Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Deposition and Characterization
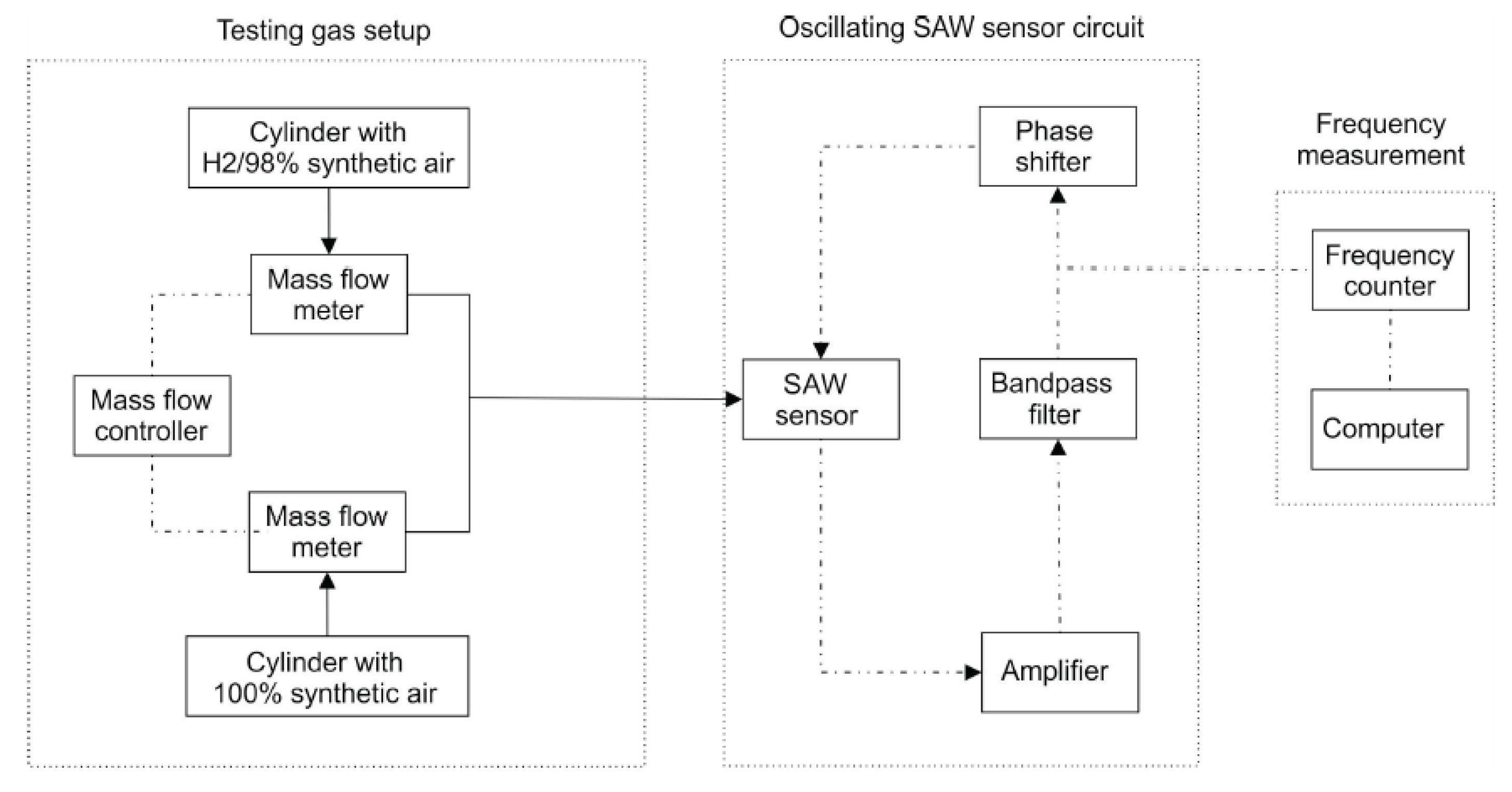
2.2. Sensor Structure and Testing
3. Results and Discussion
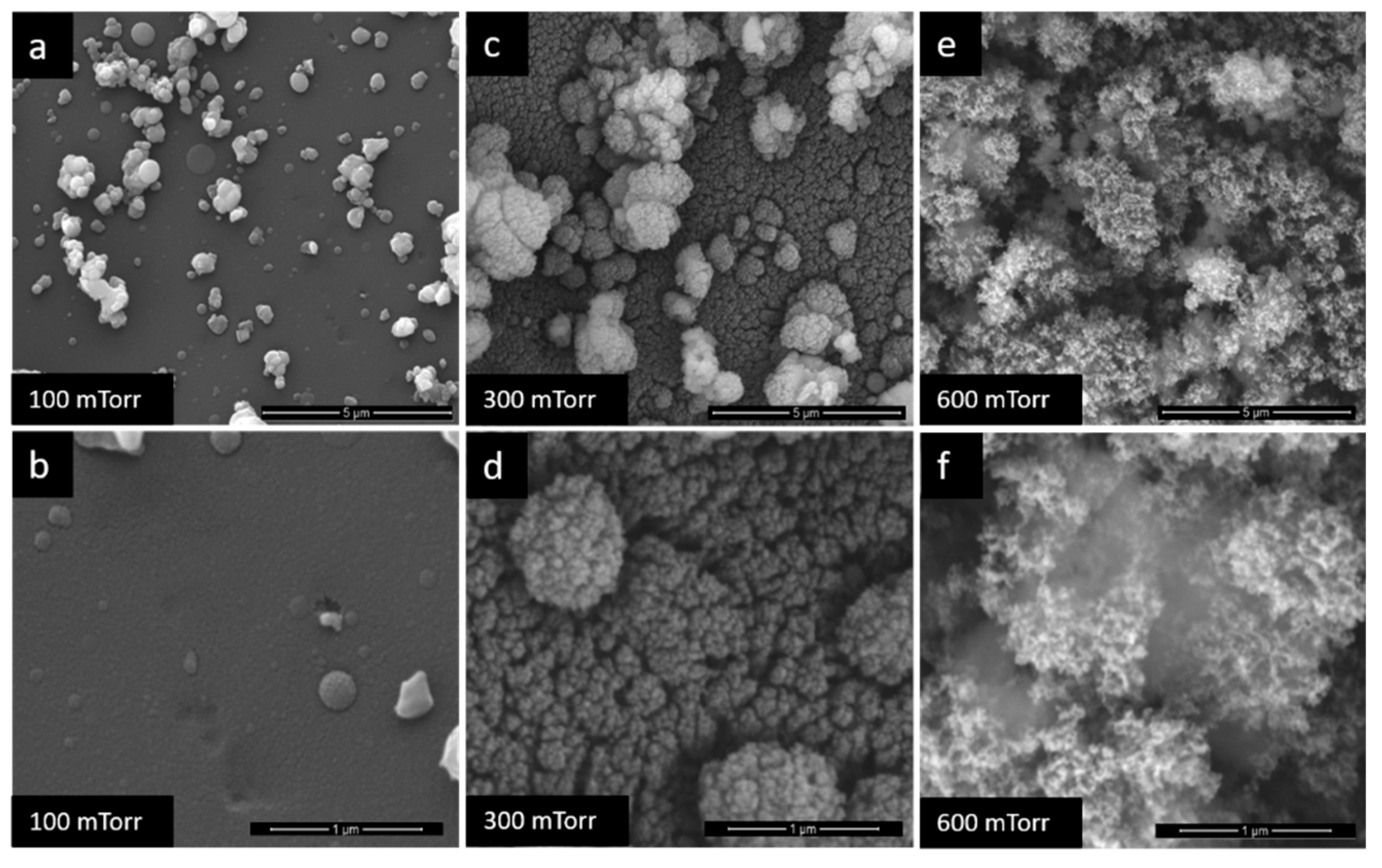
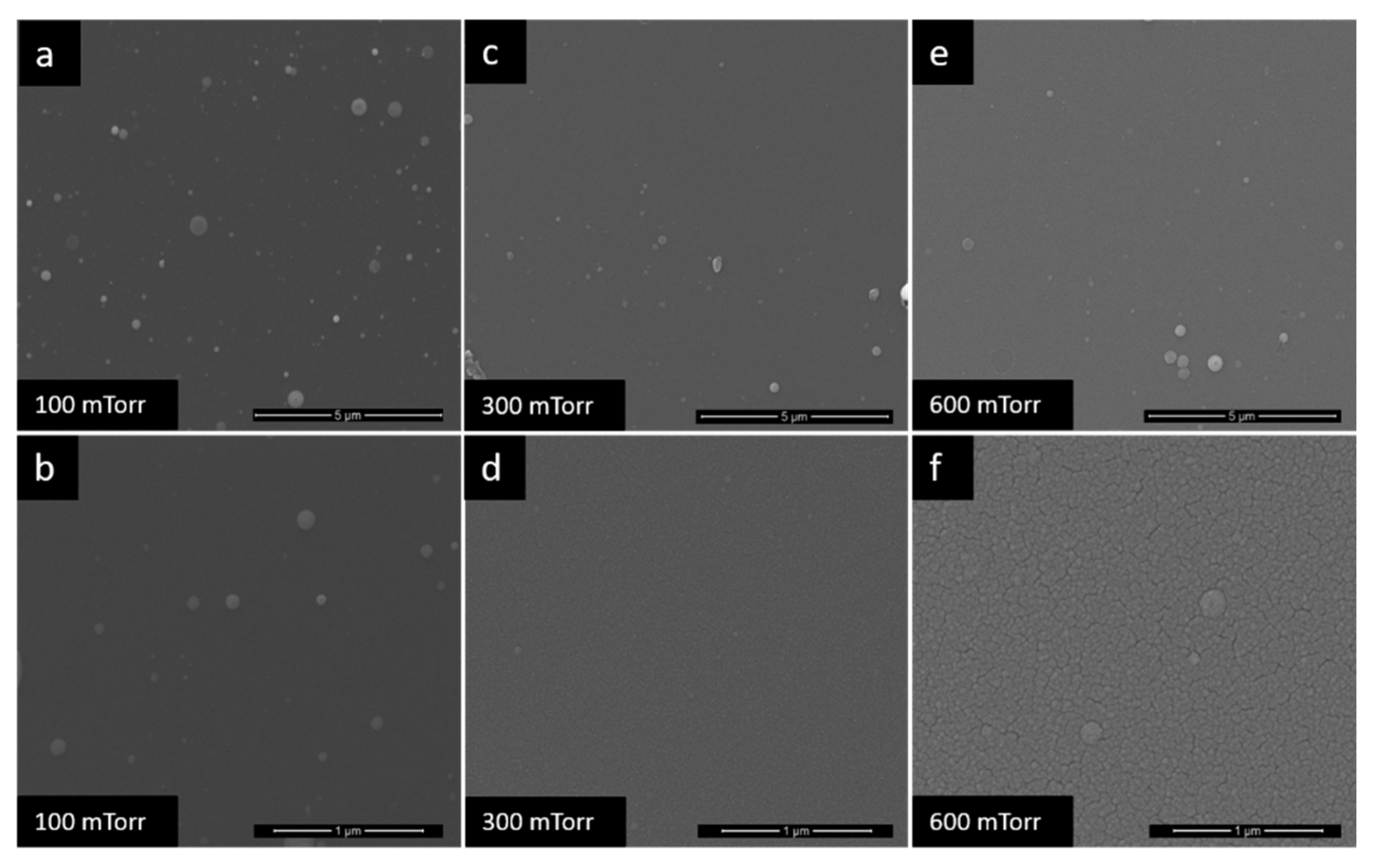
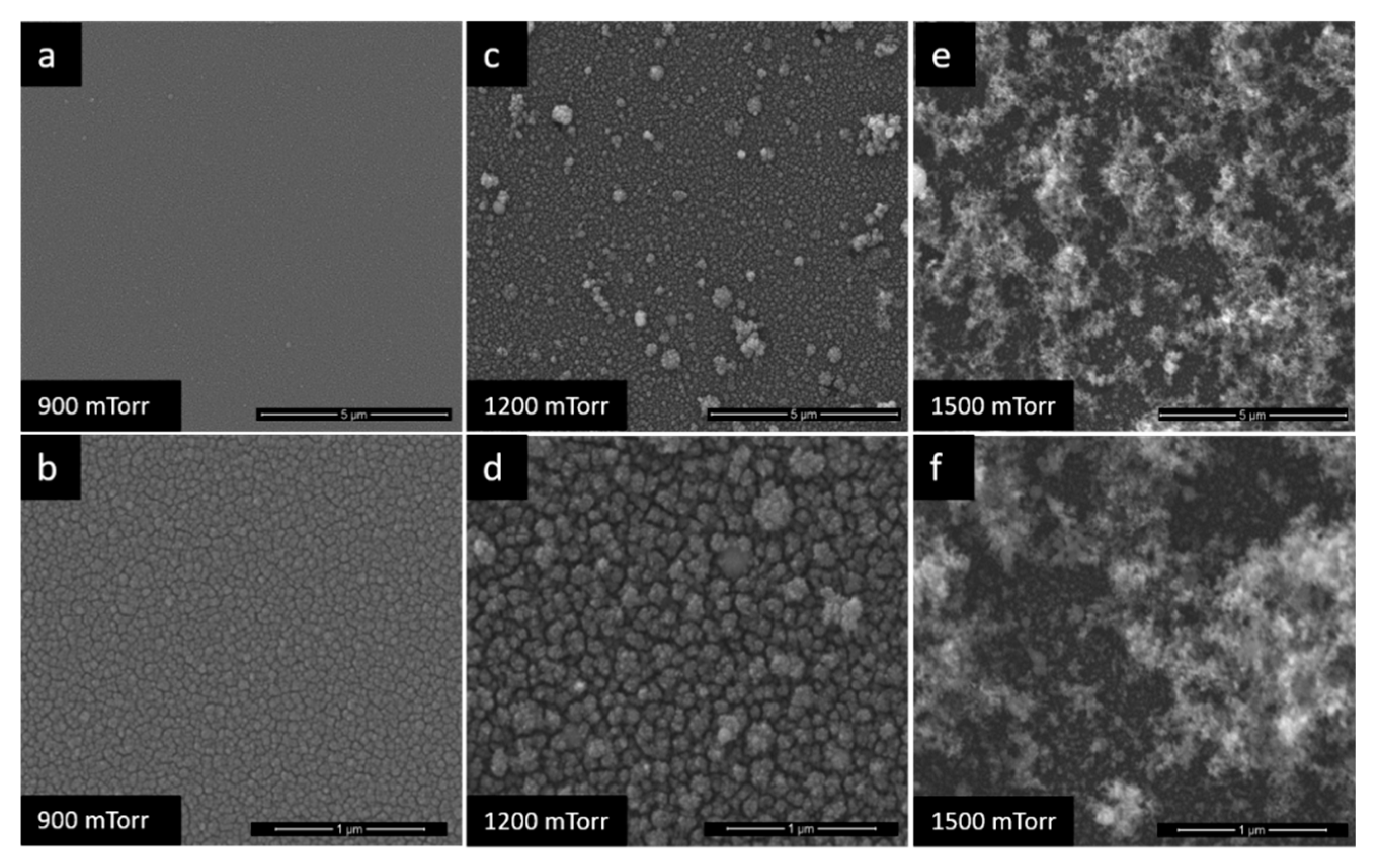
3.1. Film Morphology
3.2. Sensor Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, W.; Fu, Q.; Zhou, D.; Deng, J.; Liu, H.; Yan, G. A surface acoustic wave H2S gas sensor employing nanocrystalline SnO2 thin film. Sens. Actuators B Chem. 2013, 176, 746–752. [Google Scholar] [CrossRef]
- Chen, H.-I.; Cheng, Y.-C.; Chang, C.-H.; Chen, W.-C.; Liu, I.-P.; Lin, K.-W.; Liu, W.-C. Hydrogen sensing performance of a Pd nanoparticle/Pdfilm/GaN-based diode. Sens. Actuator B Chem. 2017, 247, 514–519. [Google Scholar] [CrossRef]
- Kunti, A.; Chowdhury, M.; Sharma, S.; Gupta, M.; Chaudhary, R. Influence of O 2 pressure on structural, morphological and optical properties of TiO 2 -SiO 2 composite thin films prepared by pulsed laser deposition. Thin Solid Films 2017, 629, 79–89. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Ouyang, C.; Wu, J.; Huang, Z.; Xu, X. Micro structural, electrical and optical properties of highly (220) oriented spinel Mn–Co–Ni–O film grown by radio frequency magnetron sputtering. Appl. Surf. Sci. 2014, 311, 443–447. [Google Scholar] [CrossRef]
- Han, Z.; Ren, J.; Zhou, J.; Zhang, S.; Zhang, Z.; Yang, L.; Yin, C. Multilayer porous Pd-WO3 composite thin films prepared by sol-gel process for hydrogen sensing. Int. J. Hydrog. Energy 2020, 45, 7223–7233. [Google Scholar] [CrossRef]
- Qadri, M.U.; Annanouch, F.E.; Aguiló, M.; Díaz, F.; Borull, J.F.; Pujol, M.C.; Llobet, E. Metal Decorated WO3 Nanoneedles Fabricated by Aerosol Assisted Chemical Vapor Deposition for Optical Gas Sensing. J. Nanosci. Nanotechnol. 2016, 16, 10125–10132. [Google Scholar] [CrossRef]
- Di, W.; Liu, F.; Lin, T.; Kong, H.; Meng, C.; Zhang, W.; Chen, Y.; Hou, Y. Influence of oxygen partial pressure on structural and electrical properties of Mn1.56Co0.96Ni0.48O4 thin films deposited by pulsed laser deposition. Appl. Surf. Sci. 2018, 447, 287–291. [Google Scholar] [CrossRef]
- Meng, L.; Wang, Z.; Yang, L.; Ren, W.; Liu, W.; Zhang, Z.; Yang, T.; Dos Santos, M. A detailed study on the Fe-doped TiO2 thin films induced by pulsed laser deposition route. Appl. Surf. Sci. 2019, 474, 211–217. [Google Scholar] [CrossRef]
- Zhang, Z.; Wong, L.M.; Zhang, Z.; Wu, Z.; Wang, S.; Chi, D.; Hong, R.; Yang, W. Pulse laser deposition of epitaxial TiO2thin films forhigh-performance ultraviolet photodetectors. Appl. Surf. Sci. 2015, 355, 398–402. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, Y.; Duan, Z.; Wang, T.; Tang, Y.; Zhao, X.; Sun, D.; Shi, W.; Wanga, F. Influence of oxygen pressure on the electrical properties of Mn-doped Bi0.5Na0.5TiO3BaTiO3 thin films by pulsed laser deposition. Ceram. Int. 2019, 45, 13518–13522. [Google Scholar] [CrossRef]
- Nyenge, R.; Swart, H.; Ntwaeaborwa, O.M. The influence of substrate temperature and deposition pressure on pulsed laser deposited thin films of CaS:Eu2+ phosphors. Phys. B Condens. Matter 2016, 480, 186–190. [Google Scholar] [CrossRef]
- Miu, D.; Bîrjega, R.; Viespe, C. Surface Acoustic Wave Hydrogen Sensors Based on Nanostructured Pd/WO3 Bilayers. Sensors 2018, 18, 3636. [Google Scholar] [CrossRef] [Green Version]
- Ballantine, D.S.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohtjen, H. Acoustic Wave Sensors, Theory, Design, and Physico-Chemical Applications; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Ricco, A.J.; Martin, S.J.; Zipperian, T.E. Surface Acoustic Wave gas sensor based on film conductivity changes. Sens. Actuator 1985, 8, 319–333. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Mei, S.; Jia, Y.; Liu, M.; Xue, X.; Yang, D. Development of a Pd/Cu nanowires coated SAW hydrogen gas sensor with fast response and recovery. Sens. Actuators B Chem. 2019, 287, 157–164. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Miu, D.; Viespe, C. Surface Acoustic Wave Sensors for Ammonia Detection at Room Temperature based on SnO2/Co3O4 Bilayers. J. Sens. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Constantinoiu, I.; Viespe, C. Hydrogen Detection with SAW Polymer/Quantum Dots Sensitive Films. Sensors 2019, 19, 4481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinoiu, I.; Viespe, C. Detection of Volatile Organic Compounds Using Surface Acoustic Wave Sensor Based on Nnaoparticles Incorporated in Polymer. Coatings 2019, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Raj, V.B.; Singh, H.; Nimal, A.; Sharma, M.; Gupta, V. Oxide thin films (ZnO, TeO2, SnO2, and TiO2) based surface acoustic wave (SAW) E-nose for the detection of chemical warfare agents. Sens. Actuator B Chem. 2013, 178, 636–647. [Google Scholar] [CrossRef]
- Dinca, V.; Viespe, C.; Brajnicov, S.; Constantinoiu, I.; Moldovan, A.; Bonciu, A.; Toader, C.N.; Ginghina, R.E.; Grigoriu, N.; Dinescu, M.; et al. MAPLE Assembled Acetylcholonesterase-Polyethylenimine Hybrid and Multilayered Interfaces for Toxic Gases Detection. Sensors 2018, 18, 4265. [Google Scholar] [CrossRef] [Green Version]
- Acar, C.; Dincer, I. The potential role of hydrogen as a sustainable transportation fuel to combat global warming. Int. J. Hydrog. Energy 2020, 45, 3396–3406. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Chandra, R. Fabrication of porous silicon filled Pd/SiC nanocauliflower thin films for high performance H2 gas sensor. Sens. Actuator B Chem. 2018, 264, 10–19. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, C.; Yang, L.; Zhang, Z.; Han, Z. Investigation of the H2 sensing properties of multilayer mesoporous pure and Pd-doped SnO2 thin film. Sens. Actuator B Chem. 2019, 283, 399–406. [Google Scholar] [CrossRef]
- Prakash, R.; Kumar, A.; Kaur, D. Pd capped W2N nano porous thin films for remarkable room temperature hydrogen gas sensing performance. Sens. Actuator B Chem. 2018, 277, 665–672. [Google Scholar] [CrossRef]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW Sensors for Chemical Vapours and Gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Zhang, H.; Wang, Z.; Xia, X.; Bao, Y.; Homewood, K.; Shao, G.; Huang, Z.; Gao, Y. Improved hydrogen sensing of (004) oriented anatase TiO2 thin films through post annealing. Int. J. Hydrog. Energy 2019, 44, 20606–20615. [Google Scholar] [CrossRef]
- Öztürk, S.; Kılınç, N. Pd thin films on flexible substrate for hydrogen sensor. J. Alloy. Compd. 2016, 674, 179–184. [Google Scholar] [CrossRef]
- Sanger, A.; Kumar, A.; Kumar, A.; Jaiswal, J.; Chandra, R. A fast response/recovery of hydrophobic Pd/V2O5thin films forhydrogen gas sensing. Sens. Actuator B Chem. 2016, 236, 16–26. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Surface Acoustic Wave Sensor with Pd/ZnO Bilayer Structure for Room Temperature Hydrogen Detection. Sensors 2017, 17, 1529. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, S.; Singh, V.; Singh, M.; Vijay, Y. Synthesis and characterization of TiO2 doped polyaniline composites for hydrogen gas sensing. Int. J. Hydrog. Energy 2011, 36, 6343–6355. [Google Scholar] [CrossRef]
- Marcu, A.; Nicolae, I.; Viespe, C. Active surface geometrical control of noise in nanowire-SAW sensors. Sens. Actuator B Chem. 2016, 231, 469–473. [Google Scholar] [CrossRef]
- Mao, S.; Zhou, H.; Wu, S.; Yang, J.; Li, Z.; Wei, X.; Wang, X.; Wang, Z.; Li, J. High performance hydrogen sensor based on Pd/TiO2 composite film. Int. J. Hydrogen Energy 2018, 43, 22727–22732. [Google Scholar] [CrossRef]
- Wei, X.B.; Yang, X.H.; Wu, T.; Pang, H.Y.; Lv, G.D.; Wu, S.H.; Li, S.B.; Chen, Z. A novel composite hydrogen sensor based on Pd nanoclusters/TiO2 nanotube arrays. IOP Conf. Ser. Mater. Sci. Eng. 2017, 167, 12041. [Google Scholar] [CrossRef] [Green Version]
- Deepti; Kumarc, H.; Tripathi, A.; Dey, A.B.; Gupta, M.; Krishna, R.; Avasthi, D.K. Improved hydrogen sensing behaviour in ion-irradiated Pd-Au alloy thin films. Sens. Actuators B Chem. 2019, 301, 127006. [Google Scholar] [CrossRef]
- Marcu, A.; Viespe, C. Surface Acoustic Wave Sensors for Hydrogen and Deuterium Detection. Sensors 2017, 17, 1417. [Google Scholar] [CrossRef] [Green Version]
- Riabinina, D.; Irissou, E.; Le Drogoff, B.; Chaker, M.; Guay, D. Influence of pressure on the Pt nanoparticle growth modes during pulsed laser ablation. J. Appl. Phys. 2010, 108, 034322. [Google Scholar] [CrossRef]
- Jakubik, W.; Powroznik, P.; Wrotniak, J.; Krzywiecki, M. Theoretical analysis of custom electrical sensitivity in SAW gas sensors with single and bi-layer structures. Sens. Actuator B Chem. 2016, 236, 1069–1074. [Google Scholar] [CrossRef]
- Fan, H.; Ge, H.; Zhang, S.; Zhang, H.; Zhu, J. Optimization of sensitivity induced by surface conductivity and sorbed mass in SAW gas sensors. Sens. Actuator B. Chem. 2012, 161, 114–123. [Google Scholar] [CrossRef]

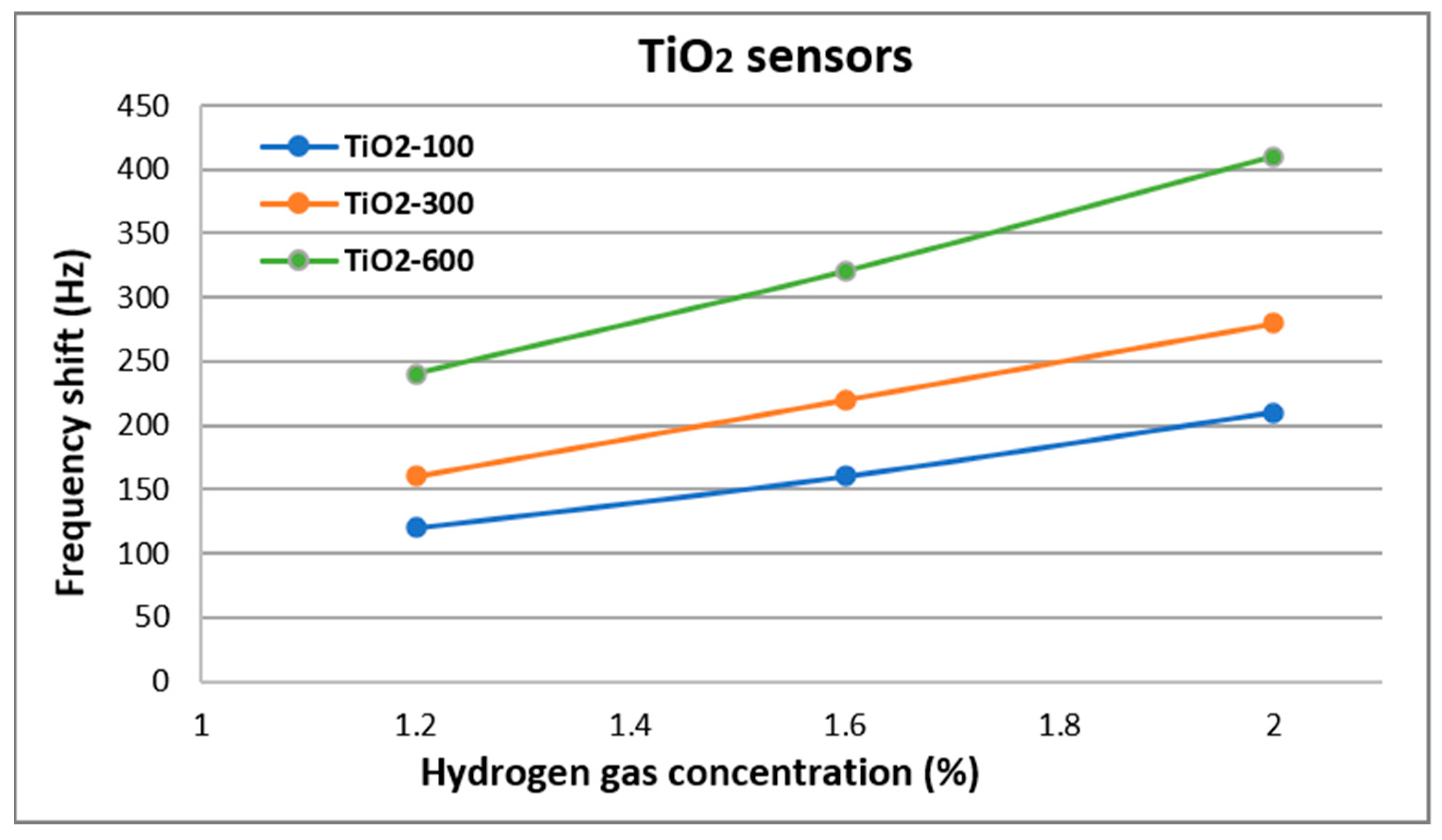
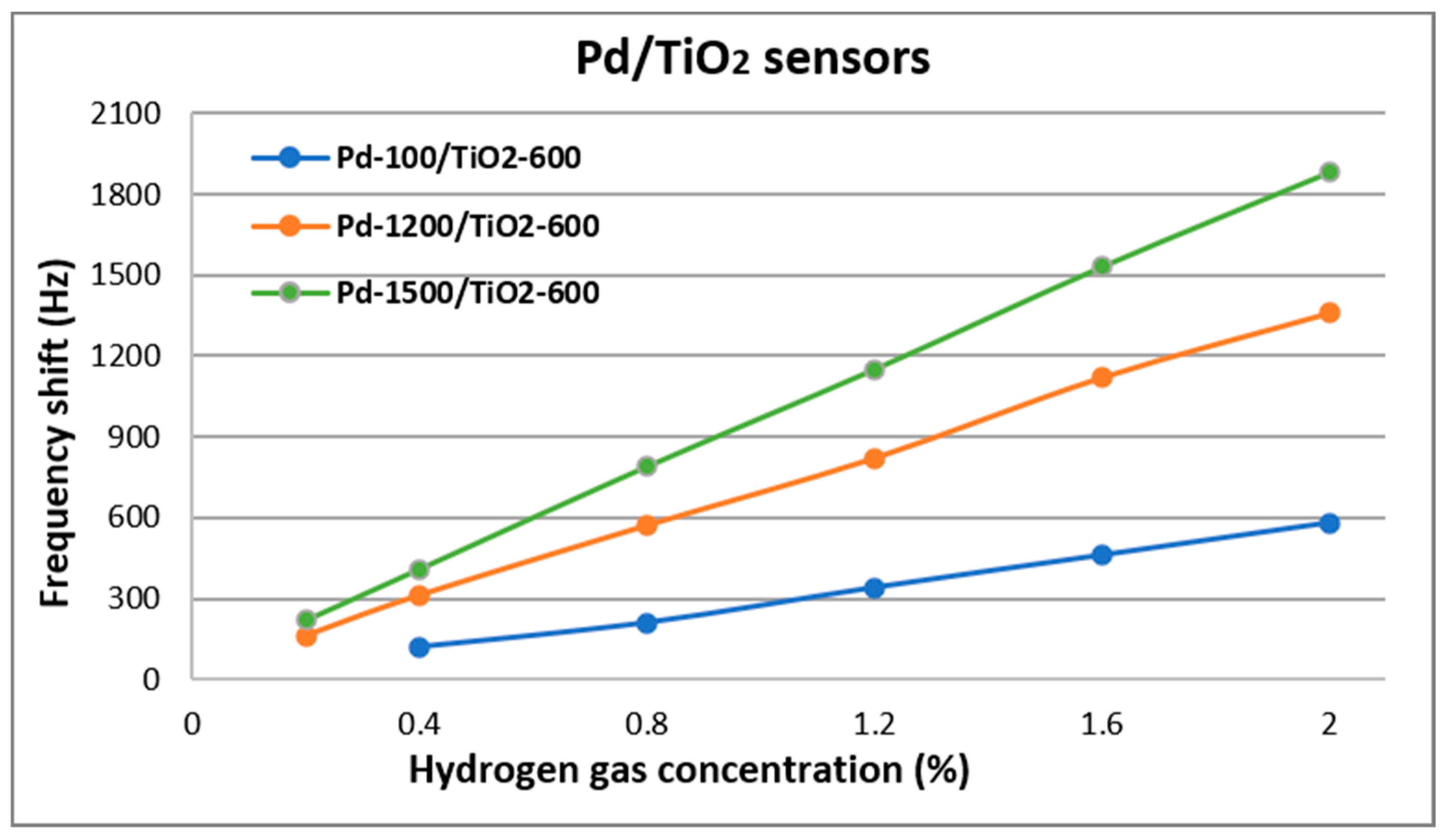
| Sensor Name | Composition of the Sensitive Layer | Deposition Pressure (mTorr) | |
|---|---|---|---|
| O2 | Ar | ||
| TiO2-100 | TiO2 | 100 | - |
| TiO2-300 | 300 | - | |
| TiO2-600 | 600 | - | |
| Pd-100/TiO2-600 | Pd/TiO2 | 600 | 100 |
| Pd-1200/TiO2-600 | 600 | 1200 | |
| Pd-1500/TiO2-600 | 600 | 1500 | |
| Sensor | Sensitivity (Δf/c) (Hz/ppm) | LOD (3 × n)/(Δf/c) (ppm) |
|---|---|---|
| TiO2-100 | 0.01 | 11807 |
| TiO2-300 | 0.01 | 8765 |
| TiO2-600 | 0.02 | 5951 |
| Pd-100/TiO2-600 | 0.02 | 4224 |
| Pd-1200/TiO2-600 | 0.07 | 1661 |
| Pd-1500/TiO2-600 | 0.10 | 1210 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials 2020, 10, 760. https://doi.org/10.3390/nano10040760
Constantinoiu I, Viespe C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials. 2020; 10(4):760. https://doi.org/10.3390/nano10040760
Chicago/Turabian StyleConstantinoiu, Izabela, and Cristian Viespe. 2020. "Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor" Nanomaterials 10, no. 4: 760. https://doi.org/10.3390/nano10040760
APA StyleConstantinoiu, I., & Viespe, C. (2020). Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials, 10(4), 760. https://doi.org/10.3390/nano10040760






