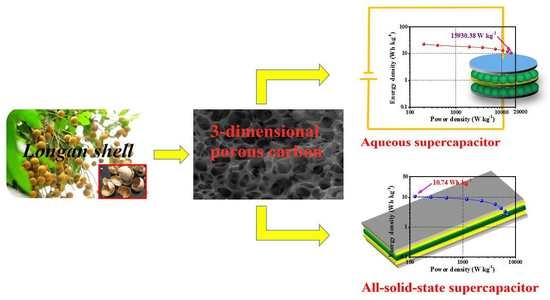
3-Dimensional Porous Carbon with High Nitrogen Content Obtained from Longan Shell and Its Excellent Performance for Aqueous and All-Solid-State Supercapacitors
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
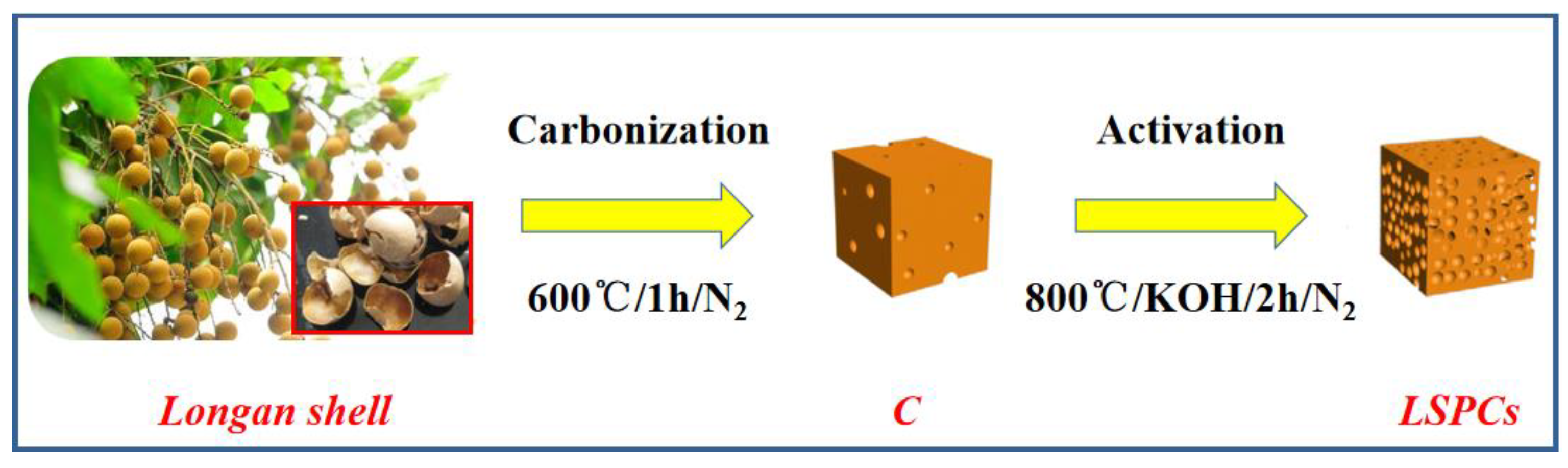
2.2. Preparation of Longan Shell 3-Dimensional Porous Carbons (LSPCs)
2.3. Physicochemical Characterization
2.4. Electrode Preparation and Electrochemical Measurement
2.4.1. Preparation of Electrode and Test in Three-Electrode System
2.4.2. Assembly and Test of Aqueous/All-Solid-State SCs
3. Results and Discussion
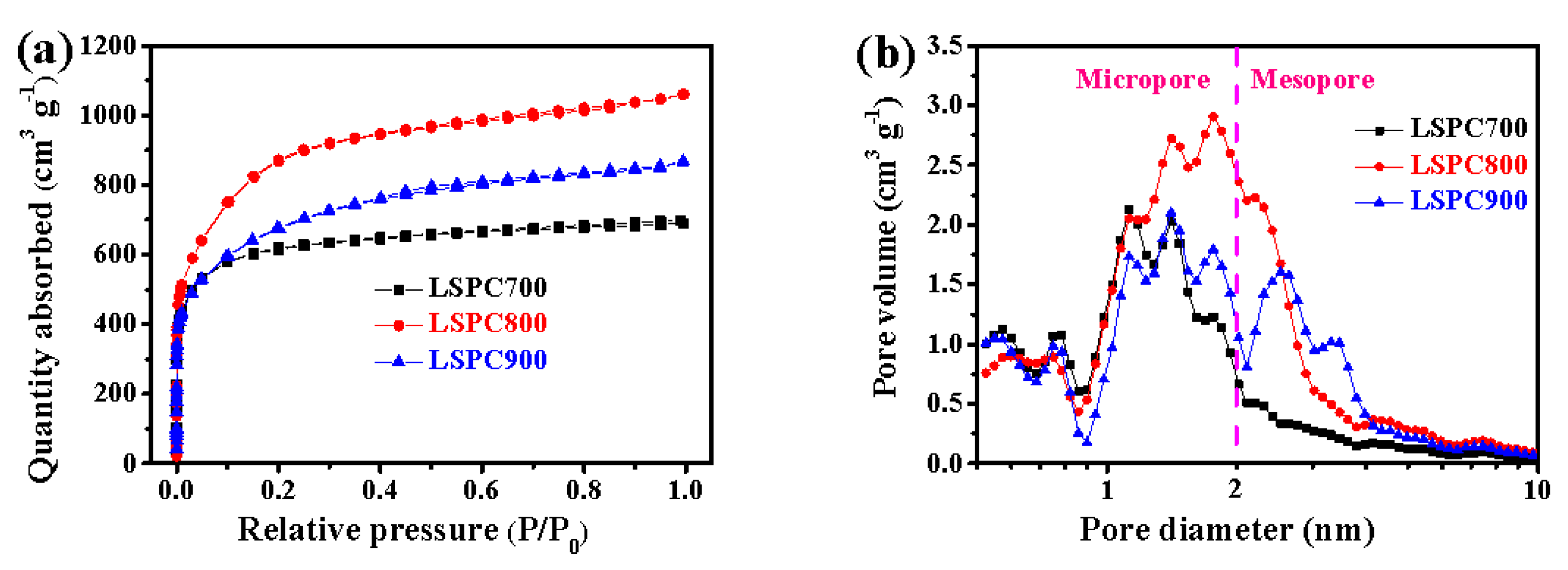
3.1. Physicochemical Characterization
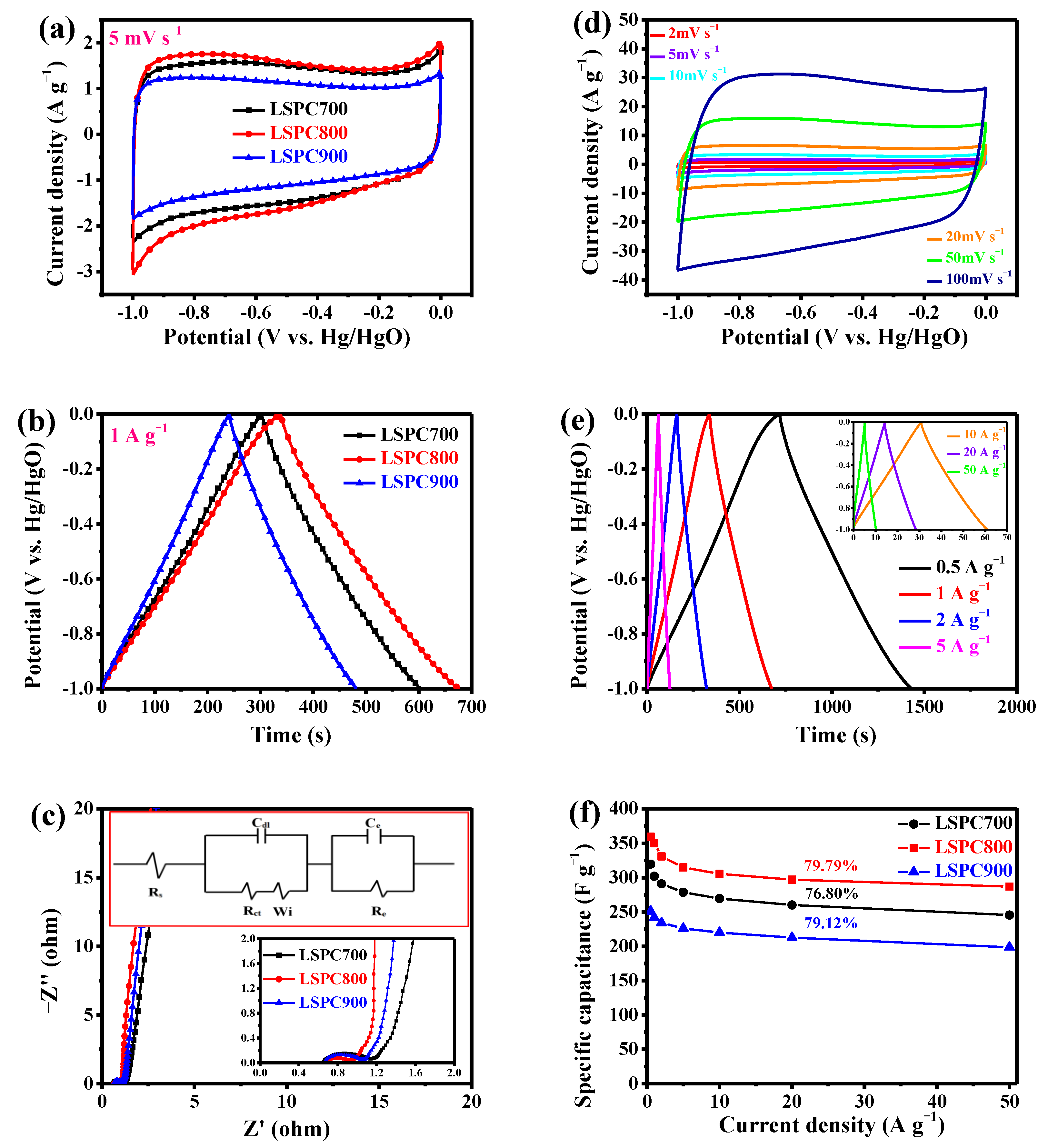
3.2. Electrochemical Behaviors of LSPCs in Three-Electrode System
3.3. Electrochemical Behaviors of LSPCs in SCs
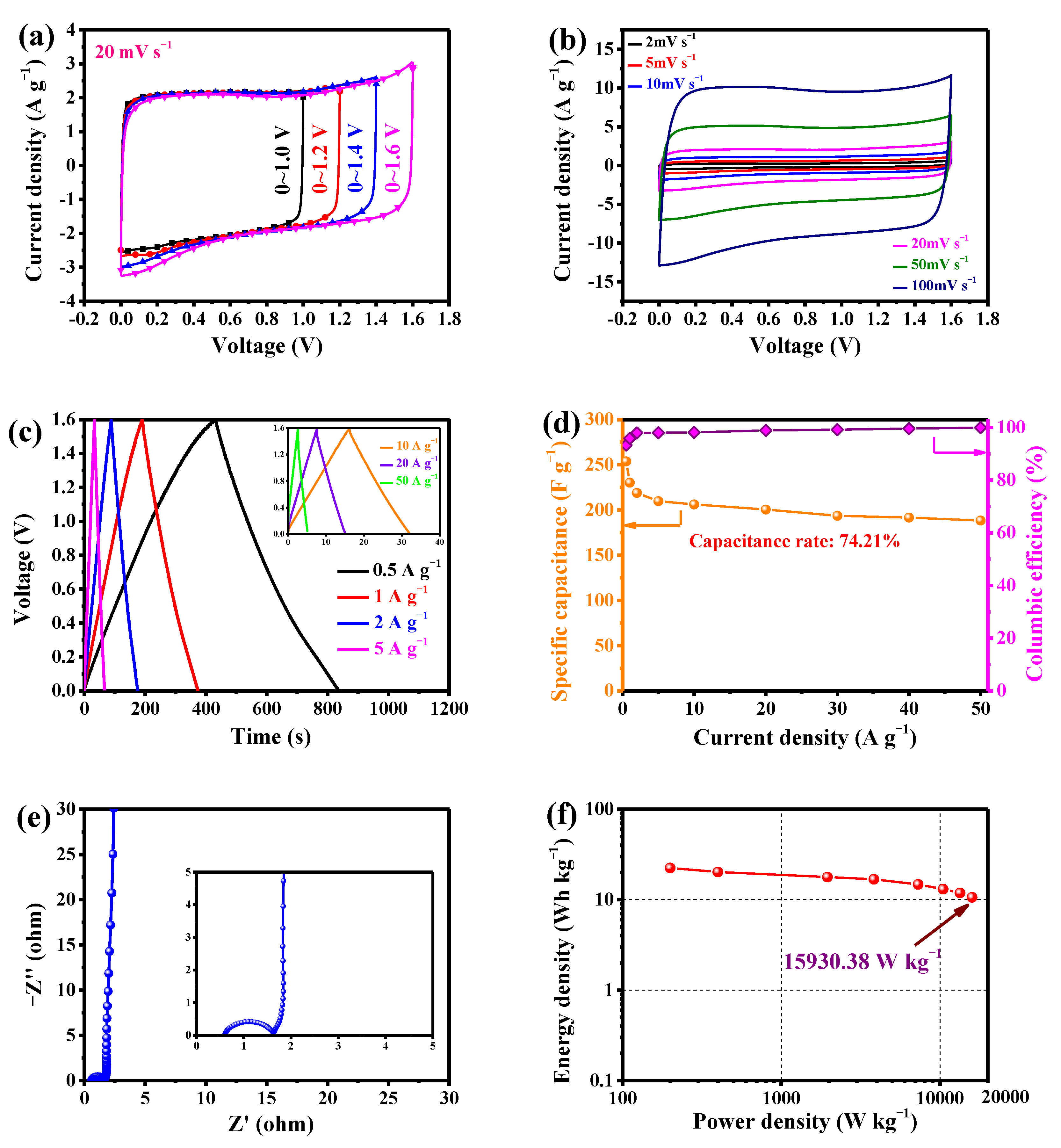
3.3.1. LSPC800-Based Aqueous SCs
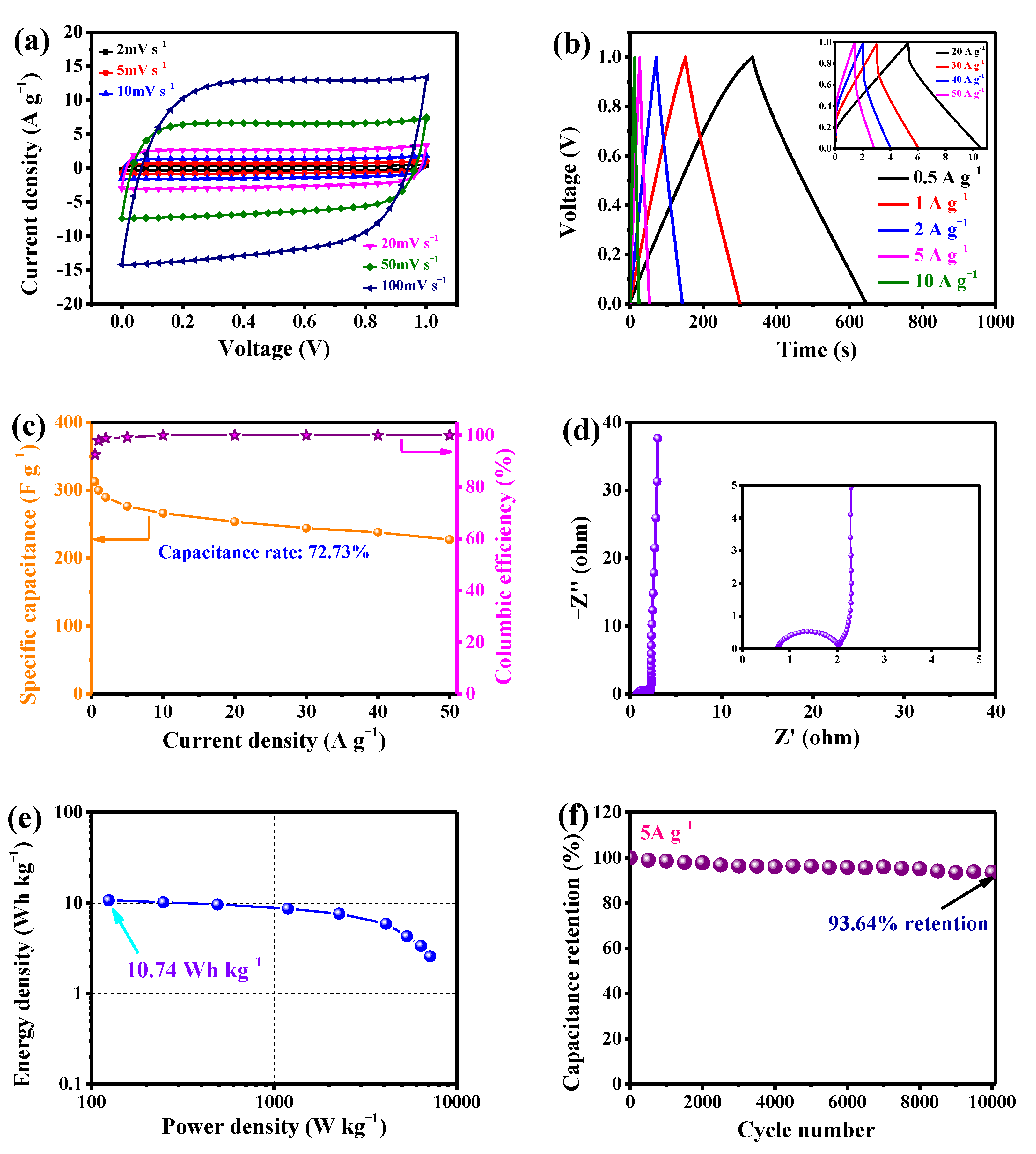
3.3.2. LSPC800-Based All-Solid-State SCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, S.; Yang, Z.; Xu, W.; Zhang, Q.; Zhao, X.; Wen, X. ACF/NiCo2S4 honeycomb-like heterostructure material: Room-temperature sulfurization and its performance in asymmetric supercapacitors. Electrochim. Acta 2019, 297, 334–343. [Google Scholar] [CrossRef]
- Enterría, M.; Pereira, M.F.R.; Martins, J.I.; Figueiredo, J.L. Hydrothermal functionalization of ordered mesoporous carbons: The effect of boron on supercapacitor performance. Carbon 2015, 95, 72–83. [Google Scholar] [CrossRef]
- Li, J.; Hao, H.; Wang, J.; Li, W.; Shen, W. Hydrogels that couple nitrogen-enriched graphene with Ni(OH)2 nanosheets for high-performance asymmetric supercapacitors. J. Alloys Compd. 2019, 782, 516–524. [Google Scholar] [CrossRef]
- Kariper, İ.A.; Meydaneri Tezel, F. Producing MoO3 thin film supercapacitor through bio-chemical bath deposition. Ceram. Int. 2019, 45, 3478–3482. [Google Scholar] [CrossRef]
- Pujari, R.B.; Patil, S.J.; Park, J.; Shanmugasundaram, A.; Lee, D.-W. Vertically aligned nanostructured FeOOH@MnO2 core shell electrode with better areal capacitance. J. Power Sources 2019, 436, 226826. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, H.; Gui, L.; Zheng, Y.; Wu, Z.; Luo, Y.; Yu, P. Construction of 0D CeO2/2D MnO2 heterostructure with high electrochemical performance. Electrochim. Acta 2019, 319, 95–100. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.J.; Thomas, J. Asymmetric Supercapacitor Electrodes and Devices. Adv. Mater. 2017, 29, 21. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, M.S.; Kim, M.-H.; Chun, J.; Kim, H.; Park, J.; Roh, K.C.; Kang, K.; Yoon, S.; et al. High-Performance Sodium-Ion Hybrid Supercapacitor Based on Nb2O5@Carbon Core-Shell Nanoparticles and Reduced Graphene Oxide Nanocomposites. Adv. Funct. Mater. 2016, 26, 3711–3719. [Google Scholar] [CrossRef]
- Guney, M.S.; Tepe, Y. Classification and assessment of energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 1187–1197. [Google Scholar] [CrossRef]
- Zhang, C.; Long, D.; Xing, B.; Qiao, W.; Zhang, R.; Zhan, L.; Liang, X.; Ling, L. The superior electrochemical performance of oxygen-rich activated carbons prepared from bituminous coal. Electrochem. Commun. 2008, 10, 1809–1811. [Google Scholar] [CrossRef]
- Van Ngo, T.; Moussa, M.; Tung, T.T.; Coghlan, C.; Losic, D. Hybridization of MOFs and graphene: A new strategy for the synthesis of porous 3D carbon composites for high performing supercapacitors. Electrochim. Acta 2020, 329, 135104. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Zhang, P.; Sun, L.; Ren, X.; Mi, H. Hierarchical hollow carbon spheres: Novel synthesis strategy, pore structure engineering and application for micro-supercapacitor. Carbon 2020, 157, 70–79. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Kim, A.; Jung, H. Nitrogen-doped mesoporous graphene with fine-tuned pore size in a few nanometer-scale for supercapacitor applications. Microporous Mesoporous Mater. 2020, 293, 109794. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Song, A.; Gao, L.; Su, L.; Shao, G. Supercapacitance of nitrogen-sulfur-oxygen co-doped 3D hierarchical porous carbon in aqueous and organic electrolyte. J. Power Sources 2017, 359, 556–567. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, Y.; Zhang, C.; Zhu, A.; Wang, T.; Tian, Y.; Yu, J.; Xing, B.; Huang, G.; Cao, Y. Effect of different pretreatment methods on sesame husk-based activated carbon for supercapacitors with aqueous and organic electrolytes. J. Mater. Sci. Mater. Electron. 2019, 30, 7873–7882. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Khosla, K.; Zhu, Z.; Lu, G.Q. Microstructure and electrochemical double-layer capacitance of carbon electrodes prepared by zinc chloride activation of sugar cane bagasse. J. Power Sources 2010, 195, 912–918. [Google Scholar] [CrossRef]
- Momodu, D.; Bello, A.; Oyedotun, K.; Ochai-Ejeh, F.; Dangbegnon, J.; Madito, M.; Manyala, N. Enhanced electrochemical response of activated carbon nanostructures from tree-bark biomass waste in polymer-gel active electrolytes. RSC Adv. 2017, 7, 37286–37295. [Google Scholar] [CrossRef]
- Liu, M.; Lu, C.; Xu, Y.; Hu, Y.; Li, J.; Zhang, H.; Zhang, Y.; Zhang, B.; Kong, L.; Liu, W.; et al. Three-Dimensional Interconnected Reticular Porous Carbon From Corn Starch By a Sample Sol-Gel Method Toward High-Performance Supercapacitors With Aqueous and Ionic Liquid Electrolytes. ACS Sustain. Chem. Eng. 2019, 7, 18690–18699. [Google Scholar] [CrossRef]
- Wei, H.; Chen, H.; Fu, N.; Chen, J.; Lan, G.; Qian, W.; Liu, Y.; Lin, H.; Han, S. Excellent electrochemical properties and large CO2 capture of nitrogen-doped activated porous carbon synthesised from waste longan shells. Electrochim. Acta 2017, 231, 403–411. [Google Scholar] [CrossRef]
- Li, S.; Yang, K.; Ye, P.; Jiang, H.; Zhang, Z.; Huang, Q.; Wang, L. Hierarchical interpenetrating rHGO-decorated NiCo2O4 nanowires architectures for high-performance supercapacitors. Appl. Surf. Sci. 2019, 473, 326–333. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xu, W.; Wang, Y.; Zhang, B.; Luo, S.; Zhou, X.; Zhang, C.; Gu, X.; Hu, C. Porous Fe2O3 nanospheres anchored on activated carbon cloth for high-performance symmetric supercapacitors. Nano Energy 2019, 57, 379–387. [Google Scholar] [CrossRef]
- Lin, J.; Yao, L.; Li, Z.; Zhang, P.; Zhong, W.; Yuan, Q.; Deng, L. Hybrid hollow spheres of carbon@CoxNi1-xMoO4 as advanced electrodes for high-performance asymmetric supercapacitors. Nanoscale 2019, 11, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.L.; Jia, D.; Tang, J.; Zhang, A.; Liu, F.; Chen, T.; Barrow, C.; Yang, W.; Liu, J. Improving the rate capability of ultrathin NiCo-LDH nanoflakes and FeOOH nanosheets on surface electrochemically modified graphite fibers for flexible asymmetric supercapacitors. J. Colloid Interface Sci. 2020, 560, 237–246. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Qin, P.; Huang, C.; Gao, B.; Pi, C.; Fu, J.; Zhang, X.; Huo, K.; Chu, P.K. Ultrathin carbon layer-encapsulated TiN nanotubes array with enhanced capacitance and electrochemical stability for supercapacitors. Appl. Surf. Sci. 2020, 503, 144293. [Google Scholar] [CrossRef]
- Yu, D.; Ma, Y.; Chen, M.; Dong, X. KOH activation of wax gourd-derived carbon materials with high porosity and heteroatom content for aqueous or all-solid-state supercapacitors. J. Colloid Interface Sci. 2019, 537, 569–578. [Google Scholar] [CrossRef]
- Patil, S.J.; Dubal, D.P.; Lee, D.-W. Gold nanoparticles decorated rGO-ZnCo2O4 nanocomposite: A promising positive electrode for high performance hybrid supercapacitors. Chem. Eng. J. 2020, 379, 122211. [Google Scholar] [CrossRef]
- Wang, K.; Xu, M.; Gu, Y.; Gu, Z.; Fan, Q.H. Symmetric supercapacitors using urea-modified lignin derived N-doped porous carbon as electrode materials in liquid and solid electrolytes. J. Power Sources 2016, 332, 180–186. [Google Scholar] [CrossRef]
- Sarigamala, K.K.; Shukla, S.; Struck, A.; Saxena, S. Rationally engineered 3D-dendritic cell-like morphologies of LDH nanostructures using graphene-based core–shell structures. Microsyst. Nanoeng. 2019, 5, s41378. [Google Scholar] [CrossRef]
- Mane, G.P.; Talapaneni, S.N.; Anand, C.; Varghese, S.; Iwai, H.; Ji, Q.; Ariga, K.; Mori, T.; Vinu, A. Preparation of Highly Ordered Nitrogen-Containing Mesoporous Carbon from a Gelatin Biomolecule and its Excellent Sensing of Acetic Acid. Adv. Funct. Mater. 2012, 22, 3596–3604. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Lee, S.; Chung, Y.G.; Chiang, W.-H.; Wu, K.C.W. Synergistic effect of metal-organic framework-derived boron and nitrogen heteroatom-doped three-dimensional porous carbons for precious-metal-free catalytic reduction of nitroarenes. Appl. Catal. B 2019, 257, 117888. [Google Scholar] [CrossRef]
- El-Khodary, S.A.; Abomohra, A.E.; El-Enany, G.M.; Aboalhassan, A.A.; Ng, D.H.L.; Wang, S.; Lian, J. Sonochemical assisted fabrication of 3D hierarchical porous carbon for high-performance symmetric supercapacitor. Ultrason. Sonochem. 2019, 58, 104617. [Google Scholar] [CrossRef]
- Zhou, J.; Lian, J.; Hou, L.; Zhang, J.; Gou, H.; Xia, M.; Zhao, Y.; Strobel, T.A.; Tao, L.; Gao, F. Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres. Nat. Commun. 2015, 6, 8503. [Google Scholar] [CrossRef] [PubMed]
- Song, L.-T.; Wu, Z.-Y.; Liang, H.-W.; Zhou, F.; Yu, Z.-Y.; Xu, L.; Pan, Z.; Yu, S.-H. Macroscopic-scale synthesis of nitrogen-doped carbon nanofiber aerogels by template-directed hydrothermal carbonization of nitrogen-containing carbohydrates. Nano Energy 2016, 19, 117–127. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Zhao, Y.; Ran, W.; He, J.; Song, Y.; Zhang, C.; Xiong, D.-B.; Gao, F.; Wu, J.; Xia, Y. Oxygen-Rich Hierarchical Porous Carbon Derived from Artemia Cyst Shells with Superior Electrochemical Performance. ACS Appl. Mater. Interfaces 2015, 7, 1132–1139. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Dong, H.; Hu, H.; Zhao, X.; Cai, Y.; Xiao, Y.; Liu, Y.; Zheng, M. Super-hierarchical porous carbons derived from mixed biomass wastes by a stepwise removal strategy for high-performance supercapacitors. J. Power Sources 2018, 377, 151–160. [Google Scholar] [CrossRef]
- Kierzek, K.; Frackowiak, E.; Lota, G.; Gryglewicz, G.; Machnikowski, J. Electrochemical capacitors based on highly porous carbons prepared by KOH activation. Electrochim. Acta 2004, 49, 515–523. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710. [Google Scholar] [CrossRef]
- Król, M.; Gryglewicz, G.; Machnikowski, J. KOH activation of pitch-derived carbonaceous materials-Effect of carbonization degree. Fuel Process. Technol. 2011, 92, 158–165. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Karthik, M.; Redondo, E.; Goikolea, E.; Roddatis, V.; Doppiu, S.; Mysyk, R. Effect of Mesopore Ordering in Otherwise Similar Micro/Mesoporous Carbons on the High-Rate Performance of Electric Double-Layer Capacitors. J. Phys. Chem. C 2014, 118, 27715–27720. [Google Scholar] [CrossRef]
- Jia, H.; Wang, S.; Sun, J.; Yin, K.; Xie, X.; Sun, L. Nitrogen-doped microporous carbon derived from a biomass waste-metasequoia cone for electrochemical capacitors. J. Alloys Compd. 2019, 794, 163–170. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. Nitrogen-doped two-dimensional porous carbon sheets derived from clover biomass for high performance supercapacitors. J. Power Sources 2017, 363, 375–383. [Google Scholar] [CrossRef]
- Lee, H.; An, K.; Chung, D.; Jung, S.; Park, Y.; Park, S.; Kim, B. Comparison studies on pore development mechanisms of activated hard carbons from polymeric resins and their applications for electrode materials. Renew. Energy 2019, 144, 116–122. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Z.; Guo, Y.; Guo, D.; Liu, G. Block copolymer derived uniform mesopores enable ultrafast electron and ion transport at high mass loadings. Nat. Commun. 2019, 10, 675. [Google Scholar] [CrossRef]
- He, D.; Niu, J.; Dou, M.; Ji, J.; Huang, Y.; Wang, F. Nitrogen and oxygen co-doped carbon networks with a mesopore-dominant hierarchical porosity for high energy and power density supercapacitors. Electrochim. Acta 2017, 238, 310–318. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, C.; Chen, P.; Yuan, D.; Guo, K. MnO2-decorated hierarchical porous carbon composites for high-performance asymmetric supercapacitors. J. Power Sources 2019, 425, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, R.; Ge, Y.; Tu, Y.; Xia, Y.; Yang, X. N- and O-doped hollow carbonaceous spheres with hierarchical porous structure for potential application in high-performance capacitance. J. Power Sources 2017, 363, 356–364. [Google Scholar] [CrossRef]
- Miao, L.; Zhu, D.; Zhao, Y.; Liu, M.; Duan, H.; Xiong, W.; Zhu, Q.; Li, L.; Lv, Y.; Gan, L. Design of carbon materials with ultramicro-, supermicro- and mesopores using solvent- and self-template strategy for supercapacitors. Microporous Mesoporous Mater. 2017, 253, 1–9. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Santhosh, R.; Adduru, J.; Rao, T.N.; Karthik, M. Activated carbon fibres as high performance supercapacitor electrodes with commercial level mass loading. Carbon 2018, 140, 465–476. [Google Scholar] [CrossRef]
- Zheng, H.; Feng, C.; Kim, S.-J.; Yin, S.; Wu, H.; Wang, S.; Li, S. Synthesis and electrochemical properties of KMn8O16 nanorods for Lithium ion batteries. Electrochim. Acta 2013, 88, 225–230. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Zhi, L.; Cao, Q.; Maier, J.; Mullen, K. Nanographene-constructed hollow carbon spheres and their favorable electroactivity with respect to lithium storage. Adv. Mater. 2010, 22, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, J.; Yang, Y.; Zheng, Q.; Liao, J.; Xie, F.; Jie, W.; Lin, D. Biomass-derived nitrogen and oxygen co-doped hierarchical porous carbon for high performance symmetric supercapacitor. J. Solid State Chem. 2018, 268, 149–158. [Google Scholar] [CrossRef]
- Tang, D.; Luo, Y.; Lei, W.; Xiang, Q.; Ren, W.; Song, W.; Chen, K.; Sun, J. Hierarchical porous carbon materials derived from waste lentinus edodes by a hybrid hydrothermal and molten salt process for supercapacitor applications. Appl. Surf. Sci. 2018, 462, 862–871. [Google Scholar] [CrossRef]
- Feng, H.; Hu, H.; Dong, H.; Xiao, Y.; Cai, Y.; Lei, B.; Liu, Y.; Zheng, M. Hierarchical structured carbon derived from bagasse wastes: A simple and efficient synthesis route and its improved electrochemical properties for high-performance supercapacitors. J. Power Sources 2016, 302, 164–173. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Rafat, M. Impact of aqueous and organic electrolytes on the supercapacitive performance of activated carbon derived from pea skin. Surf. Coat. Technol. 2018, 349, 242–250. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, T.; Zhan, X.; Long, H.; Xi, S.; Hu, H.; Tang, Z. High-performance all-solid-state flexible supercapacitors based on two-step activated carbon cloth. J. Power Sources 2014, 272, 16–23. [Google Scholar] [CrossRef]
- Tongye, W.; Xiaolin, W.; Liwen, Y.; Huaping, X.; Yong, G.; Huaming, L. A one-step moderate-explosion assisted carbonization strategy to sulfur and nitrogen dual-doped porous carbon nanosheets derived from camellia petals for energy storage. J. Power Sources 2020, 331, 373–381. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, L.; Xiao, X.; Yao, B.; Yuan, L.; Li, T.; Hu, Z.; Wang, B.; Wan, J.; Zhou, J. Flexible and cross-linked N-doped carbon nanofiber network for high performance freestanding supercapacitor electrode. Nano Energy 2015, 15, 66–74. [Google Scholar] [CrossRef]
- Balaji, S.S.; Karnan, M.; Anandhaganesh, P.; Tauquir, S.M.; Sathish, M. Performance evaluation of B-doped graphene prepared via two different methods in symmetric supercapacitor using various electrolytes. Appl. Surf. Sci. 2019, 491, 560–569. [Google Scholar] [CrossRef]

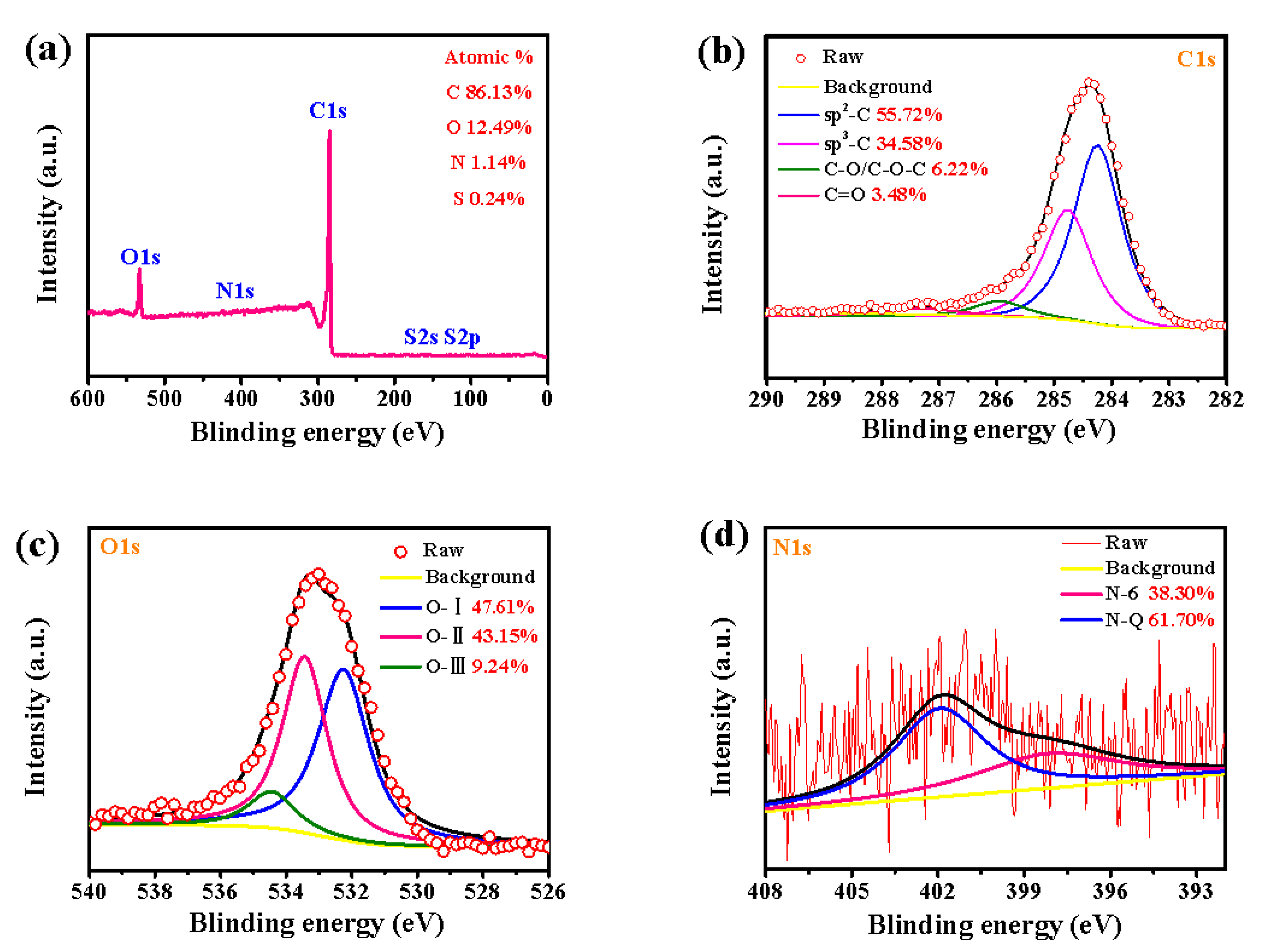



| Sample | C (%) | O (%) | N (%) | S (%) |
|---|---|---|---|---|
| LSPC700 | 83.52 | 14.88 | 1.32 | 0.28 |
| LSPC800 | 86.13 | 12.49 | 1.14 | 0.24 |
| LSPC900 | 90.31 | 8.65 | 0.86 | 0.18 |
| Sample | SBET (m2 g−1) a | Vt (cm3 g−1) b | Vmic (cm3 g−1) c | Vmes (cm3 g−1) d | Vmes/Vt (%) |
|---|---|---|---|---|---|
| LSPC700 | 2299 | 1.071 | 0.827 | 0.244 | 22.78 |
| LSPC800 | 3089 | 1.644 | 1.069 | 0.575 | 34.29 |
| LSPC900 | 2401 | 1.346 | 0.713 | 0.633 | 47.03 |
| No. | Electrode Material | Electrolyte | Voltage Window | Maximum Power Density (W kg−1) | Ref. |
|---|---|---|---|---|---|
| 1 | Hierarchical porous carbons from the mixed biomass wastes | 1 M Na2SO4 | 0–1.8 V | 9000 | [38] |
| 2 | Pig skin-derived porous carbon | EMIMBF4 | 0–3.5 V | 8750.6 | [55] |
| 3 | 3 D porous carbon from corn starch | 2 M [BMIm]BF4/AN | 0–2.6 V | ~7000 | [19] |
| 4 | Hierarchical porous carbon from waste lentinus edodes | 1 M H2SO4 | 0–1.0 V | 5000 | [56] |
| 5 | Hierarchical structured carbon derived from bagasse wastes | 6 M KOH | 0–1.0 V | 10,673 | [57] |
| 6 | Activated carbon derived from pea skin | 1M LiClO4 in EC/PC | 0–2.0 V | 17,900 | [58] |
| 7 | Longan shell 3-dimensional porous carbon | 1 M Li2SO4 | 0–1.6 V | 15,930.38 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Qu, X.; Huang, G.; Xing, B.; Zhang, F.; Li, B.; Zhang, C.; Cao, Y. 3-Dimensional Porous Carbon with High Nitrogen Content Obtained from Longan Shell and Its Excellent Performance for Aqueous and All-Solid-State Supercapacitors. Nanomaterials 2020, 10, 808. https://doi.org/10.3390/nano10040808
Liu Y, Qu X, Huang G, Xing B, Zhang F, Li B, Zhang C, Cao Y. 3-Dimensional Porous Carbon with High Nitrogen Content Obtained from Longan Shell and Its Excellent Performance for Aqueous and All-Solid-State Supercapacitors. Nanomaterials. 2020; 10(4):808. https://doi.org/10.3390/nano10040808
Chicago/Turabian StyleLiu, Yuhao, Xiaoxiao Qu, Guangxu Huang, Baolin Xing, Fengmei Zhang, Binbin Li, Chuanxiang Zhang, and Yijun Cao. 2020. "3-Dimensional Porous Carbon with High Nitrogen Content Obtained from Longan Shell and Its Excellent Performance for Aqueous and All-Solid-State Supercapacitors" Nanomaterials 10, no. 4: 808. https://doi.org/10.3390/nano10040808
APA StyleLiu, Y., Qu, X., Huang, G., Xing, B., Zhang, F., Li, B., Zhang, C., & Cao, Y. (2020). 3-Dimensional Porous Carbon with High Nitrogen Content Obtained from Longan Shell and Its Excellent Performance for Aqueous and All-Solid-State Supercapacitors. Nanomaterials, 10(4), 808. https://doi.org/10.3390/nano10040808