Discriminable Sensing Response Behavior to Homogeneous Gases Based on n-ZnO/p-NiO Composites
Abstract
1. Introduction
2. Experimental Details
2.1. Chemicals
2.2. Preparation of Materials
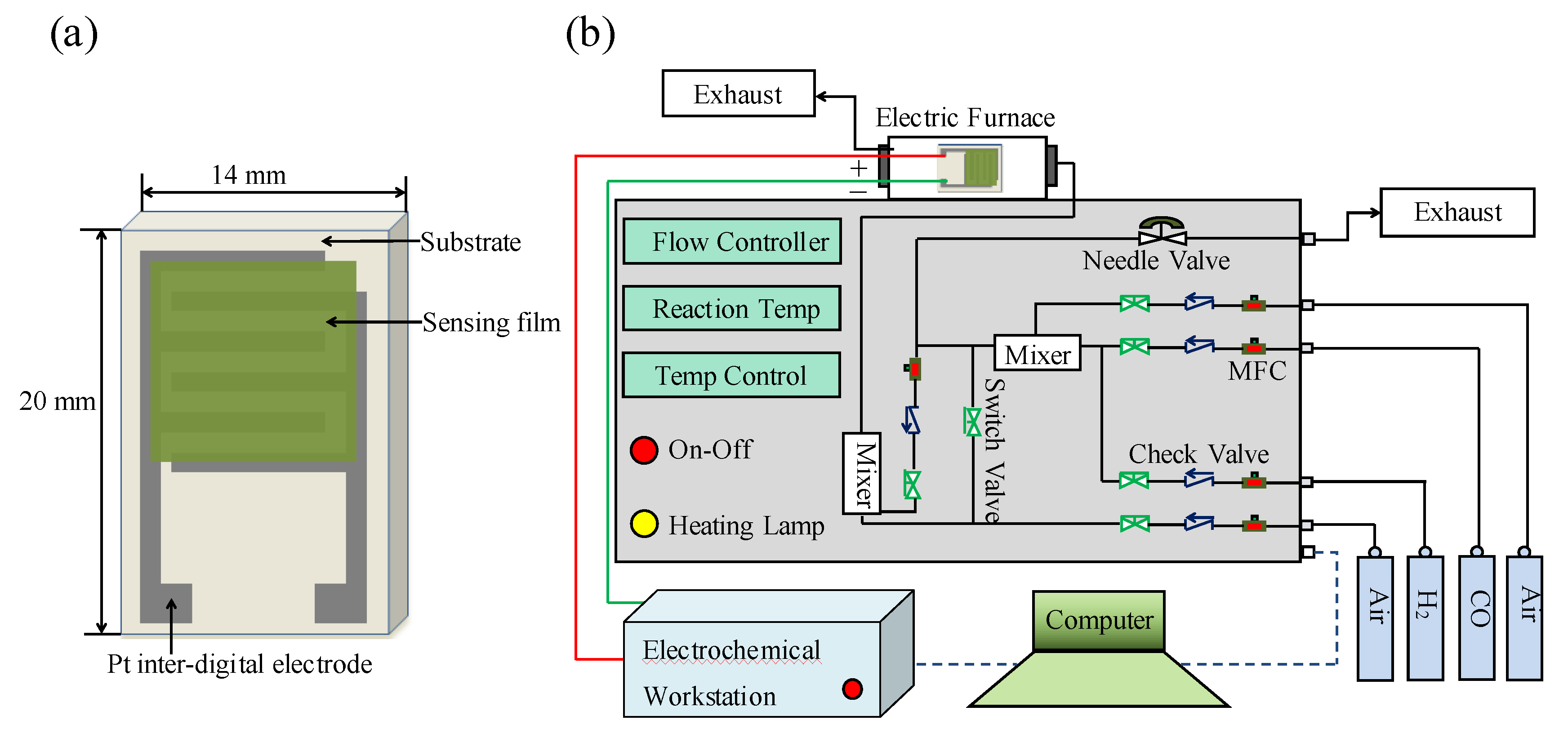
2.3. Fabrication of Gas Sensors
2.4. Gas Sensing Measurements
2.5. Material Characterizations
3. Results and Discussion
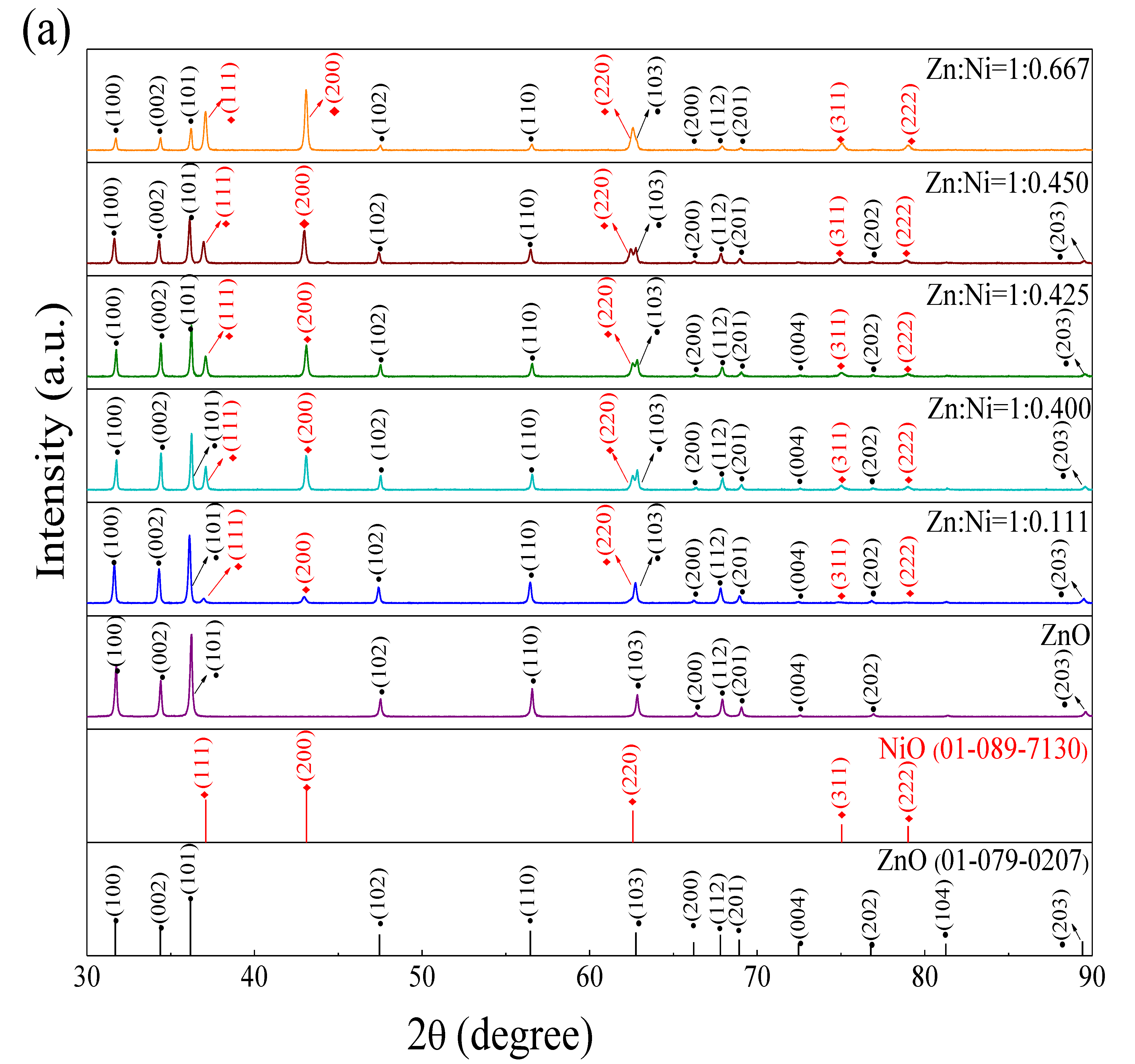
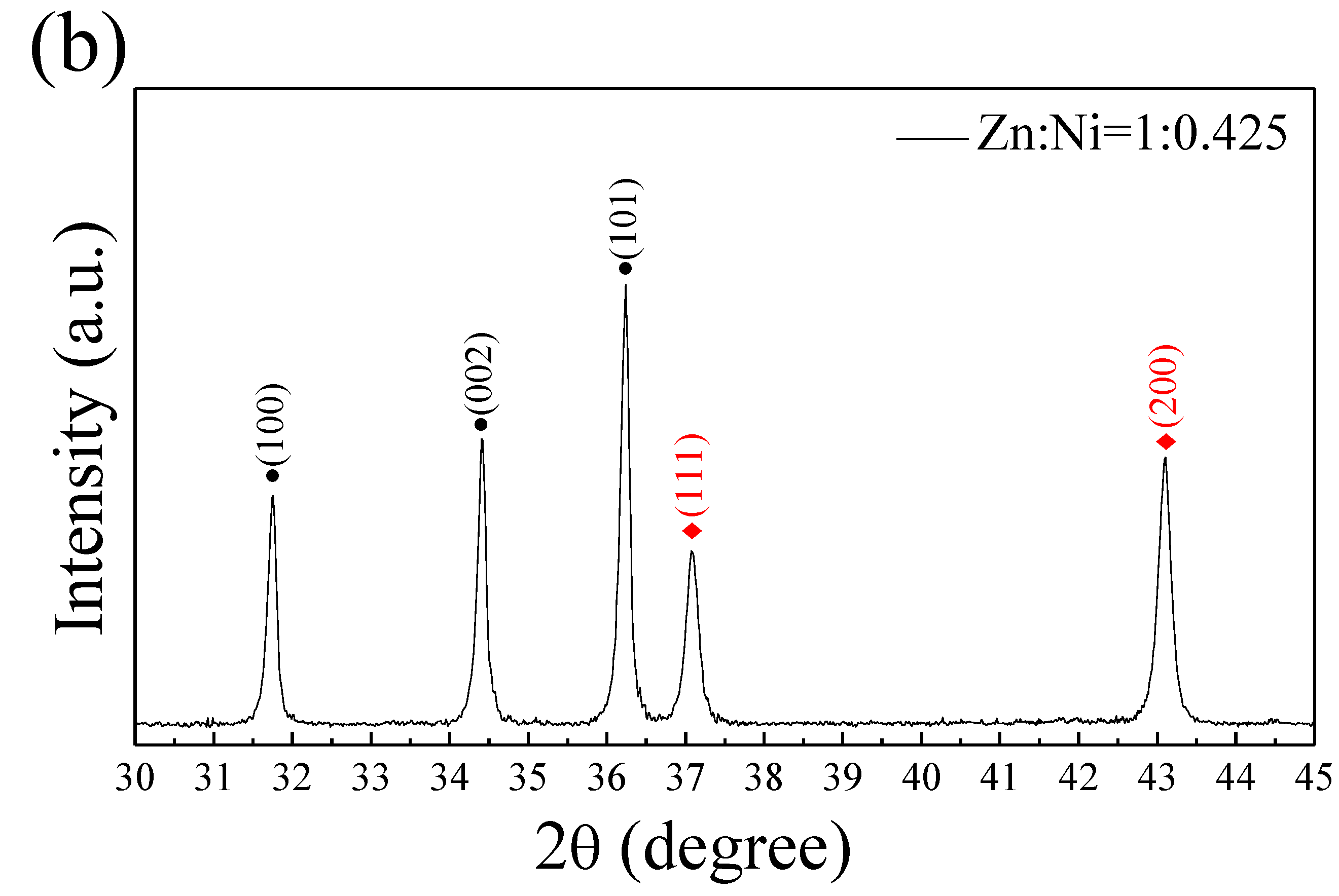
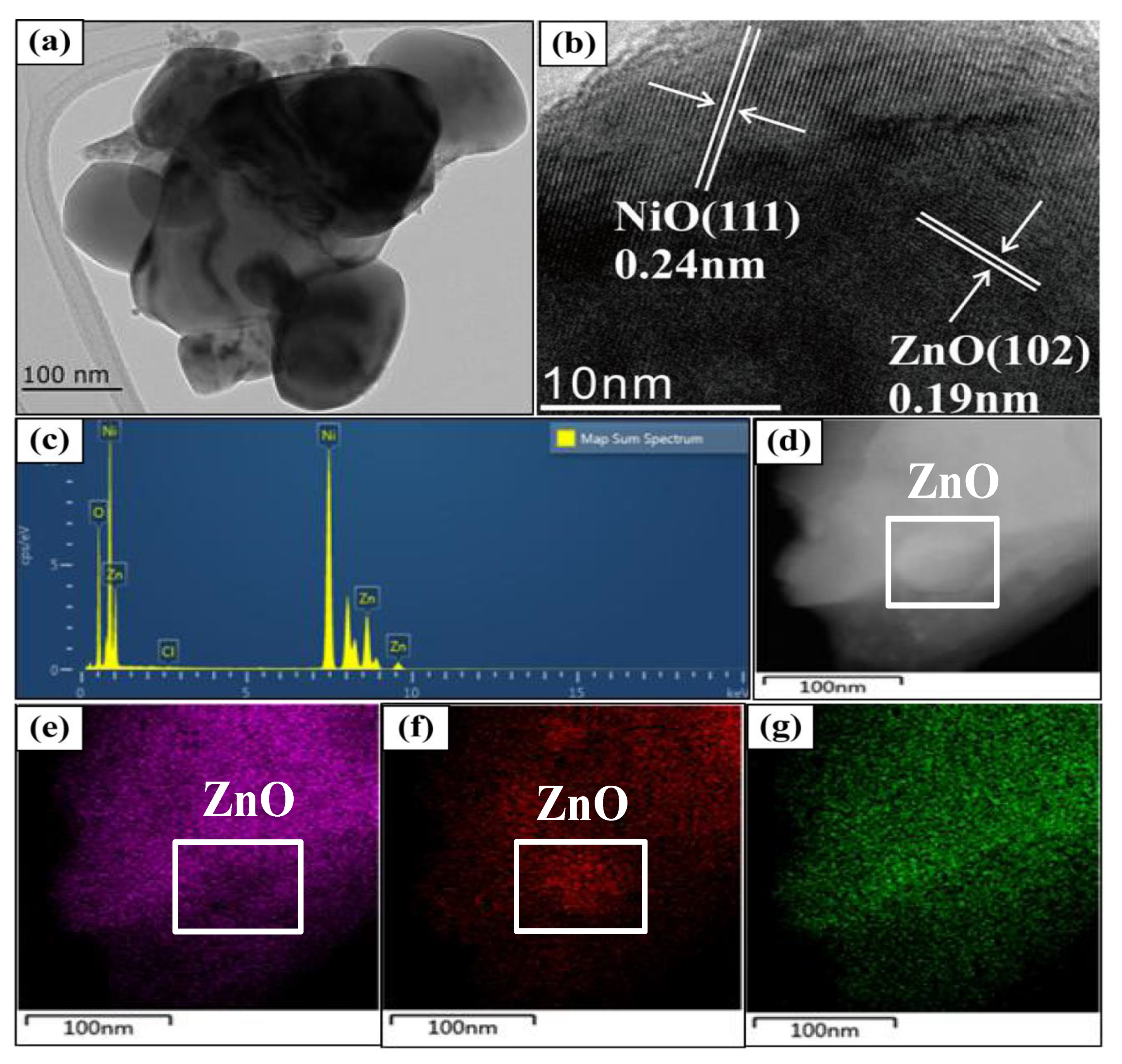
3.1. Structural and Morphological Characterization
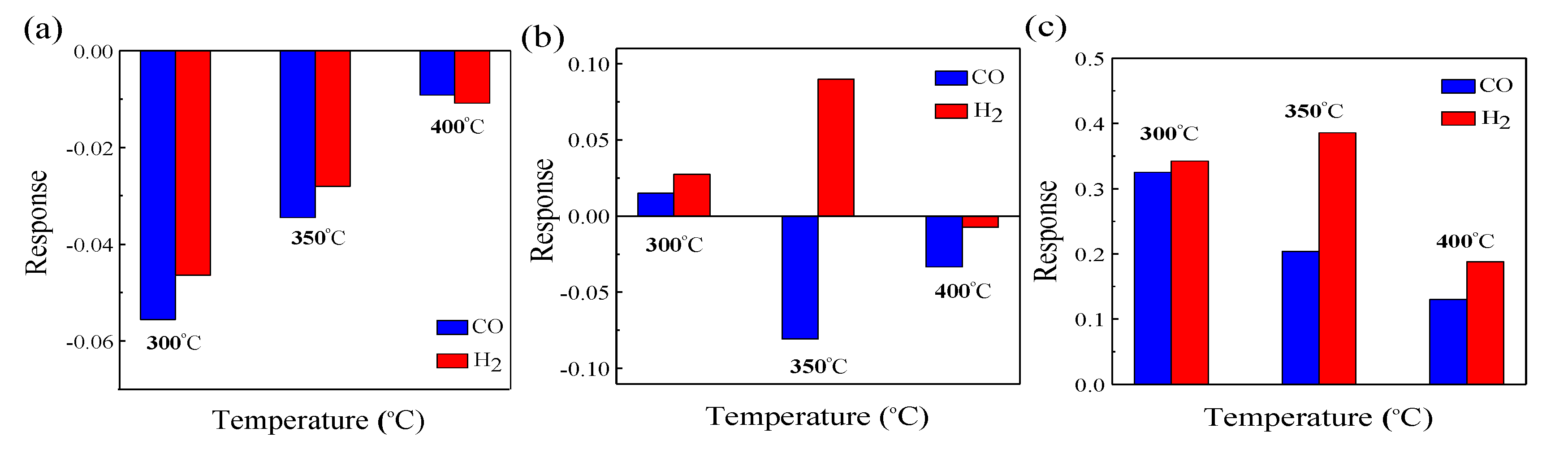
3.2. Sensing Properties of ZnO–xNiO
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koo, W.-T.; Qiao, S.; Ogata, A.F.; Jha, G.; Jang, J.-S.; Chen, V.T.; Kim, I.-T.; Penner, R.M. Accelerating Palladium Nanowire H2 Sensors Using Engineered Nanofifiltration. ACS Nano 2017, 11, 9276–9285. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gong, H.; Chow, C.L.; Guo, J.; White, T.J.; Tse, M.S.; Tan, O.K. Low-Temperature Growth of SnO2 Nanorod Arrays and Tunable n-p-n Sensing Response of a ZnO/SnO2 Heterojunction for Exclusive Hydrogen Sensors. Adv. Funct. Mater. 2011, 21, 2680–2686. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.; Umar, A.; Chen, W.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for effificient CO gas sensing applications. Sens. Actuators B 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Suman, P.H.; Kim, J.J.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Gas sensor properties of Ag- and Pd-decorated SnO micro-disks to NO2, H2 and CO: Catalyst enhanced sensor response and selectivity. Sens. Actuators B 2017, 239, 253–261. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Z.; Hu, Y.; Luo, X.; Lei, J.; Zhou, D.; Fei, L.; Wang, Y.; Gu, H. Highly Responsive Room-Temperature Hydrogen Sensing of α-MoO3, Nanoribbon Membranes. ACS Appl. Mater. Interfaces 2015, 7, 9247–9253. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wang, J.; Liu, X.; Wang, C.; Jiang, Y.; Wang, Y.; Xiao, X.; Ho, J.C.; Li, J.; Fang, Y.; et al. Rational Design of Sub-Parts per Million Specifific Gas Sensors Array Based on Metal Nanoparticles Decorated Nanowire Enhancement Mode Transistors. Nano Lett. 2013, 13, 3287–3292. [Google Scholar] [CrossRef]
- Yin, X.T.; Tao, L. Fabrication and gas sensing properties of Au-loaded SnO2 composite nanoparticles for low concentration hydrogen. J. Alloys Compd. 2017, 727, 254–259. [Google Scholar] [CrossRef]
- Han, D.M.; Zhai, L.L.; Gu, F.B.; Wang, Z.H. Highly sensitive NO2 gas sensor of ppb-level detection based on In2O3 nanobricks at lowtemperature. Sens. Actuators B 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Yin, X.T.; Guo, X.M. Selectivity and sensitivity of Pd-loaded and Fe-doped SnO2sensor for CO detection. Sens. Actuators B 2014, 200, 213–218. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Sun, M.; Gong, Z.; Guo, Y.; Yin, X.; Wu, F.; Ding, W. Gas sensing selectivity of oxygen-regulated SnO2 films with different microstructure and texture. J. Mater. Sci. Technol. 2019, 35, 2232–2237. [Google Scholar] [CrossRef]
- Kaur, M.; Dadhich, B.K.; Singh, R.; Bagwaiya, T.; Bhattacharya, S.; Debnath, A.K.; Muthe, K.P.; Gadkari, S.C. RF sputtered SnO2: NiO thin films as sub-ppm H2S sensor operable at room temperature. Sens. Actuators B 2016, 242, 389–403. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.; Li, H.; Sun, M.; Han, S.; Cai, C.; Shen, W.; Fu, Y. Ultrafast Response/Recovery and High Selectivity of the H2S Gas Sensor Based on α-Fe2O3 Nano-Ellipsoids from One-Step Hydrothermal Synthesis. ACS Appl. Mater. Interfaces 2019, 11, 12761–12769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, H.; Yan, C.; Yang, Z.; Zhu, G.; Gao, J.; Yin, F.; Wang, C. Fabrication of conductive graphene oxide-WO3 composite nanofibers by electrospinning and their enhanced acetone gas sensing properties. Sens. Actuators B 2018, 264, 128–138. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Wu, J. Layer-by-layer assembled In2O3 nanocubes/flower-like MoS2 nanofilm for room temperature formaldehyde sensing. Sens. Actuators B 2018, 273, 176–184. [Google Scholar] [CrossRef]
- Abbasi, S.; Hasanpour, M.; Ahmadpoor, F.; Sillanpää, M.; Dastan, D.; Achour, A. Application of the statistical analysis methodology for photodegradation of methyl orange using a new nanocomposite contining modified TiO2 semiconductor with SnO2. Int. J. Environ. Anal. Chem. 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.Y.; Wang, Z.; Song, Z.W.; Zhou, X.Y.; Han, N.; Chen, Y.F. Sputtered SnO2:NiO thin films on self-assembled Au nanoparticle arrays for MEMS compatible NO2 gas sensors. Sens. Actuators B 2019, 278, 28–38. [Google Scholar] [CrossRef]
- Jafari, A.; Alam, M.; Dastan, D.; Ziakhodadadian, S.; Shi, Z.; Garmestani, H.; Weidenbach, A.; Ţălu, Ş. Statistical, morphological, and corrosion behavior of PECVD derived cobalt oxide thin flms. J. Mater. Sci. Mater. Electron. 2019, 30, 21185–21198. [Google Scholar]
- Kwak, C.-H.; Woo, H.-S.; Lee, J.-H. Selective trimethylamine sensors using Cr2O3-decorated SnO2 Nanowires. Sens. Actuators B 2014, 204, 231–238. [Google Scholar] [CrossRef]
- Jafari, A.; Tahani, K.; Dastan, D.; Asgary, S.; Shi, Z.; Yin, X.-T.; Zhou, W.-D.; Garmestani, H.; Ţălu, Ş. Ion Implantation of Copper Oxide Thin Films; Statistical and Experimental Results. Surf. Interfaces 2020, 18, 100463. [Google Scholar] [CrossRef]
- Hu, Y.; Li, L.; Zhang, L.; Lv, Y. Dielectric barrier discharge plasma-assisted fabrication of g-C3N4-Mn3O4 composite for high-performance cataluminescence H2S gas sensor. Sens. Actuators B 2017, 239, 1177–1184. [Google Scholar] [CrossRef]
- Han, F.; Song, Z.; Nawaz, M.H.; Dai, M.; Han, D.; Han, L.; Fan, Y.; Xu, J.; Han, D. MoS2/ZnO-Heterostructures-Based Label-Free, Visible-Light-Excited Photoelectrochemical Sensor for Sensitive and Selective Determination of Synthetic Antioxidant Propyl Gallate. Anal. Chem. 2019, 91, 10657–10662. [Google Scholar] [CrossRef]
- Rai, P.; Kwak, W.-K.; Yu, Y.-T. Solvothermal Synthesis of ZnO Nanostructures and Their Morphology-Dependent Gas-Sensing Properties. ACS Appl. Mater. Interfaces 2013, 5, 3026–3032. [Google Scholar] [CrossRef]
- Woo, H.-S.; Kwak, C.-H.; Chung, J.-H.; Lee, J.-H. Co-Doped Branched ZnO Nanowires for Ultra selective and Sensitive Detection of Xylene. ACS Appl. Mater. Interfaces 2014, 6, 22553–22560. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Ma, J.; Cheng, X.; Li, Y.; Deng, Y.; Zhao, D. Cementing Mesoporous ZnO with Silica for Controllable and Switchable Gas Sensing Selectivity. Chem. Mater. 2019, 31, 8112–8120. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Zhang, H.; Shi, S. CO2 Sensing Behavior of Calcium-Doped ZnO Thin Film: A Study To Address the Cross-Sensitivity of CO2 in H2 and CO Environment. Langmuir 2019, 35, 10267–10275. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.J.; Yu, J.H.; Choi, G.M. The CO and H2 gas selectivity of CuO-doped SnO2-ZnO composite gas sensor. Sens. Actuators B 2002, 87, 464–470. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Leroy, E.; Julbe, A.; Kim, S.S. Design and Fabrication of Highly Selective H2 Sensors Based on SIM-1 Nanomembrane-Coated ZnO Nanowires. Sens. Actuators B 2018, 264, 410–418. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, J.; Li, J.; Zhang, N.; Hong, R.; Deng, X.; Tang, P.; Li, D. Hydrogen sensing properties of Pt-Au bimetallic nanoparticles loaded on ZnO nanorods. Sens. Actuators B 2017, 241, 895–903. [Google Scholar] [CrossRef]
- Majhi, S.M.; Rai, P.; Yu, Y.-T. Facile Approach to Synthesize Au@ZnO Core-Shell Nanoparticles and Their Application for Highly Sensitive and Selective Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 9462–9468. [Google Scholar] [CrossRef]
- Yin, X.-T.; Zhou, W.-D.; Li, J.; Lv, P.; Wang, Q.; Wang, D.; Wu, F.-Y.; Dastan, D.; Garmestani, H.; Shi, Z.; et al. Tin dioxide nanoparticles with high sensitivity and selectivity for gas sensors at sub-ppm level of hydrogen gas detection. J. Mater. Sci. Mater. Electron. 2019, 30, 14687–14694. [Google Scholar] [CrossRef]
- Guo, L.; Chen, F.; Xie, N.; Kou, X.; Wang, C.; Sun, Y.; Liu, F.; Liang, X.; Gao, Y.; Yan, X.; et al. Ultra-sensitive sensing platform based on Pt-ZnO-In2O3 nanofifibers for detection of acetone. Sens. Actuators B 2018, 272, 185–194. [Google Scholar] [CrossRef]
- Yin, X.-T.; Zhou, W.-D.; Li, J.; Wang, Q.; Wu, F.-Y.; Dastan, D.; Wang, D.; Garmestani, H.; Wang, X.-M.; Talu, S. A highly sensitivity and selectivity Pt-SnO2 nanoparticles for sensing applications at extremely low level hydrogen gas detection. J. Alloys Compd. 2019, 805, 229–236. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Yin, X.-T.; Lv, P.; Li, J.; Jafari, A.; Wu, F.-Y.; Wang, Q.; Dastan, D.; Shi, Z.; Yu, S.; Garmestani, H. Nanostructured Tungsten Trioxide Prepared at Various Growth Temperatures for Sensing Applications. J. Alloys Compd. 2020, 825, 154105. [Google Scholar] [CrossRef]
- Joy, N.A.; Nandasiri, M.I.; Rogers, P.H.; Jiang, W.; Varga, T.; Kuchibhatla, S.V.N.T.; Thevuthasan, S.; Carpenter, M.A. Selective Plasmonic Gas Sensing: H2, NO2, and CO Spectral Discrimination by a Single Au-CeO2 Nanocomposite Film. Anal. Chem. 2012, 84, 5025–5034. [Google Scholar] [CrossRef]
- Sun, G.-J.; Lee, J.K.; Choi, S.; Lee, W.I.; Kim, H.W.; Lee, C. Selective Oxidizing Gas Sensing and Dominant Sensing Mechanism of n-CaO-Decorated n-ZnO Nanorod Sensors. ACS Appl. Mater. Interfaces 2017, 9, 9975–9985. [Google Scholar] [CrossRef]
- Katoch, A.; Kim, J.-H.; Kwon, Y.J.; Kim, H.W.; Kim, S.S. Bifunctional Sensing Mechanism of SnO2-ZnO Composite Nanofibers for Drastically Enhancing the Sensing Behavior in H2 Gas. ACS Appl. Mater. Interfaces 2015, 7, 11351–11358. [Google Scholar] [CrossRef]
- Katoch, A.; Abideen, Z.U.; Kim, H.W.; Kim, S.S. Grain-Size-Tuned Highly H2-Selective Chemiresistive Sensors Based on ZnO-SnO2 Composite Nanofifibers. ACS Appl. Mater. Interfaces 2016, 8, 2486–2494. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Liu, L.; Ruan, X.; Yan, J.; Zhao, W.; Yun, J.; Wang, Y.; Qin, S.; Zhang, T. Novel SnO2@ZnO hierarchical nanostructures for highly sensitive and selective NO2 gas sensing. Sens. Actuators B 2018, 257, 714–727. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Grottrup, J.; Mishra, A.K.; de Leeuw, N.H.; Carreira, J.F.C.; Rodrigues, J.; Sedrine, N.B.; Correia, M.R.; Monteiro, T.; et al. Hybridization of Zinc Oxide Tetrapods for Selective Gas Sensing Applications. ACS Appl. Mater. Interfaces 2017, 9, 4084–4099. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization and gas sensing mechanism of n-SnO2-p-Co3O4 composite nanofibers. Sens. Actuators B 2017, 248, 500–511. [Google Scholar] [CrossRef]
- Yin, X.-T.; Dastan, D.; Wu, F.-Y.; Li, J. Facile Synthesis of SnO2/LaFeO3-XNX Composite: Photocatalytic Activity and Gas Sensing Performance. Nanomaterials 2019, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Zappa, D.; Ferroni, M.; Poli, N.; Campanini, M.; Negrea, R.; Comini, E. Branch-like NiO/ZnO heterostructures for VOC sensing. Sens. Actuators B 2018, 262, 477–485. [Google Scholar] [CrossRef]
- Xu, Q.; Ju, D.; Zhang, Z.; Yuan, S.; Zhang, J.; Xu, H.; Cao, B. Near room-temperature triethylamine sensor constructed with CuO/ZnO P-N heterostructural nanorods directly on flflat electrode. Sens. Actuators B 2016, 225, 16–23. [Google Scholar] [CrossRef]
- Sang, D.; Wang, Q.; Wang, Q.; Zhang, D.; Hu, H.; Wang, W.; Zhang, B.; Fan, Q.; Li, H. Improved electrical transport properties of an n-ZnO nanowire/p-diamond heterojunction. R. Soc. Chem. 2018, 8, 28804–28809. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Wang, Y.; Bhat, K.S.; Mahmoudi, T.; Yu, Y.T.; Suh, E.-K.; Hahn, Y.B. Improved selectivity and low concentration hydrogen gas sensor application of Pd sensitized heterojunction n-ZnO/p-NiO nanostructures. J. Alloys Compd. 2019, 797, 456–464. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Signifificant Enhancement of Hydrogen-Sensing Properties of ZnO Nanofifibers through NiO Loading. Nanomaterials 2018, 8, 902. [Google Scholar] [CrossRef]
- Dastan, D. Nanostructured Anatase Titania Thin Films Prepared by Sol-Gel Dip Coating Technique. J. At. Mol. Condens. Nano Phys. 2015, 2, 109–114. [Google Scholar]
- Dastan, D. Effect of preparation methods on the properties of titania nanoparticles: Solvothermal versus sol-gel. Appl. Phys. A 2017, 123, 699. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Wang, Y.; Zhu, B.; Zhang, S.; Huang, W.; Wang, S. CuO nanoparticle decorated ZnO nanorod sensor for low-temperature H2S detection. Mater. Sci. Eng. C 2012, 32, 2079–2085. [Google Scholar] [CrossRef]
- Liu, X.; Du, B.; Sun, Y.; Yu, M.; Yin, Y.; Tang, W.; Chen, C.; Sun, L.; Yang, B.; Ashfold, M.N.; et al. Sensitive room temperature photoluminescence-based sensing of H2S with novel CuO-ZnO nanorods. ACS Appl. Mater. Interfaces 2016, 8, 16379–16385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Li, X.; Wang, C.; Zhang, M.; Liu, A.Y. Electrospun Nanofibers of p-Type NiO/n-Type ZnO Heterojunctions with Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2915–2923. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, S.T.; Lee, H.B.; Ginting, R.T.; Oleiwi, H.F.; Yap, C.C.; Jumali, M.H.H.; Yahay, M. Automated room temperature optical absorbance CO sensor based on In-doped ZnO nanorod. Sens. Actuators B 2017, 248, 140–152. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corp.: New York, NY, USA, 1992. [Google Scholar]
- Wang, X.; Gao, M. Porous Co3O4/SnO2 quantum dots (QDs) heterostructure with abundant oxygen vacancies and Co2+ ions for highly efficient gas sensing and oxygen evolution Reaction. Nanoscale 2018, 25, 12045–12053. [Google Scholar] [CrossRef]
- Zhang, L.; He, J.; Jiao, W. Synthesis and gas sensing performance of NiO decorated SnO2 vertical standing nanotubes composite thin films. Sens. Actuators B 2019, 281, 326–334. [Google Scholar] [CrossRef]
- Gao, H.; Yu, Q.; Zhang, S.; Wang, T.; Sun, P.; Lu, H.; Liu, F.; Yan, X.; Liu, F.; Liang, X.; et al. Nanosheet-assembled NiO microspheres modified by Sn2+ ions isovalent interstitial doping for xylene gas sensors. Sens. Actuators B 2018, 269, 210–222. [Google Scholar] [CrossRef]
- Elger, A.-K.; Hess, C. Application of Raman Spectroscopy to Working Gas Sensors: From In Situ to Operando Studies. Sensors 2019, 19, 5075. [Google Scholar] [CrossRef]
- Do, T.A.T.; Giang, H.T.; van Huong, D.; Ngan, P.Q.; Thai, G.H.; Thu, D.T.; Lam, T.D. Correlation between photoluminescence spectra with gas sensing and photocatalytic activities in hierarchical ZnO nanostructures. RSC Adv. 2017, 7, 9826–9832. [Google Scholar]
- Lokesh, K.; Kavitha, G.; Manikandan, E.; Mani, G.K.; Kaviyarasu, K.; Rayappan, J.B.B.; Ladchumananandasivam, R.; Aanand, J.S.; Jayachandran, M.; Maaza, M. Effective Ammonia Detection Using n-ZnO/p-NiO Heterostructured Nanofibers. IEEE Sens. J. 2016, 16, 2477–2483. [Google Scholar] [CrossRef]
- Barsan, N.; Hübner, M.; Weimar, U. Conduction mechanisms in SnO2 based polycrystalline thick film gas sensors exposed to CO and H2 in different oxygen backgrounds. Sens. Actuators B 2011, 157, 510–517. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, R.; Liu, S.; Song, J.; Chen, J.; Dong, B.; Song, H. NiO@ZnO Heterostructured Nanotubes: Coelectrospinning Fabrication, Characterization, and Highly Enhanced Gas Sensing Properties. Inorg. Chem. 2012, 51, 7733–7740. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, D.; Li, X. Ultrasensitive NO2 Detection Utilizing Mesoporous ZnSe/ZnO Heterojunction-Based Chemiresistive-Type Sensors. ACS Appl. Mater. Interfaces 2019, 11, 29029–29040. [Google Scholar] [CrossRef] [PubMed]
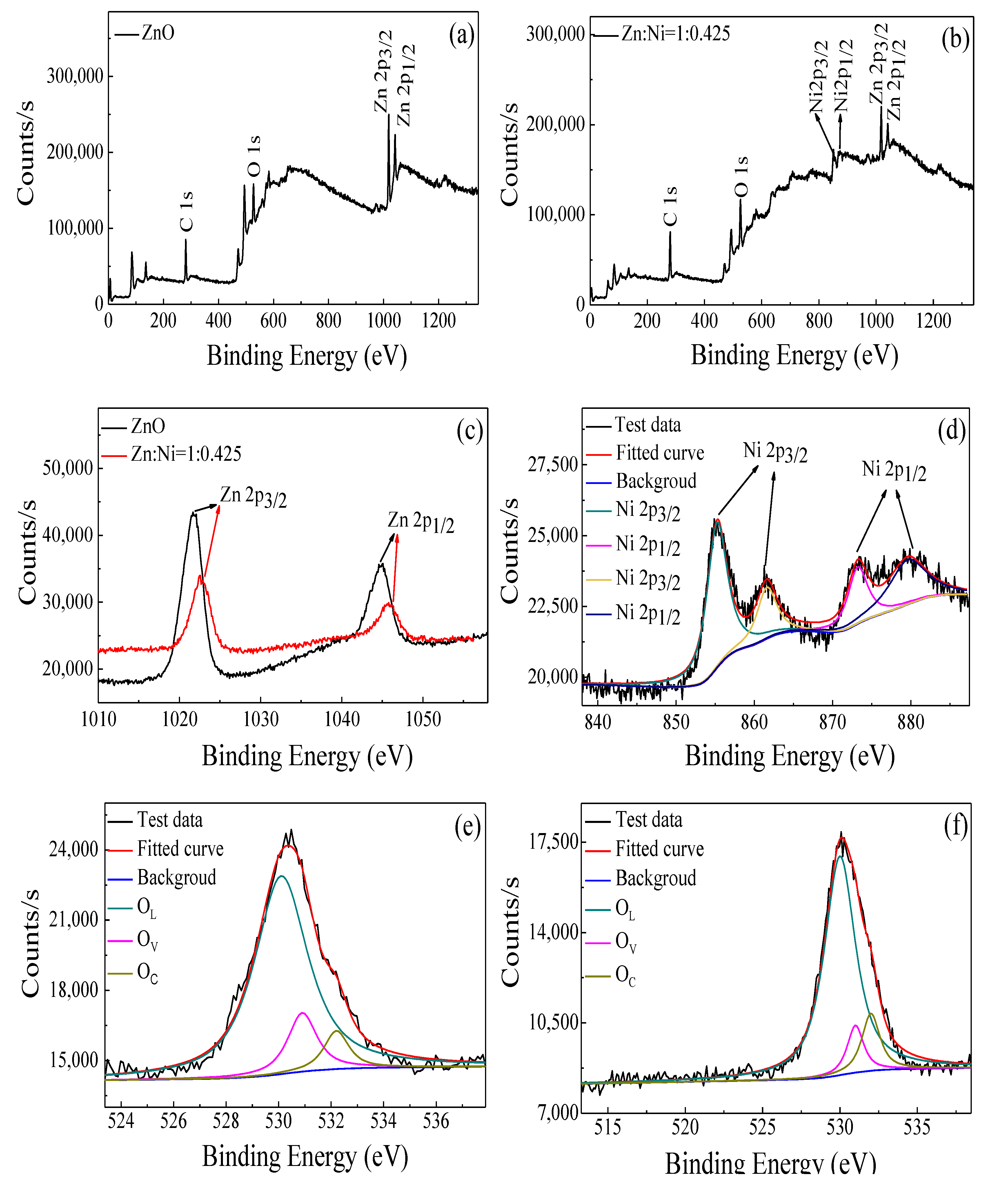
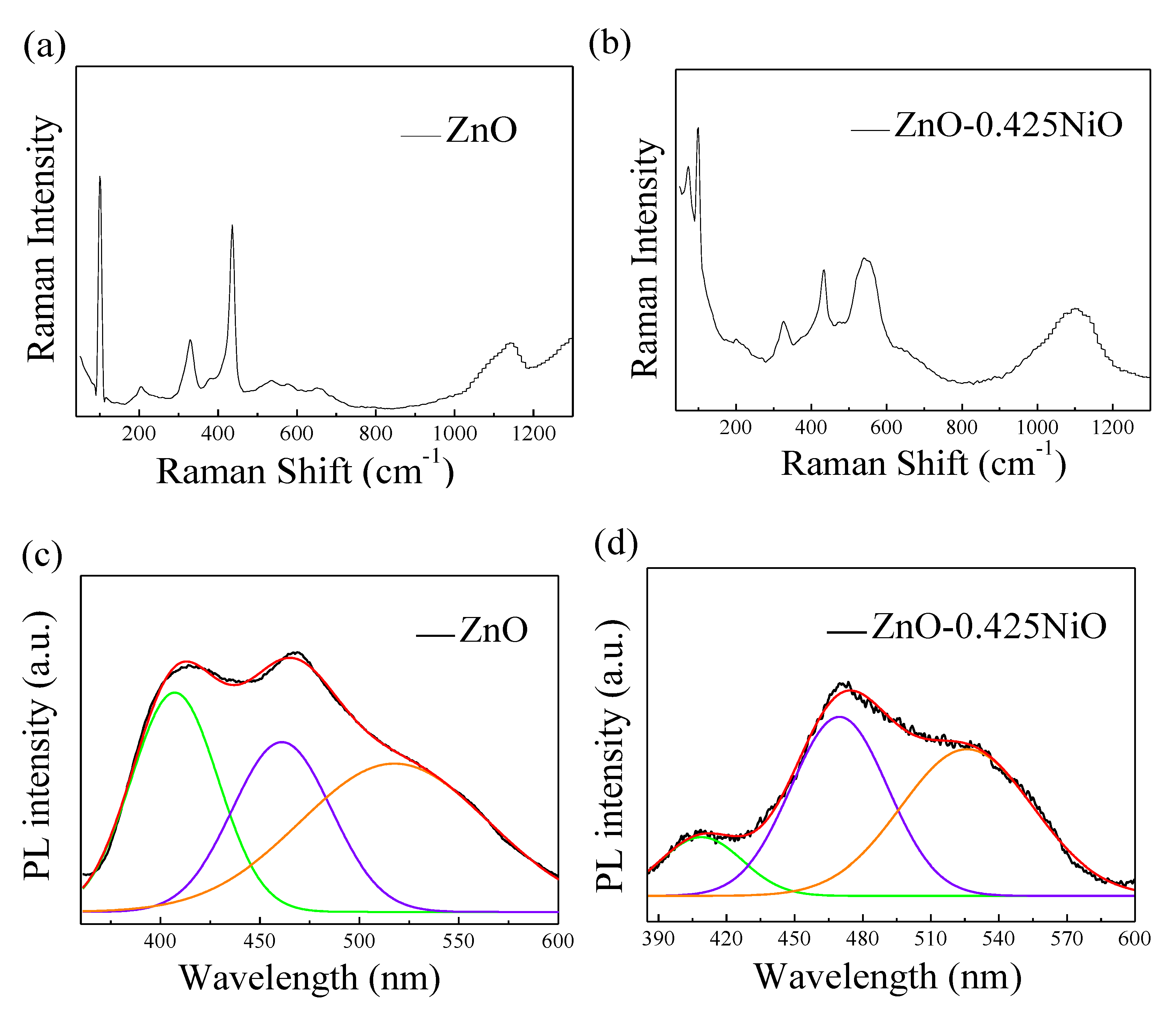
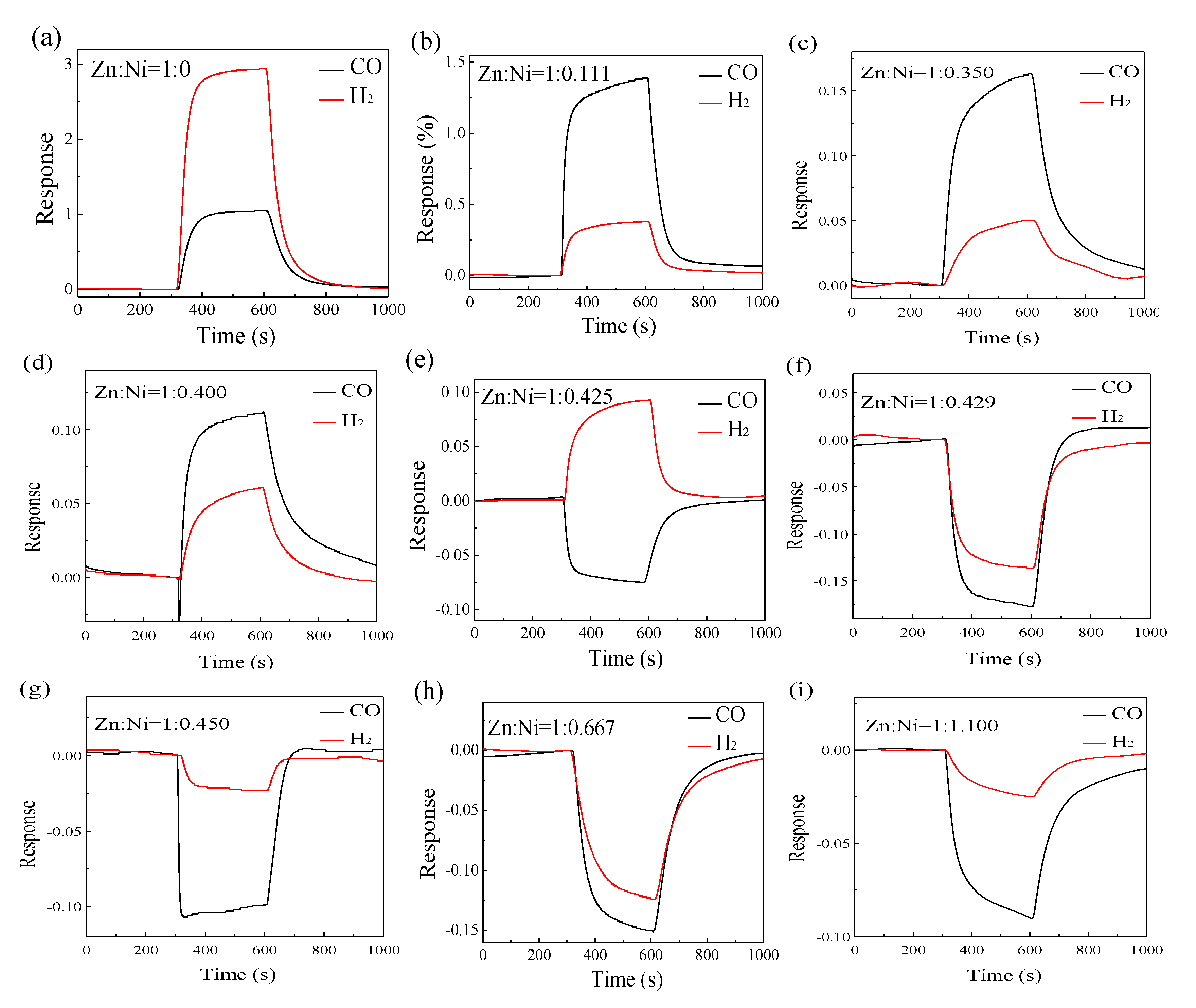
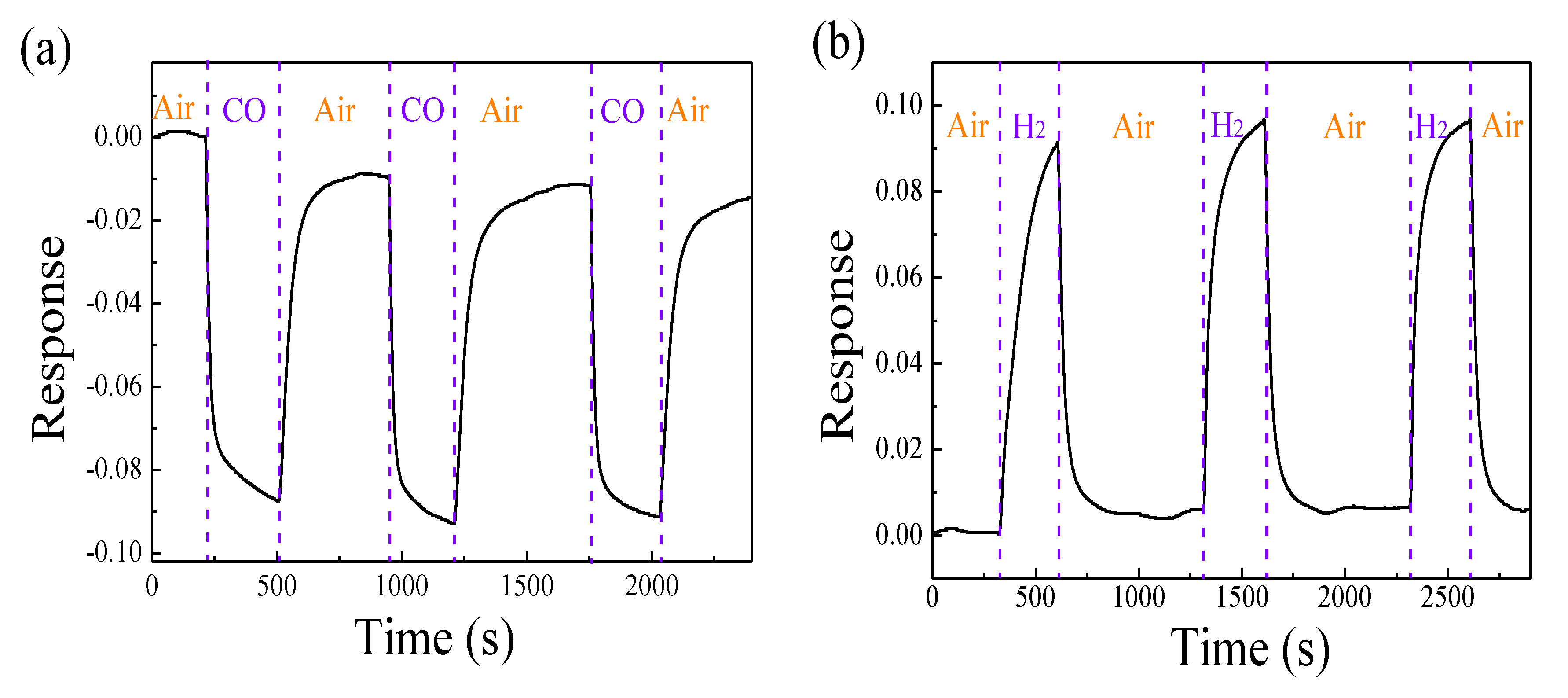
| Metal Oxide | Diffraction Planes | Diffraction Angles (deg.) | FWHM (β) | Crystallite Size (nm) | Average Size (nm) |
|---|---|---|---|---|---|
| ZnO | (100) | 31.69 | 0.1314 | 62.24 | 63.42 |
| (002) | 34.38 | 0.1248 | 65.99 | ||
| (101) | 36.18 | 0.1334 | 62.04 | ||
| NiO | (111) | 37.09 | 0.1877 | 44.21 | 44.61 |
| (200) | 43.09 | 0.1879 | 45.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.-D.; Dastan, D.; Li, J.; Yin, X.-T.; Wang, Q. Discriminable Sensing Response Behavior to Homogeneous Gases Based on n-ZnO/p-NiO Composites. Nanomaterials 2020, 10, 785. https://doi.org/10.3390/nano10040785
Zhou W-D, Dastan D, Li J, Yin X-T, Wang Q. Discriminable Sensing Response Behavior to Homogeneous Gases Based on n-ZnO/p-NiO Composites. Nanomaterials. 2020; 10(4):785. https://doi.org/10.3390/nano10040785
Chicago/Turabian StyleZhou, Wen-Dong, Davoud Dastan, Jing Li, Xi-Tao Yin, and Qi Wang. 2020. "Discriminable Sensing Response Behavior to Homogeneous Gases Based on n-ZnO/p-NiO Composites" Nanomaterials 10, no. 4: 785. https://doi.org/10.3390/nano10040785
APA StyleZhou, W.-D., Dastan, D., Li, J., Yin, X.-T., & Wang, Q. (2020). Discriminable Sensing Response Behavior to Homogeneous Gases Based on n-ZnO/p-NiO Composites. Nanomaterials, 10(4), 785. https://doi.org/10.3390/nano10040785






