Preparation and Evaluation of Nanofibrous Hydroxypropyl Cellulose and β-Cyclodextrin Polyurethane Composite Mats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PUUS
2.3. Preparation of Spinning Solutions
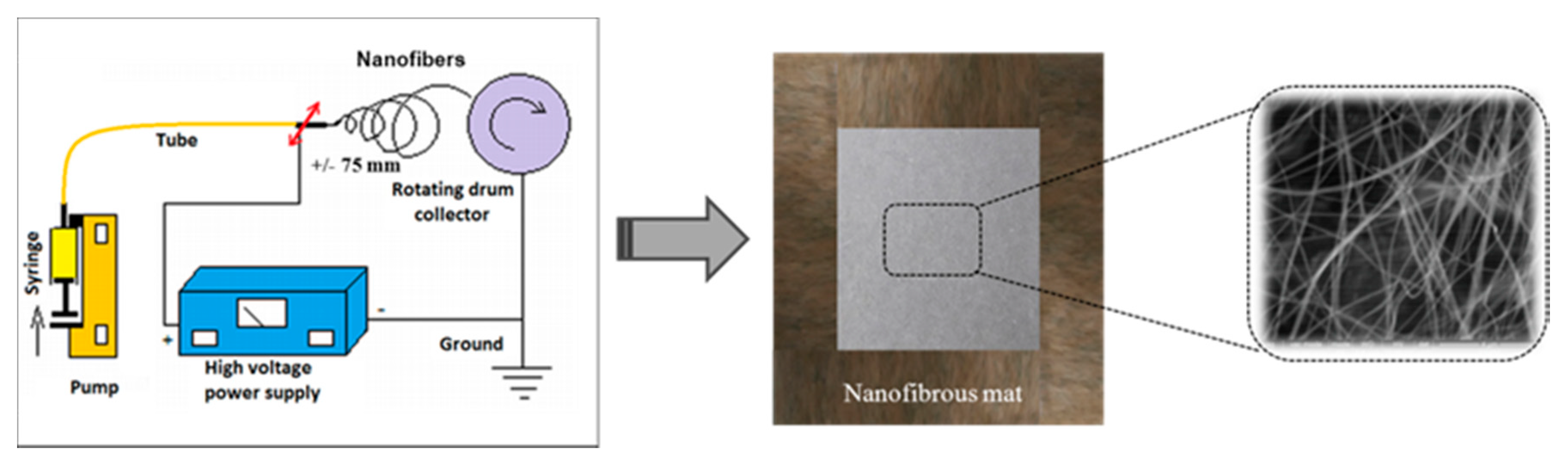
2.4. Electrospinning Process
2.5. Methods
2.5.1. Fourier Transform Infrared-Attenuated Total Reflectance Spectroscopy (FTIR-ATR)
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. Atomic Force Microscopy (AFM)
2.5.4. Stress–Strain Measurements
2.5.5. Static Contact Angles Measurements
2.5.6. Dynamic Vapors Sorption (DVS)
2.5.7. Differential Scanning Calorimetry Analysis (DSC)
2.5.8. Biological Study
3. Results
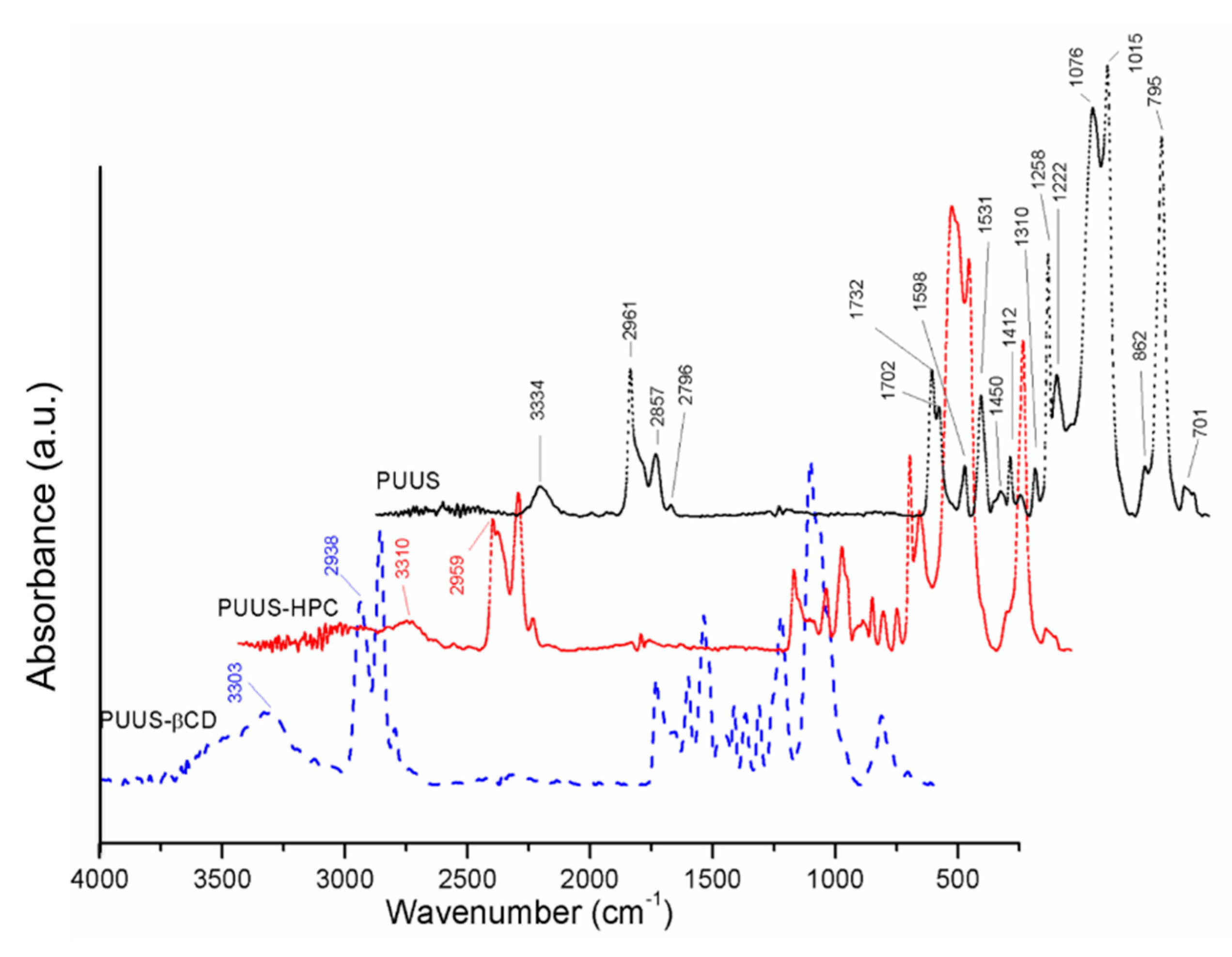
3.1. FTIR-ATR Characterization
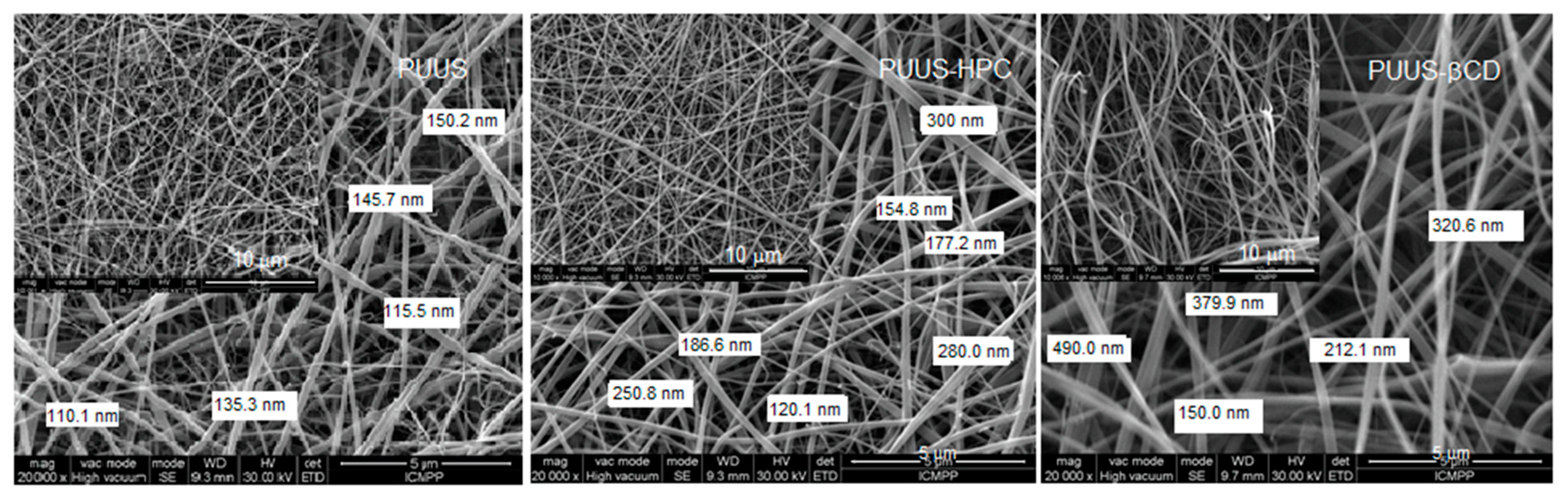
3.2. Scanning Electron Microscopy Analysis (SEM)
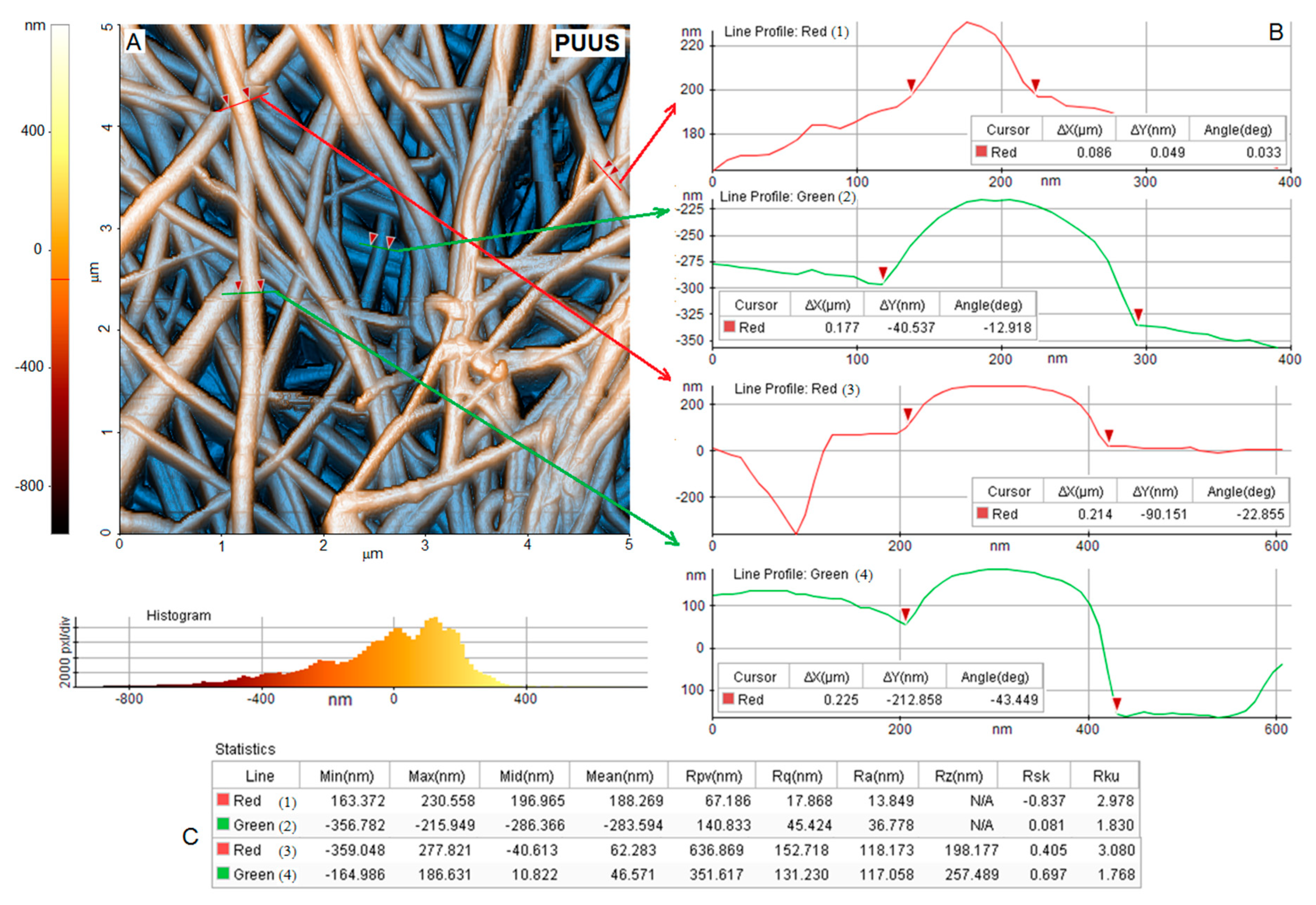
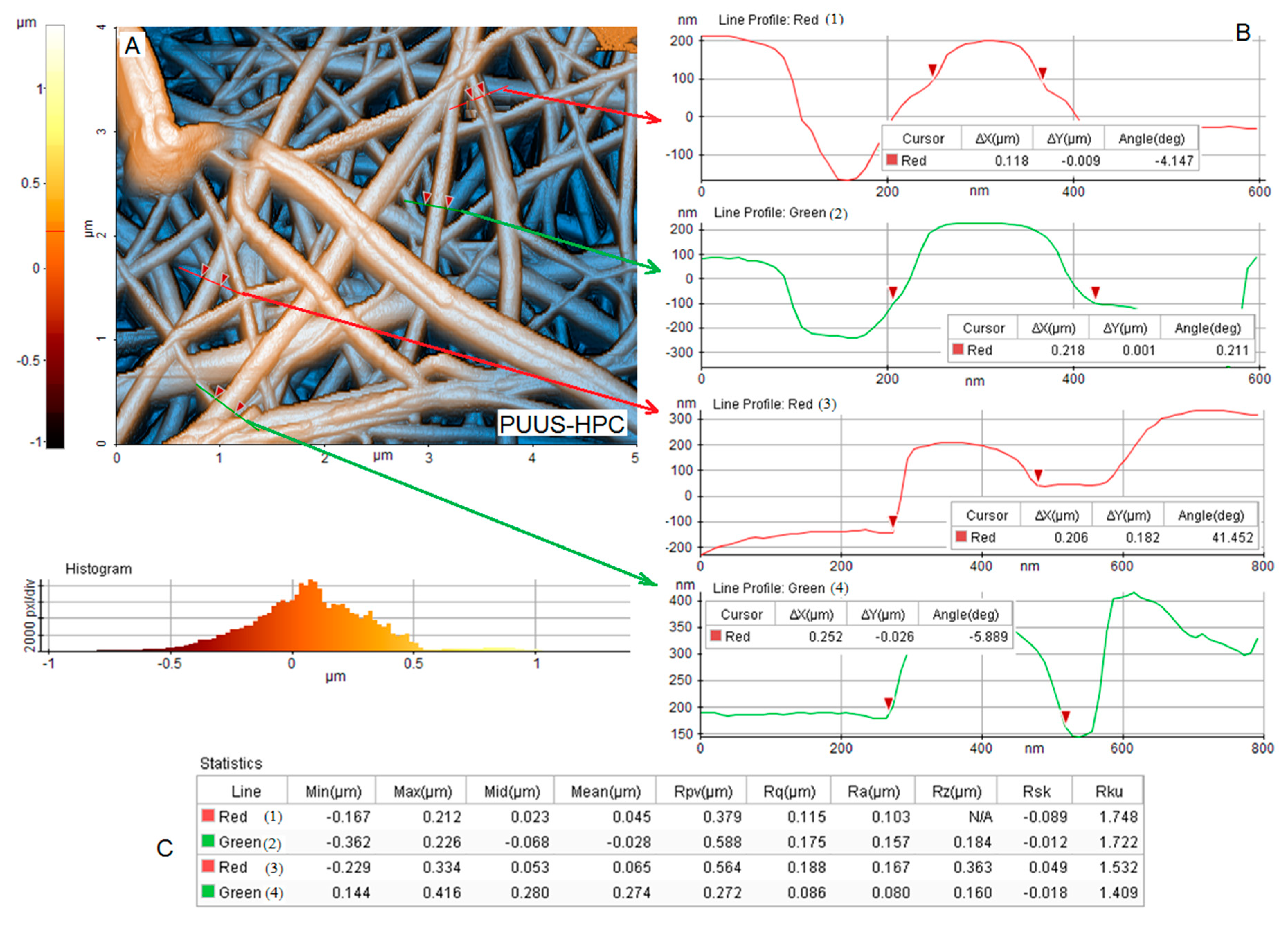
3.3. Atomic Force Microscopy Analysis (AFM)
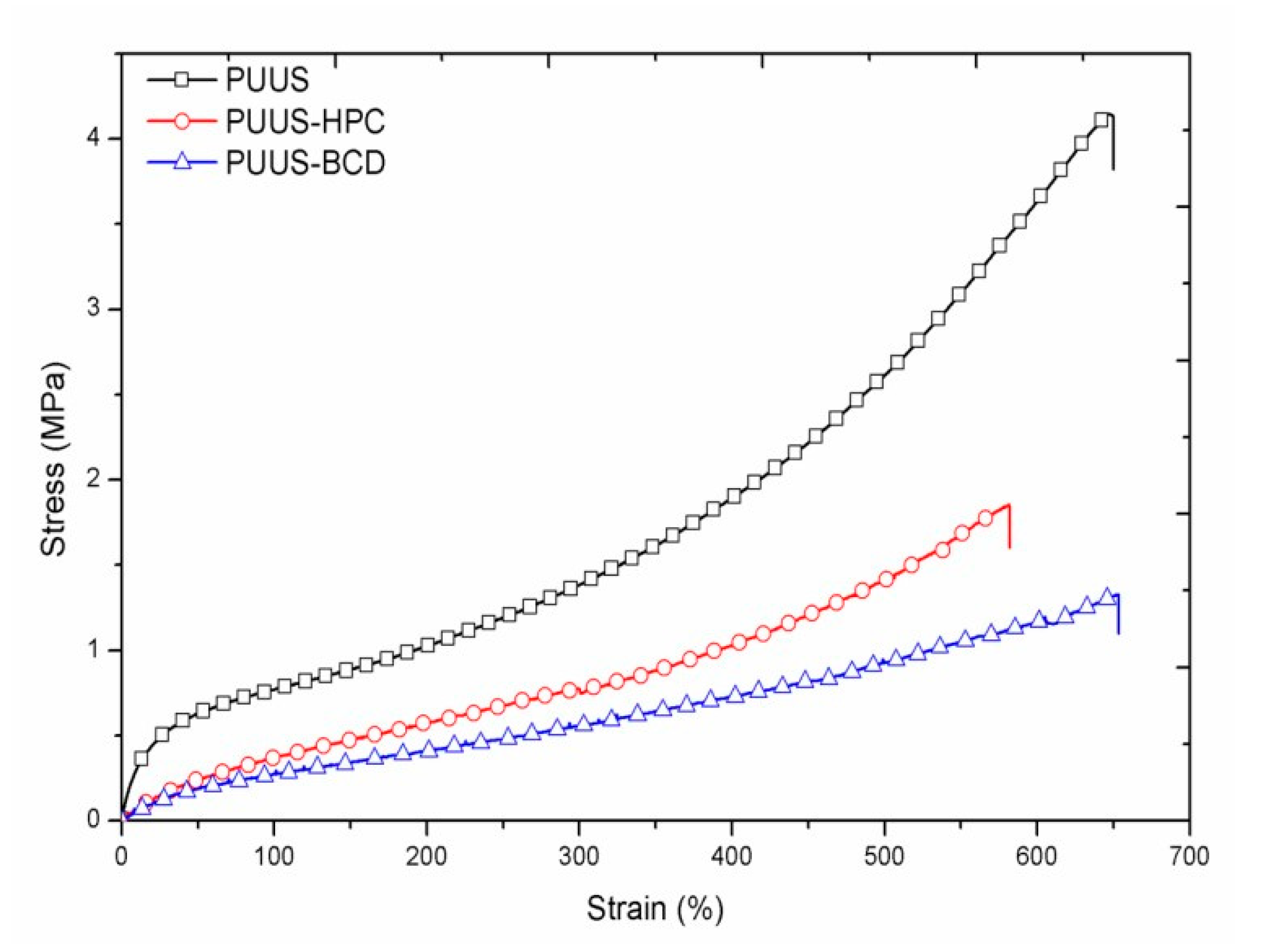
3.4. Mechanical Properties
3.5. Static Contact Angle Determinations
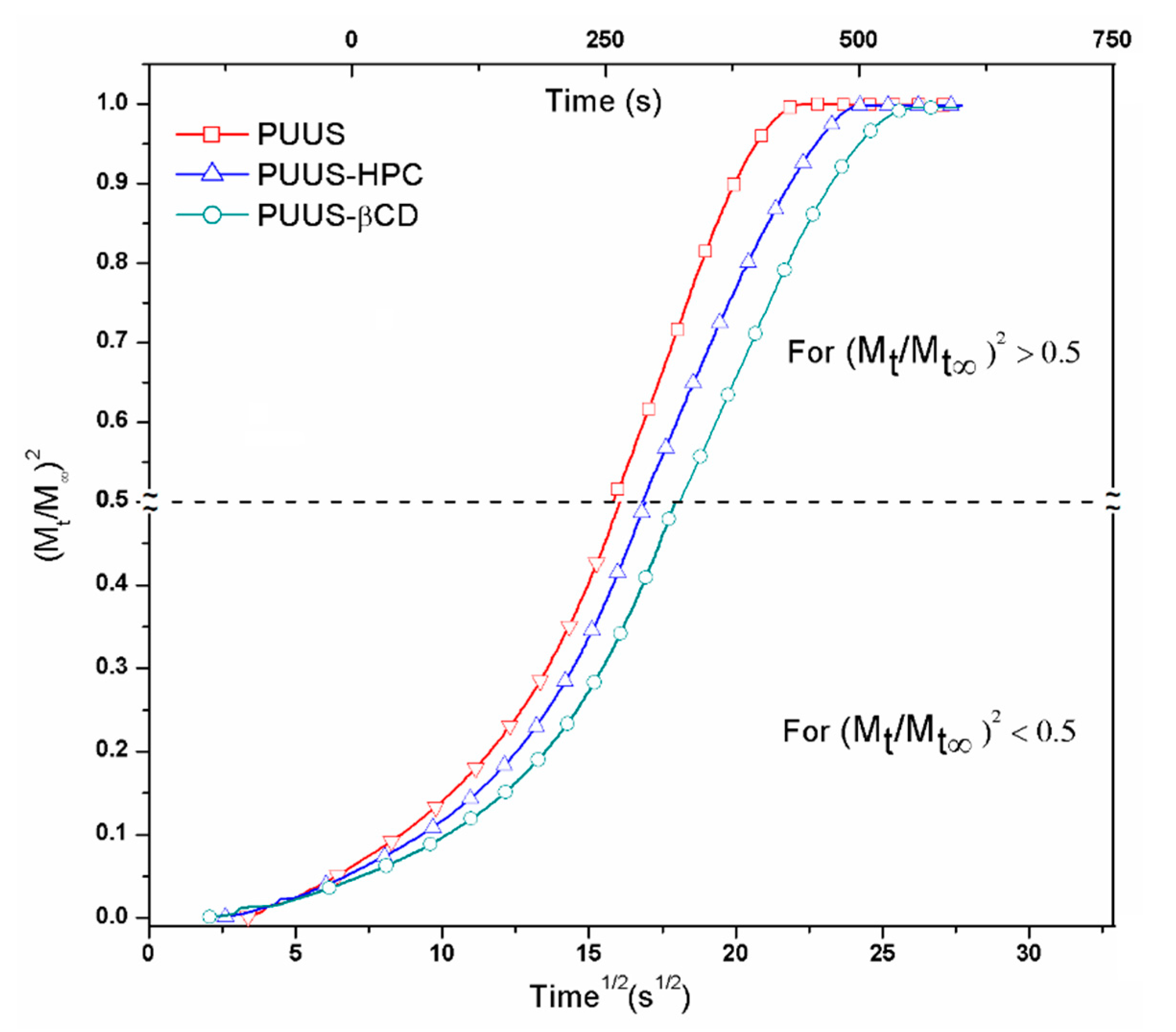
3.6. Dynamic Vapour Sorption Analysis
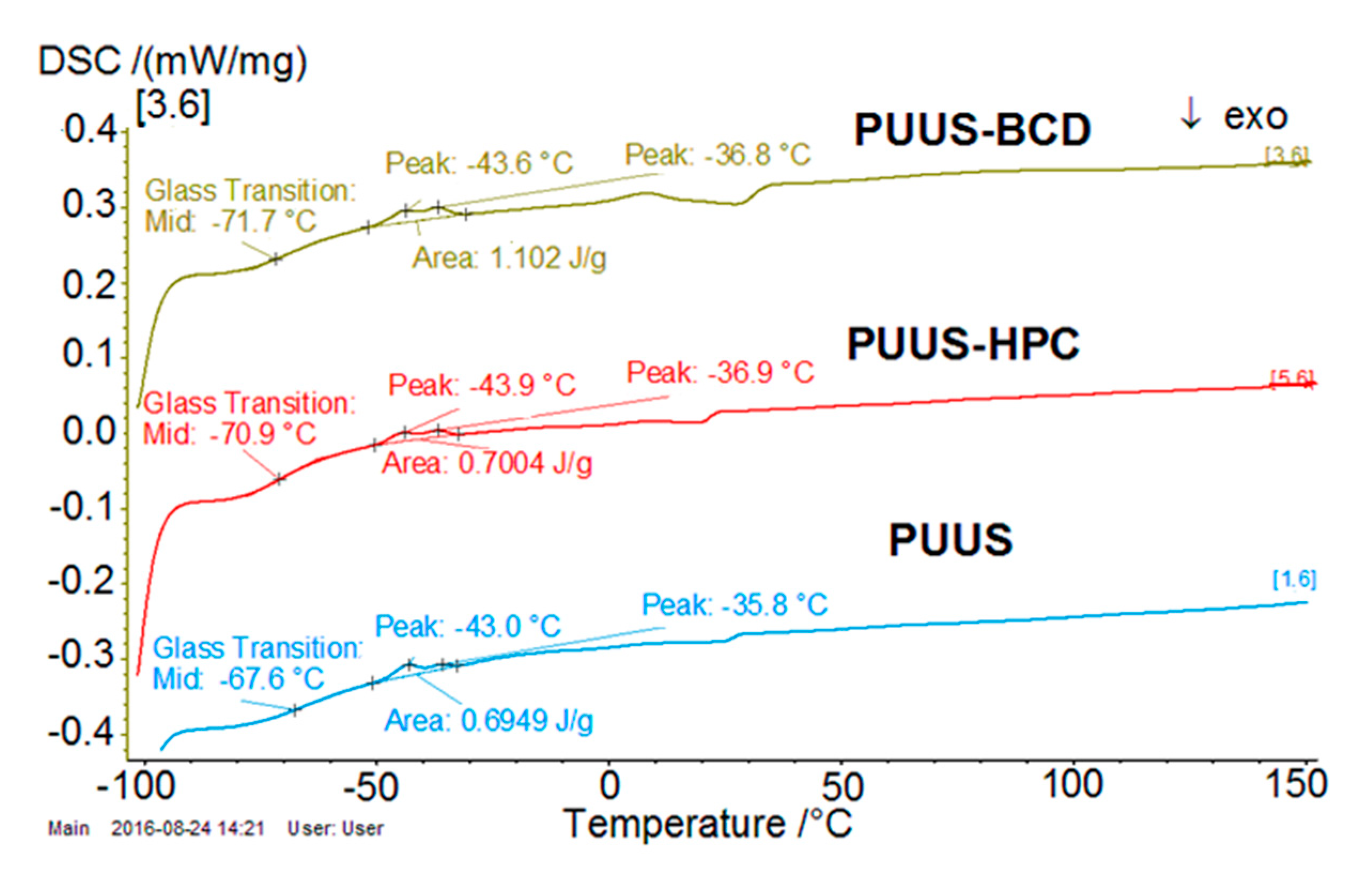
3.7. DSC Analysis
3.8. Biocompatibility Assessments
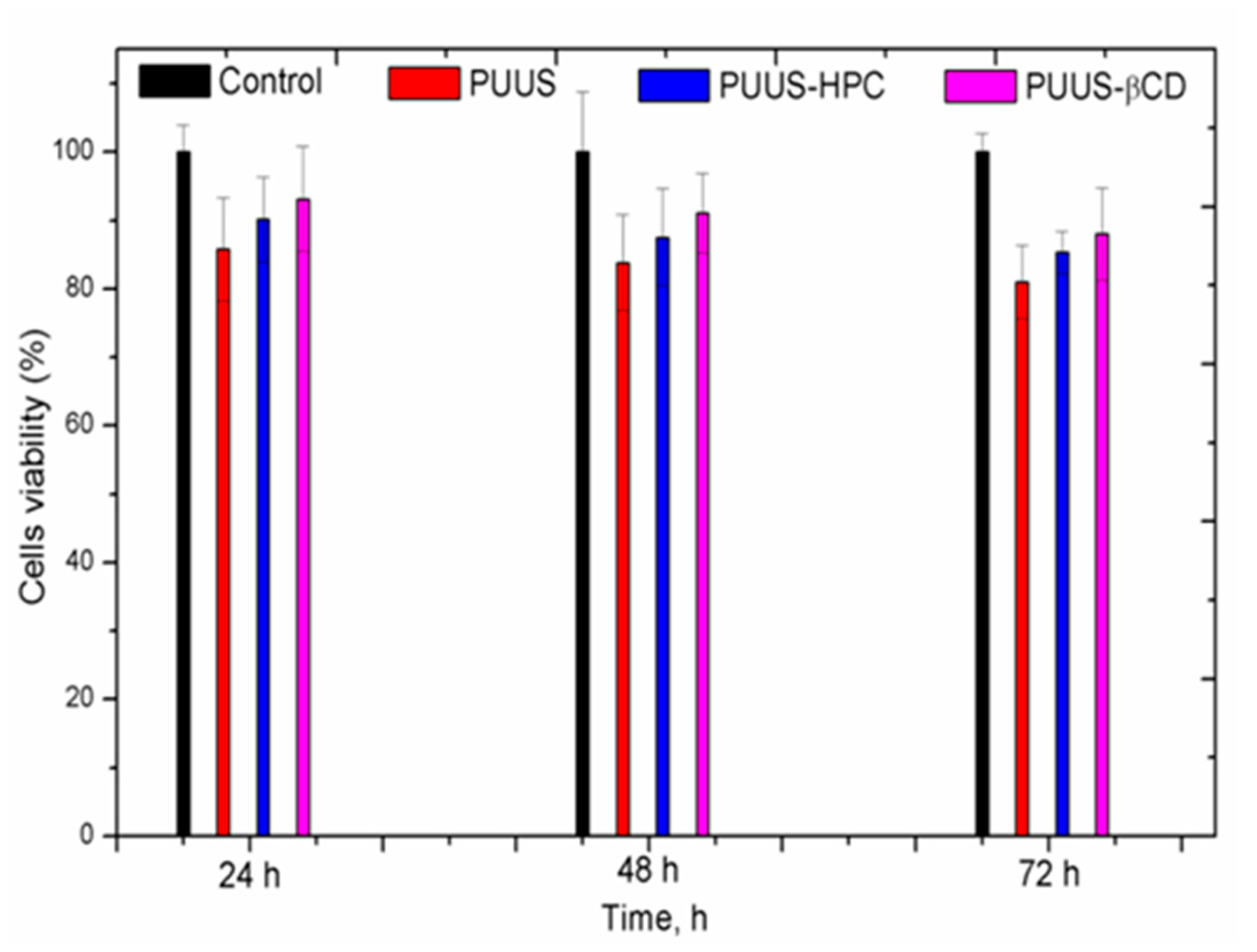
3.8.1. In Vitro Cytotoxicity Tests
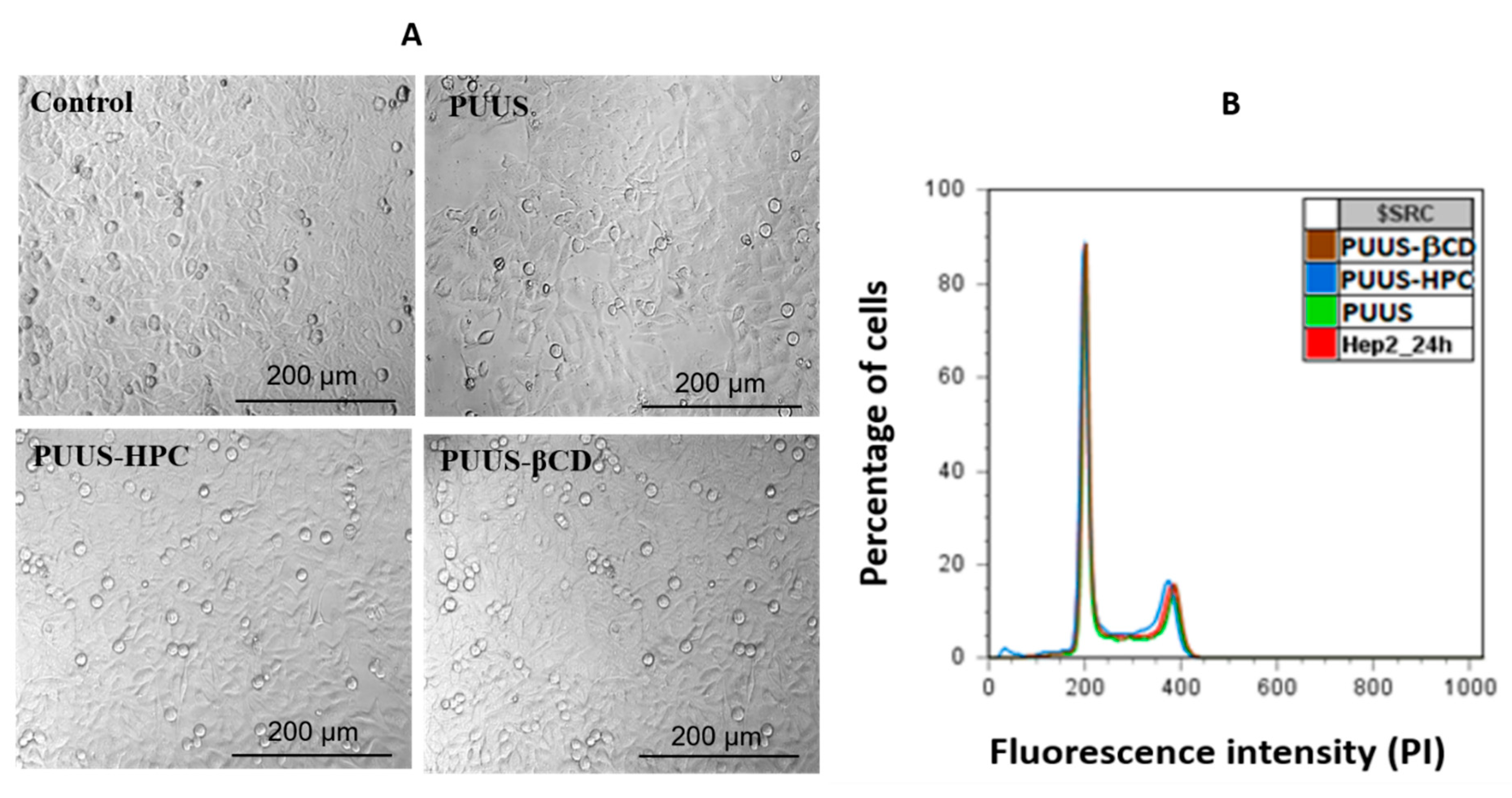
3.8.2. In Vitro Comparison of Cells Morphology and Cell Cycle Analysis
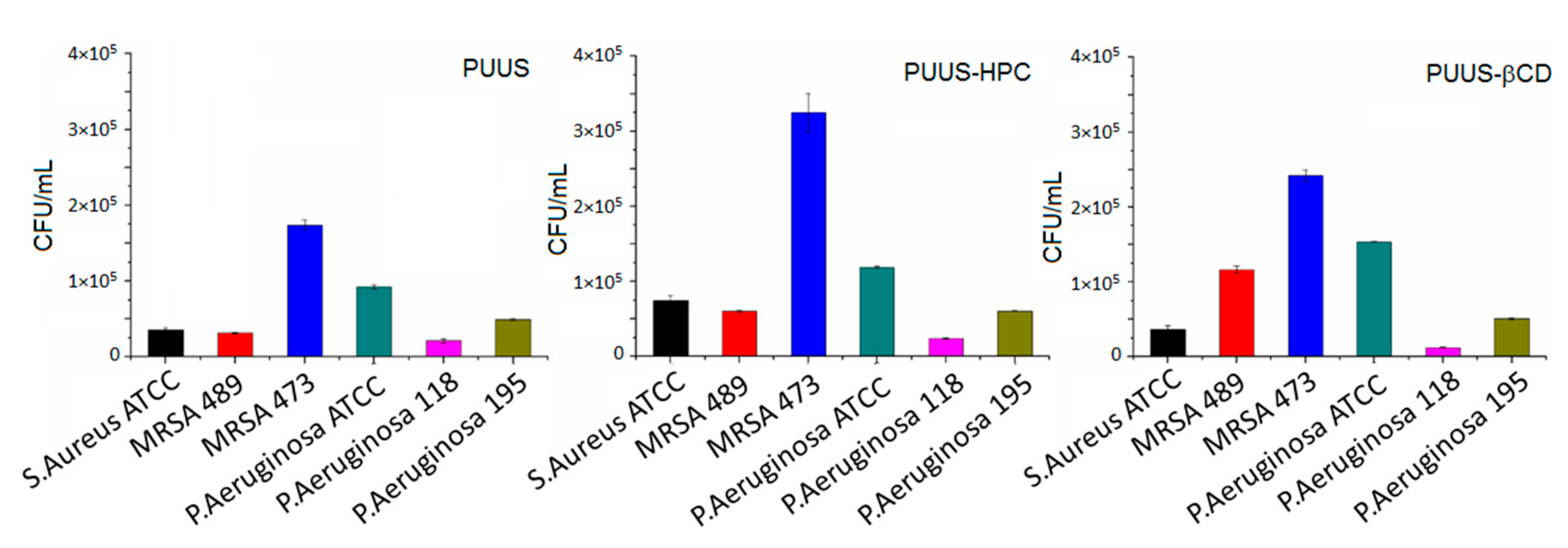
3.8.3. Microbial Adhesion Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Zhijiang, C.; Ping, X.; Shiqi, H.; Cong, Z. Soy protein nanoparticles modified bacterial cellulose electrospun nanofiber membrane scaffold by ultrasound induced self-assembly technique: Characterization and cytocompatibility. Cellulose 2019, 26, 6133–6150. [Google Scholar] [CrossRef]
- Oprea, A.E.; Ficai, A.; Andronescu, E. Electrospun nanofibers for tissue engineering applications. In Materials for Biomedical Engineering: Nanobiomaterials in Tissue Engineering; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–95. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotech. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun nanofibers: Recent applications in drug delivery and cancer therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wan, L.S.; Liu, Z.M.; Huang, X.J.; Xu, Z.K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Huang, F.; Wei, Q. Electrospun nanofibers for enzyme immobilization. In Electrospinning: Nanofabrication and Applications; Ding, B., Wang, X., Yu, J., Eds.; William Andrew: Norwich, NY, USA, 2019; pp. 765–781. [Google Scholar] [CrossRef]
- Joseph, J.; Patel, R.; Wenham, A.; Smith, J.R. Biomedical applications of polyurethane materials and coatings. Trans. IMF 2018, 96, 121–129. [Google Scholar] [CrossRef]
- Ni, H.; Daum, J.L.; Soucek, M.; Simonsick, W.J. Cycloaliphatic polyester based high solids polyurethane coatings: I. The effect of difunctional alcohols. J. Coat. Technol. 2002, 74, 49. [Google Scholar] [CrossRef]
- Vessot, S.; Andrieu, J.; Laurent, P.; Galy, J.; Gérard, J.F. Air convective drying and curing of polyurethane-based paints on sheet molding compound surfaces. J. Coat. Technol. 1998, 70, 67–76. [Google Scholar] [CrossRef]
- Yang, S.-R.; Kwon, O.-J.; Kim, D.-H.; Park, J.-S. Characterization of the polyurethane foam using alginic acid as a polyol. Fiber. Polym. 2007, 8, 257–262. [Google Scholar] [CrossRef]
- Zhao, P.Z.; Wang, Y.S.; Zhu, J.H.; Hua, X.Y.; Wen, Q.Z. Characterization of graded polyurethane elastomer by FTIR. Sci. China Ser. B Chem. 2008, 51, 58. [Google Scholar] [CrossRef]
- Stokes, K.; McVenes, R.; Andersion, J.M. Polyurethane elastomer biostability. J. Biomater. Appl. 1995, 9, 321–354. [Google Scholar] [CrossRef] [PubMed]
- Klempner, D.; Frisch, K. Advances in Urethane Science and Technology; Rapra Publisher: Akron, OH, USA, 2001. [Google Scholar]
- Desai, S.D.; Emanuel, A.L.; Sinha, V.K. Biomaterial based polyurethane adhesive for bonding rubber and wood joints. J. Polym. Res. 2003, 10, 275–281. [Google Scholar] [CrossRef]
- Vlad, S.; Butnaru, M.; Filip, D.; Macocinschi, D.; Nistor, A.; Gradinaru, L.M.; Ciobanu, C. Polyetherurethane membranes modified with renewable resource as a potential candidate for biomedical applications. Dig. J. Nanomater. Biostruct. 2010, 5, 1089–1100. [Google Scholar]
- Vlad, S.; Ciobanu, C.; Butnaru, M.; Macocinschi, D.; Filip, D.; Gradinaru, L.M.; Mandru, M. Preparation of polyurethane microspheres by electrospray technique. Dig. J. Nanomater. Biostruct. 2011, 6, 643–652. [Google Scholar]
- Macocinschi, D.; Filip, D.; Vlad, S.; Cristea, M.; Butnaru, M. Segmented biopolyurethanes for medical applications. J. Mater. Sci. Mater. Med. 2009, 20, 1659–1668. [Google Scholar] [CrossRef]
- Vlad, S.; Filip, D.; Macocinschi, D.; Spiridon, I.; Nistor, A.; Gradinaru, L.M.; Musteata, V.E. New polyetherurethanes based on cellulose derivative for biomedical applications. j. Optoelectron. Adv. Mater. 2010, 4, 407–414. [Google Scholar]
- Gradinaru, L.M.; Mandru, M.; Ciobanu, C.; Filip, D.; Macocinschi, D.; Vlad, S. Influence of diisocyanate structure on the properties of some polyetherurethanes based on renewable resources. J. Optoelectron. Adv. Mater. 2011, 13, 286–292. [Google Scholar]
- Lin, L.; Ma, J.; Mei, Q.; Cai, B.; Chen, J.; Zuo, Y.; Zou, Q.; Li, J.; Li, Y. Elastomeric polyurethane foams incorporated with nanosized hydroxyapatite fillers for plastic reconstruction. Nanomaterials 2018, 8, 972. [Google Scholar] [CrossRef]
- Yin, C.; Rozet, S.; Okamoto, R.; Kondo, M.; Tamada, Y.; Tanaka, T.; Hattori, H.; Tanaka, M.; Sato, H.; Iino, S. Physical properties and in vitro biocompatible evaluation of silicone-modified polyurethane nanofibers and films. Nanomaterials 2019, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Akduman, C.; Kumbasar, E.P.A. Electrospun polyurethane nanofibers. In Aspects of Polyurethanes; Ylmaz, F., Ed.; Books on Demand: Norderstedt, Germany, 2017; pp. 17–52. [Google Scholar] [CrossRef]
- Khil, M.-S.; Cha, D.-I.; Kim, H.-Y.; Kim, I.-S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. Electrospun nanofibrous materials for wound healing applications. In Electrospun Materials for Tissue Engineering and Biomedical Applications, 1st ed.; Uyar, T., Kny, E., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 147–177. [Google Scholar] [CrossRef]
- Kiliç, E.; Yakar, A.; Bayramgil, N.P. Preparation of electrospun polyurethane nanofiber mats for the release of doxorubicine. J. Mater. Sci. Mater. Med. 2018, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qiu, L.; Ke, Q.; He, C.; Mo, X. Electrospinning thermoplastic polyurethane-contained collagen nanofibers for tissue-engineering applications. J. Biomater. Sci. Polym. Ed. 2009, 20, 1513–1536. [Google Scholar] [CrossRef]
- Mani, M.P.; Jaganathan, S.K.; Faudzi, A.A.M.; Sunar, M.S. Engineered electrospun polyurethane composite patch combined with bi-functional components rendering high strength for cardiac tissue engineering. Polymers 2019, 11, 705. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Green electrospining of hydroxypropyl cellulose nanofibres for drug delivery applications. J. Nanosci. Nanotechnol. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Du, J.; Gan, S.; Bian, Q.; Fu, D.; Wei, Y.; Wang, K.; Lin, Q.; Chen, W.; Huang, D. Preparation and characterization of porous hydroxyapatite/b-cyclodextrin based polyurethane composite scaffolds for bone tissue engineering. J. Biomater. Appl. 2018, 33, 402–409. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Electrospun crosslinked polycyclodextrin nanofibers: Highly efficient molecular filtration thru host-guest inclusion complexation. Sci. Rep. 2017, 7, 7369. [Google Scholar] [CrossRef]
- Chabalala, M.B.; Seshabela, B.C.; Van Hulle, S.W.H.; Mamba, B.B.; Mhlanga, S.D.; Nxumalo, E.N. Cyclodextrin-based nanofibers and membranes: Fabrication, properties and applications. In Cyclodextrin: A Versatile Ingredient; Arora, P., Dhingra, N., Eds.; IntechOpen: London, UK, 2018; pp. 135–168. [Google Scholar] [CrossRef][Green Version]
- Tugui, C.; Vlad, S.; Iacob, M.; Varganici, C.D.; Pricop, L.; Cazacu, M. Interpenetrating poly(urethane-urea)-polydimethylsiloxane networks designed as active elements in electromechanical transducers. Polym. Chem. 2016, 7, 2709–2719. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- AL-Rubiae, M.S. Polymer- nanoparticles composites for the reduction of the bacterial adherence to surfaces. Iraqi J. Biotechnol. 2016, 15, 17–24. [Google Scholar]
- Zhao, H.; Hao, T.-H.; Hu, G.-H.; Shi, D.; Huang, D.; Jiang, T.; Zhang, Q.-C. Preparation and characterization of polyurethanes with cross-linked siloxane in the side chain by sol-gel reactions. Materials 2017, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Pergal, M.V.; Dzunuzovic, J.V.; Poreba, R.; Micic, D.; Stefanov, P.; Pezo, L.; Spírková, M. Surface and thermomechanical characterization of polyurethane networks based on poly(dimethylsiloxane) and hyperbranched polyester. Express Polym. Lett. 2013, 7, 806–820. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Brogueira, P.; de Pinho, M.N. Sub-micron tailoring of bi-soft segment asymmetric polyurethane membrane surfaces with enhanced hemocompatibility properties. Colloids Surf. B 2011, 86, 21–27. [Google Scholar] [CrossRef]
- Lim, H.; Hoag, S.W. Plasticizer effects on physical–mechanical properties of solvent cast soluplus® films. AAPS Pharm. Sci. Tech. 2013, 14, 903–910. [Google Scholar] [CrossRef]
- Stanciu, A.; Bulacovschi, V.; Lungu, M.; Vlad, S.; Balint, S.; Oprea, S. Mechanical behaviour of crosslinked poly(ester-siloxane) urethanes. Eur. Polym. J. 1999, 35, 2039–2044. [Google Scholar] [CrossRef]
- Huyer, L.D.; Zhang, B.; Korolj, A.; Montgomery, M.; Drecun, S.; Conant, G.; Zhao, Y.; Reis, L.; Radisic, M. Highly elastic and moldable polyester biomaterial for cardiac tissue engineering applications. ACS Biomater. Sci. Eng. 2016, 2, 780–788. [Google Scholar] [CrossRef]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian cell behavior on hydrophobic substrates: Influence of surface properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Milkovic, L.; Cipak, A.; Mozetic, M.; Recek, N.; Zarkovic, N.; Vesel, A. Cell adhesion on hydrophobic polymer surfaces. Mater. Technol. 2012, 46, 53–56. [Google Scholar]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion behaviors on superhydrophobic surfaces. Chem. Commun. 2014, 50, 3900–3913. [Google Scholar] [CrossRef]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Balik, C.M. On the extraction of diffusion coefficients from gravimetric data for sorption of small molecules by polymer thin film. Macromolecules 1996, 29, 3025–3029. [Google Scholar] [CrossRef]
- Darie, R.N.; Vlad, S.; Anghel, N.; Doroftei, F.; Tamminen, T.; Spiridon, I. New PP/PLA/cellulose composites: Effect of cellulose functionalization on accelerated weathering behaviour. Polym. Adv. Technol. 2015, 26, 941–952. [Google Scholar] [CrossRef]
- Vlad, S.; Spiridon, I.; Grigoras, C.V.; Drobota, M.; Nistor, A. Thermal, mechanical and wettability properties of some branched polyetherurethane elastomers. e-Polymers 2009, 9, 1. [Google Scholar] [CrossRef]
- Rahmawati, R.; Nozaki, S.; Kojio, K.; Takahara, A.; Shinohara, N.; Yamasak, S. Microphase-separated structure and mechanical properties of cycloaliphatic diisocyanate-based thiourethane elastomers. Polym. J. 2019, 51, 265–273. [Google Scholar] [CrossRef]
- Khodir, W.K.A.; Razak, A.H.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and characterization of gentamicin sulfate in the collagen added electrospun nanofibers for skin regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef]
- Cianfruglia, L.; Minnelli, C.; Laudadio, E.; Scirè, A.; Armeni, T. Side effects of curcumin: Epigenetic and antiproliferative implications for normal dermal fibroblast and breast cancer cells. Antioxidants 2019, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, D.; Li, W.; Yu, J.; Chen, Y. Effect of size, shape, and surface modification on cytotoxicity of gold nanoparticles to human HEp-2 and Canine MDCK cells. J. Nanomater. 2012. [Google Scholar] [CrossRef]
- Tudose, M.; Anghel, E.M.; Culita, D.C.; Somacescu, S.; Calderon-Moreno, J.; Tecuceanu, V.; Dumitrascu, F.D.; Dracea, O.; Popa, M.; Marutescu, L.; et al. Covalent coupling of tuberculostatic agents and graphene oxide: A promising approach for enhancing and extending their antimicrobial applications. Appl. Surf. Sci. 2019, 471, 553–565. [Google Scholar] [CrossRef]
- Ding, M.; Li, J.; Fu, X.; Zhou, J.; Tan, H.; Gu, Q.; Fu, Q. Synthesis, degradation, and cytotoxicity of multiblock poly (ε-caprolactone urethane) s containing gemini quaternary ammonium cationic groups. Biomacromolecules 2009, 10, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Stachewicz, U.; Szewczyk, P.; Kruk, A.; Barber, A.H.; Czyrska-Filemonowicz, A. Pore shape and size dependence on cells growth into electrospun fiber scaffolds for tissue engineering: 2D and 3D analyses using SEM and FIB-SEM tomography. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell. Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Denchai, A.; Tartarini, D.; Mele, E. Cellular response to surface morphology: Electrospinning and computational modelling. Front. Bioeng. Biotechnol. 2018, 6, 155. [Google Scholar] [CrossRef]
- Gritsch, L.; Meng, D.; Boccaccini, A.R. Nanostructured biocomposites, for tissue engineering scaffolds. In Biomedical Composites, 2nd ed.; Ambrosio, L., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 501–542. [Google Scholar] [CrossRef]
- Darouiche, R.O. Device-associated infections: A macroproblem that starts with microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]

| Sample | PUUS | PUUS-HPC | PUUS-βCD | |
|---|---|---|---|---|
| Electrospinning condition | Voltage (kV) | 15 | ||
| Flow rate (mL h−1) | 0.5 | |||
| Needle size (gauge) | 22 | |||
| Spinning time (h) | 10 | |||
| Collector-needle distance (mm) | 80 | |||
| Tensile strength (MPa) | 4.14 ± 0.01 | 1.84 ± 0.008 | 1.32 ± 0.004 | |
| Elongation at break (%) | 650 ± 17 | 580 ± 24 | 650 ± 13 | |
| Tensile modulus (MPa) | 3.90 ± 0.01 | 0.87 ± 0.001 | 0.73 ± 0.002 | |
| Toughness (MPa) | 11.57 ± 0.8 | 4.80 ± 0.5 | 4.16 ± 0.1 | |
| Sample | * K1 × 103 (s−1) | * K2 × 103 (s−1) | l × 102 (cm) | D1 × 106 (cm2 s−1) | D2 × 106 (cm2 s−1) | Contact Angle (°) | Tg (°C) |
|---|---|---|---|---|---|---|---|
| PUUS | 0.804 | −7.360 | 9 | 1.2781 | 6.04 | 88.61 ± 0.32 | −67.6 |
| PUUS-HPC | 0.836 | −16.38 | 9 | 1.3298 | 13.44 | 85.40 ± 0.12 | −70.9 |
| PUUS-βCD | 1.393 | −55.66 | 9 | 2.2149 | 45.68 | 84.83 ± 0.17 | −71.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradinaru, L.M.; Barbalata-Mandru, M.; Drobota, M.; Aflori, M.; Spiridon, M.; Gradisteanu Pircalabioru, G.; Bleotu, C.; Butnaru, M.; Vlad, S. Preparation and Evaluation of Nanofibrous Hydroxypropyl Cellulose and β-Cyclodextrin Polyurethane Composite Mats. Nanomaterials 2020, 10, 754. https://doi.org/10.3390/nano10040754
Gradinaru LM, Barbalata-Mandru M, Drobota M, Aflori M, Spiridon M, Gradisteanu Pircalabioru G, Bleotu C, Butnaru M, Vlad S. Preparation and Evaluation of Nanofibrous Hydroxypropyl Cellulose and β-Cyclodextrin Polyurethane Composite Mats. Nanomaterials. 2020; 10(4):754. https://doi.org/10.3390/nano10040754
Chicago/Turabian StyleGradinaru, Luiza Madalina, Mihaela Barbalata-Mandru, Mioara Drobota, Magdalena Aflori, Maria Spiridon, Gratiela Gradisteanu Pircalabioru, Coralia Bleotu, Maria Butnaru, and Stelian Vlad. 2020. "Preparation and Evaluation of Nanofibrous Hydroxypropyl Cellulose and β-Cyclodextrin Polyurethane Composite Mats" Nanomaterials 10, no. 4: 754. https://doi.org/10.3390/nano10040754
APA StyleGradinaru, L. M., Barbalata-Mandru, M., Drobota, M., Aflori, M., Spiridon, M., Gradisteanu Pircalabioru, G., Bleotu, C., Butnaru, M., & Vlad, S. (2020). Preparation and Evaluation of Nanofibrous Hydroxypropyl Cellulose and β-Cyclodextrin Polyurethane Composite Mats. Nanomaterials, 10(4), 754. https://doi.org/10.3390/nano10040754








