Photocatalytic Performance and Degradation Pathway of Rhodamine B with TS-1/C3N4 Composite under Visible Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of TS-1/C3N4 Composites
2.3. Characterization of TS-1/C3N4 Composites
2.4. Photocatalytic Test
2.4.1. Photocatalytic Degradation of RhB
2.4.2. Photocatalytic Degradation of Dimethyl Sulfoxide (DMSO)
2.5. Photoelectrochemical Measurement
2.6. Trapping Experiments of Active Oxidized Species
3. Results and Discussion
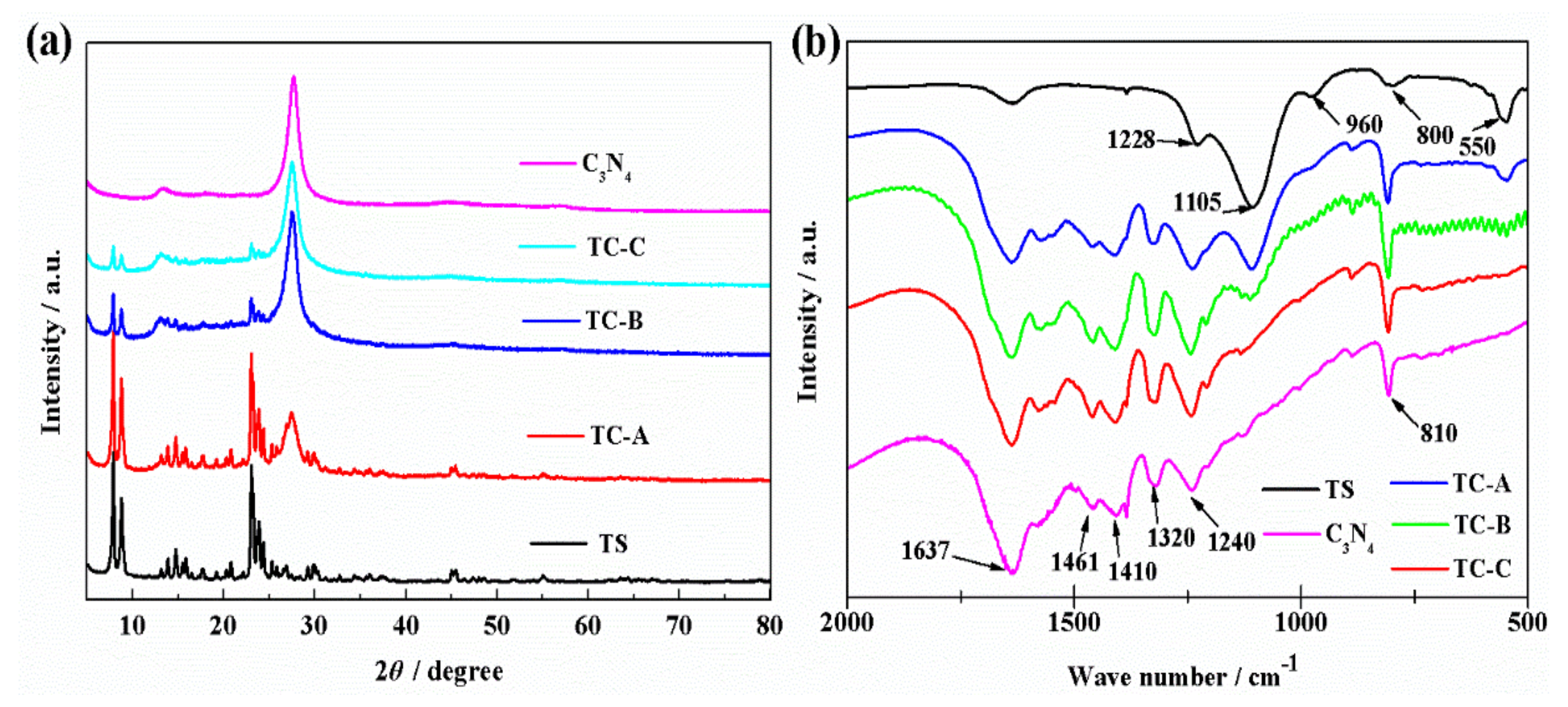
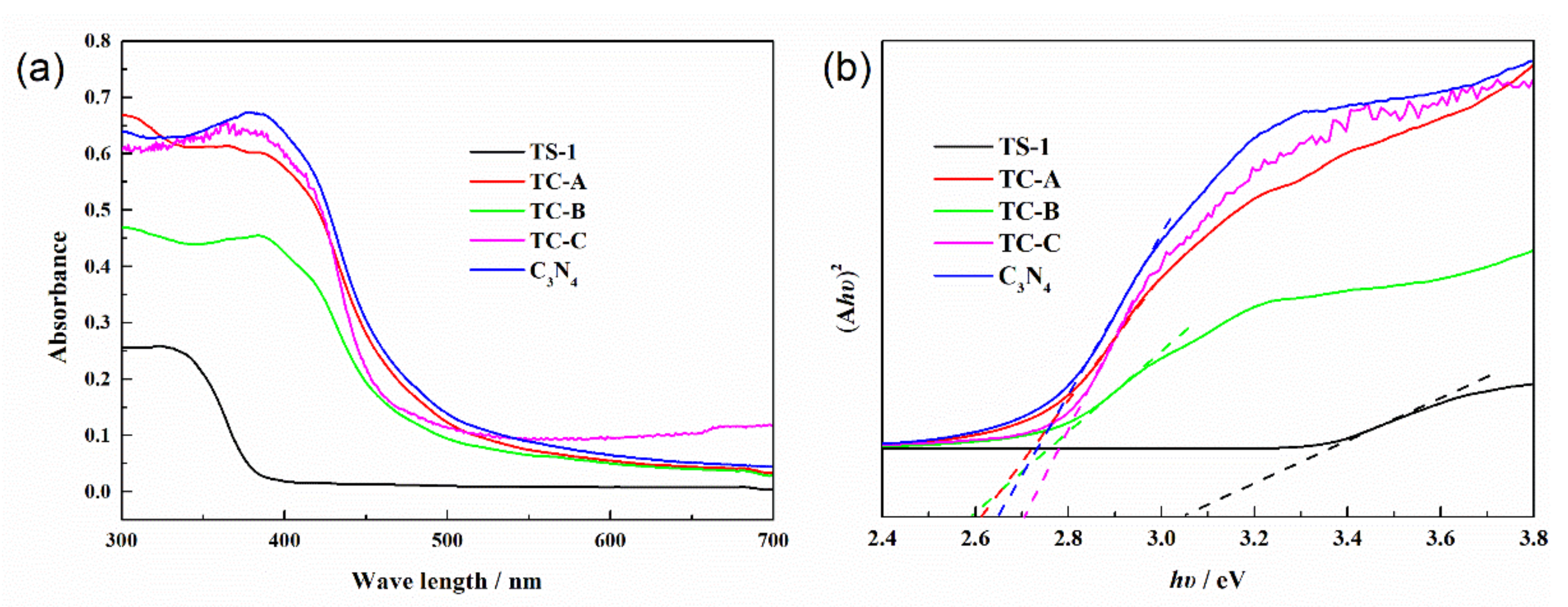
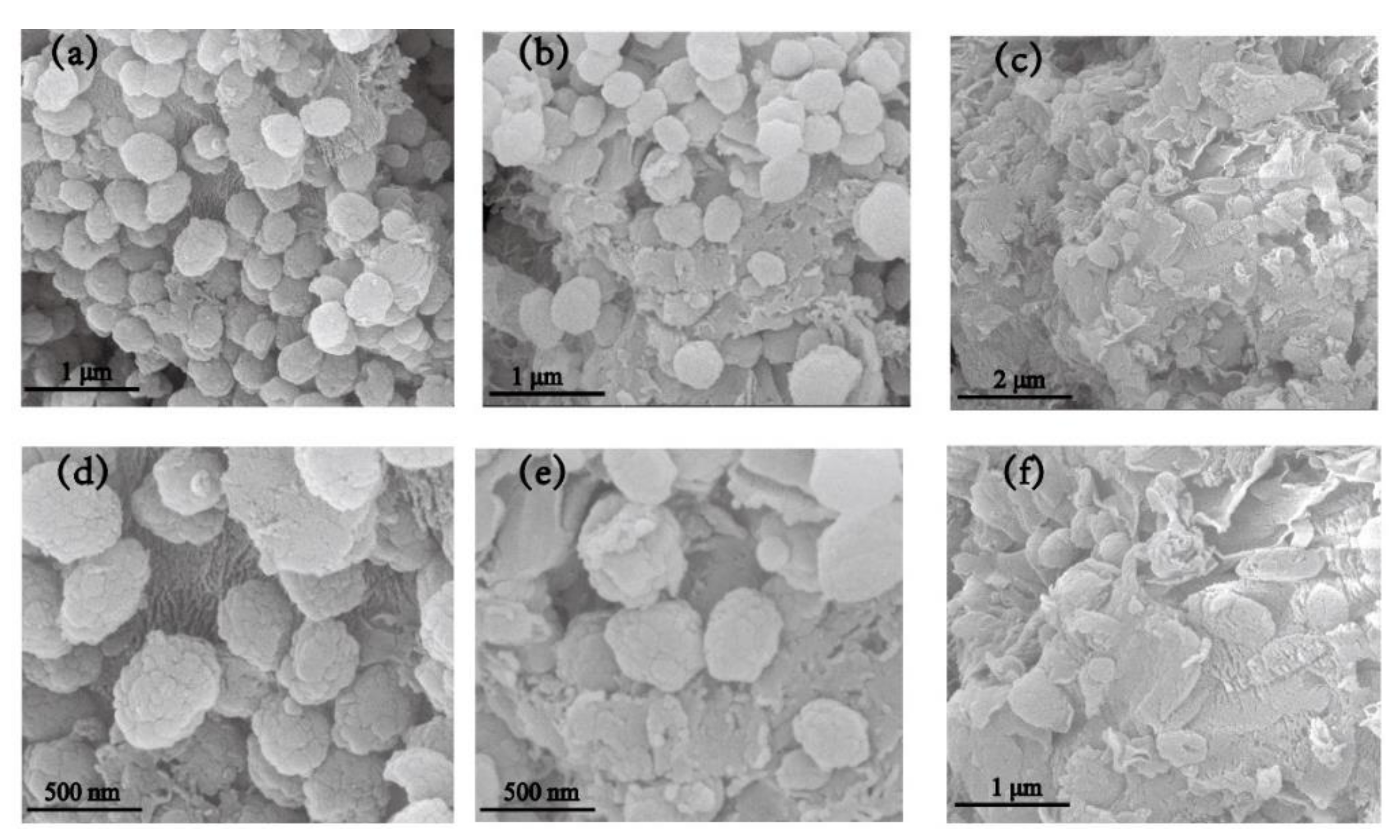
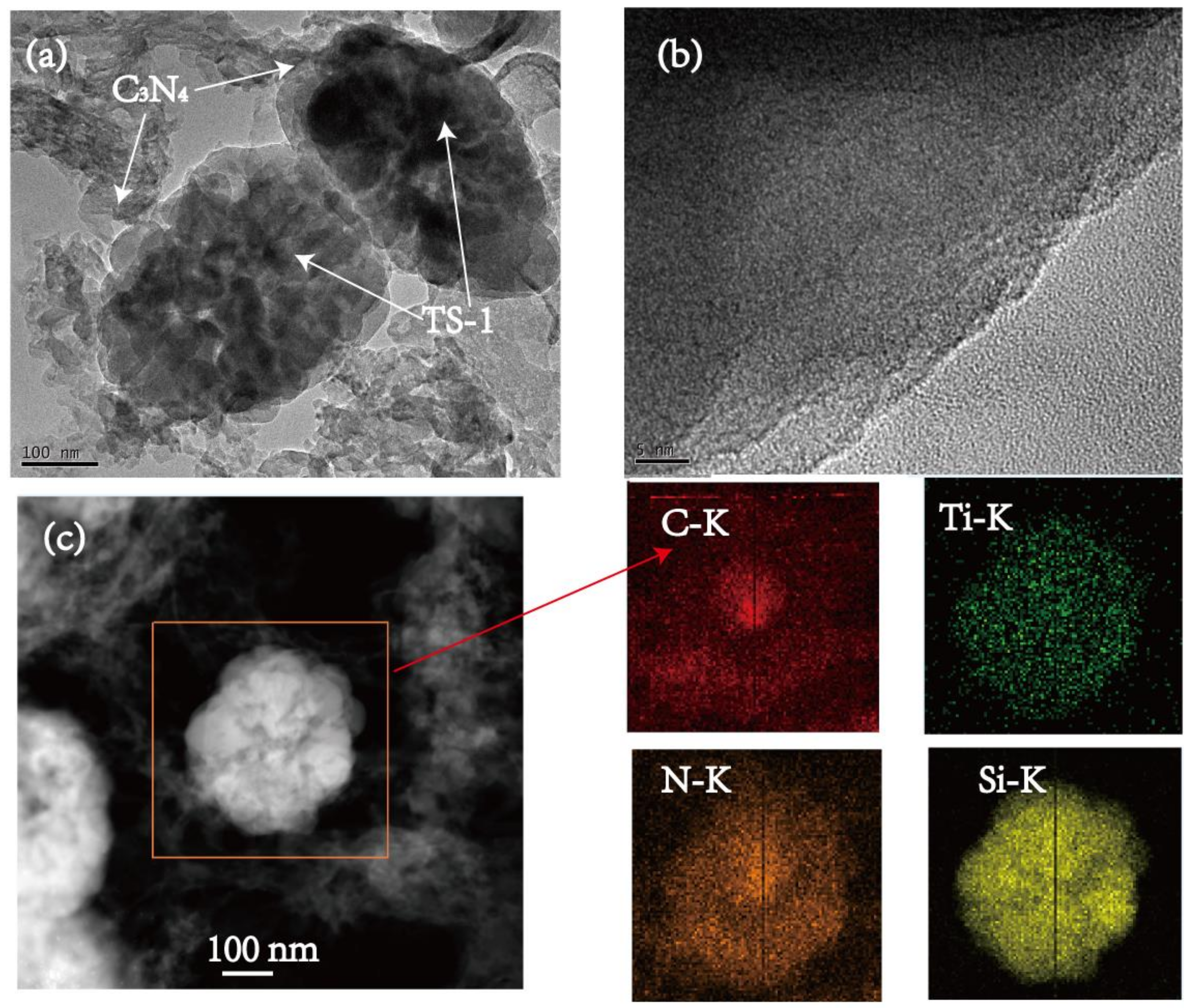
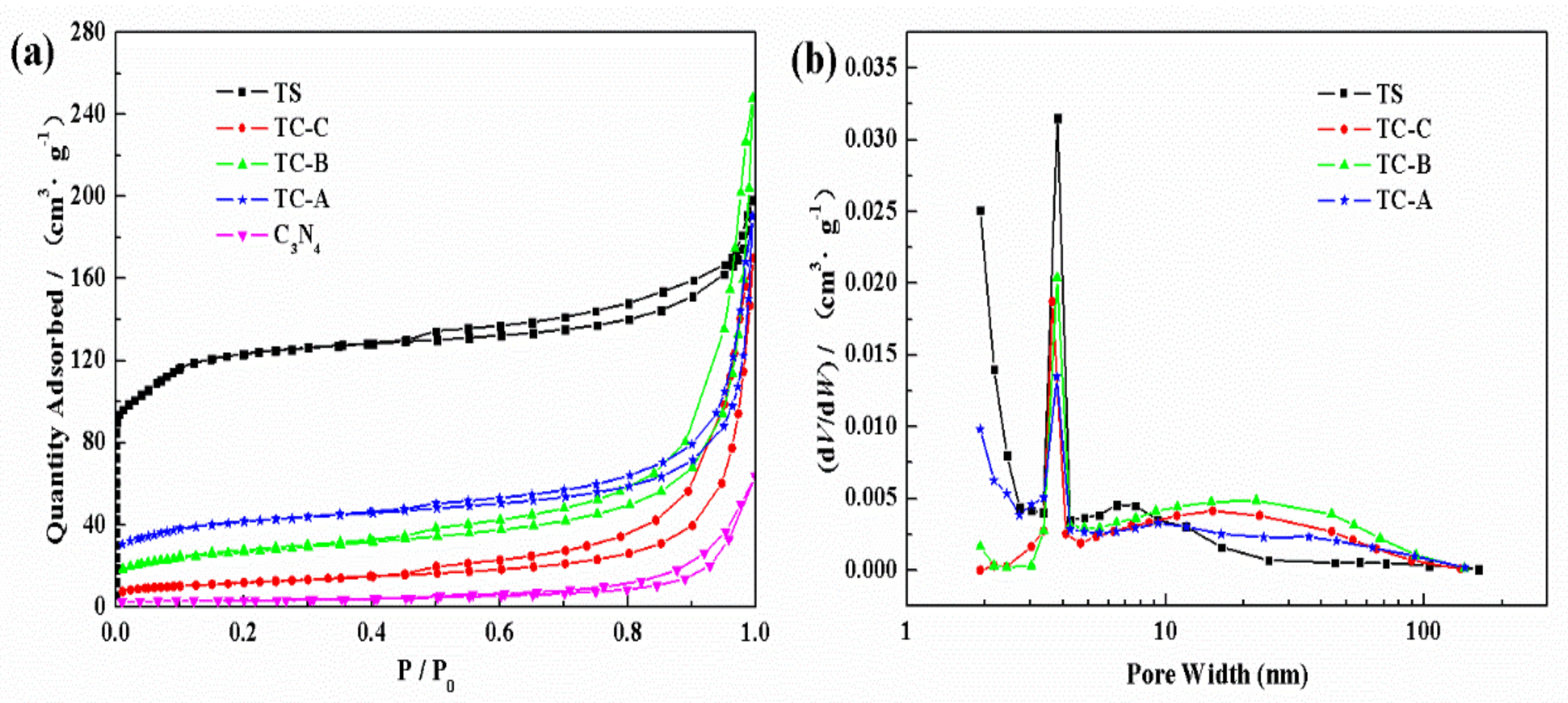
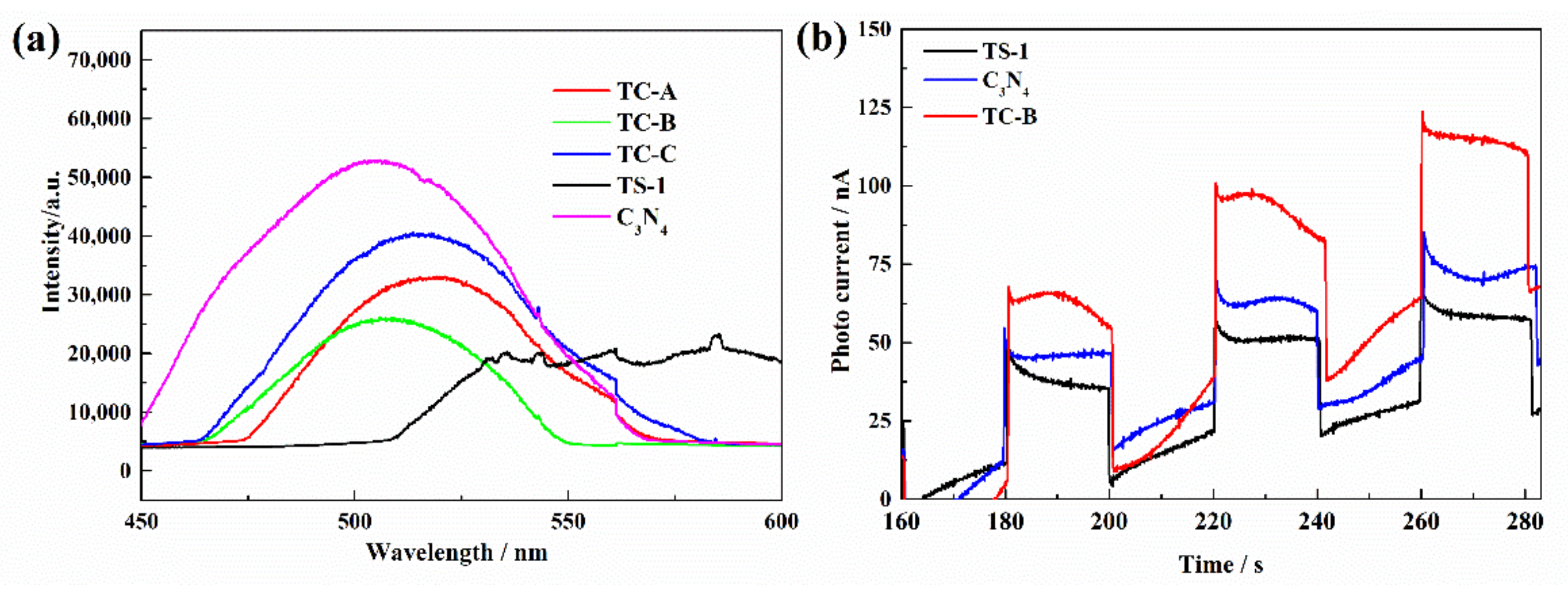
3.1. Characterization of TS-1/C3N4 Composites
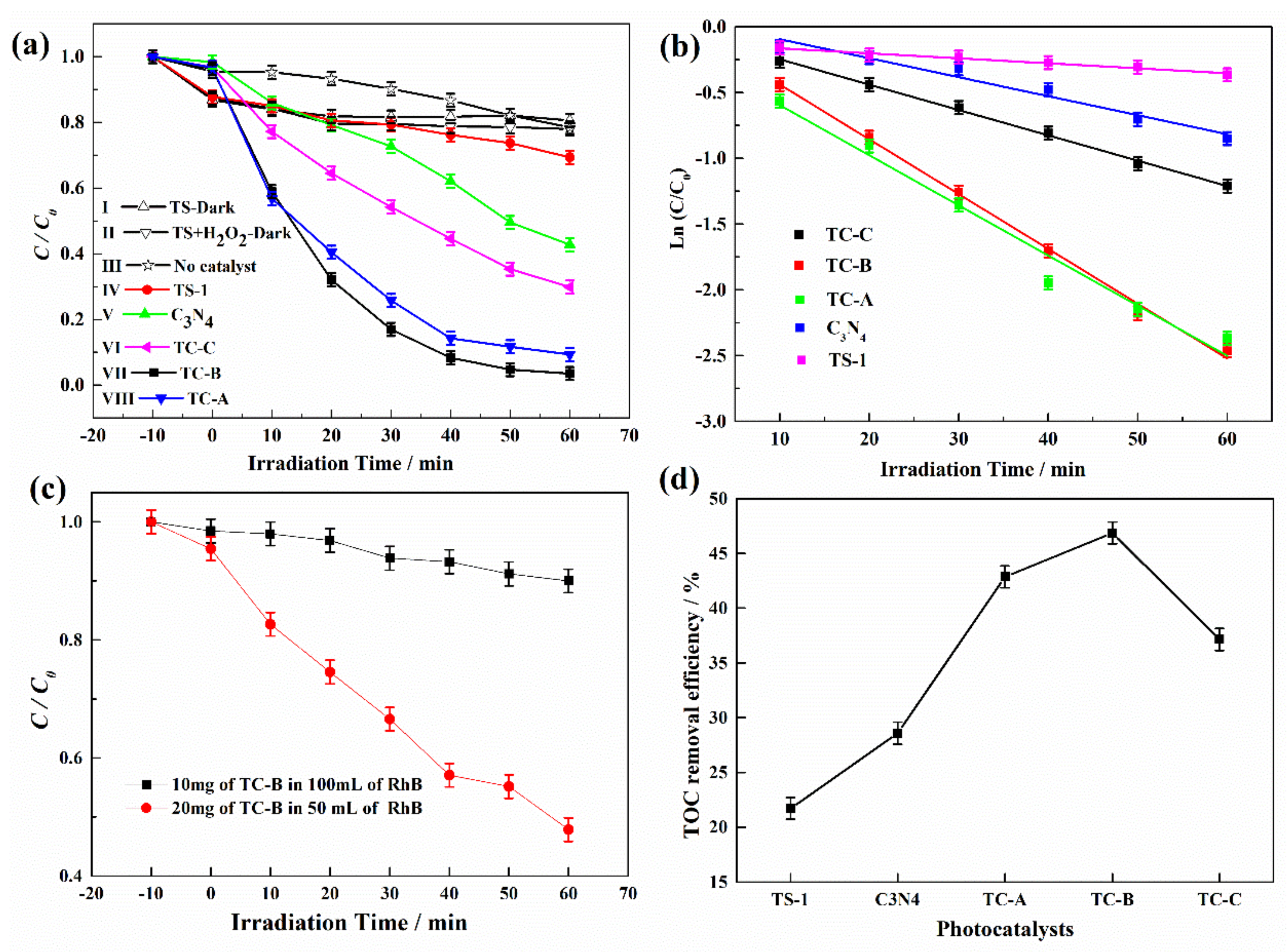
3.2. Photocatalytic RhB Degradation Performance of TS-1/C3N4 Composites
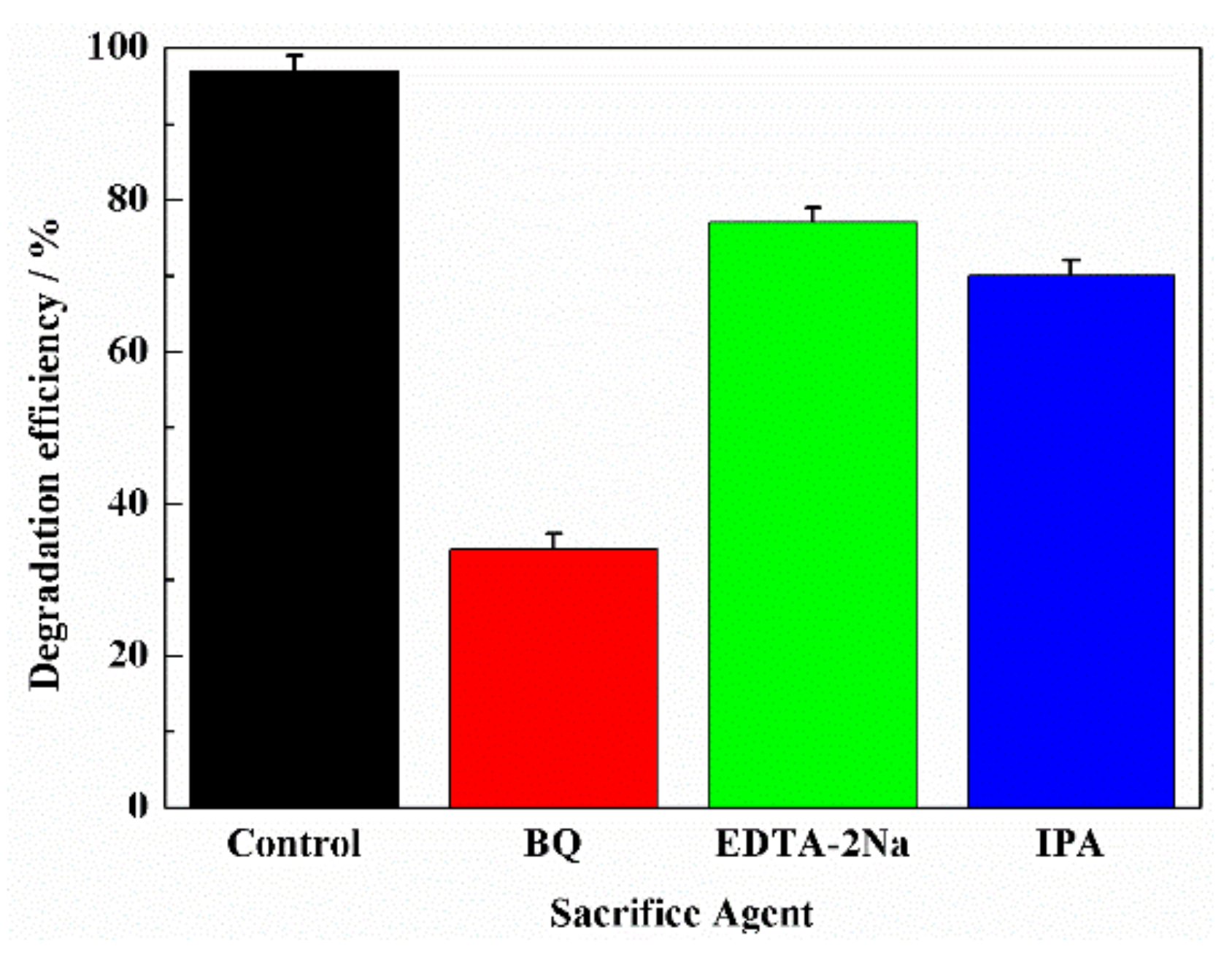
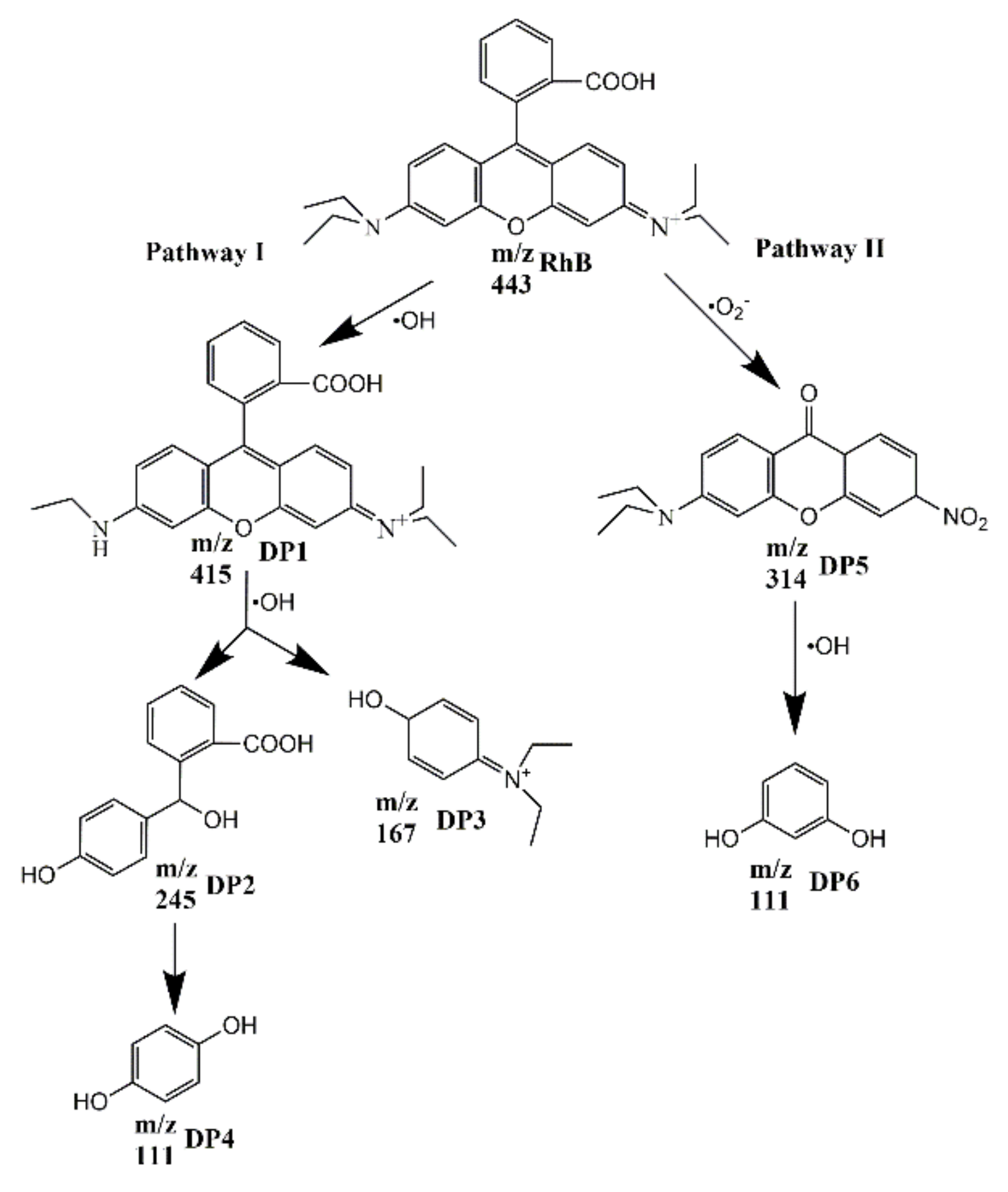
3.3. Photocatalytic Degradation Pathways of RhB
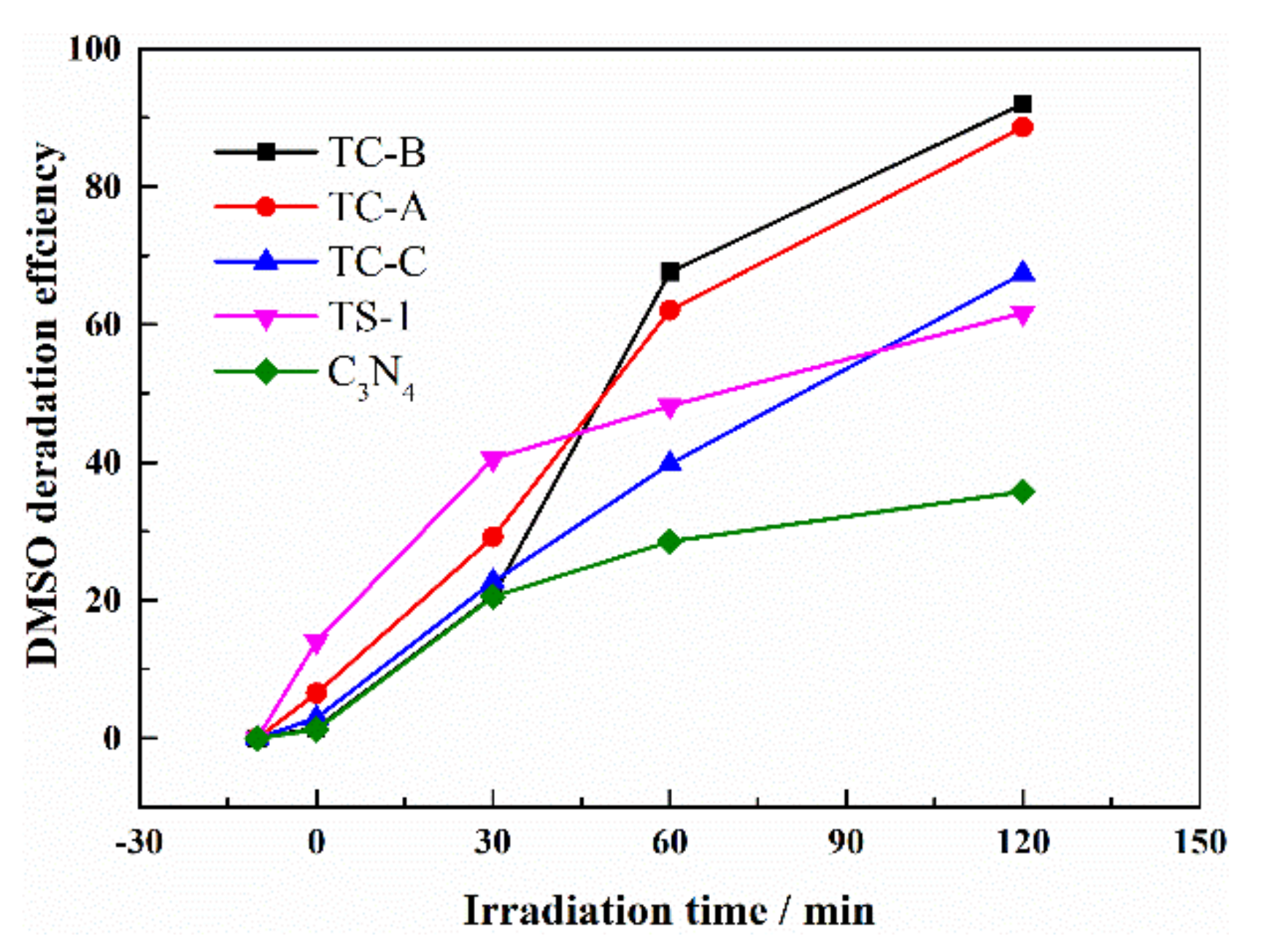
3.4. Extensive Applicability of TS-1/C3N4 Composites as Photocatalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Pan, N.; Wang, D.; Yang, J.; Guo, Z.; Yuan, W. A Si–O–Si bridge assembled from 3-mercaptopropyltrimethoxysilane and silicon carbide for effective charge transfer in photocatalysis. J. Mater. Sci. 2018, 53, 12432–12440. [Google Scholar] [CrossRef]
- Chauhan, M.; Soni, K.; Karthik, P.E.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Promising visible-light driven hydrogen production from water on a highly efficient CuCo2S4 nanosheet photocatalyst. J. Mater. Chem. A 2019, 7, 6985–6994. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng, G. Modulation of Bi2MoO6-based materials for photocatalytic water splitting and environmental application: A critical review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef]
- Fan, Z.; Meng, F.; Gong, J.; Li, H.; Ding, Z.; Ding, B. One-step hydrothermal synthesis of mesoporous Ce-doped anatase TiO2 nanoparticles with enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 11866–11872. [Google Scholar] [CrossRef]
- Xi, L.; Shang, J.; Wang, L.; Feng, H.; Hao, W.; Wang, T.; Du, Y.E. Enhanced photocatalytic activity of Bi24O31Br10: Constructing heterojunction with BiOI. J. Mater. Sci. Technol. 2017, 33, 281–284. [Google Scholar]
- Wang, Y.; Zhang, L.; Zhang, X.; Zhang, Z.; Tong, Y.; Li, F.; Wu, C.S.; Wang, X. Openmouthed β-SiC hollow-sphere with highly photocatalytic activity for reduction of CO2 with H2O. Appl. Catal. B Environ. 2017, 206, 158–167. [Google Scholar] [CrossRef]
- Huang, Y.; Fu, M.; He, T. Synthesis of g-C3N4/BiVO4 nanocomposite photocatalyst and its application in photocatalytic reduction of CO2. Acta Phys. Chim. Sin. 2015, 31, 991–993. [Google Scholar]
- Mills, A.; O’Rourke, C. Photocatalytic organic synthesis in an NMR tube: C—C coupling of phenoxyacetic acid and acrylamide. Catal. Today 2014, 230, 256–264. [Google Scholar] [CrossRef]
- Melcher, J.; Feroz, S.; Bahnemann, D. Comparing photocatalytic activities of commercially available iron-doped and iron-undoped aeroxide TiO2 P25 powders. J. Mater. Sci. 2017, 52, 6341–6348. [Google Scholar] [CrossRef]
- Mukherjee, P.; Bhaumik, A.; Kumar, R. Eco-friendly, Selective Hydroxylation of C-7 aromatic compounds catalyzed by TS-1/H2O2 system under solvent-free solid-liquid-liquid-type triphase conditions. Ind. Eng. Chem. Res. 2015, 46, 8657–8664. [Google Scholar] [CrossRef]
- Wang, W.; Yu, F.; Yun, G.; Guo, Y.; Gong, X.Q.; Lu, G. Preparation of lamellar-stacked TS-1 and its catalytic performance for the ammoximation of butanone with H2O2. J. Mater. Sci. 2018, 53, 4034–4045. [Google Scholar] [CrossRef]
- Xue, Y.; Zuo, G.; Wen, Y.; Wei, H.; Liu, M.; Wang, X.; Li, B. Seed-assisted synthesis of TS-1 crystals containing Al with high catalytic performances in cyclohexanone ammoximation. RSC Adv. 2019, 9, 2386–2394. [Google Scholar] [CrossRef]
- Song, Z.; Xiang, F.; Nan, S.; Dong, L.; Li, Y.; Liu, Y.; Chen, X.; Zhou, X.; Chen, D.; Yang, C. Propene epoxidation with H2 and O2 on Au/TS-1 catalyst: Cost-effective synthesis of small-sized mesoporous TS-1 and its unique performance. Catal. Today 2018, in press. [Google Scholar] [CrossRef]
- Heinrich, S.; Plettig, M.; Klemm, E. Role of the Ti(IV)-superoxide species in the selective oxidation of alkanes with hydrogen peroxide in the gas phase on titanium silicalite-1: An in situ EPR investigation. Catal. Lett. 2011, 141, 251–258. [Google Scholar] [CrossRef]
- Hsu, H.; Selvin, R.; Roselin, L.; Kumari, P.; Cao, J.-W. Photocatalytic efficiency of nanocrystalline zeolite TS-1 for the degradation of methyl orange. React. Kinet. Catal. Lett. 2009, 98, 265–272. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H.; Yi, C. Heterogeneous photocatalytic degradation of organic contaminant in water over high-activity Fe-TS-1 under the radiation of solar light. Optik 2018, 158, 1460–1469. [Google Scholar] [CrossRef]
- Li, N.; Yang, B.; Liu, M.; Chen, Y.; Zhou, J. Synergetic photo-epoxidation of propylene with molecular oxygen over bimetallic Au–Ag/TS-1 photocatalysts. Chin. J. Catal. 2017, 38, 831–843. [Google Scholar] [CrossRef]
- On, D.; Lutic, D.; Kaliaguine, S. An example of mesostructured zeolitic material: UL-TS-1. Microporous Mesoporous Mater. 2001, 44, 435–444. [Google Scholar]
- Wu, Q.; Wang, H.; Yi, C. Preparation of photo-Fenton heterogeneous catalyst (Fe-TS-1 zeolite) and its application in typical azo dye decoloration. J. Photochem. Photobiol. A 2018, 356, 138–149. [Google Scholar] [CrossRef]
- Lv, Q.; Li, G.; Lu, H.; Cai, W.; Huang, H.; Cheng, C. Preparation of magnetic zeolite γ-Fe2O3/TS-1 with core/shell structure and application in photocatalytic degradation. Microporous Mesoporous Mater. 2015, 203, 202–207. [Google Scholar] [CrossRef]
- Ye, X.; Cui, Y.; Qiu, X.; Wang, X. Selective oxidation of benzene to phenol by Fe-CN/TS-1 catalysts under visible light irradiation. Appl. Catal. B 2014, 152, 383–389. [Google Scholar] [CrossRef]
- Adepu, A.; Katta, V.; Narayanan, V. Synthesis, characterization, and photocatalytic degradation of Rhodamine B dye under sunlight irradiation of porous titanosilicate (TS)/bismuth vanadate (BiVO4) nanocomposite hybrid catalyst. New J. Chem. 2017, 41, 2498–2504. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Z.; Peng, Y.; Yuan, W. A simple route to significant enhancement of photocatalytic water oxidation on BiVO4 by heterojunction with SiC. Chem. Eng. J. 2015, 281, 102–108. [Google Scholar] [CrossRef]
- Du, C.; Cui, N.; Li, L.; Hua, Z.; Shi, J. A large-surface-area TS-1 nanocatalyst: A combination of nanoscale particle sizes and hierarchical micro/mesoporous structures. RSC Adv. 2019, 9, 9694–9699. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Liu, J.; Fan, X.; Wang, B.; Wang, M.; Ren, W.; Wang, J.; Li, M.; Shi, J. Brand new P-doped g-C3N4: Enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light. J. Mater. Chem. A 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Ding, K.; Wen, L.; Huang, M.; Zhang, Y.; Lu, Y.; Chen, Z. How does the B,F-monodoping and B/F-codoping affect the photocatalytic water-splitting performance of g-C3N4. Phys. Chem. Chem. Phys. 2016, 18, 19217. [Google Scholar] [CrossRef]
- Kong, H.; Won, D.; Kim, J.; Woo, S. Sulfur-doped g-C3N4/BiVO4 composite photocatalyst for water oxidation under visible light. Chem. Mater. 2016, 28, 1318–1324. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K.; Xiao, W.; Cheng, B. Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4-Pt nanocomposite photocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 11492–11501. [Google Scholar] [CrossRef]
- Xue, J.; Ma, S.; Zhou, Y.; Zhang, Z.; He, M. Facile photochemical synthesis of Au/Pt/g-C3N4 with plasmon-enhanced photocatalytic activity for antibiotic degradation. ACS Appl. Mater. Interfaces 2015, 7, 9630–9637. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, C.; Cheng, Z.; Wang, H.; Dou, Y.; Shi, W.; Shao, M.; Hui, H.; Yang, L.; Kang, Z. High-performance NiO/g-C3N4 composites for visible-light-driven photocatalytic overall water splitting. Inorg. Chem. Front. 2018, 5, 1646–1652. [Google Scholar] [CrossRef]
- Xu, H.; Gan, Z.; Zhou, W.; Ding, Z.; Zhang, X. A metal-free 3C-SiC/g-C3N4 composite with enhanced visible light photocatalytic activity. RSC Adv. 2017, 7, 40028–40033. [Google Scholar] [CrossRef]
- Fang, Z.; Rong, H.; Zhou, L.; Pang, Q. In-situ synthesis of CdS/g-C3N4 hybrid nanocomposites with enhanced visible photocatalytic activity. J. Mater. Sci. 2015, 50, 3057–3064. [Google Scholar] [CrossRef]
- Ye, W.; Shao, Y.; Hu, X.; Liu, C.; Sun, C. Highly enhanced photoreductive degradation of polybromodiphenyl ethers with g-C3N4/TiO2 under visible light irradiation. Nanomaterials 2017, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, W.; Dong, C.; Xing, M.; Tan, X.; Zhang, J. Eco-friendly one-pot synthesis of well-adorned mesoporous g-C3N4 with efficiently enhanced visible light photocatalytic activity. Catal. Sci. Technol. 2017, 7, 1726–1734. [Google Scholar] [CrossRef]
- Ren, B.; Chen, L.I.; Yuan, X.; Wang, F. Determination and correlation of melamine solubility. J. Chem. Ind. Eng. 2003, 54, 1001–1003. [Google Scholar]
- Li, J.; Ran, M.; Chen, P.; Cui, W.; Li, J.; Sun, Y.; Jiang, G.; Zhou, Y.; Dong, F. Controlling the secondary pollutant on B-doped g-C3N4 during photocatalytic NO removal: A combined DRIFTS and DFT investigation. Catal. Sci. Technol. 2019, 9, 4531–4537. [Google Scholar] [CrossRef]
- Lei, Z.; Quan, D.; Fu, W.; Tang, T.; Dong, P.; He, M.; Chen, Q. CoMo catalyst on zeolite TS-1 nanorod assemblies with high activity in the hydrodesulfurization of 4,6-dimethyldibenzothiophene. J. Catal. 2018, 359, 130–142. [Google Scholar]
- Zhuang, C.; Tang, L.; Yu, Z.; Peng, T.; Zhang, Y.; Li, L.; Zhou, Y.; Zou, Z. Hollow BiVO4/Bi2S3 cruciate heterostructures with enhanced visible-light photoactivity. Catal. Sci. Technol. 2019, 9, 182–187. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Yao, W.; Chen, K.; Chen, D. One step synthesis of P-doped g-C3N4 with the enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2018, 430, 309–315. [Google Scholar] [CrossRef]
- Xin, H.; Zhao, J.; Xu, S.; Li, J.; Zhang, W.; Guo, X.; Hensen, E.; Yang, Q.; Li, C. Enhanced catalytic oxidation by hierarchically structured TS-1 zeolite. J. Phys. Chem. C 2010, 114, 6553–6559. [Google Scholar] [CrossRef]
- Deng, X.; Wang, Y.; Shen, L.; Wu, H.; Liu, Y.; He, M. Low-cost synthesis of titanium silicalite-1 (TS-1) with highly catalytic oxidation performance through a controlled hydrolysis process. Ind. Eng. Chem. Res. 2013, 52, 1190–1196. [Google Scholar] [CrossRef]
- John, F.; Jacob, J.; Thomas, J.K.; Solomon, S. Electrical and optical properties of nano-crystalline RE-Ti-Nb-O6 (RE = Dy, Er, Gd, Yb) synthesized through a modified combustion method. J. Asian Ceram. Soc. 2018, 5, 151–159. [Google Scholar] [CrossRef][Green Version]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-based heterostructured photocatalysts. Adv. Energy Mater. 2017, 8, 1701503. [Google Scholar] [CrossRef]
- Erdogan, B.; Arbag, H.; Yasyerli, N. SBA-15 supported mesoporous Ni and Co catalysts with high coke resistance for dry reforming of methane. Int. J. Hydrog. Energy 2018, 43, 1396–1405. [Google Scholar] [CrossRef]
- Vitova, T.; Hormes, J.; Falk, M.; Buse, K. Site-selective investigation of site symmetry and site occupation of iron in Fe-doped lithium niobate crystals. J. Appl. Phys. 2009, 105, 382–387. [Google Scholar] [CrossRef]
- Yang, J.; Peng, Y.; Yang, B.; Li, P. Enhanced photocatalytic degradation of Rhodamine B over metal-free SiC/C3N4 heterostructure under visible light irradiation. Mater. Res. Express 2018, 5, 085511. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, D.; Chen, J.; Guo, L.; Mao, S.S. Enhanced photocatalytic hydrogen evolution over graphitic carbon nitride modified with Ti-activated mesoporous silica. Appl. Catal. A Gen. 2016, 521, 111–117. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H.; Jia, Y.; Zhou, G. Kinetics of the acid orange 7 degradation in the photocatalytic system of UV/H2O2/TS-1. J. Water Process Eng. 2017, 19, 106–111. [Google Scholar] [CrossRef]
- Safari, M.; Nikazar, M.; Dadvar, M. Photocatalytic degradation of methyl tert-butyl ether (MTBE) by Fe-TiO2 nanoparticles. J. Ind. Eng. Chem. 2013, 19, 1697–1702. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zeng, X.; Huang, B.; Gao, S.; Lu, J. Synergetic effect of Ti3+ and oxygen doping on enhancing photoelectrochemical and photocatalytic properties of TiO2/g-C3N4 heterojunctions. ACS Appl. Mater. Interfaces 2017, 9, 11577–11586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shao, C.; Li, X.; Wang, X.; Guo, X.; Liu, Y. Three dimensional hierarchical heterostructures of g-C3N4 nanosheets/TiO2 nanofibers: Controllable growth via gas-solid reaction and enhanced photocatalytic activity under visible light. J. Hazard. Mater. 2018, 344, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Cai, W.; Zhou, J.; Li, Z. Enhanced photocatalytic performance and degradation pathway of Rhodamine B over hierarchical double-shelled zinc nickel oxide hollow sphere heterojunction. Appl. Surf. Sci. 2018, 430, 549–560. [Google Scholar] [CrossRef]
- Khan, A.; Mehmood, A.; Sayed, M.; Nazar, M.; Ismail, B.; Khan, R.; Ullah, H.; Abdur Rehman, H.; Khan, A.; Khan, A. Influence of acids, bases and surfactants on the photocatalytic degradation of a model dye rhodamine B. J. Mol. Liq. 2017, 236, 395–403. [Google Scholar] [CrossRef]

| Composites | Mass Percentage (%) | m(TS-1):m(C3N4) | |
|---|---|---|---|
| C | N | ||
| TC-A | 23.90 | 39.59 | 1:2 |
| TC-B | 30.10 | 49.79 | 1:4 |
| TC-C | 32.83 | 54.31 | 1:7 |
| Irradiation Time (min) | m/z | |||||
|---|---|---|---|---|---|---|
| 443 (RhB) | 415 (DP1) | 314 (DP5) | 245 (DP2) | 167 (DP3) | 111 (DP4/DP6) | |
| 0 | ◯ * | ◯ | / | / | / | / |
| 10 | ◯ | ◯ | / | / | / | |
| 20 | ◯ | ◯ | ◯ | ◯ | ||
| 30 | ◯ | ◯ * | ◯ | / | ◯ | ◯ |
| 40 | ◯ | ◯ | ◯ | / | ◯ | ◯ |
| 50 | / | / | ◯ | / | ◯ * | ◯ |
| 60 | / | / | ◯ * | / | ◯ | ◯ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhu, H.; Peng, Y.; Li, P.; Chen, S.; Yang, B.; Zhang, J. Photocatalytic Performance and Degradation Pathway of Rhodamine B with TS-1/C3N4 Composite under Visible Light. Nanomaterials 2020, 10, 756. https://doi.org/10.3390/nano10040756
Yang J, Zhu H, Peng Y, Li P, Chen S, Yang B, Zhang J. Photocatalytic Performance and Degradation Pathway of Rhodamine B with TS-1/C3N4 Composite under Visible Light. Nanomaterials. 2020; 10(4):756. https://doi.org/10.3390/nano10040756
Chicago/Turabian StyleYang, Jingjing, Hongqing Zhu, Yuan Peng, Pengxi Li, Shuyan Chen, Bing Yang, and Jinzhong Zhang. 2020. "Photocatalytic Performance and Degradation Pathway of Rhodamine B with TS-1/C3N4 Composite under Visible Light" Nanomaterials 10, no. 4: 756. https://doi.org/10.3390/nano10040756
APA StyleYang, J., Zhu, H., Peng, Y., Li, P., Chen, S., Yang, B., & Zhang, J. (2020). Photocatalytic Performance and Degradation Pathway of Rhodamine B with TS-1/C3N4 Composite under Visible Light. Nanomaterials, 10(4), 756. https://doi.org/10.3390/nano10040756





