Functional CdS-Au Nanocomposite for Efficient Photocatalytic, Photosensitizing, and Two-Photon Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. CdS NPs
2.1.2. CdS-Au Hybrid Nanosystems
2.1.3. Au Nanostructures
2.2. Characterization Methods
2.2.1. Morphology and Spectroscopy Characterization
2.2.2. Photocatalytic Activity Measurements
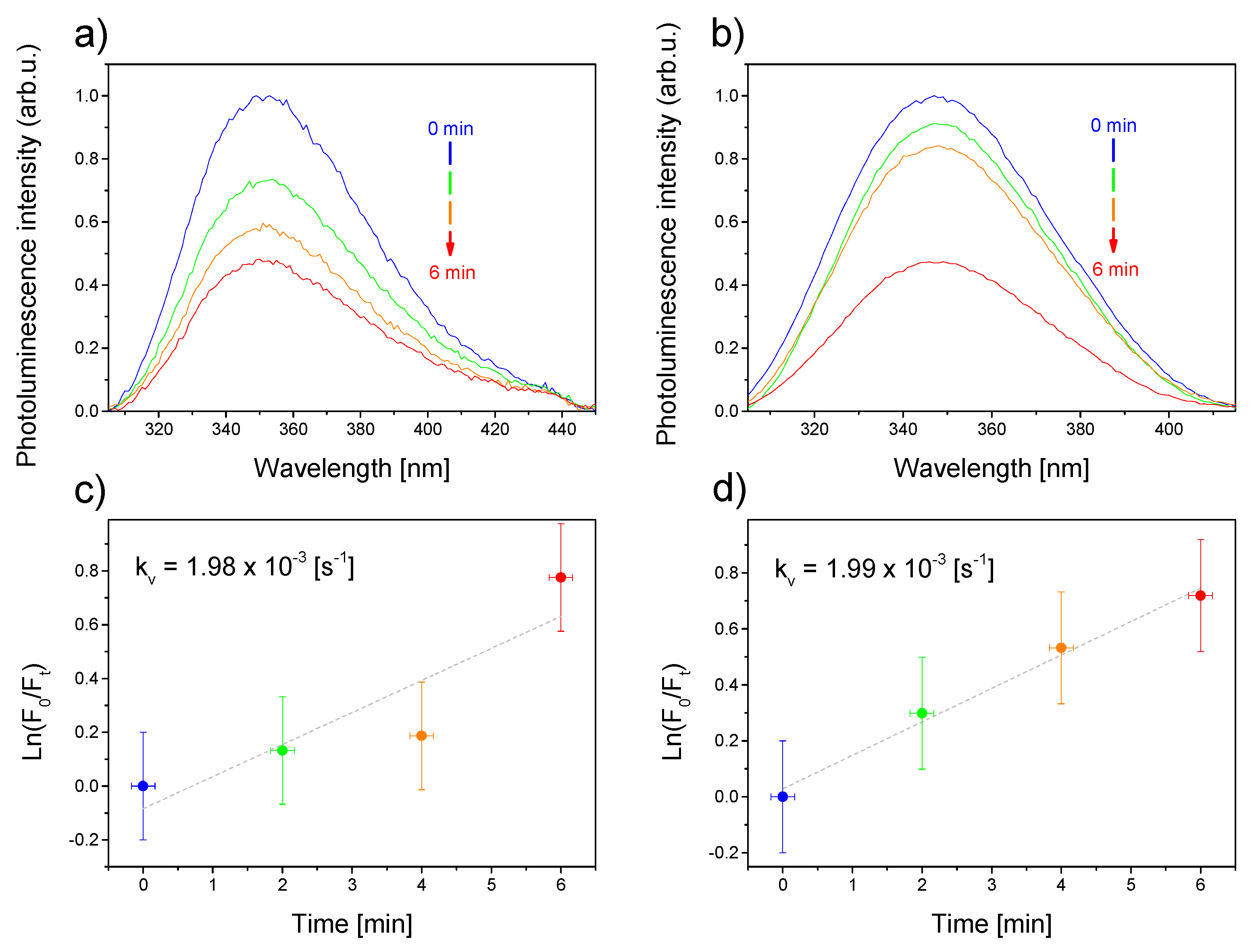
2.2.3. Reactive Oxygen Species Generation Measurements
2.2.4. Two-Photon Absorption Cross-Section Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wawrzyńczyk, D.; Bazylińska, U.; Lamch, Ł.; Kulbacka, J.; Szewczyk, A.; Bednarkiewicz, A.; Wilk, K.A.; Samoć, M. Förster Resonance Energy Transfer-Activated Processes in Smart Nanotheranostics Fabricated in a Sustainable Manner. ChemSusChem 2018, 12, 706–719. [Google Scholar]
- Wang, H.; Liang, X.; Wang, J.; Jiao, S.; Xue, D. Multifunctional inorganic nanomaterials for energy applications. Nanoscale 2019, 12, 14–42. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Singhania, T.; Singh, A.; Sharma, S.; Rani, S.; Neogy, A.; Yadav, S.R.; Sangal, V.K.; Garg, N. Photocatalytic Degradation of Bisphenol-A using N, Co Codoped TiO2 Catalyst under Solar Light. Sci. Rep. 2019, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zeng, G.; Huang, D.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Olowoyo, J.O.; Kumar, M.; Jain, S.L.; Babalola, J.O.; Vorontsov, A.V.; Kumar, U. Insights into Reinforced Photocatalytic Activity of the CNT–TiO2 Nanocomposite for CO2 Reduction and Water Splitting. J. Phys. Chem. C 2018, 123, 367–378. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, M.; Lv, X.; Liu, Y.-Y.; Shi, C.; Dai, Y.; Wang, A.; Majima, T. Insight into iron group transition metal phosphides (Fe2P, Co2P, Ni2P) for improving photocatalytic hydrogen generation. Appl. Catal. B Environ. 2019, 246, 330–336. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated Charge Carriers and Reactive Oxygen Species in ZnO/Au Hybrid Nanostructures with Enhanced Photocatalytic and Antibacterial Activity. J. Am. Chem. Soc. 2013, 136, 750–757. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, K.-D.; Hsu, Y.-J. Plasmon-mediated charge dynamics and photoactivity enhancement for Au-decorated ZnO nanocrystals. J. Mater. Chem. A 2018, 6, 4286–4296. [Google Scholar] [CrossRef]
- Costi, R.; Saunders, A.; Elmalem, E.; Salant, A.; Banin, U. Visible Light-Induced Charge Retention and Photocatalysis with Hybrid CdSe-Au Nanodumbbells. Nano Lett. 2008, 8, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Carlson, M.T.; Stolarczyk, J.; Feldmann, J. Electron Transfer Rate vs Recombination Losses in Photocatalytic H2 Generation on Pt-Decorated CdS Nanorods. ACS Energy Lett. 2016, 1, 1137–1142. [Google Scholar] [CrossRef]
- Naskar, S.; Schlosser, A.; Miethe, J.F.; Steinbach, F.; Feldhoff, A.; Bigall, N.C. Site-Selective Noble Metal Growth on CdSe Nanoplatelets. Chem. Mater. 2015, 27, 3159–3166. [Google Scholar] [CrossRef]
- Kuo, M.-Y.; Hsiao, C.-F.; Chiu, Y.-H.; Lai, T.-H.; Fang, M.-J.; Wu, J.-Y.; Chen, J.-W.; Wu, C.-L.; Wei, K.-H.; Yuan, L.; et al. Au@Cu2O core@shell nanocrystals as dual-functional catalysts for sustainable environmental applications. Appl. Catal. B Environ. 2019, 242, 499–506. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Hsu, Y.-J. Au@Cu7S4 yolk@shell nanocrystal-decorated TiO2 nanowires as an all-day-active photocatalyst for environmental purification. Nano Energy 2017, 31, 286–295. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Huang, B. Fabrication and Characterization of Visible-Light-Driven Plasmonic Photocatalyst Ag/AgCl/TiO2 Nanotube Arrays. J. Phys. Chem. C 2009, 113, 16394–16401. [Google Scholar] [CrossRef]
- Vaiano, V.; Paez, C.A.J.; Matarangolo, M.; Navío, J.; Hidalgo, M.C. UV and visible-light driven photocatalytic removal of caffeine using ZnO modified with different noble metals (Pt, Ag and Au). Mater. Res. Bull. 2019, 112, 251–260. [Google Scholar] [CrossRef]
- Han, S.; Hu, L.; Gao, N.; Al-Ghamdi, A.A.; Fang, X. Efficient Self-Assembly Synthesis of Uniform CdS Spherical Nanoparticles-Au Nanoparticles Hybrids with Enhanced Photoactivity. Adv. Funct. Mater. 2014, 24, 3725–3733. [Google Scholar] [CrossRef]
- Dana, J.; Maity, P.; Ghosh, H.N. Hot-electron transfer from the semiconductor domain to the metal domain in CdSe@CdS{Au} nano-heterostructures. Nanoscale 2017, 9, 9723–9731. [Google Scholar] [CrossRef]
- Wang, P.; Sheng, Y.; Wang, F.; Yu, H. Synergistic effect of electron-transfer mediator and interfacial catalytic active-site for the enhanced H2 -evolution performance: A case study of CdS-Au photocatalyst. Appl. Catal. B Environ. 2018, 220, 561–569. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef]
- He, Y.; Zhong, Y.; Su, Y.; Lu, Y.; Jiang, Z.; Peng, F.; Xu, T.; Su, S.; Huang, Q.; Fan, C.; et al. Water-Dispersed Near-Infrared-Emitting Quantum Dots of Ultrasmall Sizes for In Vitro and In Vivo Imaging. Angew. Chem. Int. Ed. 2011, 50, 5695–5698. [Google Scholar] [CrossRef]
- Wawrzyńczyk, D. Two-photon absorption in penicillamine capped CdS tetrapods. J. Mater. Chem. C 2017, 5, 1724–1729. [Google Scholar] [CrossRef]
- Wawrzynczyk, M.; Szeremeta, J.; Samoc, M.; Nyk, M. Optical nonlinearities of colloidal InP@ZnS core-shell quantum dots probed by Z-scan and two-photon excited emission. APL Mater. 2015, 3, 116108. [Google Scholar] [CrossRef]
- Wawrzynczyk, M.; Szeremeta, J.; Samoc, M.; Nyk, M. Third-Order Nonlinear Optical Properties of Infrared Emitting PbS and PbSe Quantum Dots. J. Phys. Chem. C 2016, 120, 21939–21945. [Google Scholar] [CrossRef]
- Achtstein, A.W.; Ballester, A.; Movilla, J.L.; Hennig, J.; Climente, J.I.; Prudnikau, A.; Antanovich, A.; Scott, R.; Artemyev, M.; Planelles, J.; et al. One- and Two-Photon Absorption in CdS Nanodots and Wires: The Role of Dimensionality in the One- and Two-Photon Luminescence Excitation Spectrum. J. Phys. Chem. C 2015, 119, 1260–1267. [Google Scholar] [CrossRef]
- Szeremeta, J.; Nyk, M.; Wawrzynczyk, M.; Samoc, M. Wavelength dependence of nonlinear optical properties of colloidal CdS quantum dots. Nanoscale 2013, 5, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Nyk, M.; Wawrzynczyk, D.; Szeremeta, J.; Samoc, M. Spectrally resolved size-dependent third-order nonlinear optical properties of colloidal CdSe quantum dots. Appl. Phys. Lett. 2012, 100, 041102. [Google Scholar] [CrossRef]
- Scott, R.; Achtstein, A.W.; Prudnikau, A.; Antanovich, A.; Christodoulou, S.; Moreels, I.; Artemyev, M.; Woggon, U. Two Photon Absorption in II–VI Semiconductors: The Influence of Dimensionality and Size. Nano Lett. 2015, 15, 4985–4992. [Google Scholar] [CrossRef]
- Moloney, M.P.; Govan, J.; Loudon, A.; Mukhina, M.; Gun’Ko, Y.K. Preparation of chiral quantum dots. Nat. Protoc. 2015, 10, 558–573. [Google Scholar] [CrossRef]
- Makarov, N.S.; Drobizhev, M.; Rebane, A. Two-photon absorption standards in the 550–1600 nm excitation wavelength range. Opt. Express 2008, 16, 4029–4047. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, B.; Yin, Z.; Liu, Z.; Zhang, H. Phosphine-Free, Low-Temperature Synthesis of Tetrapod-Shaped CdS and Its Hybrid with Au Nanoparticles. Small 2014, 10, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-X.; Liu, M.-D.; Li, C.-X.; Hong, S.; Zheng, D.-W.; Liu, X.-H.; Chen, S.; Cheng, H.; Zhang, X.-Z. A metal-semiconductor nanocomposite as an efficient oxygen-independent photosensitizer for photodynamic tumor therapy. Nanoscale Horiz. 2017, 2, 349–355. [Google Scholar] [CrossRef]
- Mokari, T.; Rothenberg, E.; Popov, I.; Costi, R.; Banin, U. Selective Growth of Metal Tips onto Semiconductor Quantum Rods and Tetrapods. Science 2004, 304, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Menagen, G.; Mocatta, D.; Salant, A.; Popov, I.; Dorfs, D.; Banin, U. Selective Gold Growth on CdSe Seeded CdS Nanorods. Chem. Mater. 2008, 20, 6900–6902. [Google Scholar] [CrossRef]
- Vaneski, A.; Susha, A.S.; Berr, M.; Jaeckel, F.; Feldmann, J.; Rodríguez-Fernández, J.; Rogach, A.L. Hybrid Colloidal Heterostructures of Anisotropic Semiconductor Nanocrystals Decorated with Noble Metals: Synthesis and Function. Adv. Funct. Mater. 2011, 21, 1547–1556. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Band Gap Implications on Nano-TiO2 Surface Modification with Ascorbic Acid for Visible Light-Active Polypropylene Coated Photocatalyst. Nanomaterials 2018, 8, 599. [Google Scholar] [CrossRef]
- Kassab, K. Photophysical and photosensitizing properties of selected cyanines. J. Photochem. Photobiol. B Biol. 2002, 68, 15–22. [Google Scholar] [CrossRef]
- Wawrzyńczyk, D.; Cichy, B.; Stek, W.; Nyk, M. The role of l-cysteine and introduced surface defects in reactive oxygen species generation by ZnO nanoparticles. Dalton Trans. 2018, 47, 8320–8329. [Google Scholar] [CrossRef]
- Waiskopf, N.; Ben-Shahar, Y.; Galchenko, M.; Carmel, I.; Moshitzky, G.; Soreq, H.; Banin, U.; Moshitsky, G. Photocatalytic Reactive Oxygen Species Formation by Semiconductor-Metal Hybrid Nanoparticles. Toward Light-Induced Modulation of Biological Processes. Nano Lett. 2016, 16, 4266–4273. [Google Scholar] [CrossRef]
- Shaviv, E.; Schubert, O.; Alves-Santos, M.; Goldoni, G.; Di Felice, R.; Vallée, F.; Del Fatti, N.; Banin, U.; Sönnichsen, C. Absorption Properties of Metal-Semiconductor Hybrid Nanoparticles. ACS Nano 2011, 5, 4712–4719. [Google Scholar] [CrossRef] [PubMed]
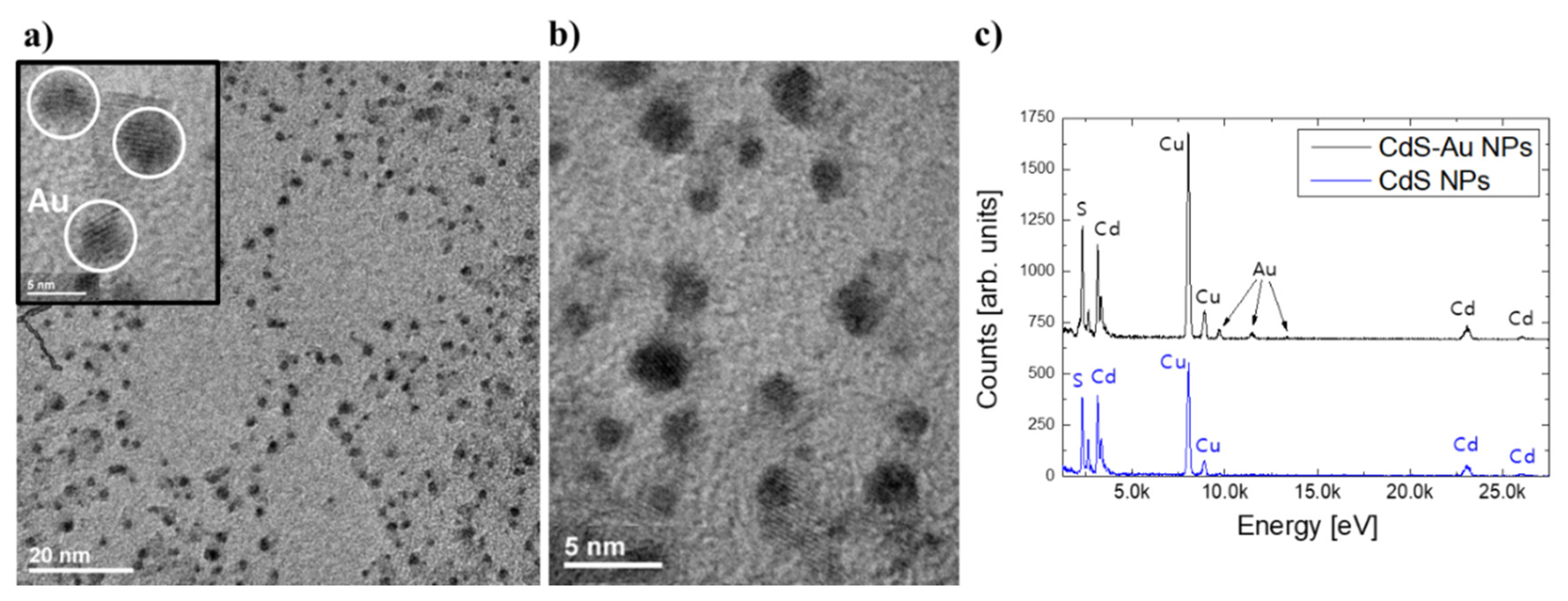
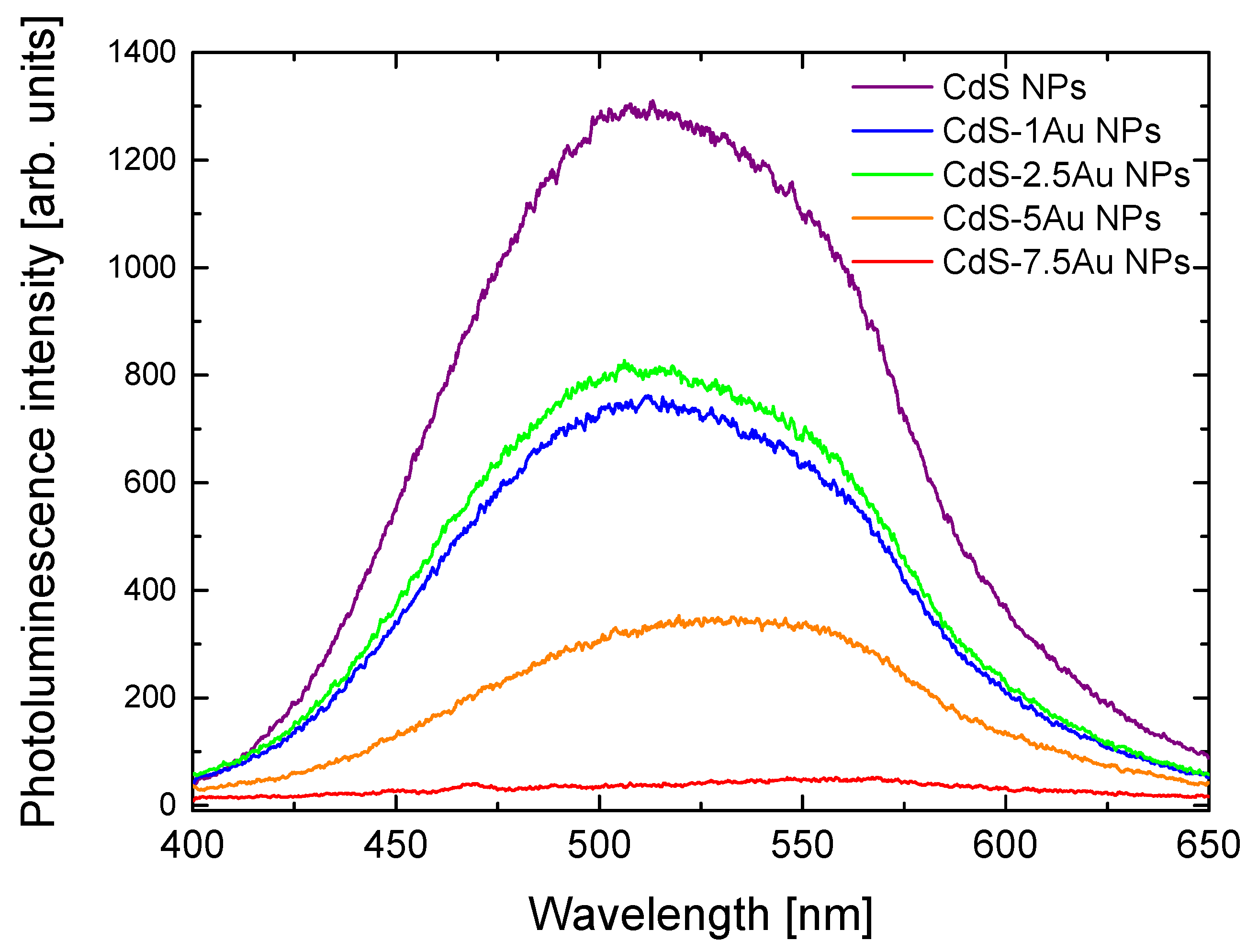
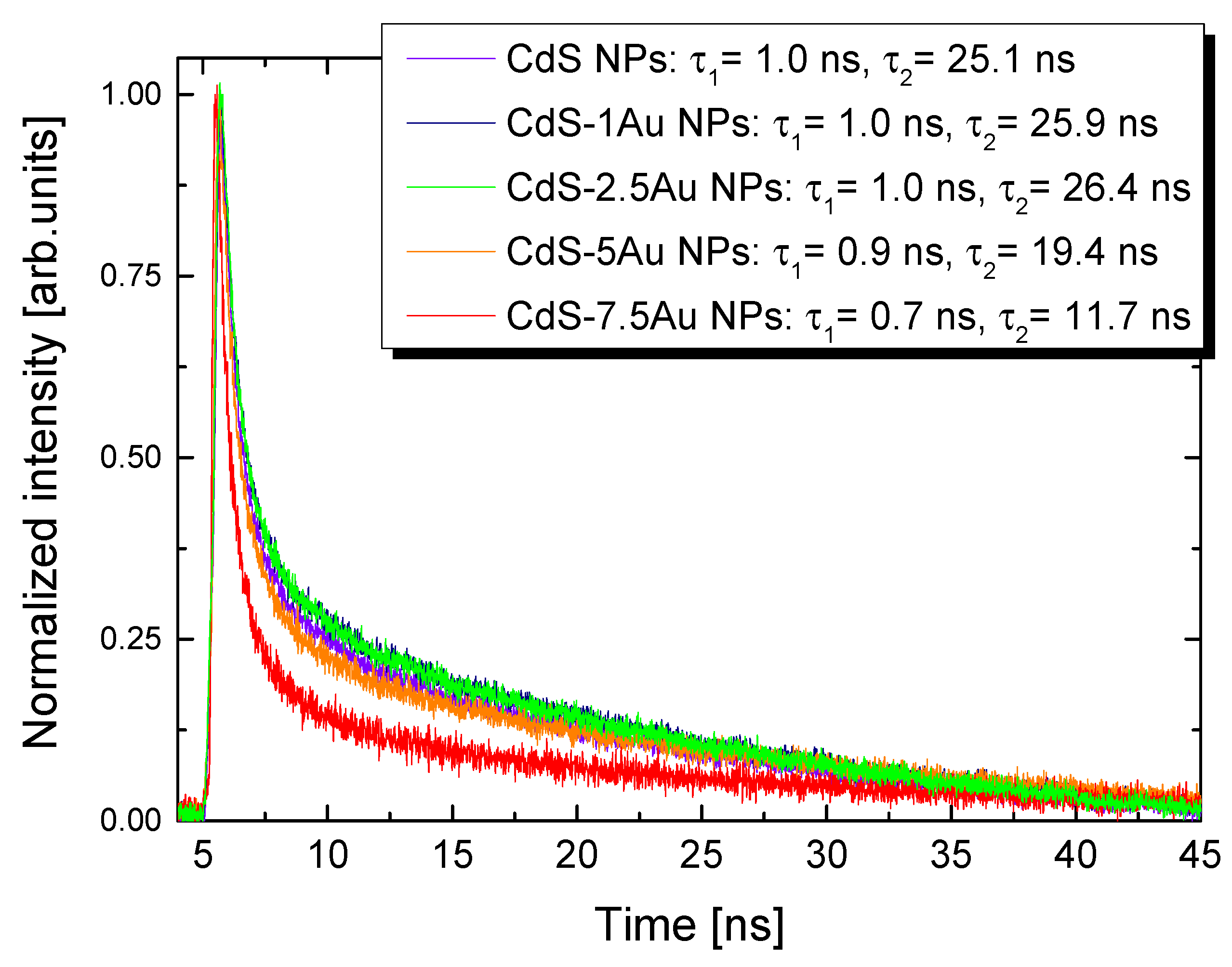
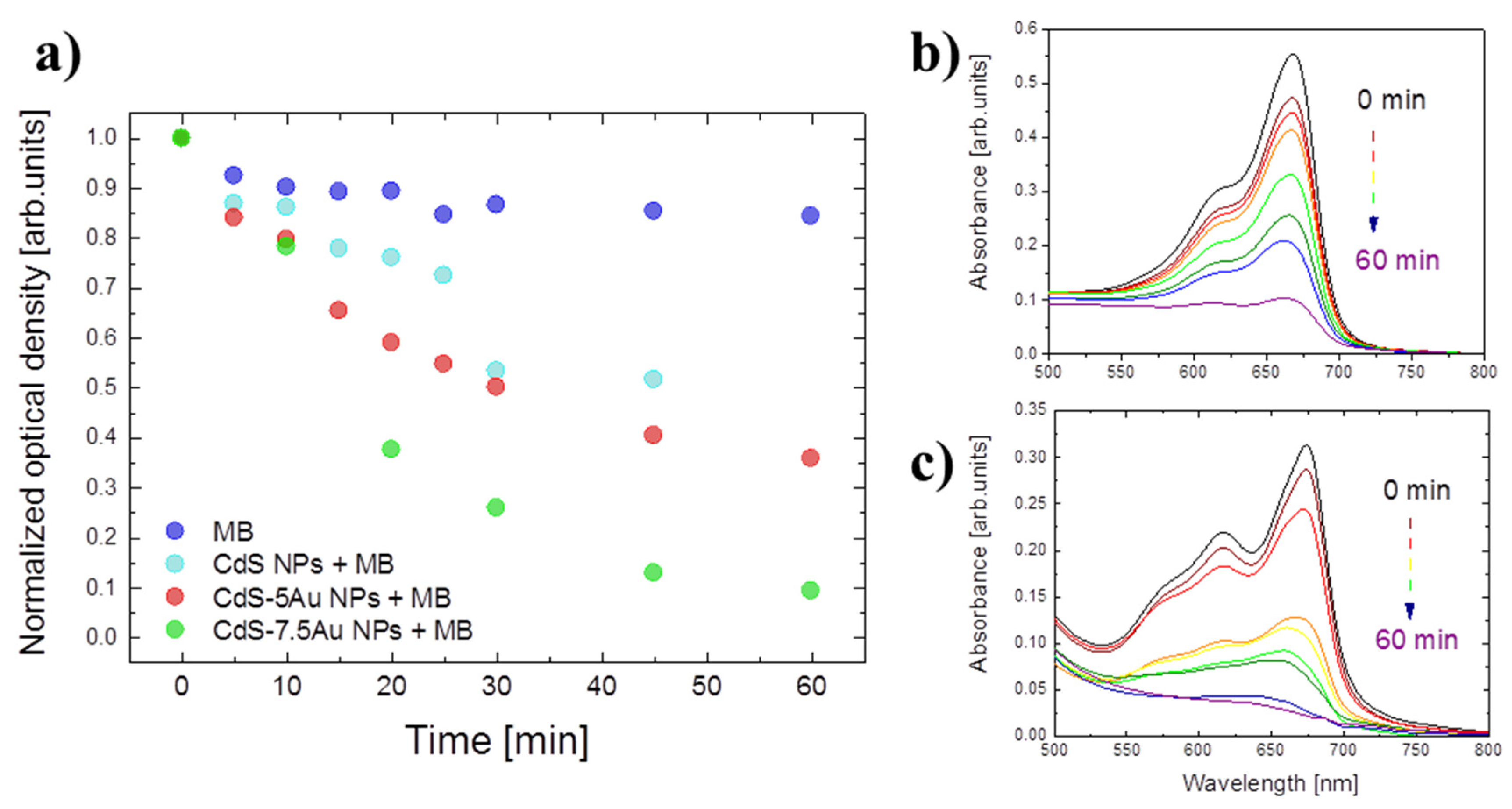
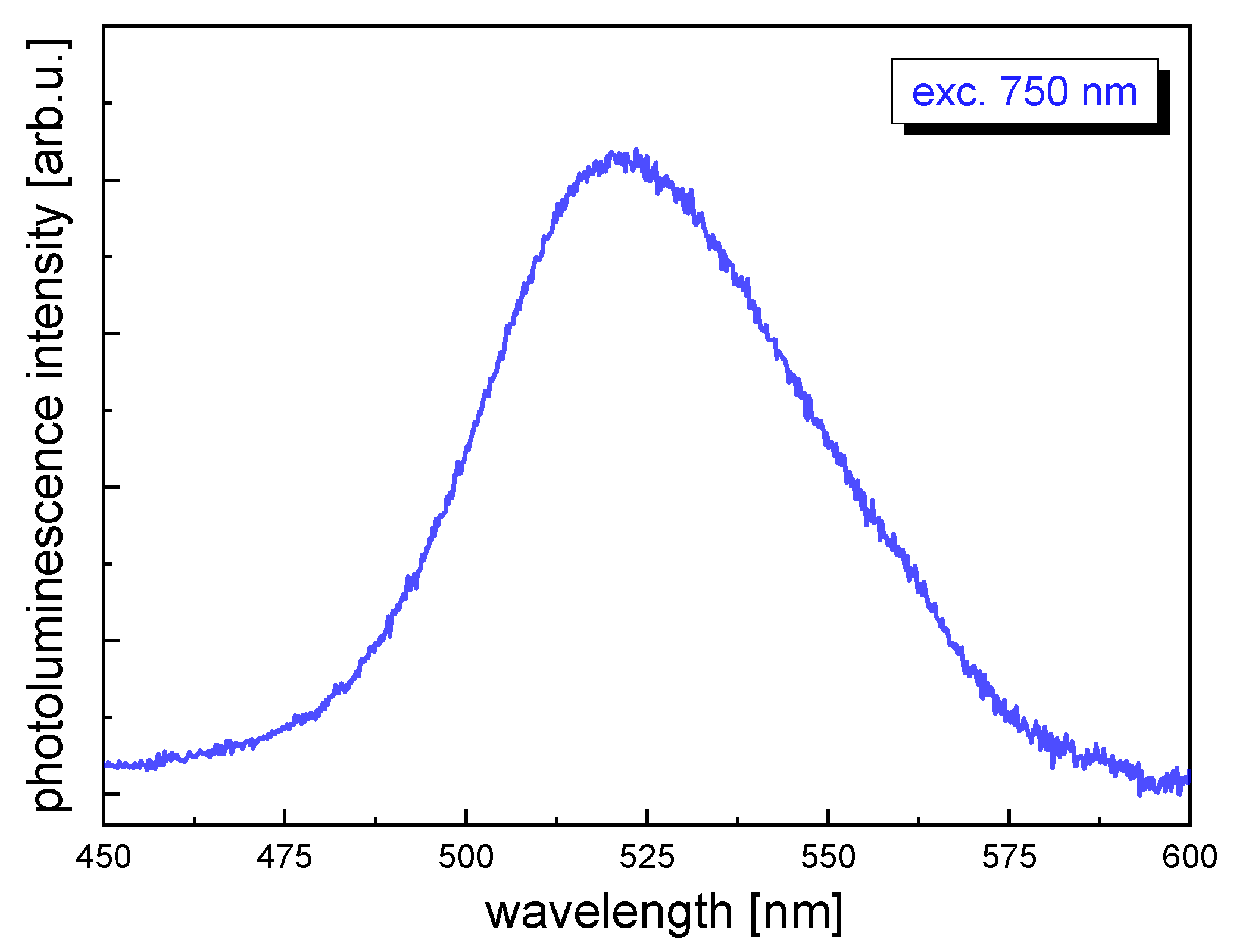
| Sample | exc. λ (nm) | σ2 (GM) | σ2 × QY (GM) | σ2/MW (GM) | Ref. |
|---|---|---|---|---|---|
| CdS | 700 | 6 × 102 | 3.66 × 102 | 1× 10−3 | |
| 725 | 5.7 × 102 | 3.42 × 102 | 0.6 × 10−3 | [23] | |
| 750 | 2.3 × 102 | 1.38 × 102 | 0.2 × 10−3 | ||
| CdS-5Au | 700 | 7.0 × 103 | 7.0 × 102 | 9 × 10−3 | this work |
| 725 | 15.8 × 103 | 1.58 × 103 | 20 × 10−3 | ||
| 750 | 6.9 × 103 | 6.90 × 102 | 9 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrot, K.C.; Wawrzyńczyk, D.; Bezkrovnyi, O.; Kępiński, L.; Cichy, B.; Samoć, M.; Nyk, M. Functional CdS-Au Nanocomposite for Efficient Photocatalytic, Photosensitizing, and Two-Photon Applications. Nanomaterials 2020, 10, 715. https://doi.org/10.3390/nano10040715
Nawrot KC, Wawrzyńczyk D, Bezkrovnyi O, Kępiński L, Cichy B, Samoć M, Nyk M. Functional CdS-Au Nanocomposite for Efficient Photocatalytic, Photosensitizing, and Two-Photon Applications. Nanomaterials. 2020; 10(4):715. https://doi.org/10.3390/nano10040715
Chicago/Turabian StyleNawrot, Katarzyna C., Dominika Wawrzyńczyk, Oleksii Bezkrovnyi, Leszek Kępiński, Bartłomiej Cichy, Marek Samoć, and Marcin Nyk. 2020. "Functional CdS-Au Nanocomposite for Efficient Photocatalytic, Photosensitizing, and Two-Photon Applications" Nanomaterials 10, no. 4: 715. https://doi.org/10.3390/nano10040715
APA StyleNawrot, K. C., Wawrzyńczyk, D., Bezkrovnyi, O., Kępiński, L., Cichy, B., Samoć, M., & Nyk, M. (2020). Functional CdS-Au Nanocomposite for Efficient Photocatalytic, Photosensitizing, and Two-Photon Applications. Nanomaterials, 10(4), 715. https://doi.org/10.3390/nano10040715







