Biogenic-Mediated Synthesis of Mesoporous Cu2O/CuO Nano-Architectures of Superior Catalytic Reductive towards Nitroaromatics
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Preparative Route for Pomegranate Seeds Extract (PSE)
2.2.2. Bulk Synthesis of CuO (Bulk Fabricated CuO)
2.2.3. Biogenic Synthesis of Copper Oxide Nanoparticles (PSE-Capped Cu2O/CuO)
2.3. Reduction of Nitrobenzene
2.4. Characterization
3. Results and Discussion
3.1. X-Ray Diffraction (XRD)
3.2. FTIR Spectroscopy
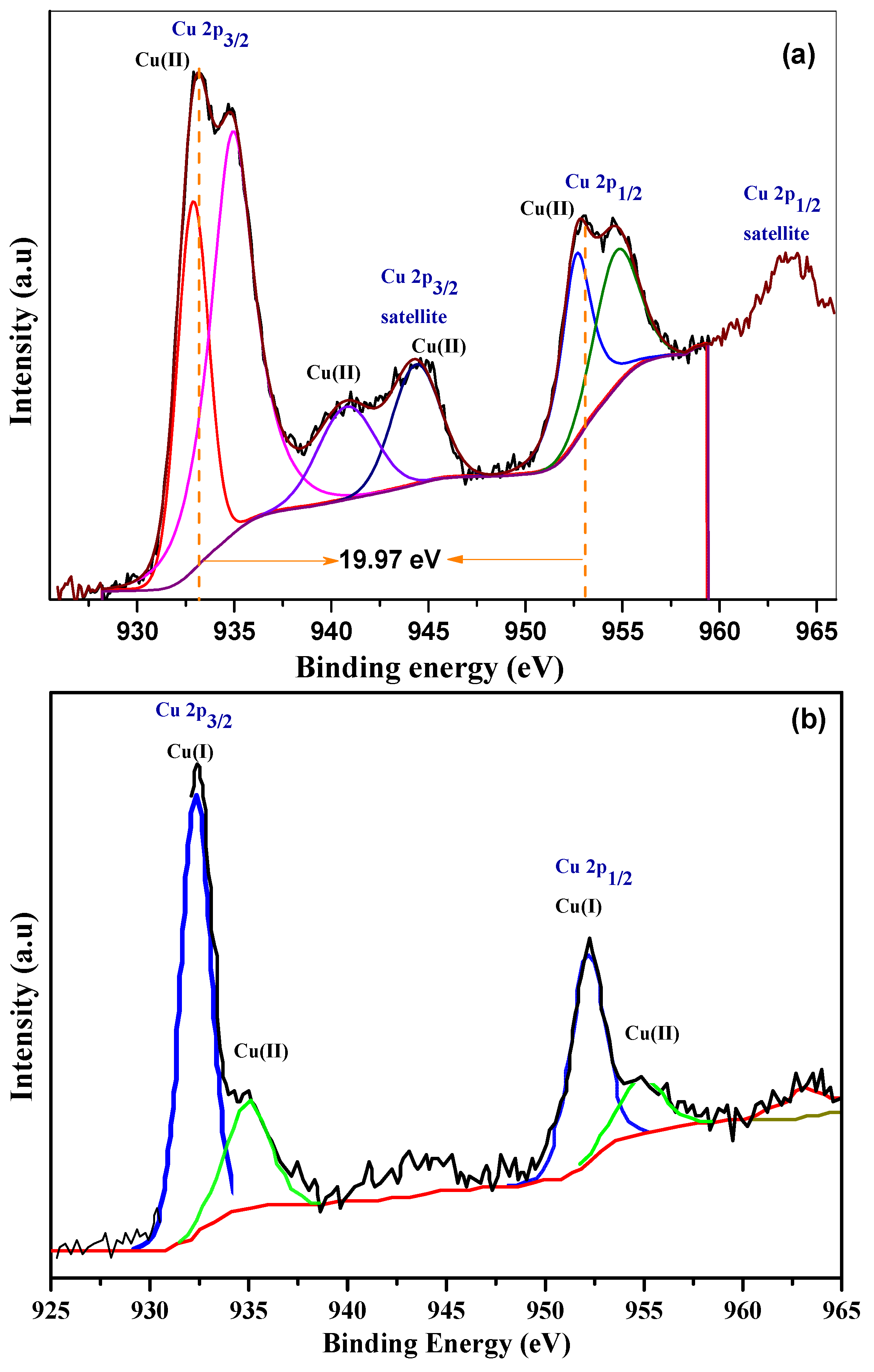
3.3. X-Ray Photoelectron Spectroscopy (XPS)
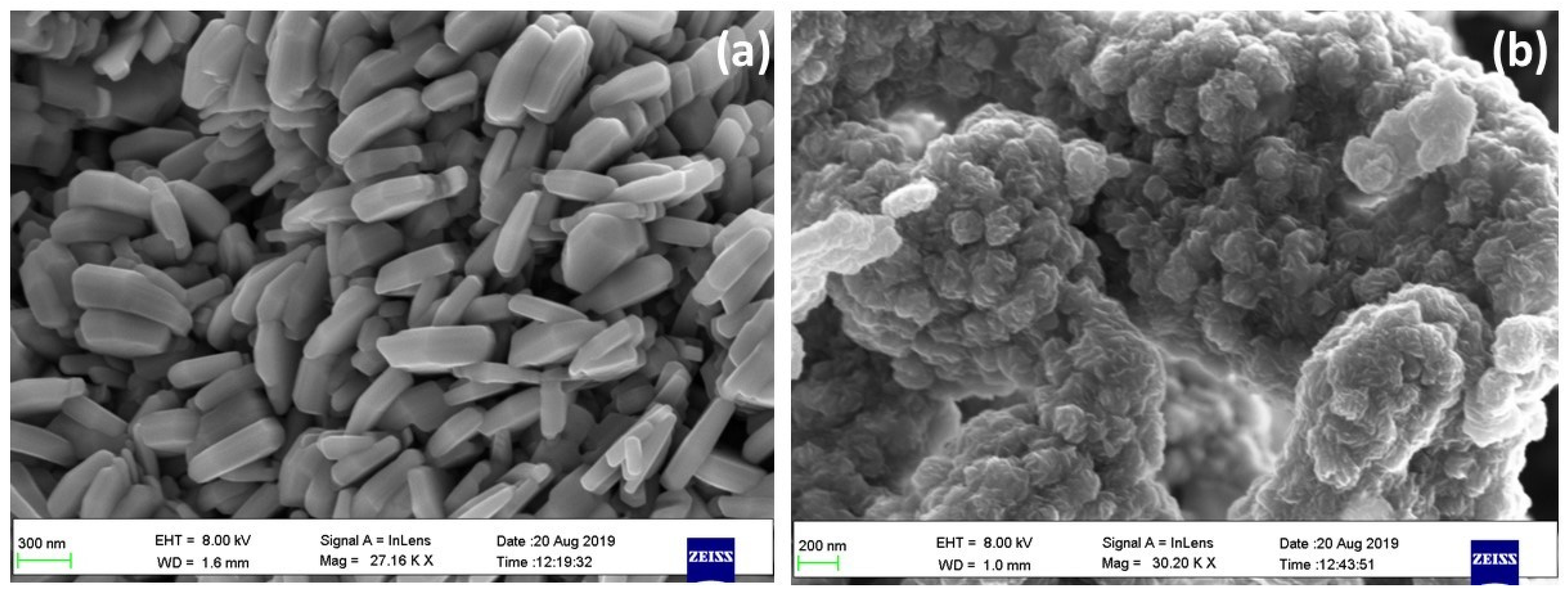
3.4. Morphological Features Using FESEM
3.5. Surface Characteristics
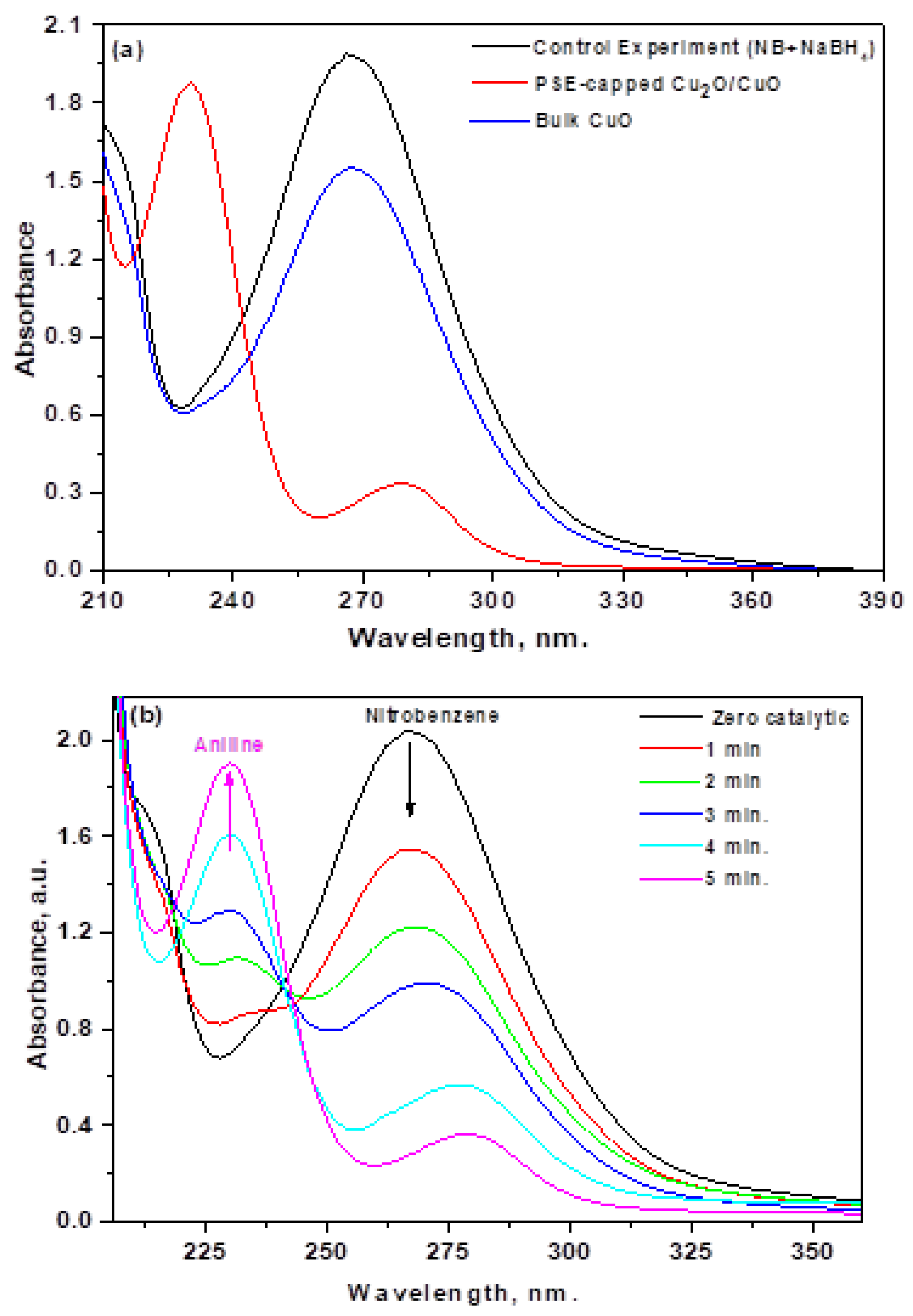
3.6. Catalytic Properties
3.6.1. Influence of Reaction Time
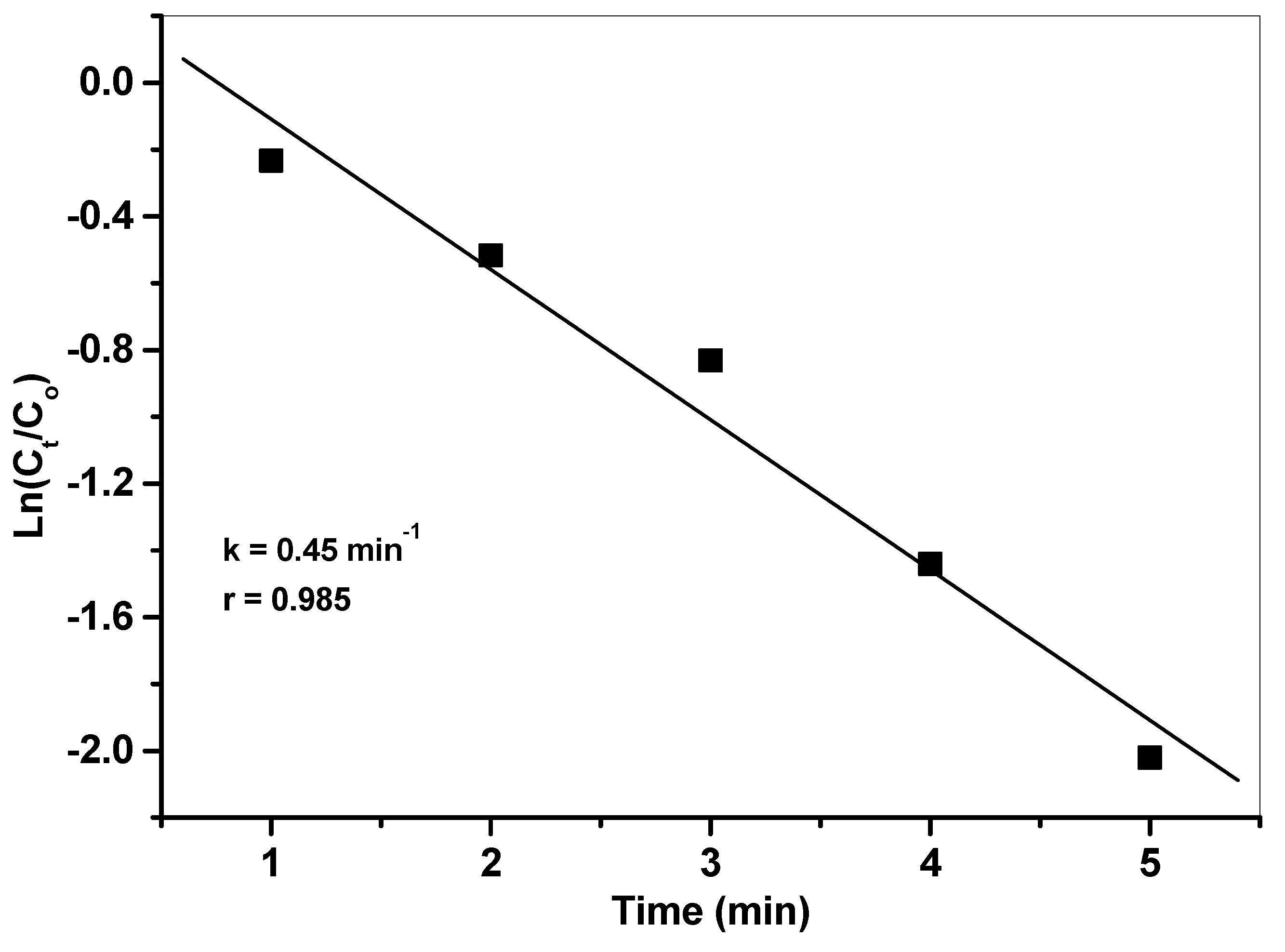
3.6.2. Kinetics Studies
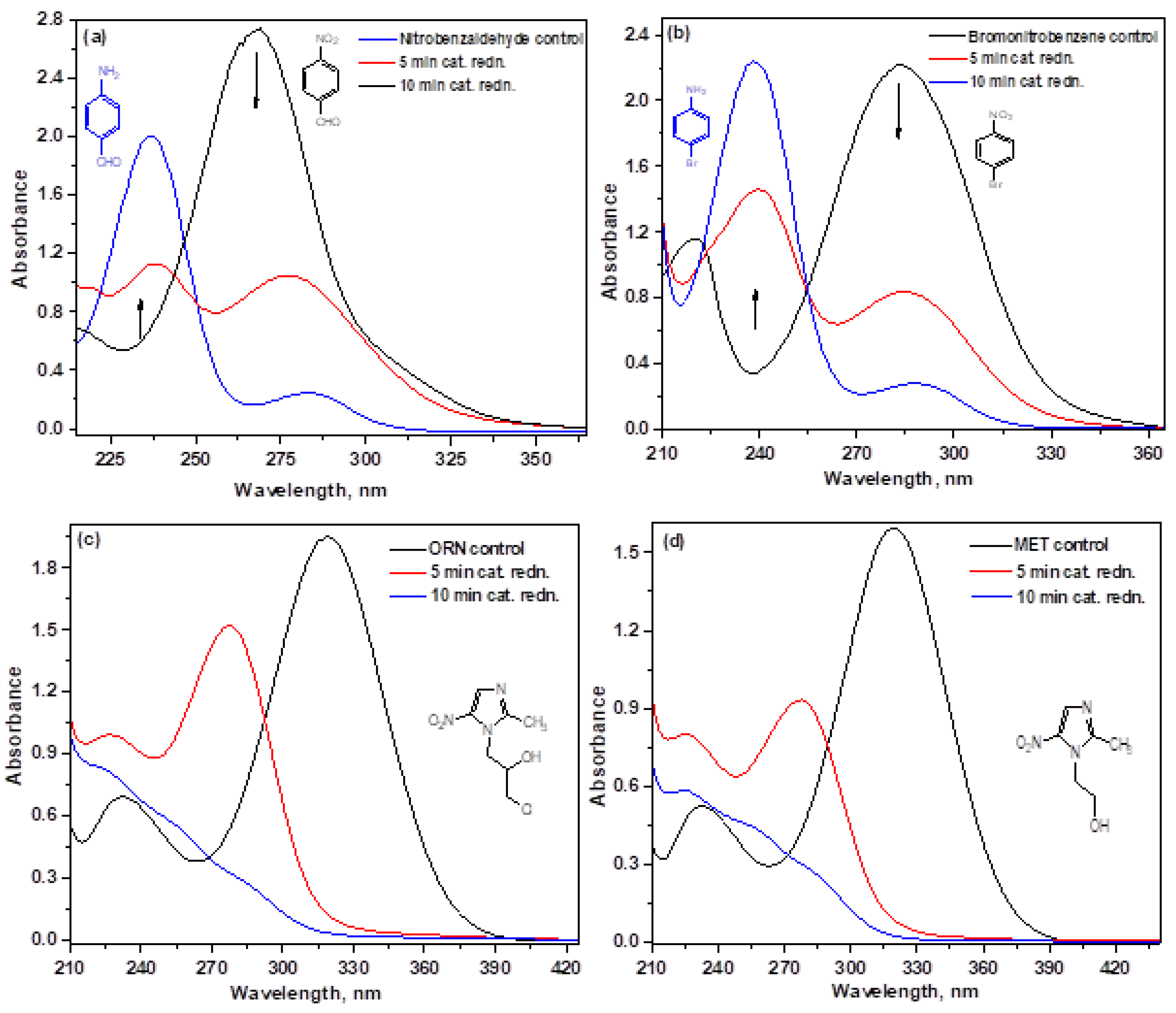
3.6.3. Influence of Various Substrates
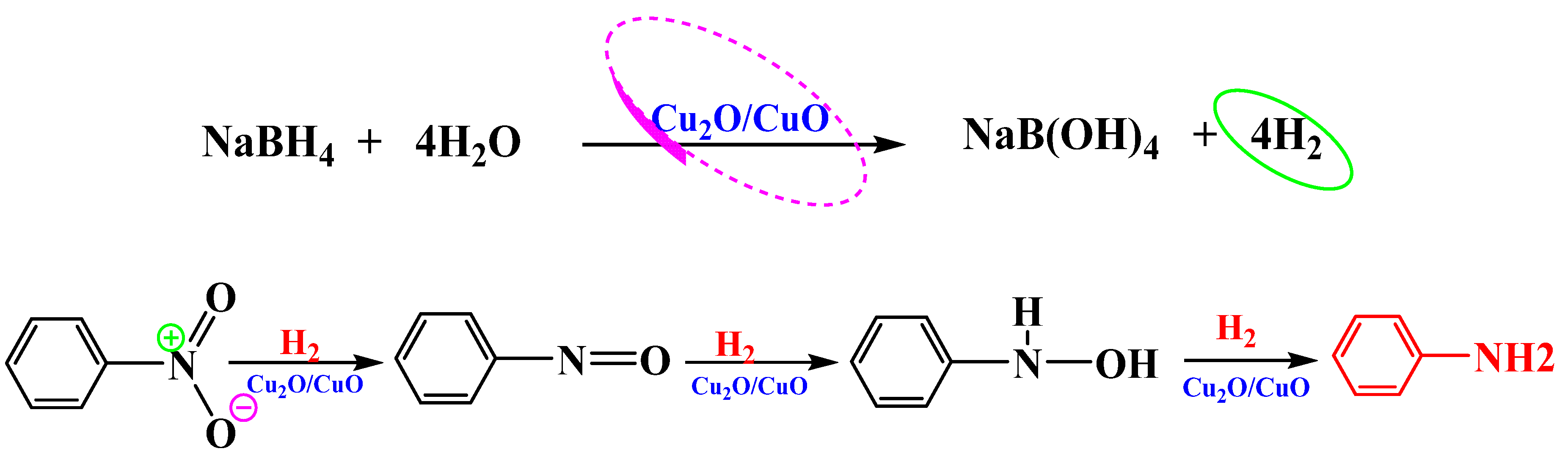
3.6.4. Reaction Mechanism
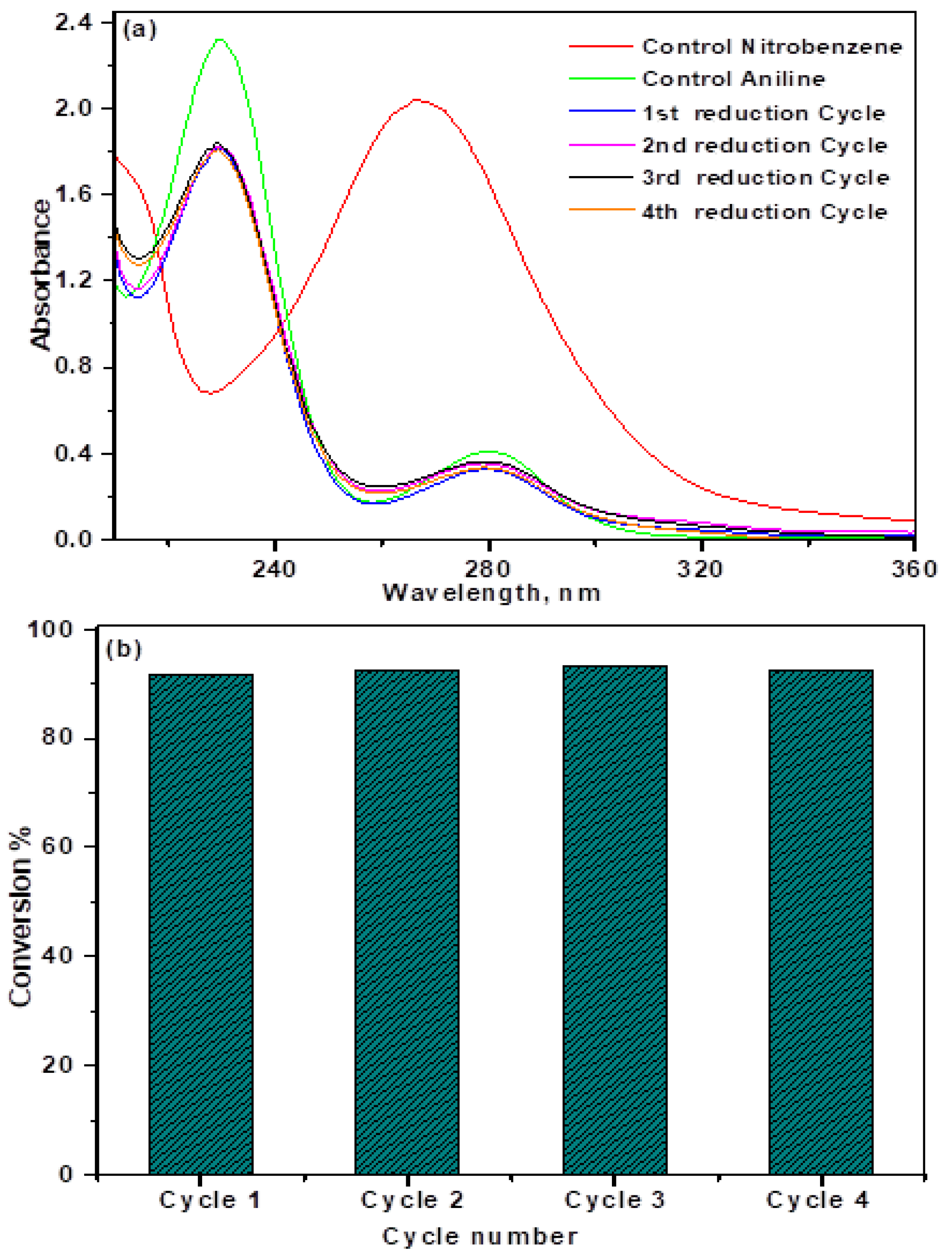
3.6.5. Catalyst Recycling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qazi, U.Y.; Javaid, R. A Review on Metal Nanostructures: Preparation Methods and Their Potential Applications. Adv. Nanoparticles 2016, 5, 27–43. [Google Scholar] [CrossRef]
- El-Shobaky, G.A.; Radwan, N.R.; El-Shall, M.S.; Turky, A.; Hassan, H.M. The role of preparation of CuO–NiO system on its physicochemical surface and catalytic properties. Colloids Surf. A Physicochem. Eng. Asp. 2007, 311, 161–169. [Google Scholar] [CrossRef]
- Guo, T.; Yao, M.; Lin, Y.-H.; Nan, C.-W. A comprehensive review on synthesis methods for transition-metal oxide nanostructures. CrystEngComm. 2015, 17, 3551–3585. [Google Scholar] [CrossRef]
- Wan, X.; Wang, J.; Zhu, L.; Tang, J. Gas sensing properties of Cu2O and its particle size and morphology-dependent gas-detection sensitivity. J. Mater. Chem. A 2014, 2, 13641–13647. [Google Scholar] [CrossRef]
- Akimoto, K.; Ishizuka, S.; Yanagita, M.; Nawa, Y.; Paul, G.K.; Sakurai, T. Thin film deposition of Cu2O and application for solar cells. Sol. Energy 2006, 80, 715–722. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Zhu, W. Shape Evolution and Size-Controllable Synthesis of Cu2O Octahedra and Their Morphology-Dependent Photocatalytic Properties. J. Phys. Chem. B 2006, 110, 13829–13834. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Zhao, W.; Fu, W.; Yang, H.; Tian, C.; Li, M.; Li, Y.; Zhang, L.; Sui, Y.; Zhou, X.; Chena, H.; et al. Synthesis of Nanostructured Cuprous Oxide and Its Performance as Humidity and Temperature Sensor. CrystEngComm 2011, 13, 2871–2877. [Google Scholar] [CrossRef]
- Yadav, B.C.; Yadav, A.K. Synthesis of Nanostructured Cuprous Oxide and Its Performance as Humidity and Temperature Sensor. Int. J. Green Nanotechnol. Mater. Sci. Eng. 2009, 1, M16–M31. [Google Scholar] [CrossRef]
- Deng, X.; Wang, C.; Zhou, E.; Huang, J.; Shao, M.; Wei, X.Q.; Liu, X.; Ding, M.; Xu, X. One-Step Solvothermal Method to Prepare Ag/Cu2O Composite with Enhanced Photocatalytic Properties. Nanoscale Res. Lett. 2016, 11, 29. [Google Scholar] [CrossRef]
- Sui, Y.; Zeng, Y.; Zheng, W.T.; Liu, B.; Zou, B.; Yang, H. Synthesis of polyhedron hollow structure Cu2O and their gas-sensing properties. Sens. Actuators B Chem. 2012, 171, 135–140. [Google Scholar] [CrossRef]
- Gou, L.; Murphy, C.J. Controlling the size of Cu2O nanocubes from 200 to 25 nm. J. Mater. Chem. 2004, 14, 735–738. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, T.; Gu, Q.; Cheng, G.; Zheng, R.T. Shape control mechanism of cuprous oxide nanoparticles in aqueous colloidal solutions. Powder Technol. 2012, 227, 35–42. [Google Scholar] [CrossRef]
- Huang, M.H.-Y.; Chiu, C.-Y. Achieving polyhedral nanocrystal growth with systematic shape control. J. Mater. Chem. A 2013, 1, 8081. [Google Scholar] [CrossRef]
- Gou, L.; Murphy, C. Solution-Phase Synthesis of Cu2O Nanocubes. Nano Lett. 2003, 3, 231–234. [Google Scholar] [CrossRef]
- Sun, S.; Deng, D.; Kong, C.; Gao, Y.; Yang, S.; Song, X.; Ding, B.; Yang, Z. Seed-mediated synthesis of polyhedral 50-facet Cu2O architectures. CrystEngComm 2011, 13, 5993–5997. [Google Scholar] [CrossRef]
- Kumar, R.V.; Mastai, Y.; Diamant, Y.; Gedanken, A. Sonochemical synthesis of amorphous Cu and nanocrystalline Cu2O embedded in a polyaniline matrix. J. Mater. Chem. 2001, 11, 1209–1213. [Google Scholar] [CrossRef]
- Nunes, D.; Santos, L.; Duarte, P.; Pimentel, A.; Pinto, J.V.; Barquinha, P.; Carvalho, P.; Fortunato, E.; Martins, R. Room Temperature Synthesis of Cu2O Nanospheres: Optical Properties and Thermal Behavior. Microsc. Microanal. 2014, 21, 108–119. [Google Scholar] [CrossRef]
- Singh, Z.; Singh, Z. CTAB Surfactant Assisted and High pH Nano-Formulations of CuO Nanoparticles Pose Greater Cytotoxic and Genotoxic Effects. Sci. Rep. 2019, 9, 5880. [Google Scholar] [CrossRef]
- Won, Y.-H.; Stanciu, L. Cu2O and Au/Cu2O Particles: Surface Properties and Applications in Glucose Sensing. Sensors 2012, 12, 13019–13033. [Google Scholar] [CrossRef]
- Booth, G. Ullman’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2002. [Google Scholar]
- Ley, S.V.; Thomas, A.W. Modern synthetic methods for copper-mediated C(aryl)[bond]O, C(aryl)[bond]N, and C(aryl)[bond]S bond formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef] [PubMed]
- Reymond, S.; Cossy, J. Copper-Catalyzed Diels−Alder Reactions. Chem. Rev. 2008, 108, 5359–5406. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Radiman, S.; Tabet, N.; Daud, A.R. Synthesis and optical properties of CuO nanostructures obtained via a novel thermal decomposition method. J. Alloys Compd. 2011, 509, 8761–8769. [Google Scholar] [CrossRef]
- Dubal, D.; Dhawale, D.S.; Salunkhe, R.; Jamdade, V.; Lokhande, C.D. Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J. Alloys Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Ethiraj, A.S.; Kang, D.J. Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett. 2012, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, X. Solution-phase synthesis of CuO hierarchical nanosheets at near-neutral pH and near-room temperature. Mater. Lett. 2007, 61, 2222–2226. [Google Scholar] [CrossRef]
- Zhu, C.; Osherov, A.; Panzer, M.J. Surface chemistry of electrodeposited Cu2O films studied by XPS. Electrochimica Acta 2013, 111, 771–778. [Google Scholar] [CrossRef]
- Kulkarni, P.; Mahamuni, S.; Chandrachood, M.; Mulla, I.; Sinha, A.P.B.; Nigavekar, A.S.; Kulkarni, S.K. Photoelectron spectroscopic studies on a silicon interface with Bi2Sr2CaCu2BO8+δhighTcsuperconductor. J. Appl. Phys. 1990, 67, 3438–3442. [Google Scholar] [CrossRef]
- Essawy, A.A. Silver imprinted zinc oxide nanoparticles: Green synthetic approach, characterization and efficient sunlight-induced photocatalytic water detoxification. J. Clean. Prod. 2018, 183, 1011–1020. [Google Scholar] [CrossRef]
- Alrowaili, Z.A.; Alsohaimi, I.H.; Betiha, M.A.; Essawy, A.A.; Mousa, A.A.; Alruwaili, S.F.; Hassan, H.M.; Mosa, A.A. Green fabrication of silver imprinted titania / silica nanospheres as robust visible light-induced photocatalytic wastewater purification. Mater. Chem. Phys. 2020, 241, 122403. [Google Scholar] [CrossRef]
- Sau, T.K.; Pal, A.; Pal, T. Size Regime Dependent Catalysis by Gold Nanoparticles for the Reduction of Eosin. J. Phys. Chem. B 2001, 105, 9266–9272. [Google Scholar] [CrossRef]
- Wan, X.-K.; Wang, J.-Q.; Nan, Z.-A.; Wang, Q.-M. Ligand effects in catalysis by atomatically precise gold nanoclusters. Sci. Adv. 2017, 3, e1701823. [Google Scholar] [CrossRef] [PubMed]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef] [PubMed]
- Fountoulaki, S.; Daikopoulou, V.; Gkizis, P.L.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I. Mechanistic Studies of the Reduction of Nitroarenes by NaBH4 or Hydrosilanes Catalyzed by Supported Gold Nanoparticles. ACS Catal. 2014, 4, 3504–3511. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Z.; Li, L.; Guo, L.; Yang, S. Two-dimensional structure Au nanosheets are super active for the catalytic reduction of 4-nitrophenol. Phys. Chem. Chem. Phys. 2015, 17, 14656–14661. [Google Scholar] [CrossRef] [PubMed]


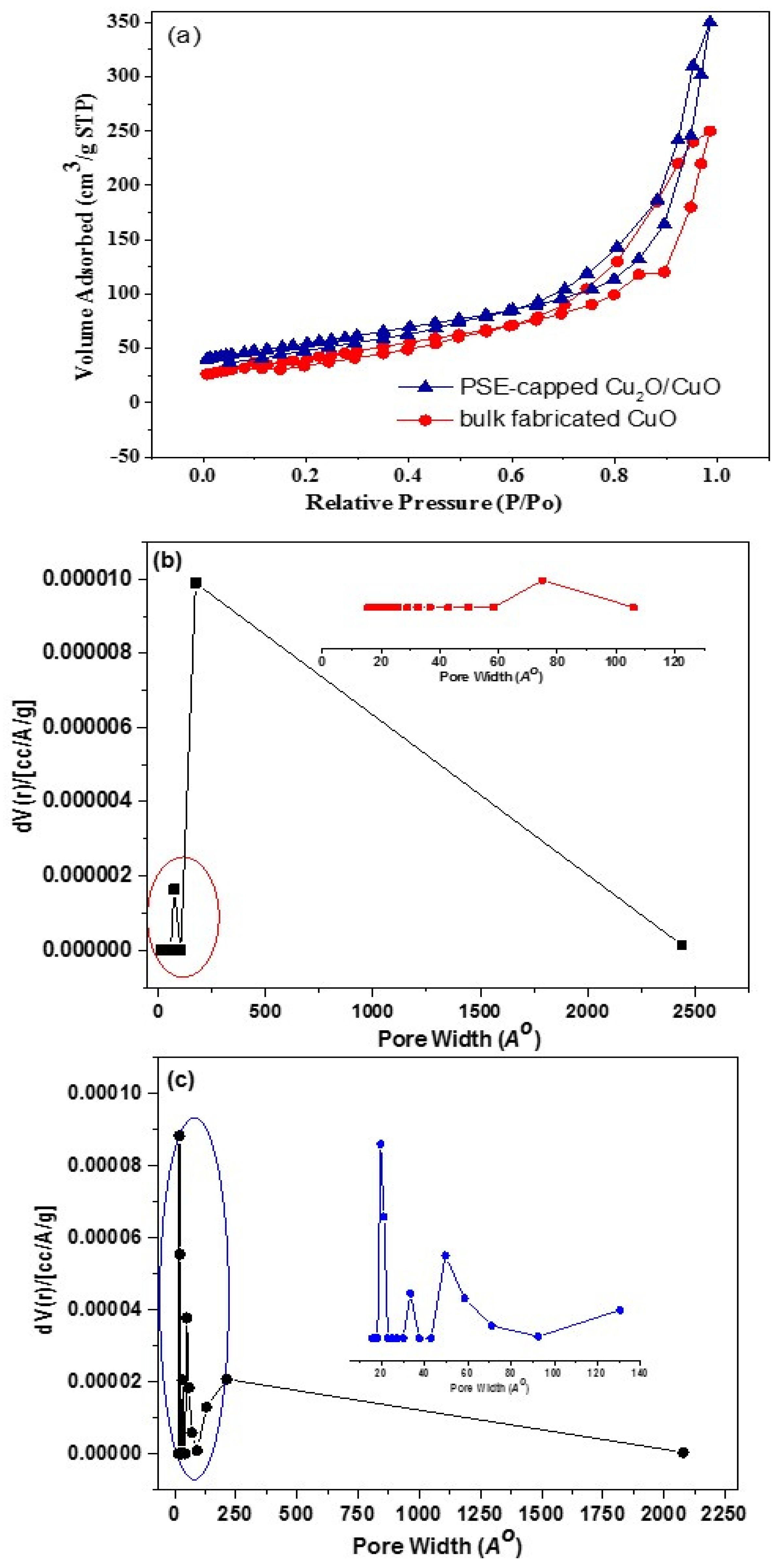
| Catalysts | SBETa /m2 g−1 | VBJHc /cm3 g−1 | DBJHb /nm |
|---|---|---|---|
| Bulk fabricated CuO | 9.2 | 0.0081 | 15.2 |
| PSE-capped Cu2O/CuO | 20.1 | 0.0362 | 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhumaimess, M.S.; Essawy, A.A.; Kamel, M.M.; Alsohaimi, I.H.; Hassan, H.M.A. Biogenic-Mediated Synthesis of Mesoporous Cu2O/CuO Nano-Architectures of Superior Catalytic Reductive towards Nitroaromatics. Nanomaterials 2020, 10, 781. https://doi.org/10.3390/nano10040781
Alhumaimess MS, Essawy AA, Kamel MM, Alsohaimi IH, Hassan HMA. Biogenic-Mediated Synthesis of Mesoporous Cu2O/CuO Nano-Architectures of Superior Catalytic Reductive towards Nitroaromatics. Nanomaterials. 2020; 10(4):781. https://doi.org/10.3390/nano10040781
Chicago/Turabian StyleAlhumaimess, Mosaed S., Amr A. Essawy, Mahmoud M. Kamel, Ibrahim Hotan Alsohaimi, and Hassan M. A. Hassan. 2020. "Biogenic-Mediated Synthesis of Mesoporous Cu2O/CuO Nano-Architectures of Superior Catalytic Reductive towards Nitroaromatics" Nanomaterials 10, no. 4: 781. https://doi.org/10.3390/nano10040781
APA StyleAlhumaimess, M. S., Essawy, A. A., Kamel, M. M., Alsohaimi, I. H., & Hassan, H. M. A. (2020). Biogenic-Mediated Synthesis of Mesoporous Cu2O/CuO Nano-Architectures of Superior Catalytic Reductive towards Nitroaromatics. Nanomaterials, 10(4), 781. https://doi.org/10.3390/nano10040781








