Thermal Decomposition Behavior and Thermal Safety of Nitrocellulose with Different Shape CuO and Al/CuO Nanothermites
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Bamboo Leaf-like CuO(b) and Al/CuO(b)
2.3. Preparation of Flaky-shaped CuO(f) and Al/CuO(f)
2.4. Samples Characterization
2.5. Measurement of Thermal Decomposition Properties and Compatibility
3. Results and Discussion
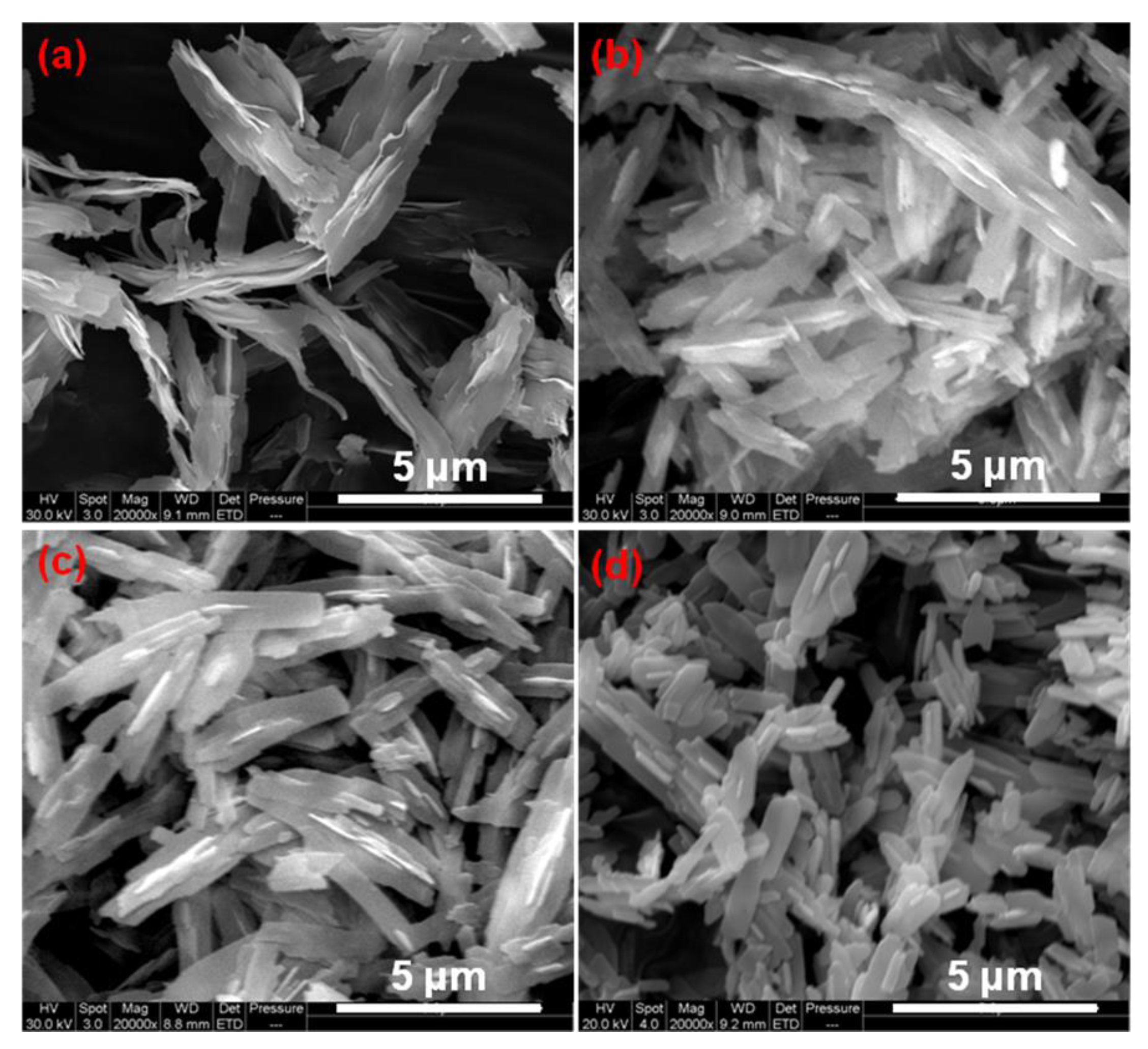
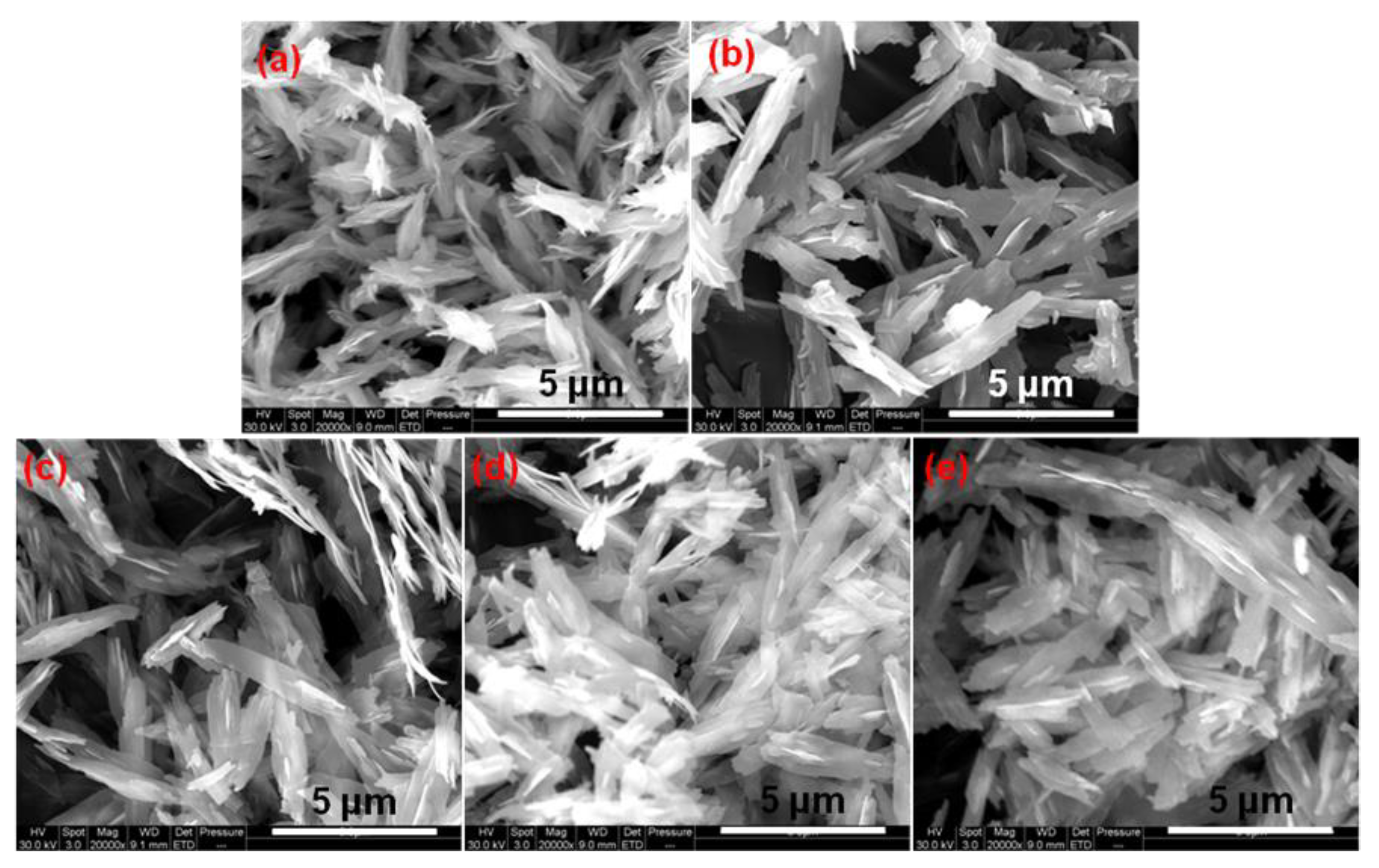
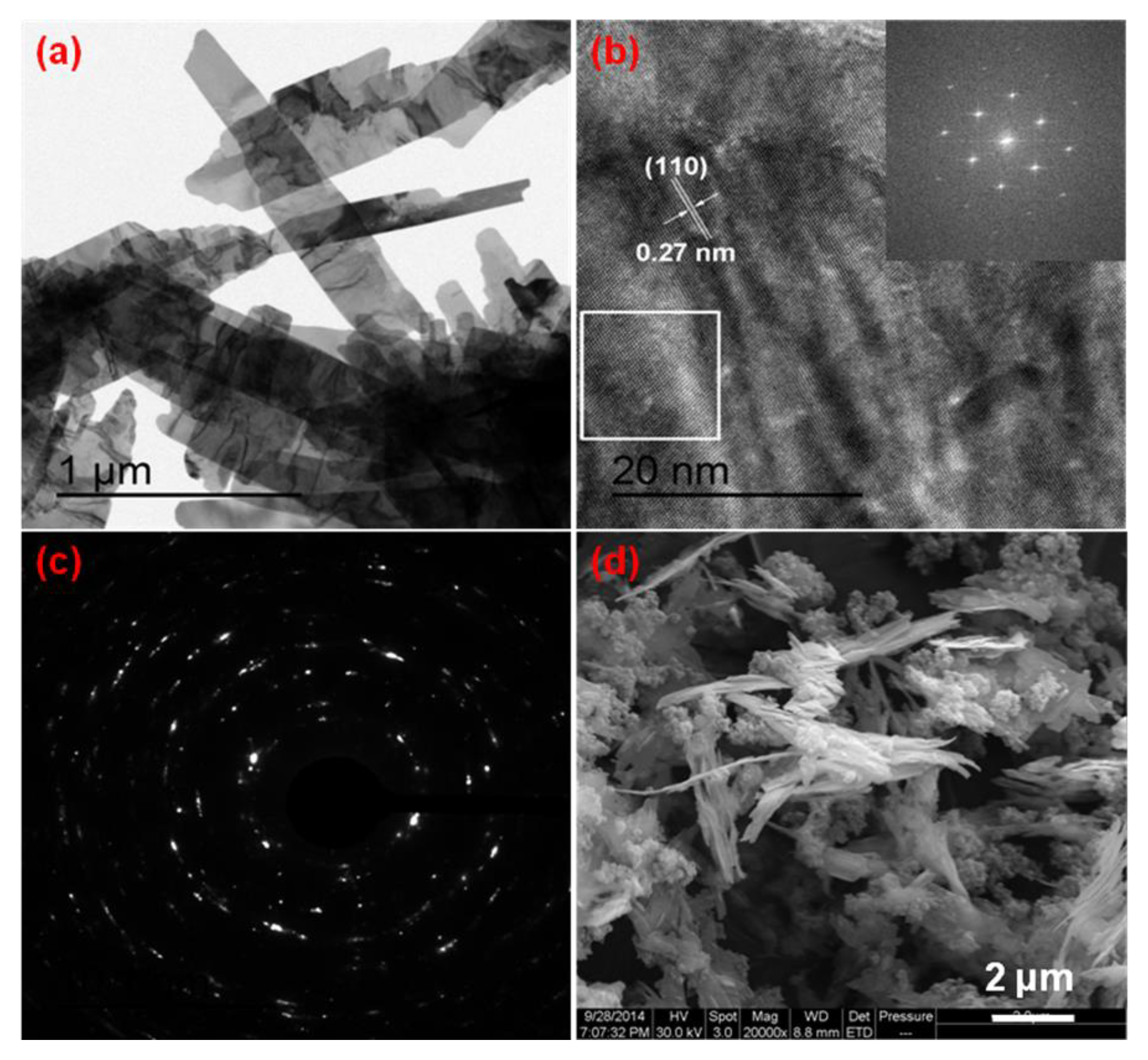
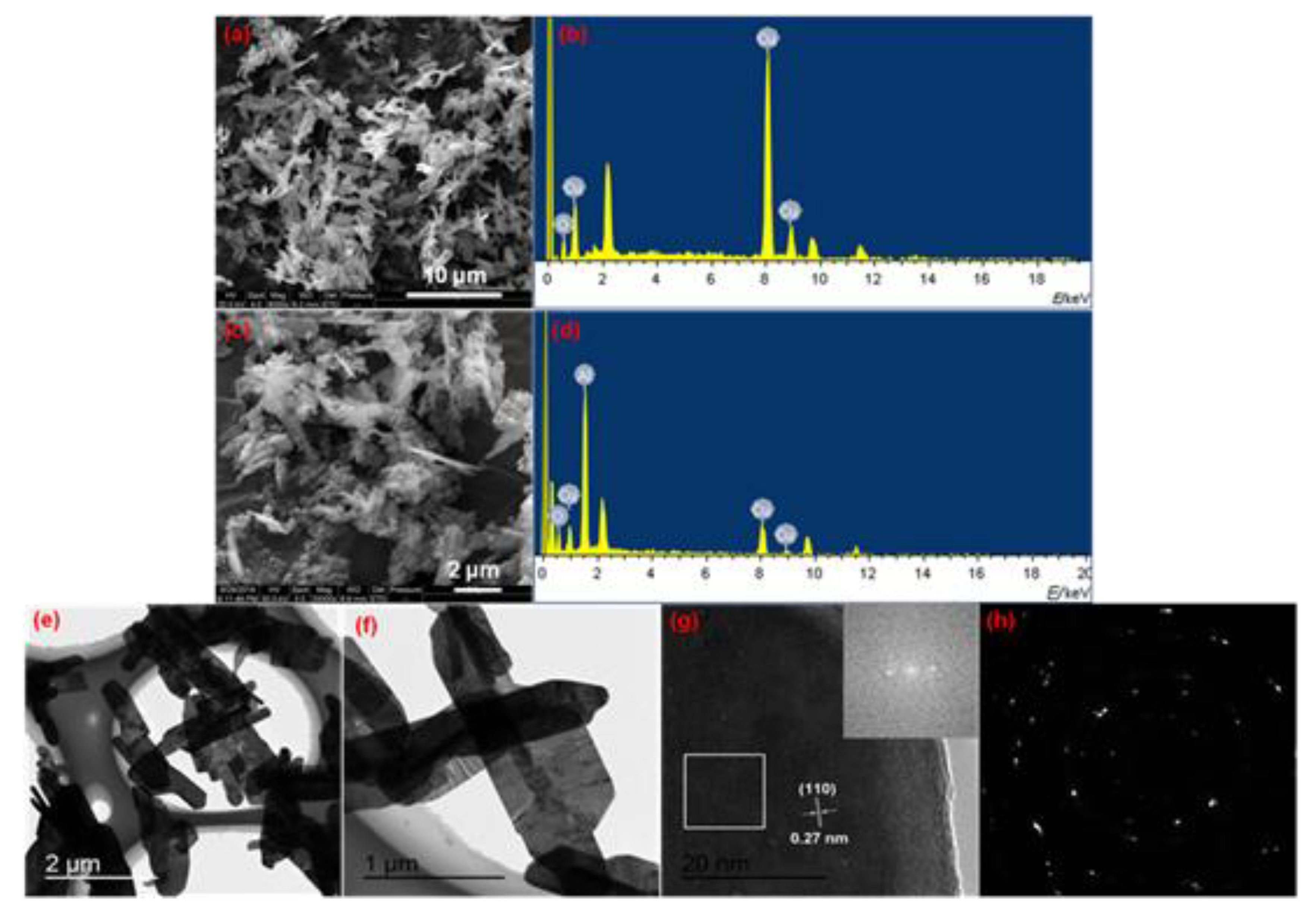
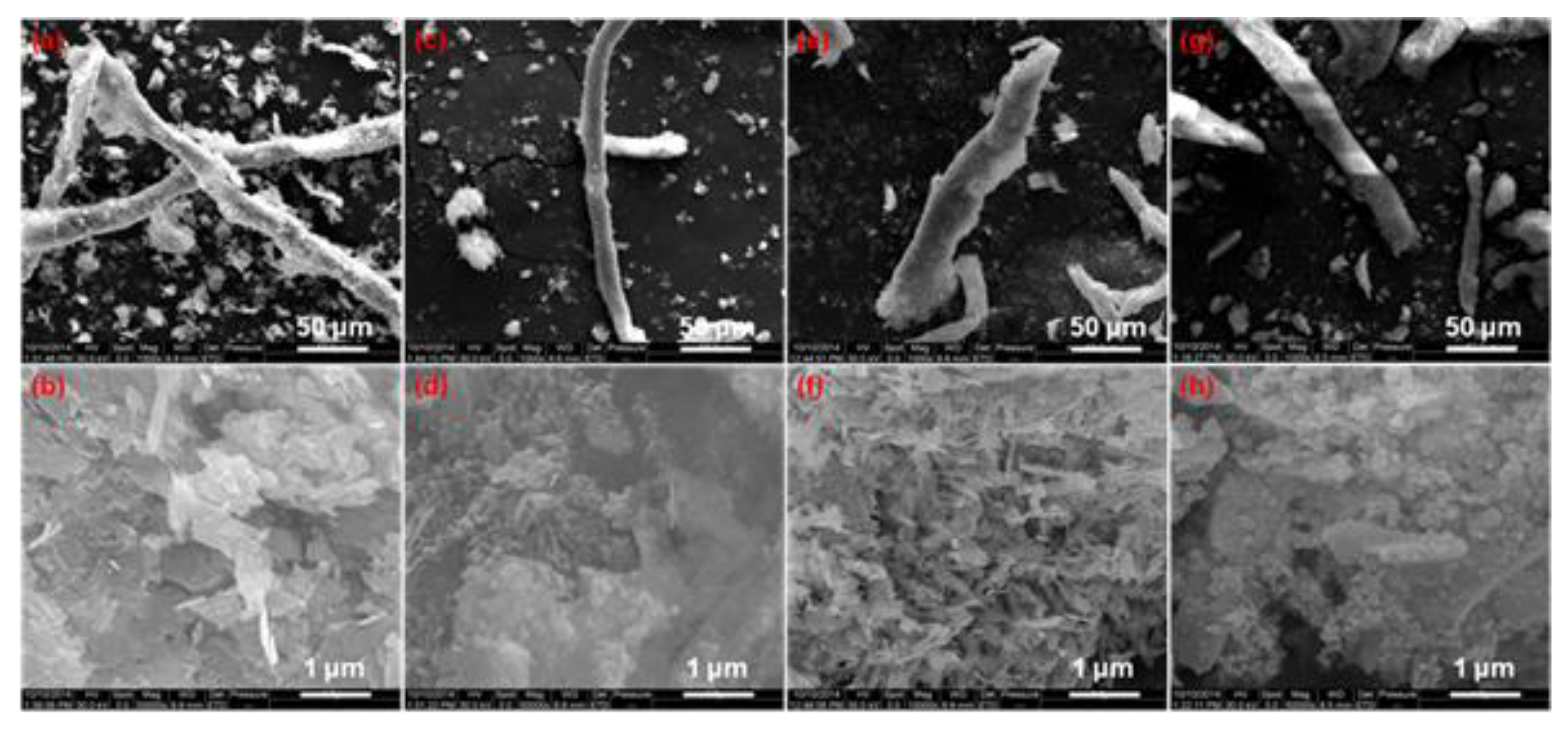
3.1. Morphology and Structure
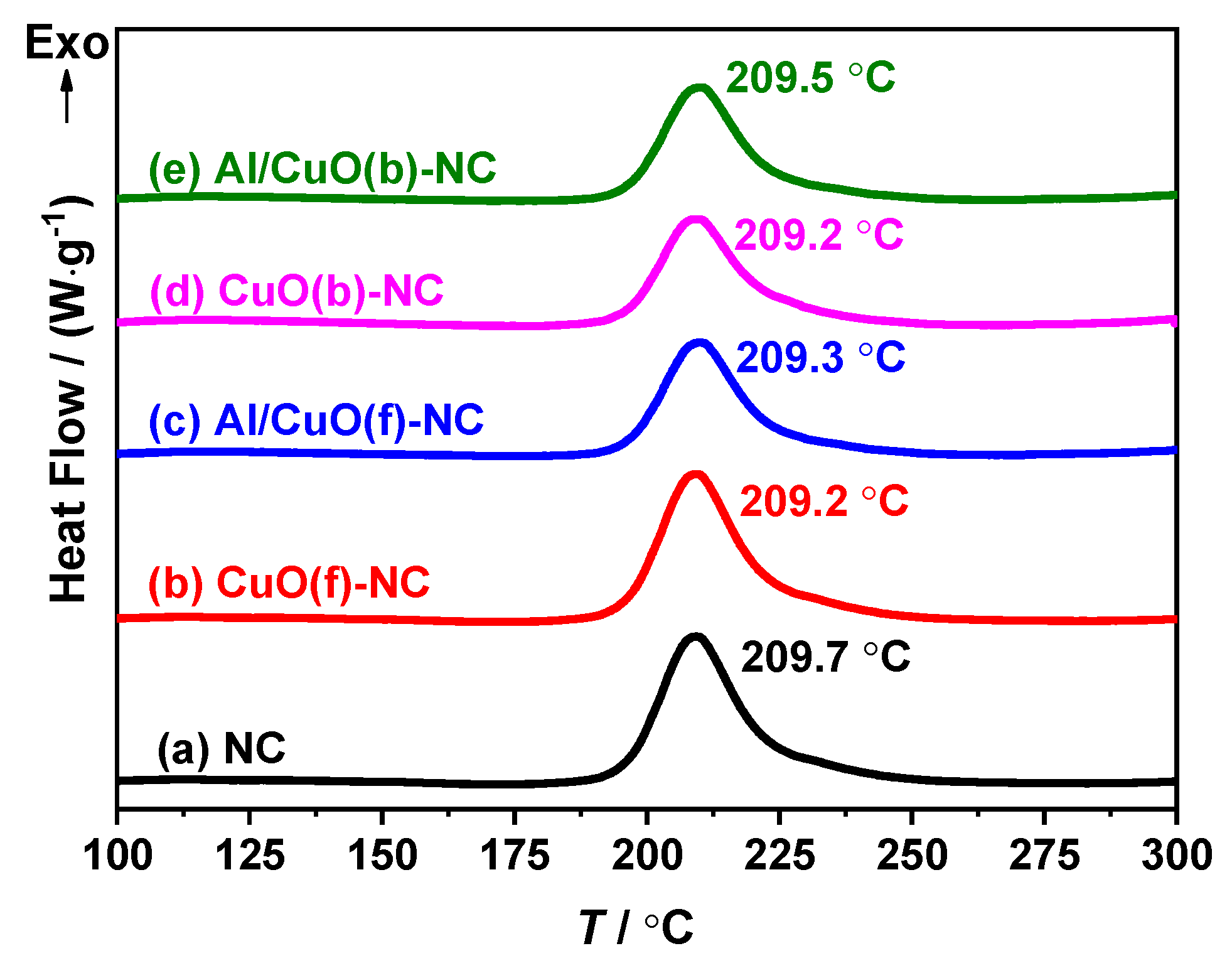
3.2. Effect of CuO and Al/CuO on Thermal Decomposition of NC
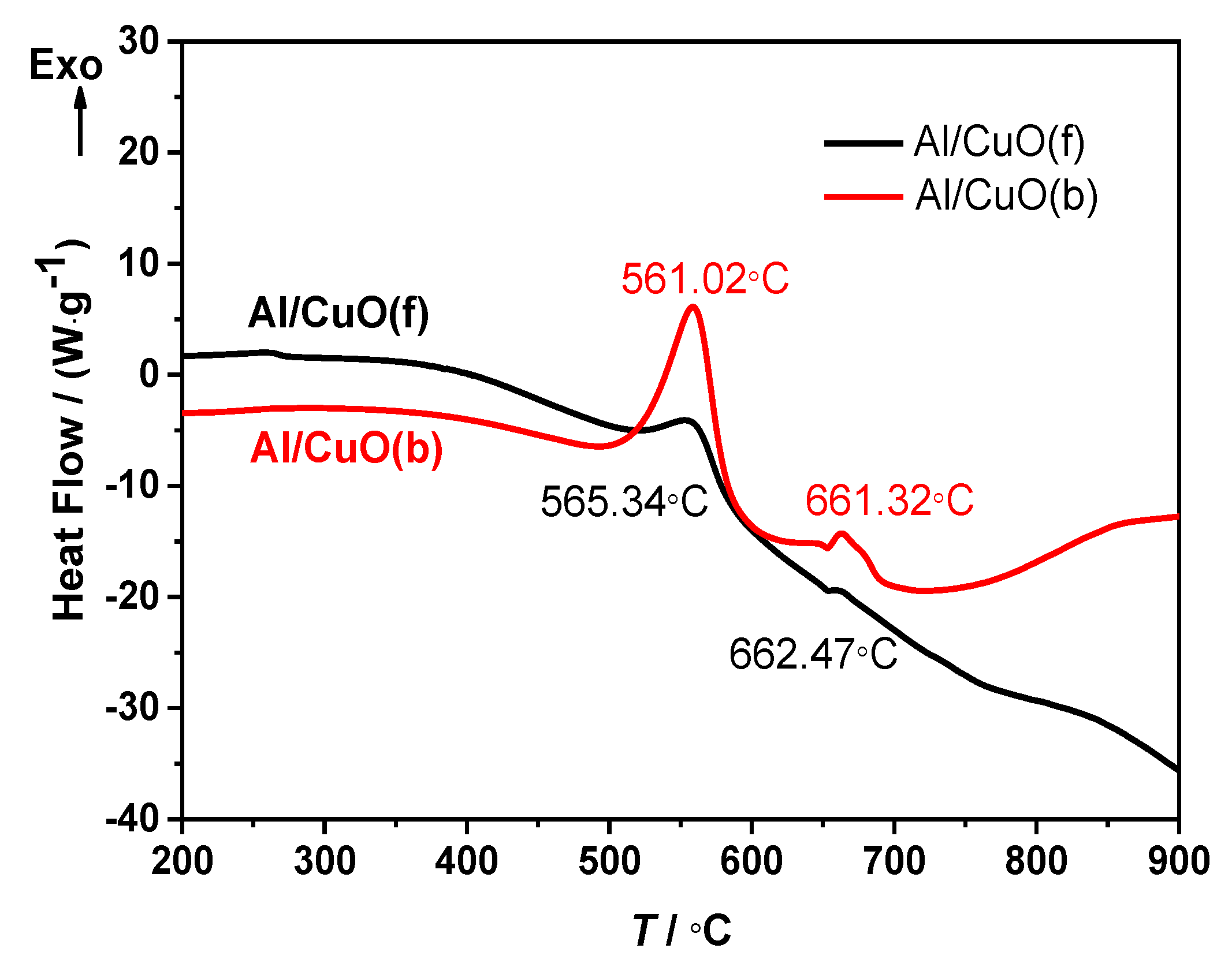
3.2.1. Compatibility Analysis
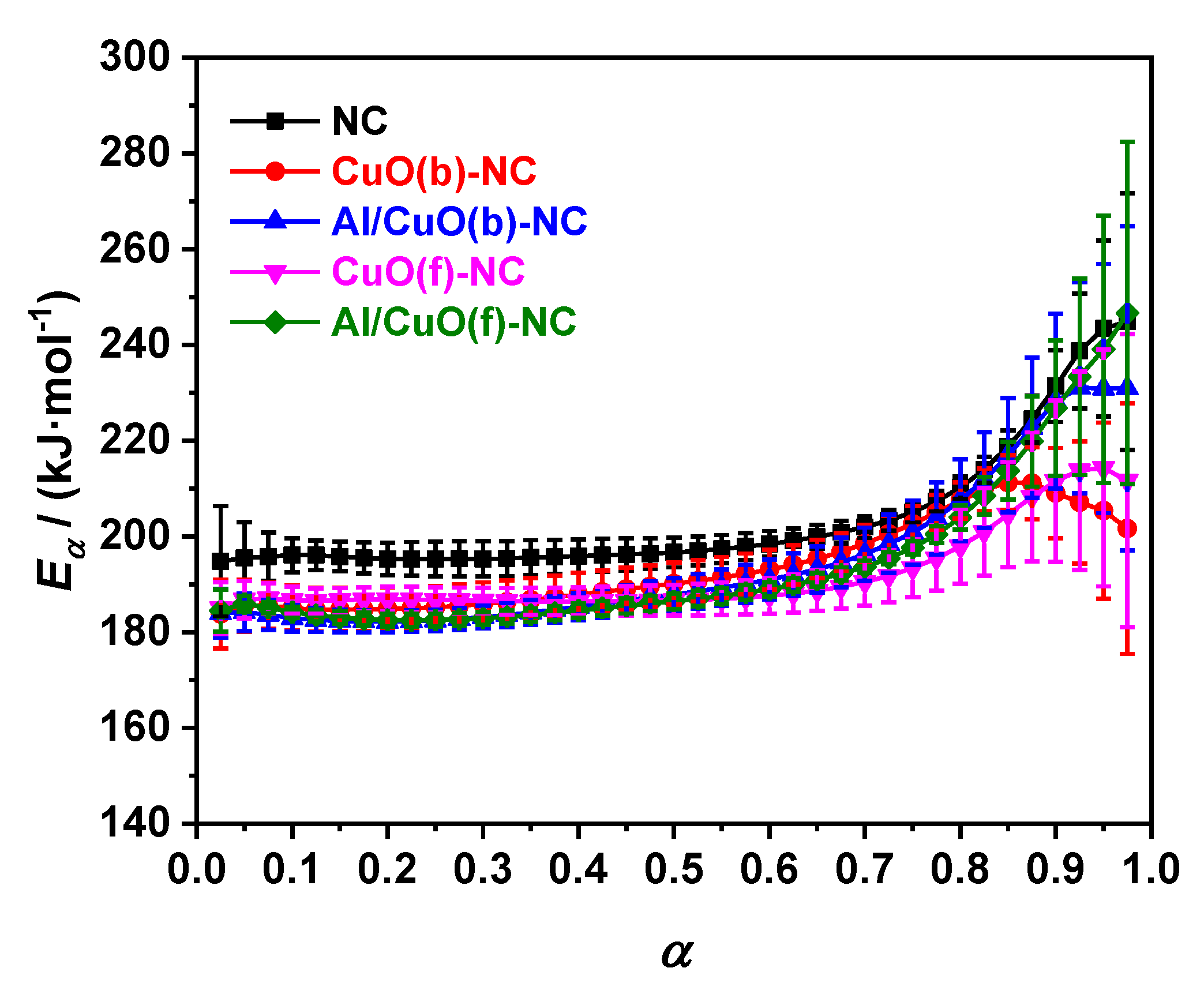
3.2.2. Non-isothermal Kinetic Analysis
3.2.3. Thermal Safety Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jia, S.; Wang, Y.; Liu, X.; Zhao, S.; Zhao, W.; Huang, Y.; Li, Z.; Lin, Z. Hierarchically porous CuO nano-labyrinths as binder-free anodes for long-life and high-rate lithium ion batteries. Nano Energy 2019, 59, 229–236. [Google Scholar] [CrossRef]
- Tan, G.; Wu, F.; Yuan, Y.; Chen, R.; Zhao, T.; Yao, Y.; Qian, J.; Liu, J.; Ye, Y.; Shahbazian-Yassar, R.; et al. Freestanding three-dimensional core–shell nanoarrays for lithium-ion battery anodes. Nat. Commun. 2016, 7, 11774. [Google Scholar] [CrossRef] [PubMed]
- Nakate, U.T.; Lee, G.H.; Ahmad, R.; Patil, P.; Hahn, Y.-B.; Yu, Y.T.; Suh, E.-k. Nano-bitter gourd like structured CuO for enhanced hydrogen gas sensor application. Int. J. Hydrog. Energy 2018, 43, 22705–22714. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Si, F.; Yao, C.; Du, M.; Hussain, I.; Kim, H.; Huang, S.; Lin, Z.; Hayat, W. Persulfate non-radical activation by nano-CuO for efficient removal of chlorinated organic compounds: Reduced graphene oxide-assisted and CuO (0 0 1) facet-dependent. Chem. Eng. J. 2019, 356, 178–189. [Google Scholar] [CrossRef]
- Wang, L.; Hou, J.; Liu, S.; Carrier, A.J.; Guo, T.; Liang, Q.; Oakley, D.; Zhang, X. CuO nanoparticles as haloperoxidase-mimics: Chloride-accelerated heterogeneous Cu-Fenton chemistry for H2O2 and glucose sensing. Sensor Actuat. B-CH. 2019, 287, 180–184. [Google Scholar] [CrossRef]
- Wang, Z.; Qureshi, N.; Yasin, S.; Mukhin, A.; Ressouche, E.; Zherlitsyn, S.; Skourski, Y.; Geshev, J.; Ivanov, V.; Gospodinov, M.; et al. Magnetoelectric effect and phase transitions in CuO in external magnetic fields. Nat. Commun. 2016, 7, 10295. [Google Scholar] [CrossRef]
- Pandas, H.M.; Fazli, M. Preparation and application of La2O3 and CuO nano particles as catalysts for ammonium perchlorate thermal decomposition. Propell. Explos. Pyrot. 2018, 43, 1096–1104. [Google Scholar] [CrossRef]
- Dolgachev, V.; Khaneft, A.; Mitrofanov, A. Ignition of organic explosive materials by a copper oxide film absorbing a laser pulse. Propell. Explos. Pyrot. 2018, 43, 992–998. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, S.; Tao, B.; Liu, X.; Lin, K.; Yang, Y.; Fan, R.; Xia, D.; Hao, D. Catalytic decomposition of ammonium perchlorate on hollow mesoporous CuO microspheres. Vacuum 2019, 159, 105–111. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Xu, J.; Ma, X.; Ye, Y.; Yang, G.; Zhang, K. In situ preparation of explosive embedded CuO/Al/CL20 nanoenergetic composite with enhanced reactivity. Chem. Eng. J. 2018, 354, 885–895. [Google Scholar] [CrossRef]
- Sharma, J.K.; Srivastava, P.; Singh, G.; Akhtar, M.S.; Ameen, S. Catalytic thermal decomposition of ammonium perchlorate and combustion of composite solid propellants over green synthesized CuO nanoparticles. Thermochim. Acta 2015, 614, 110–115. [Google Scholar] [CrossRef]
- Chowdhury, S.; Sullivan, K.; Piekiel, N.; Zhou, L.; Zachariah, M.R. Diffusive vs explosive reaction at the nanoscale. J. Phys. Chem C 2010, 114, 9191–9195. [Google Scholar] [CrossRef]
- Glavier, L.; Nicollet, A.; Jouot, F.; Martin, B.; Barberon, J.; Renaud, L.; Rossi, C. Nanothermite/RDX-based miniature device for impact ignition of high explosives. Propell. Explos. Pyrot. 2018, 42, 308–317. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X. Al/CuO composite coatings with nanorods structure assembled by electrophoretic deposition for enhancing energy released. Vacuum 2019, 163, 216–223. [Google Scholar] [CrossRef]
- Kim, K.J.; Cho, M.H.; Kim, J.H.; Kim, S.H. Effect of paraffin wax on combustion properties and surface protection of Al/CuO-based nanoenergetic composite pellets. Combust. Flame 2018, 198, 169–175. [Google Scholar] [CrossRef]
- Courty, L.; Lagrange, J.-F.; Gillard, P.; Boulnois, C. Laser ignition of a low vulnerability propellant based on nitrocellulose: Effects of Ar and N2 surrounding atmospheres. Propell. Explos. Pyrot. 2018, 43, 986–991. [Google Scholar] [CrossRef]
- Wei, R.; He, Y.; Zhang, Z.; He, J.; Yuen, R.; Wang, J. Effect of different humectants on the thermal stability and fire hazard of nitrocellulose. J. Therm. Anal. Calorim. 2018, 133, 1291–1307. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M. A simplified method to predict the heat release rate of industrial nitrocellulose materials. Appl. Sci. 2018, 8, 910. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Chelouche, S.; Derradji, M.; Bessa, W.; Mezroua, A. A promising energetic polymer from Posidonia oceanica brown algae: Synthesis, characterization, and kinetic modeling. Macromol. Chem. Phys. 2019, 220, 1900358. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F. Analytical methods for stability assessment of nitrate esters-based propellants. Crit. Rev. Anal. Chem. 2019, 49, 415–438. [Google Scholar] [CrossRef]
- Betzler, F.M.; Klapötke, T.M.; Sproll, S. Energetic nitrogen-rich polymers based on cellulose. Cent. Eur. J. Energy Mater. 2011, 8, 157–171. [Google Scholar]
- Luo, L.; Jin, B.; Xiao, Y.; Zhang, Q.; Chai, Z.; Huang, Q.; Chu, S.; Peng, R. Study on the isothermal decomposition kinetics and mechanism of nitrocellulose. Polym. Test. 2019, 75, 337–343. [Google Scholar] [CrossRef]
- Trache, D.; Maggi, F.; Palmucci, I.; DeLuca, L.T. Thermal behavior and decomposition kinetics of composite solid propellants in the presence of amide burning rate suppressants. J. Therm. Anal. Calorim. 2018, 132, 1601–1615. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F. Stabilizers for nitrate ester-based energetic materials and their mechanism of action: A state-of-the-art review. J. Mater. Sci. 2018, 53, 100–123. [Google Scholar] [CrossRef]
- Chelouche, S.; Trache, D.; Tarchoun, A.F.; Khimeche, K. Effect of organic eutectic on nitrocellulose stability during artificial aging. J. Energy Mater. 2019, 37, 387–406. [Google Scholar] [CrossRef]
- Chelouche, S.; Trache, D.; Tarchoun, A.F.; Abdelaziz, A.; Khimeche, K.; Mezroua, A. Organic eutectic mixture as efficient stabilizer for nitrocellulose: Kinetic modeling and stability assessment. Thermochim. Acta 2019, 673, 78–91. [Google Scholar] [CrossRef]
- Zhao, N.; Li, J.; Gong, H.; An, T.; Zhao, F.; Yang, A.; Hu, R.; Ma, H. Effects of α-Fe2O3 nanoparticles on the thermal behavior and non-isothermal decomposition kinetics of nitrocellulose. J. Anal. Appl. Pyrol. 2016, 120, 165–173. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, N.; Zhang, T.; Gong, H.; Ma, H.; An, T.; Zhao, F.; Hu, R. Compatibility and thermal decomposition mechanism of nitrocellulose/Cr2O3 nanoparticles studied using DSC and TG-FTIR. RSC Adv. 2019, 9, 3927–3937. [Google Scholar] [CrossRef]
- Chelouche, S.; Trache, D.; Tarchoun, A.F.; Abdelaziz, A.; Khimeche, K. Compatibility assessment and decomposition kinetics of nitrocellulose with eutectic mixture of organic stabilizers. J. Energy Mater. 2020, 38, 48–67. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Chelouche, S.; Khimeche, K. New insights on the compatibility of nitrocellulose with aniline-based compounds. Propell. Explos. Pyrot. 2019, 44, 970–979. [Google Scholar] [CrossRef]
- Yousef, M.A.; Hudson, M.K.; Berry, B.C. Study on the compatibility of azo-tetrazolate high-energy materials using DSC. J. Therm. Anal. Calorim. 2018, 133, 1481–1490. [Google Scholar] [CrossRef]
- Huang, H.; Shi, Y.; Yang, J.; Li, B. Compatibility study of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) with some energetic materials and inert materials. J. Energy Mater. 2015, 33, 66–72. [Google Scholar] [CrossRef]
- Liu, Z. Thermal Analyses for Energetic Materials; National Defense Industry Press: Beijing, China, 2008; pp. 21–22. [Google Scholar]
- An, T.; Zhao, F.; Pei, Q.; Xiao, L.; Xu, S.; Gao, H.; Xing, X. Preparation, characterization and combustion catalytic activity of nanopartical super thermites. Chin. J. Inorg. Chem. 2011, 27, 231–238. [Google Scholar]
- Zhao, F.; Yi, J.; An, T.; Wang, Y.; Hong, W. Combustion Catalysts for Solid Propellant; National Defense Industry Press: Beijing, China, 2016; pp. 90–120. [Google Scholar]
- Prentice, D.; Pantoya, M.L.; Clapsaddle, B.J. Effect of nanocomposite synthesis on the combustion performance of a ternary thermite. J. Phys. Chem. B 2005, 109, 20180–20185. [Google Scholar] [CrossRef] [PubMed]
- Shende, R.; Subramanian, S.; Hasan, S.; Apperson, S.; Thiruvengadathan, R.; Gangopadhyay, K.; Gangopadhyay, S.; Redner, P.; Kapoor, D.; Nicolich, S.; et al. Nanoenergetic composites of CuO nanorods, nanowires, and Al-nanoparticles. Propell. Explos. Pyrot. 2008, 33, 122–130. [Google Scholar] [CrossRef]
- Criado, J.M.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Critical study of the isoconversional methods of kinetic analysis. J. Therm. Anal. Calorim. 2008, 92, 199–203. [Google Scholar] [CrossRef]
- Ma, H.; Song, J.; Hu, R.; Pan, Q.; Wang, Y. Non-isothermal decomposition kinetics, thermal behavior and computational detonation properties on 4-amino-1,2,4-triazol-5-one (ATO). J. Anal. Appl. Pyrol. 2008, 83, 145–150. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Trache, D. Comments on “Thermal degradation behavior of hypochlorite-oxidized starch nanocrystals under different oxidized levels”. Carbohydr. Polym. 2016, 151, 535–537. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Nanoclay nucleation effect in the thermal stabilization of a polymer nanocomposite: A kinetic mechanism change. J. Phys. Chem. C 2012, 116, 11797–11807. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, R.; Xie, Y.; Li, F. The estimation of critical temperatures of thermal explosion for energetic materials using non-isothermal DSC. Thermochim. Acta 1994, 244, 171–176. [Google Scholar]
- Yi, J.; Zhao, F.; Wang, B.; Liu, Q.; Zhou, C.; Hu, R.; Ren, Y.; Xu, S.; Xu, K.; Ren, X. Thermal behaviors, nonisothermal decomposition reaction kinetics, thermal safety and burning rates of BTATz-CMDB propellant. J. Hazard. Mater. 2010, 181, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Muravyev, N.V.; Kiselev, V.G. Cheaper, faster, or better: Are simple estimations of safety parameters of hazardous materials reliable? J. Hazard. Mater. 2017, 334, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Gao, S.; Zhao, F.; Shi, Q.; Zhang, T.; Zhang, J. Thermal Analysis Kinetics, 2nd ed.; Science Press: Beijing, China, 2008; pp. 148–167. [Google Scholar]
- Peng, L.; Yao, Q.; Wang, J.; Li, Z.; Zhu, Q.; Li, X. Pyrolysis of RDX and its derivatives via reactive molecular dynamics simulations. Acta Phys. Chim. Sin. 2017, 33, 745–754. [Google Scholar]

| Method | β/(°C∙min−1) | NC | CuO(b)-NC | Al/CuO(b)-NC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea/(kJ·mol−1) | lg(A/s−1) | r | Ea/(kJ·mol−1) | lg(A/s−1) | r | Ea/(kJ·mol−1) | lg(A/s−1) | r | ||
| MacCallum-Tanner | 5.0 | 208.0 ± 3.5 | 20.6 ± 0.4 | 0.9983 | 180.3 ± 5.3 | 17.5 ± 0.5 | 0.9927 | 176.2 ± 5.1 | 17.1 ± 0.4 | 0.9917 |
| 10.0 | 205.4 ± 4.2 | 20.3 ± 0.4 | 0.9984 | 183.3 ± 5.8 | 17.9 ± 0.2 | 0.9935 | 184.3 ± 4.7 | 18.0 ± 0.3 | 0.9920 | |
| 15.0 | 209.3 ± 3.4 | 20.7 ± 0.4 | 0.9988 | 187.3 ± 5.7 | 18.3 ± 0.4 | 0.9930 | 184.2 ± 5.8 | 18.0 ± 0.4 | 0.9925 | |
| 20.0 | 209.3 ± 2.7 | 20.7 ± 0.6 | 0.9987 | 182.6 ± 4.8 | 17.8 ± 0.6 | 0.9958 | 184.6 ± 5.7 | 18.0 ± 0.2 | 0.9927 | |
| 25.0 | 211.8 ± 5.1 | 20.9 ± 0.4 | 0.9990 | 192.6 ± 4.8 | 18.9 ± 0.4 | 0.9934 | 181.7 ± 4.4 | 17.7 ± 0.4 | 0.9943 | |
| 30.0 | 210.1 ± 4.5 | 20.8 ± 0.4 | 0.9982 | 187.7 ± 4.1 | 18.4 ± 0.3 | 0.9962 | 182.3 ± 4.3 | 17.8 ± 0.3 | 0.9959 | |
| Šatava-Šesták | 5.0 | 204.6 ± 3.9 | 20.2 ± 0.4 | 0.9983 | 178.4 ± 3.2 | 17.3 ± 0.6 | 0.9927 | 174.6 ± 3.7 | 16.9 ± 0.4 | 0.9917 |
| 10.0 | 202.1 ± 5.2 | 19.9 ± 0.6 | 0.9984 | 181.2 ± 4.0 | 17.7 ± 0.5 | 0.9935 | 182.2 ± 6.0 | 17.8 ± 0.3 | 0.9920 | |
| 15.0 | 205.8 ± 5.4 | 20.4 ± 0.3 | 0.9988 | 185.0 ± 5.1 | 18.1 ± 0.3 | 0.9930 | 182.1 ± 6.7 | 17.8 ± 0.2 | 0.9925 | |
| 20.0 | 205.8 ± 4.2 | 20.3 ± 0.3 | 0.9987 | 180.6 ± 4.7 | 17.6 ± 0.5 | 0.9958 | 182.5 ± 6.3 | 17.8 ± 0.4 | 0.9927 | |
| 25.0 | 208.1 ± 3.6 | 20.6 ± 0.5 | 0.9990 | 190.0 ± 5.6 | 18.7 ± 0.3 | 0.9934 | 179.8 ± 5.3 | 17.5 ± 0.2 | 0.9943 | |
| 30.0 | 206.6 ± 5.6 | 20.4 ± 0.4 | 0.9982 | 185.4 ± 5.0 | 18.1 ± 0.3 | 0.9962 | 180.3 ± 4.9 | 17.6 ± 0.3 | 0.9959 | |
| Agrawal | 5.0 | 207.2 ± 5.6 | 20.5 ± 0.5 | 0.9982 | 179.7 ± 4.0 | 17.5 ± 0.3 | 0.9920 | 175.7 ± 4.5 | 17.1 ± 0.3 | 0.9909 |
| 10.0 | 204.5 ± 5.3 | 20.2 ± 0.6 | 0.9983 | 182.6 ± 4.8 | 17.8 ± 0.5 | 0.9929 | 183.6 ± 4.0 | 17.9 ± 0.3 | 0.9913 | |
| 15.0 | 208.4 ± 4.8 | 20.6 ± 0.4 | 0.9987 | 186.5 ± 5.8 | 18.3 ± 0.5 | 0.9924 | 183.4 ± 3.4 | 17.9 ± 0.2 | 0.9919 | |
| 20.0 | 208.3 ± 4.0 | 20.6 ± 0.6 | 0.9986 | 181.9 ± 3.5 | 17.8 ± 0.3 | 0.9954 | 183.8 ± 4.6 | 18.0 ± 0.3 | 0.9920 | |
| 25.0 | 210.7 ± 5.2 | 20.8 ± 0.6 | 0.9990 | 191.7 ± 4.4 | 18.8 ± 0.5 | 0.9928 | 180.9 ± 3.6 | 17.6 ± 0.2 | 0.9938 | |
| 30.0 | 209.0 ± 4.7 | 20.7 ± 0.6 | 0.9981 | 186.8 ± 6.7 | 18.3 ± 0.5 | 0.9958 | 181.5 ± 4.8 | 17.7 ± 0.4 | 0.9955 | |
| General integral | 5.0 | 205.8 ± 6.2 | 19.0 ± 0.4 | 0.9985 | 178.3 ± 6.7 | 16.0 ± 0.6 | 0.9919 | 174.3 ± 5.3 | 15.6 ± 0.2 | 0.9908 |
| 10.0 | 203.3 ± 6.08 | 18.7 ± 0.3 | 0.9983 | 181.3 ± 6.9 | 16.4 ± 0.3 | 0.9928 | 182.3 ± 5.9 | 16.5 ± 0.3 | 0.9912 | |
| 15.0 | 207.2 ± 4.9 | 19.1 ± 0.5 | 0.9987 | 185.3 ± 6.8 | 16.8 ± 0.5 | 0.9923 | 182.2 ± 6.1 | 16.5 ± 0.3 | 0.9918 | |
| 20.0 | 207.2 ± 5.3 | 19.1 ± 0.4 | 0.9986 | 180.7 ± 3.8 | 16.3 ± 0.5 | 0.9954 | 182.7 ± 5.7 | 16.5 ± 0.4 | 0.9920 | |
| 25.0 | 209.6 ± 6.2 | 19.3 ± 0.6 | 0.9989 | 190.6 ± 4.4 | 17.4 ± 0.3 | 0.9928 | 179.8 ± 4.3 | 16.2 ± 0.3 | 0.9937 | |
| 30.0 | 208.0 ± 4.7 | 19.2 ± 0.5 | 0.9981 | 185.8 ± 3.5 | 16.9 ± 0.4 | 0.9958 | 180.4 ± 5.9 | 16.3 ± 0.4 | 0.9955 | |
| Universal integral | 5.0 | 207.2 ± 4.3 | 20.5 ± 0.6 | 0.9982 | 179.7 ± 6.9 | 17.5 ± 0.4 | 0.9920 | 175.7 ± 3.9 | 17.1 ± 0.2 | 0.9909 |
| 10.0 | 204.5 ± 5.3 | 20.2 ± 0.3 | 0.9983 | 182.6 ± 6.5 | 17.8 ± 0.5 | 0.9929 | 183.6 ± 4.8 | 17.9 ± 0.2 | 0.9913 | |
| 15.0 | 208.4 ± 4.6 | 20.6 ± 0.5 | 0.9987 | 186.5 ± 5.1 | 18.3 ± 0.3 | 0.9924 | 183.4 ± 5.7 | 17.9 ± 0.4 | 0.9919 | |
| 20.0 | 208.3 ± 3.9 | 20.6 ± 0.4 | 0.9986 | 181.9 ± 6.1 | 17.8 ± 0.3 | 0.9954 | 183.8 ± 3.5 | 18.0 ± 0.4 | 0.9920 | |
| 25.0 | 210.7 ± 5.4 | 20.8 ± 0.6 | 0.9990 | 191.7 ± 6.1 | 18.8 ± 0.4 | 0.9928 | 180.9 ± 5.5 | 17.7 ± 0.2 | 0.9938 | |
| 30.0 | 209.0 ± 6.1 | 20.7 ± 0.4 | 0.9981 | 186.8 ± 5.6 | 18.3 ± 0.5 | 0.9958 | 181.5 ± 4.4 | 17.7 ± 0.4 | 0.9955 | |
| Mean | 207.5 ± 4.8 | 20.2 ± 0.5 | 184.5 ± 5.2 | 17.8 ± 0.4 | 181.1 ± 5.0 | 17.4 ± 0.3 | ||||
| Flynn-Wall-Ozawa | 185.7 ± 1.9 (EeO) | 0.9998 | 178.7 ± 3.6 (EeO) | 0.9919 | 176.3 ± 5.3 (EeO) | 0.9982 | ||||
| 197.6 ± 6.4 (EpO) | 0.9979 | 187.0 ± 4.8 (EpO) | 0.9987 | 183.6 ± 2.6 (EpO) | 0.9996 | |||||
| Kissinger | 199.7 ± 6.8 (EK) | 19.8 ± 0.7 | 0.9977 | 188.6 ± 5.0 (EK) | 18.6 ± 0.6 | 0.9986 | 185.0 ± 2.7 (EK) | 18.2 ± 0.3 | 0.9996 | |
| Mean(EeO, EpO, EK) | 194.3 ± 5.0 | 184.8 ± 4.5 | 181.6 ± 3.5 | |||||||
| Method | β/(°C∙min−1) | CuO(f)-NC | Al/CuO(f)-NC | ||||
|---|---|---|---|---|---|---|---|
| Ea/(kJ·mol−1) | lg(A/s−1) | r | Ea/(kJ·mol−1) | lg(A/s−1) | r | ||
| MacCallum-Tanner | 5.0 | 190.4 ± 6.6 | 18.6 ± 0.4 | 0.9917 | 177.6 ± 5.7 | 17.2 ± 0.6 | 0.9889 |
| 10.0 | 197.2 ± 4.0 | 19.3 ± 0.4 | 0.9921 | 177.7 ± 3.5 | 17.2 ± 0.6 | 0.9920 | |
| 15.0 | 192.0 ± 5.8 | 18.8 ± 0.5 | 0.9928 | 176.3 ± 4.7 | 17.1 ± 0.5 | 0.9936 | |
| 20.0 | 202.3 ± 6.8 | 19.9 ± 0.6 | 0.9932 | 187.8 ± 4.9 | 18.4 ± 0.4 | 0.9952 | |
| 25.0 | 189.1 ± 6.1 | 18.4 ± 0.5 | 0.9925 | 179.9 ± 3.9 | 17.5 ± 0.4 | 0.9930 | |
| 30.0 | 198.0 ± 3.7 | 19.4 ± 0.6 | 0.9941 | 190.6 ± 5.5 | 18.7 ± 0.4 | 0.9942 | |
| Šatava-Šesták | 5.0 | 187.9 ± 6.7 | 18.4 ± 0.5 | 0.9917 | 175.9 ± 3.3 | 17.1 ± 0.5 | 0.9889 |
| 10.0 | 194.4 ± 5.7 | 19.1 ± 0.4 | 0.9921 | 176.0 ± 4.5 | 17.1 ± 0.2 | 0.9920 | |
| 15.0 | 189.5 ± 5.3 | 18.5 ± 0.5 | 0.9928 | 174.7 ± 3.3 | 17.0 ± 0.4 | 0.9936 | |
| 20.0 | 199.2 ± 4.1 | 19.6 ± 0.5 | 0.9932 | 185.5 ± 6.1 | 18.2 ± 0.5 | 0.9952 | |
| 25.0 | 186.7 ± 6.7 | 18.2 ± 0.5 | 0.9925 | 178.0 ± 6.1 | 17.3 ± 0.5 | 0.9930 | |
| 30.0 | 195.1 ± 5.1 | 19.1 ± 0.5 | 0.9941 | 188.1 ± 3.8 | 18.4 ± 0.3 | 0.9942 | |
| Agrawal | 5.0 | 189.7 ± 4.7 | 18.6 ± 0.5 | 0.9910 | 177.1 ± 6.6 | 17.2 ± 0.3 | 0.9879 |
| 10.0 | 196.4 ± 3.7 | 19.3 ± 0.6 | 0.9915 | 177.1 ± 5.4 | 17.2 ± 0.5 | 0.9913 | |
| 15.0 | 191.2 ± 6.4 | 18.7 ± 0.5 | 0.9921 | 175.6 ± 5.3 | 17.1 ± 0.3 | 0.9929 | |
| 20.0 | 201.3 ± 5.5 | 19.8 ± 0.5 | 0.9926 | 187.0 ± 3.8 | 18.3 ± 0.3 | 0.9947 | |
| 25.0 | 188.2 ± 5.1 | 18.4 ± 0.5 | 0.9918 | 179.1 ± 3.7 | 17.4 ± 0.5 | 0.9923 | |
| 30.0 | 196.9 ± 3.2 | 19.3 ± 0.4 | 0.9936 | 189.7 ± 3.5 | 18.6 ± 0.3 | 0.9937 | |
| General integral | 5.0 | 188.4 ± 5.3 | 17.1 ± 0.5 | 0.9909 | 175.6 ± 4.6 | 15.7 ± 0.3 | 0.9878 |
| 10.0 | 195.1 ± 4.7 | 17.8 ± 0.6 | 0.9914 | 175.8 ± 4.2 | 15.8 ± 0.3 | 0.9912 | |
| 15.0 | 190.1 ± 6.1 | 17.3 ± 0.5 | 0.9921 | 174.4 ± 5.3 | 15.6 ± 0.4 | 0.9929 | |
| 20.0 | 200.3 ± 6.5 | 18.4 ± 0.5 | 0.9925 | 185.9 ± 5.8 | 16.9 ± 0.5 | 0.9947 | |
| 25.0 | 187.2 ± 3.8 | 16.9 ± 0.4 | 0.9918 | 178.0 ± 5.0 | 16.0 ± 0.6 | 0.9922 | |
| 30.0 | 196.0 ± 5.5 | 17.9 ± 0.6 | 0.9936 | 188.6 ± 3.2 | 17.2 ± 0.4 | 0.9936 | |
| Universal integral | 5.0 | 201.3 ± 4.1 | 19.8 ± 0.6 | 0.9926 | 177.1 ± 5.5 | 17.2 ± 0.4 | 0.9879 |
| 10.0 | 196.4 ± 3.4 | 19.3 ± 0.4 | 0.9915 | 177.1 ± 5.0 | 17.2 ± 0.4 | 0.9913 | |
| 15.0 | 190.1 ± 4.0 | 17.3 ± 0.4 | 0.9921 | 175.6 ± 6.5 | 17.1 ± 0.4 | 0.9929 | |
| 20.0 | 191.2 ± 6.3 | 18.7 ± 0.5 | 0.9921 | 187.0 ± 6.8 | 18.3 ± 0.3 | 0.9947 | |
| 25.0 | 188.2 ± 3.5 | 18.4 ± 0.6 | 0.9918 | 179.1 ± 6.7 | 17.4 ± 0.3 | 0.9923 | |
| 30.0 | 196.9 ± 4.1 | 19.3 ± 0.5 | 0.9936 | 189.7 ± 3.4 | 18.6 ± 0.3 | 0.9937 | |
| Mean | 193.5 ± 5.1 | 18.7 ± 0.5 | 180.6 ± 4.9 | 17.3 ± 0.4 | |||
| Flynn-Wall-Ozawa | 177.8 ± 5.1 (EeO) | 0.9983 | 171.5 ± 6.8 (EeO) | 0.9968 | |||
| 183.7 ± 8.3 (EpO) | 0.9959 | 185.9 ± 3.9 (EpO) | 0.9991 | ||||
| Kissinger | 185.1 ± 8.7 (EK) | 18.2 ± 1.0 | 0.9956 | 187.4 ± 4.1 (EK) | 18.5 ± 0.4 | 0.9990 | |
| Mean(EeO, EpO, EK) | 182.2 ± 7.4 | 181.6 ± 4.9 | |||||
| Sample | Ea/(kJ·mol−1) | lg(A/s−1) | Te0/°C | Tp0/°C | Tbe0/°C | Tbp0/°C | ΔS≠/(J∙mol−1∙K−1) | ΔH≠/(kJ∙mol−1) | ΔG≠/(kJ∙mol−1) |
|---|---|---|---|---|---|---|---|---|---|
| NC | 207.5 | 20.2 | 181.8 | 197.0 | 191.4 | 206.7 | 138.4 | 199.7 | 134.6 |
| CuO(b)-NC | 184.5 | 17.8 | 176.4 | 189.3 | 180.2 | 193.5 | 91.7 | 188.6 | 146.2 |
| Al/CuO(b)-NC | 181.1 | 17.4 | 169.3 | 184.4 | 178.6 | 194.3 | 84.5 | 185.0 | 146.3 |
| CuO(f)-NC | 193.5 | 18.7 | 178.6 | 190.0 | 189.3 | 206.3 | 109.1 | 185.0 | 133.9 |
| Al/CuO(f)-NC | 180.6 | 17.3 | 152.9 | 191.8 | 161.3 | 201.9 | 83.2 | 187.4 | 148.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, E.; Zhao, N.; Qin, Z.; Ma, H.; Li, H.; Xu, S.; An, T.; Yi, J.; Zhao, F. Thermal Decomposition Behavior and Thermal Safety of Nitrocellulose with Different Shape CuO and Al/CuO Nanothermites. Nanomaterials 2020, 10, 725. https://doi.org/10.3390/nano10040725
Yao E, Zhao N, Qin Z, Ma H, Li H, Xu S, An T, Yi J, Zhao F. Thermal Decomposition Behavior and Thermal Safety of Nitrocellulose with Different Shape CuO and Al/CuO Nanothermites. Nanomaterials. 2020; 10(4):725. https://doi.org/10.3390/nano10040725
Chicago/Turabian StyleYao, Ergang, Ningning Zhao, Zhao Qin, Haixia Ma, Haijian Li, Siyu Xu, Ting An, Jianhua Yi, and Fengqi Zhao. 2020. "Thermal Decomposition Behavior and Thermal Safety of Nitrocellulose with Different Shape CuO and Al/CuO Nanothermites" Nanomaterials 10, no. 4: 725. https://doi.org/10.3390/nano10040725
APA StyleYao, E., Zhao, N., Qin, Z., Ma, H., Li, H., Xu, S., An, T., Yi, J., & Zhao, F. (2020). Thermal Decomposition Behavior and Thermal Safety of Nitrocellulose with Different Shape CuO and Al/CuO Nanothermites. Nanomaterials, 10(4), 725. https://doi.org/10.3390/nano10040725




