Alteration of the Mitochondrial Effects of Ceria Nanoparticles by Gold: An Approach for the Mitochondrial Modulation of Cells Based on Nanomedicine
Abstract
1. Introduction
2. Materials and Methods
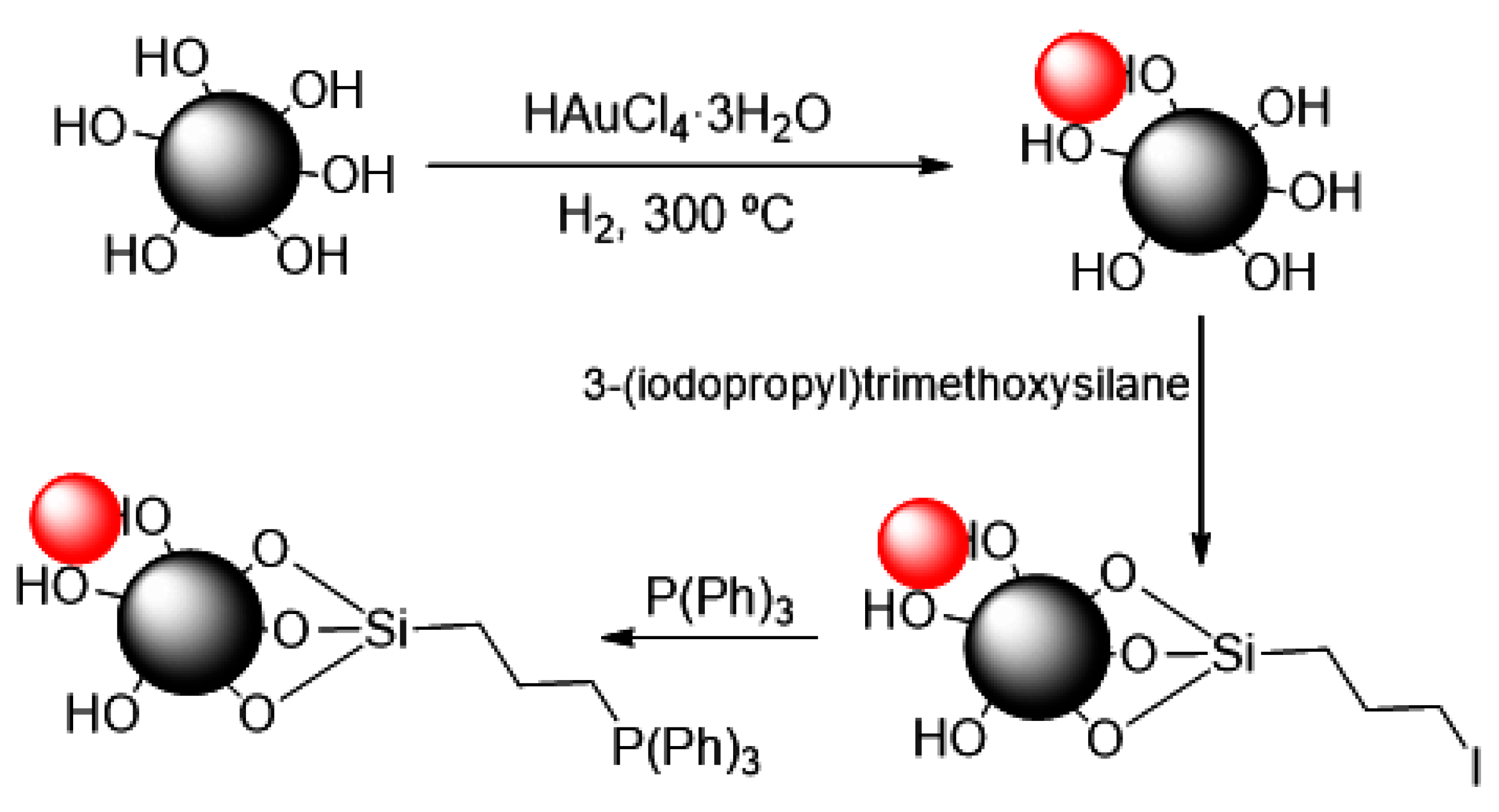
2.1. Synthesis and Characterization of NPs
2.1.1. Preparation of 3–Iodopropyl-Functionalized AuCeO2 (I–AuCeO2)
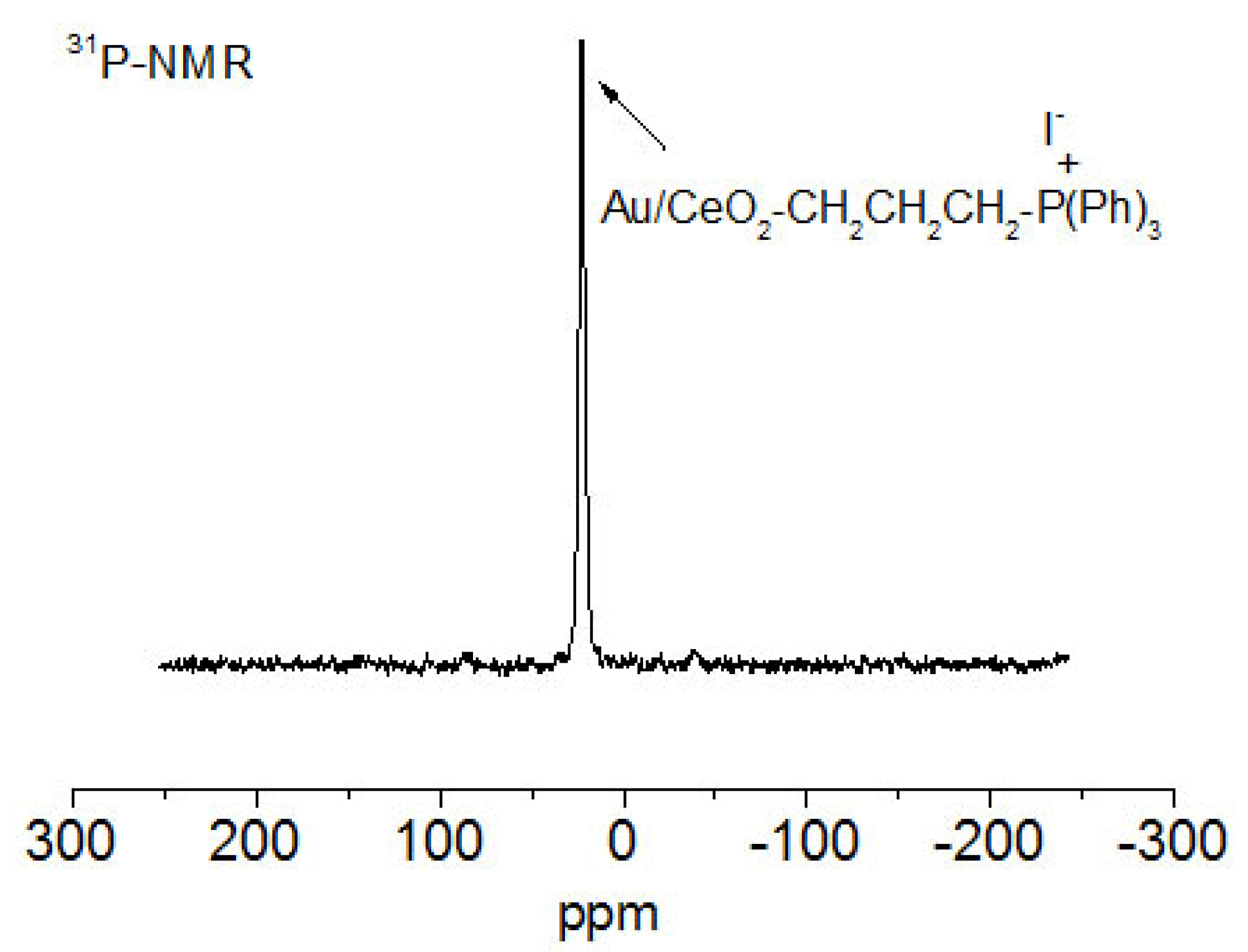
2.1.2. Preparation of Triphenylphosphonium-Functionalized AuCeO2 (TPP–AuCeO2)
2.2. Cell Culture
2.2.1. Cellular Viability and Proliferation
2.2.2. Cellular Uptake and Internalization of Conjugate by Confocal Microscopy
2.2.3. Measurement of the Mitochondrial O2 Consumption
2.2.4. Measurement of Mitochondrial Membrane Potential (∆Ψm) and Total ROS Production
2.2.5. ATP Determination
2.2.6. Real-Time PCR
2.2.7. Western Blot
2.3. Statistical Analysis
3. Results
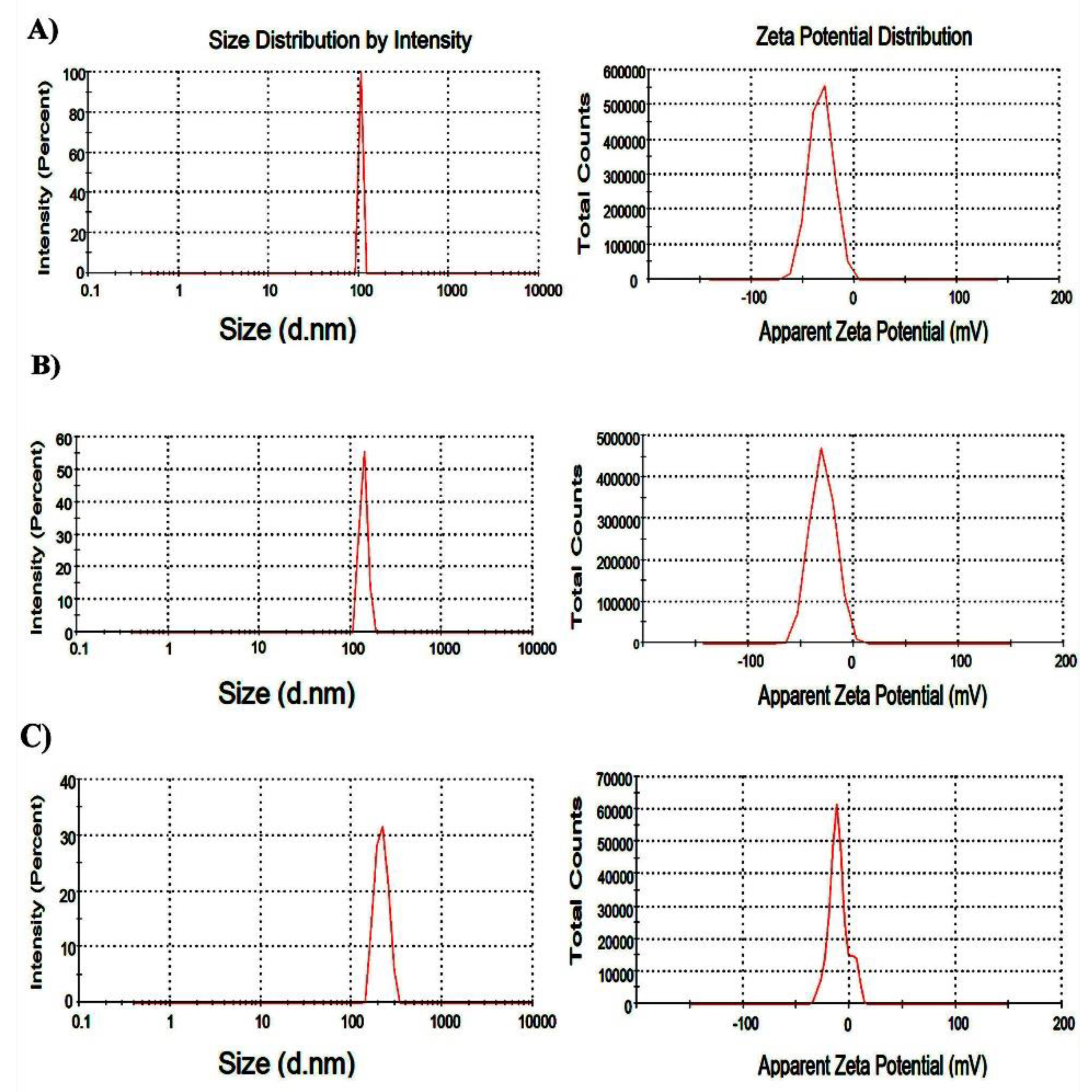
3.1. Synthesis and Characterization of Nanoparticles
3.2. Cell Culture Studies
3.2.1. Cellular Viability and Proliferation
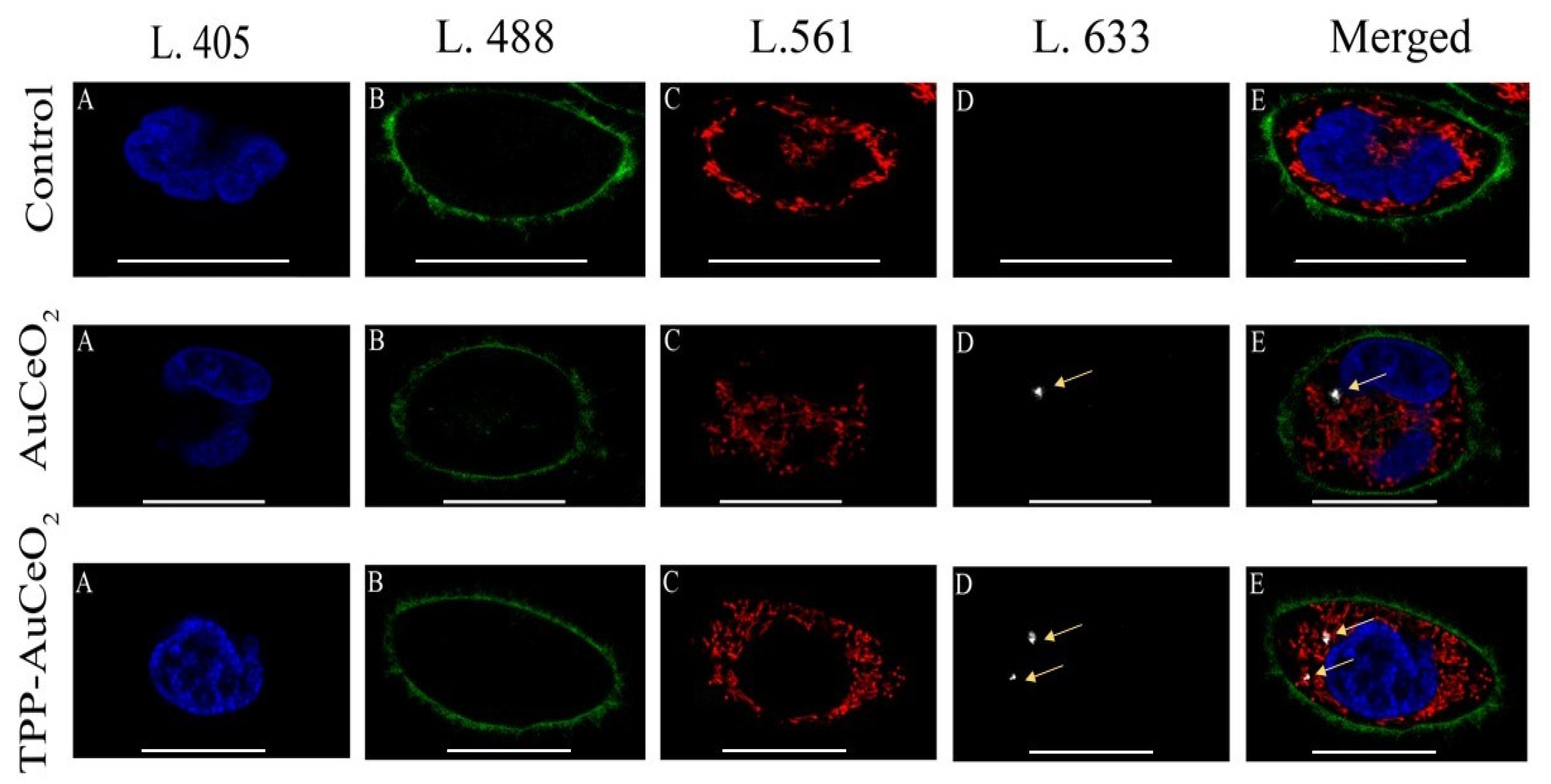
3.2.2. Cellular Uptake and Internalization of Conjugate by Confocal Microscopy
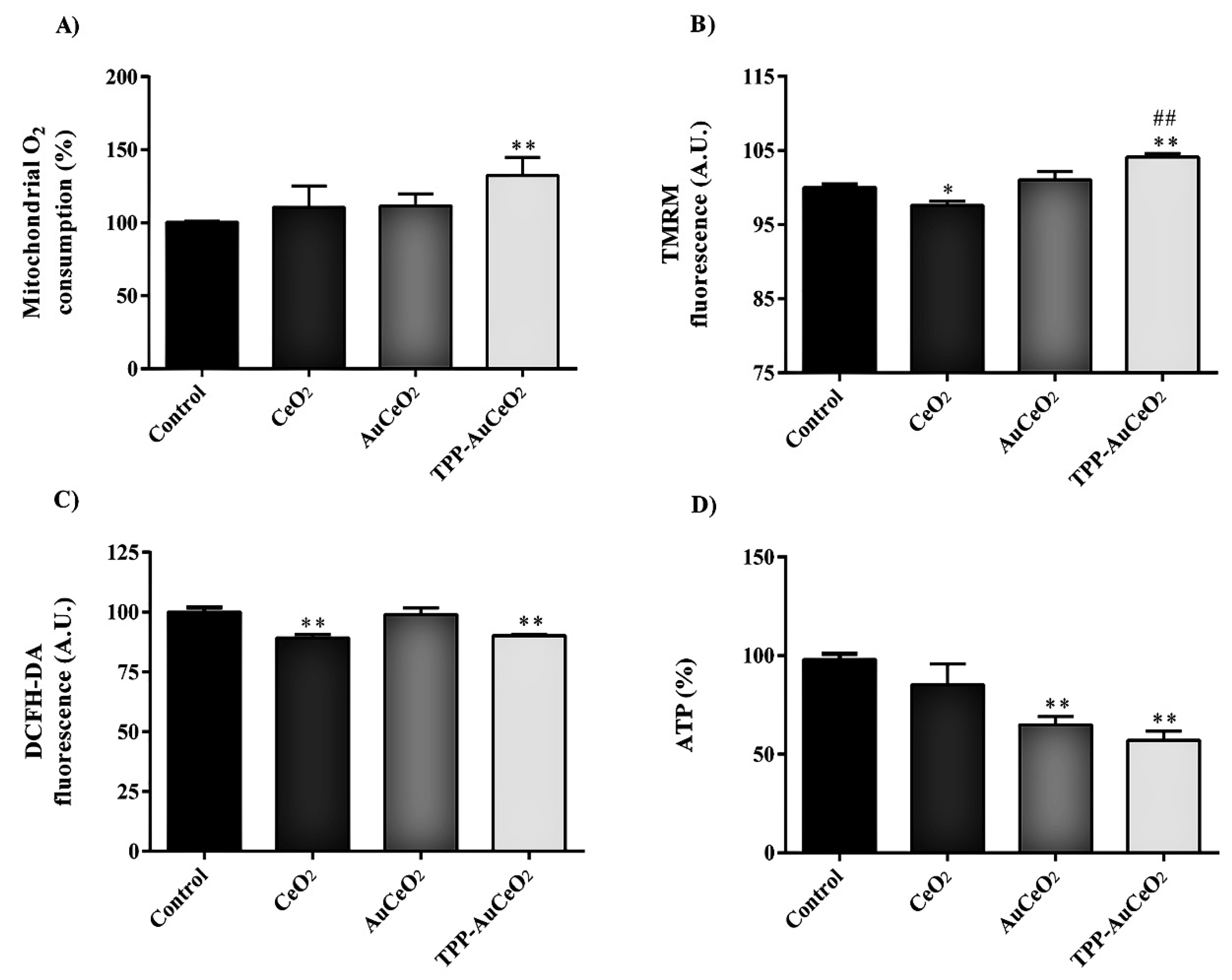
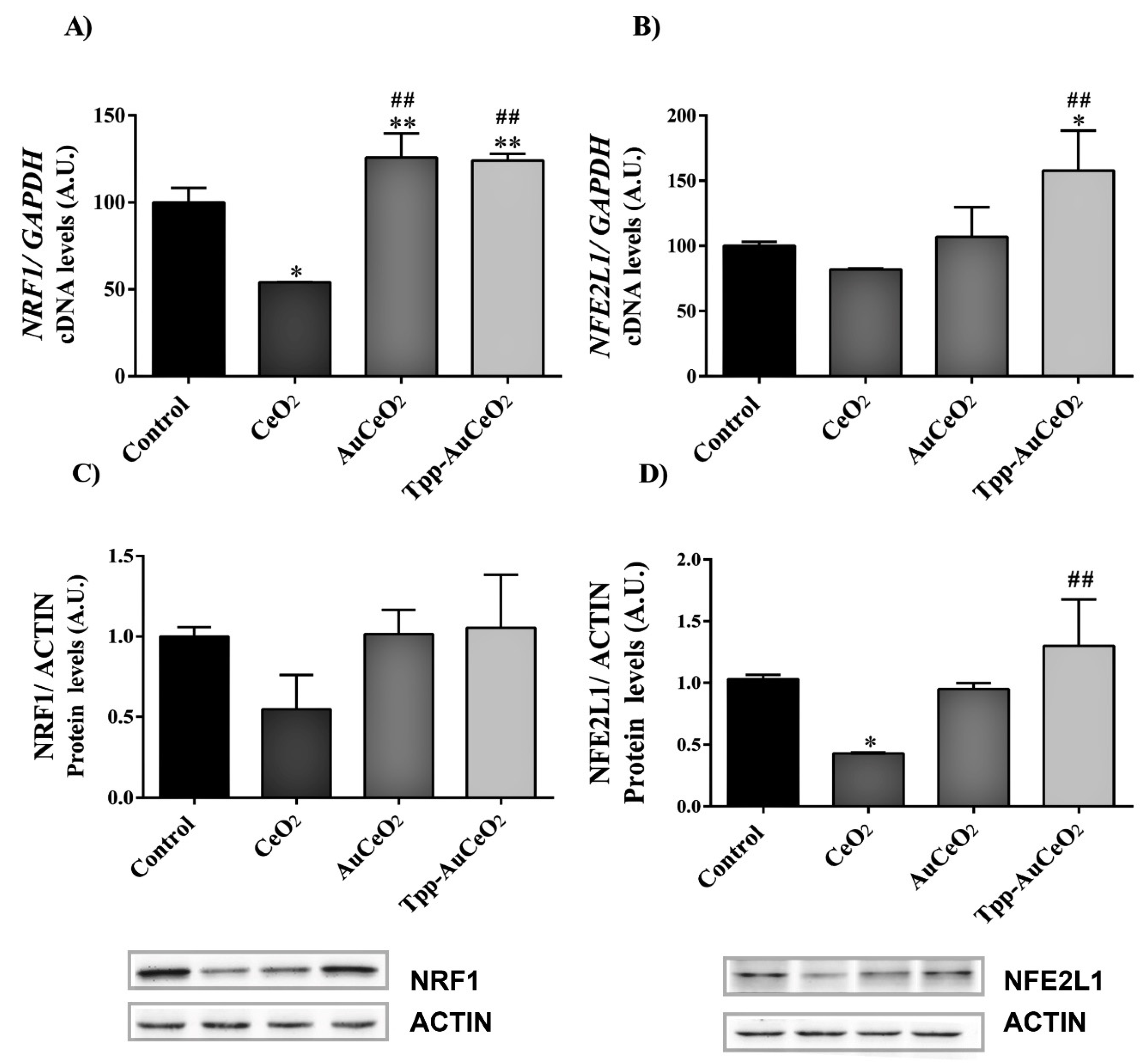
3.2.3. Mitochondrial Functional Studies after Nanoparticles Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fan, C.-Q.; Dong, H.; Wang, S.-M.; Yang, X.-C.; Yang, S. Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed. 2017, 12, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Mathe, D.; Kovács, N.; Horvath, I.; Jauregui-Osoro, M.; De Rosales, R.T.M.; Mullen, G.E.D.; Wong, W.; Yan, Y.; Krüger, D.; et al. Synthesis, characterization, and application of Core–Shell Co0.16Fe2.84O4@NaYF4(Yb, Er) and Fe3O4@NaYF4(Yb, Tm) nanoparticle as trimodal (MRI, PET/SPECT, and optical) imaging agents. Bioconjugate Chem. 2015, 27, 319–328. [Google Scholar] [CrossRef]
- Martín, R.; Menchón, C.; Apostolova, N.; Victor, V.M.; Alvaro, M.; Herance, J.R.; García, H. Nano-jewels in biology. Gold and platinum on diamond nanoparticles as antioxidant systems against cellular oxidative stress. ACS Nano 2010, 4, 6957–6965. [Google Scholar]
- Diab, M.S.; Dkhil, M.A.M.; Bauomy, A.A.; Al-Quraishy, S. Antioxidant and hepatoprotective role of gold nanoparticles against murine hepatic schistosomiasis. Int. J. Nanomed. 2015, 10, 7467–7475. [Google Scholar] [CrossRef]
- Hou, J.; Yu, X.; Shen, Y.; Shi, Y.; Su, C.; Zhao, L. Triphenyl phosphine-functionalized chitosan nanoparticles enhanced antitumor efficiency through targeted delivery of doxorubicin to mitochondria. Nanoscale Res. Lett. 2017, 12, 158. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cha, M.-Y.; Kim, K.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- D’Hollander, A.; Jans, H.; Velde, G.V.; Verstraete, C.; Massa, S.; Devoogdt, N.; Stakenborg, T.; Muyldermans, S.; Lagae, L.; Himmelreich, U. Limiting the protein corona: A successful strategy for in vivo active targeting of anti-HER2 nanobody-functionalized nanostars. Biomaterials 2017, 123, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Charbgoo, F.; Bin Ahmad, M.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron localization determines defect formation on ceria substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Lazar, S.; Freitag, B.; Egoavil, R.; Verbeeck, J.; Put, S.; Strauven, Y.; Van Tendeloo, G. High resolution mapping of surface reduction in ceria nanoparticles. Nanoscale 2011, 3, 3385. [Google Scholar] [CrossRef] [PubMed]
- Maldotti, A.; Juárez, R.; Molinari, A.; García, H. Photoinduced reactivity of Au–H intermediates in alcohol oxidation by gold nanoparticles supported on ceria. Chem. Sci. 2011, 2, 1831–1834. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.; Vallabani, N.V.S.; Shutthanandan, V.; Bowden, M.; Karakoti, A.S.; Singh, S. Gold core/ceria shell-based redox active nanozyme mimicking the biological multienzyme complex phenomenon. J. Colloid Interface Sci. 2018, 513, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Grady, D.; Halloran, B.; Cummings, S.; Leveille, S.; Wells, L.; Black, D.; Byl, N. 1,25-Dihydroxyvitamin D 3 and muscle strength in the elderly: A randomized controlled trial. J. Clin. Endocrinol. Metab. 1991, 73, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Herance, J.R.; García, H.; Gutiérrez-Carcedo, P.; Navalón, S.; Pineda-Lucena, A.; Palomino-Schätzlein, M.; Gutierrez, P.C. A translational approach to assess the metabolomic impact of stabilized gold nanoparticles by NMR spectroscopy. Analyst 2019, 144, 1265–1274. [Google Scholar] [CrossRef]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, Y.; Saita, E.; Suzuki-Sugihara, N.; Kamiya, T.; Taguchi, C.; Iida, K.; Kondo, K. Terminalia bellirica extract inhibits low-density lipoprotein oxidation and macrophage inflammatory response in vitro. Antioxidants 2016, 5, 20. [Google Scholar] [CrossRef]
- Chithrani, D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Menchon, C.; Martin, R.; Apostolova, N.; Victor, V.M.; Alvaro, M.; Herance, J.R.; García, H. Gold nanoparticles supported on nanoparticulate ceria as a powerful agent against intracellular oxidative stress. Small 2012, 8, 1895–1903. [Google Scholar] [CrossRef]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale Metal–Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Z.-Y.; Liu, C.; Zeng, Y.-P.; Hao, Y.; Gu, Y.; Wang, W.-D.; Li, R. PEGylated ceria nanoparticles used for radioprotection on human liver cells under γ-ray irradiation. Free Radic. Boil. Med. 2015, 87, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kyosseva, S.V.; McGinnis, J.F. Cerium oxide nanoparticles as promising ophthalmic therapeutics for the treatment of retinal diseases. World J. Ophthalmol. 2015, 5, 23–30. [Google Scholar] [CrossRef]
- Fiorani, L.; Passacantando, M.; Santucci, S.; Di Marco, S.; Bisti, S.; Maccarone, R. Cerium Oxide Nanoparticles Reduce Microglial Activation and Neurodegenerative Events in Light Damaged Retina. PLoS ONE 2015, 10, e0140387. [Google Scholar] [CrossRef]
- Chen, B.-H.; Babu, K.S.; Anandkumar, M.; Tsai, T.; Kao, T.; Inbaraj, B.S. Cytotoxicity and antibacterial activity of gold-supported cerium oxide nanoparticles. Int. J. Nanomed. 2014, 9, 5515–5531. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2015, 24, 547–553. [Google Scholar] [CrossRef]
- Lyakhovich, A.; Graifer, D. Mitochondria-mediated oxidative stress: Old target for new drugs. Curr. Med. Chem. 2015, 22, 3040–3053. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Xiao, H.; Lü, F.; Stewart, D.; Zhang, Y. Mechanisms underlying chemopreventive effects of flavonoids via multiple signaling nodes within Nrf2-ARE and AhR-XRE gene regulatory networks. Curr. Chem. Boil. 2013, 7, 151–176. [Google Scholar] [CrossRef]
- Reinecke, F.; Smeitink, J.A.; Van Der Westhuizen, F. OXPHOS gene expression and control in mitochondrial disorders. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2009, 1792, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Dao, V.T.-V.; Daiber, A.; Maghzal, G.J.; Di Lisa, F.; Kaludercic, N.; Leach, S.; Cuadrado, A.; Jaquet, V.; Seredenina, T.; et al. Reactive oxygen-related diseases: Therapeutic targets and emerging clinical indications. Antioxid. Redox Signal. 2015, 23, 1171–1185. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; De Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Rovira-Llopis, S.; Asiri, A.M.; Baldovi, H.G.; Navalón, S.; Victor, V.M.; García, H.; Herance, J.R. Ceria nanoparticles with rhodamine B as a powerful theranostic agent against intracellular oxidative stress. RSC Adv. 2015, 5, 79423–79432. [Google Scholar] [CrossRef]
- Patel, P.; Kansara, K.; Singh, R.; Shukla, R.; Singh, S.; Dhawan, A.; Kumar, A. Cellular internalization and antioxidant activity of cerium oxide nanoparticles in human monocytic leukemia cells. Int. J. Nanomed. 2018, 13, 39–41. [Google Scholar] [CrossRef]
- Zhitomirsky, B.; Farber, H.; Assaraf, Y.G. LysoTracker and MitoTracker Red are transport substrates of P-glycoprotein: Implications for anticancer drug design evading multidrug resistance. J. Cell. Mol. Med. 2018, 22, 2131–2141. [Google Scholar] [CrossRef]
- Yang, L.; Nienhaus, G.U.; Shang, L. Mechanistic aspects of fluorescent gold nanocluster internalization by live HeLa cells. Nanoscale 2013, 5, 1537. [Google Scholar] [CrossRef]
- Torrano, A.; Blechinger, J.; Osseforth, C.; Argyo, C.; Reller, A.; Bein, T.; Michaelis, J.; Bräuchle, C. A fast analysis method to quantify nanoparticle uptake on a single cell level. Nanomedicine 2013, 8, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.B.; Dong, J.; Bischof, J.C. Cellular uptake and nanoscale localization of gold nanoparticles in cancer using label-free confocal raman microscopy. Mol. Pharm. 2010, 8, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.J. Assays for determination of protein concentration. Curr. Protoc. Pharmacol. 2016, 73, A.3A.1–A.3A.32. [Google Scholar] [CrossRef] [PubMed]
- Hebbe, V.; Londez, A.; Goujon-Ginglinger, C.; Meyer, F.; Uziel, J.; Jugé, S.; Lacour, J. NMR enantiodifferentiation of triphenylphosphonium salts by chiral hexacoordinated phosphate anions. Tetrahedron Lett. 2003, 44, 2467–2471. [Google Scholar] [CrossRef]
- Belli, V.; Guarnieri, D.; Biondi, M.; Della Sala, F.; Netti, P.A. Dynamics of nanoparticle diffusion and uptake in three-dimensional cell cultures. Colloids Surf. B: Biointerfaces 2017, 149, 7–15. [Google Scholar] [CrossRef]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radic. Boil. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Brand, M.D.; Esteves, T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef]
- Satoh, J.-I.; Kawana, N.; Yamamoto, Y. Pathway analysis of ChIP-Seq-based NRF1 target genes suggests a logical hypothesis of their involvement in the pathogenesis of neurodegenerative diseases. Gene Regul. Syst. Boil. 2013, 7, 139–152. [Google Scholar] [CrossRef]
- Biswas, M.; Chan, J.Y. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol. Appl. Pharmacol. 2009, 244, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Han, J.W.; Chan, J.Y. Nuclear Factor Erythroid-2 Like 1 (NFE2L1): Structure, function and regulation. Gene 2016, 584, 17–25. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Carcedo, P.; Navalón, S.; Simó, R.; Setoain, X.; Aparicio-Gómez, C.; Abasolo, I.; Victor, V.M.; García, H.; Herance, J.R. Alteration of the Mitochondrial Effects of Ceria Nanoparticles by Gold: An Approach for the Mitochondrial Modulation of Cells Based on Nanomedicine. Nanomaterials 2020, 10, 744. https://doi.org/10.3390/nano10040744
Gutiérrez-Carcedo P, Navalón S, Simó R, Setoain X, Aparicio-Gómez C, Abasolo I, Victor VM, García H, Herance JR. Alteration of the Mitochondrial Effects of Ceria Nanoparticles by Gold: An Approach for the Mitochondrial Modulation of Cells Based on Nanomedicine. Nanomaterials. 2020; 10(4):744. https://doi.org/10.3390/nano10040744
Chicago/Turabian StyleGutiérrez-Carcedo, Patricia, Sergio Navalón, Rafael Simó, Xavier Setoain, Carolina Aparicio-Gómez, Ibane Abasolo, Victor Manuel Victor, Hermenegildo García, and José Raúl Herance. 2020. "Alteration of the Mitochondrial Effects of Ceria Nanoparticles by Gold: An Approach for the Mitochondrial Modulation of Cells Based on Nanomedicine" Nanomaterials 10, no. 4: 744. https://doi.org/10.3390/nano10040744
APA StyleGutiérrez-Carcedo, P., Navalón, S., Simó, R., Setoain, X., Aparicio-Gómez, C., Abasolo, I., Victor, V. M., García, H., & Herance, J. R. (2020). Alteration of the Mitochondrial Effects of Ceria Nanoparticles by Gold: An Approach for the Mitochondrial Modulation of Cells Based on Nanomedicine. Nanomaterials, 10(4), 744. https://doi.org/10.3390/nano10040744








