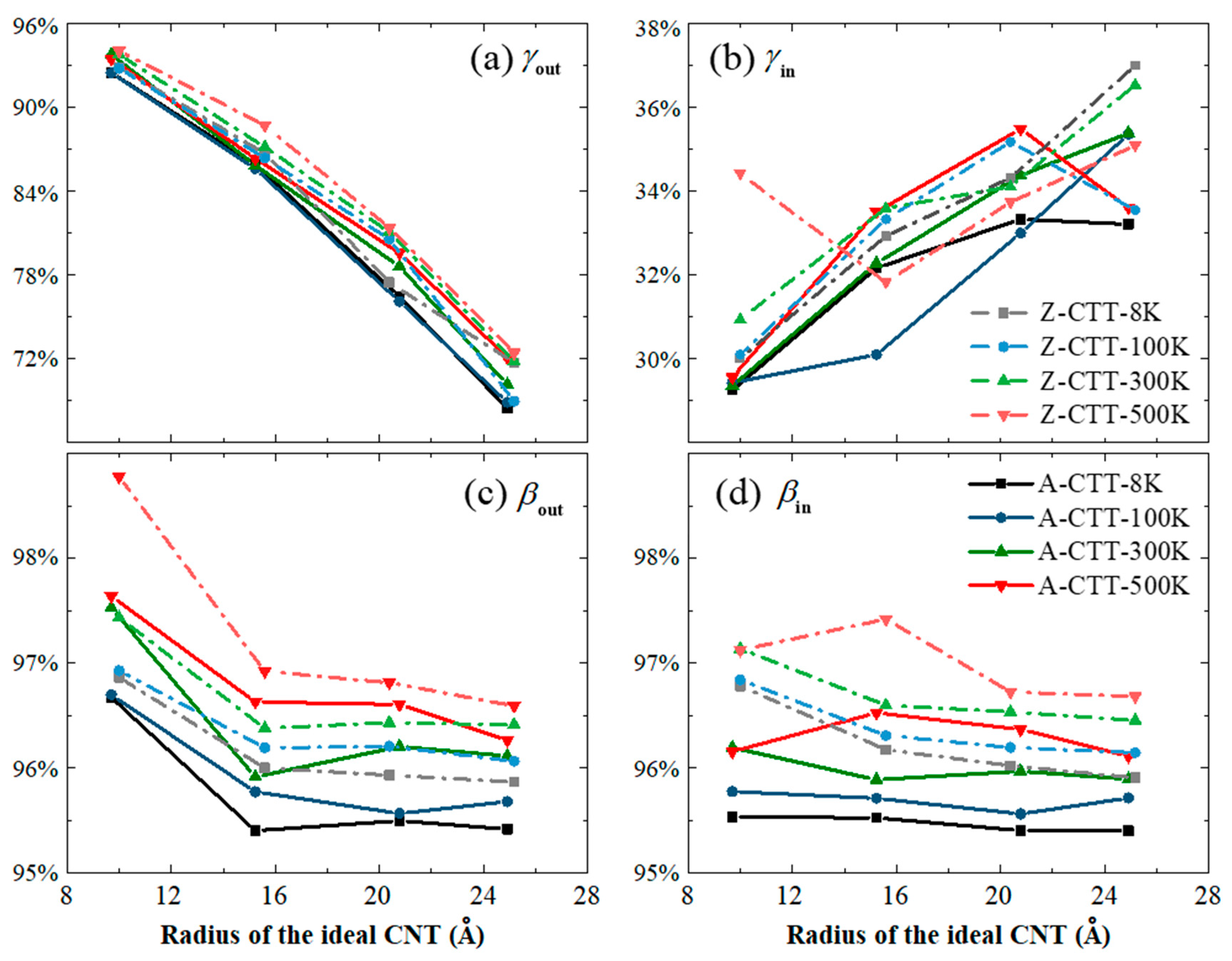

| CTT | r0 (Å) | A0 (Å2) | Aout (Å2) | γout (%) | Ain (Å2) | γin (%) | C0 (Å) | Cout (Å) | βout (%) | Cin (Å) | βin (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A-9 | 6.23 | 121.91 | 143.17 | 117.43 | 42.78 | 35.09 | 39.14 | 43.18 | 110.31 | 37.71 | 96.34 |
| A-10 | 6.92 | 150.51 | 161.37 | 107.21 | 49.21 | 32.70 | 43.49 | 45.21 | 103.95 | 41.14 | 94.59 |
| A-12 | 8.31 | 216.74 | 211.44 | 97.56 | 65.38 | 30.17 | 52.19 | 51.73 | 99.13 | 49.68 | 95.19 |
| A-14 | 9.69 | 295.00 | 272.86 | 92.49 | 86.28 | 29.25 | 60.89 | 58.86 | 96.67 | 58.17 | 95.53 |
| A-22 | 15.23 | 728.47 | 625.74 | 85.90 | 234.32 | 32.17 | 95.68 | 91.28 | 95.40 | 91.40 | 95.52 |
| A-30 | 20.76 | 1354.60 | 1035.73 | 76.46 | 451.48 | 33.33 | 130.47 | 124.59 | 95.50 | 124.47 | 95.40 |
| A-36 | 24.92 | 1950.63 | 1334.81 | 68.43 | 647.88 | 33.21 | 156.56 | 149.39 | 95.42 | 149.36 | 95.40 |
| Z-13 | 5.20 | 84.80 | 107.22 | 126.45 | —— | —— | 32.64 | 37.14 | 113.78 | —— | —— |
| Z-16 | 6.39 | 128.45 | 147.06 | 114.49 | —— | —— | 40.18 | 43.29 | 107.76 | —— | —— |
| Z-18 | 7.19 | 162.57 | 177.74 | 109.34 | —— | —— | 45.20 | 47.65 | 105.42 | —— | —— |
| Z-19 | 7.59 | 181.13 | 185.63 | 102.48 | —— | —— | 47.71 | 49.07 | 102.86 | —— | —— |
| Z-20 | 7.99 | 200.70 | 202.43 | 100.86 | 63.64 | 31.71 | 50.22 | 50.63 | 100.82 | 48.31 | 96.20 |
| Z-25 | 9.99 | 313.59 | 291.29 | 92.89 | 94.09 | 30.00 | 62.78 | 60.81 | 96.86 | 60.75 | 96.78 |
| Z-39 | 15.59 | 763.16 | 661.67 | 86.70 | 251.30 | 32.93 | 97.93 | 94.01 | 96.00 | 94.18 | 96.17 |
| Z-51 | 20.38 | 1305.04 | 1010.96 | 77.47 | 447.89 | 34.32 | 128.06 | 122.85 | 95.93 | 122.97 | 96.02 |
| Z-63 | 25.18 | 1991.43 | 1428.29 | 71.72 | 736.97 | 37.01 | 158.19 | 151.65 | 95.87 | 151.72 | 95.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Cai, H.; Hu, C.; Shi, J.; Li, Z.; Cai, K. Analogous Diamondene Nanotube Structure Prediction Based on Molecular Dynamics and First-Principle Calculations. Nanomaterials 2020, 10, 846. https://doi.org/10.3390/nano10050846
Zhou X, Cai H, Hu C, Shi J, Li Z, Cai K. Analogous Diamondene Nanotube Structure Prediction Based on Molecular Dynamics and First-Principle Calculations. Nanomaterials. 2020; 10(5):846. https://doi.org/10.3390/nano10050846
Chicago/Turabian StyleZhou, Xin, Haifang Cai, Chunwei Hu, Jiao Shi, Zongli Li, and Kun Cai. 2020. "Analogous Diamondene Nanotube Structure Prediction Based on Molecular Dynamics and First-Principle Calculations" Nanomaterials 10, no. 5: 846. https://doi.org/10.3390/nano10050846
APA StyleZhou, X., Cai, H., Hu, C., Shi, J., Li, Z., & Cai, K. (2020). Analogous Diamondene Nanotube Structure Prediction Based on Molecular Dynamics and First-Principle Calculations. Nanomaterials, 10(5), 846. https://doi.org/10.3390/nano10050846






