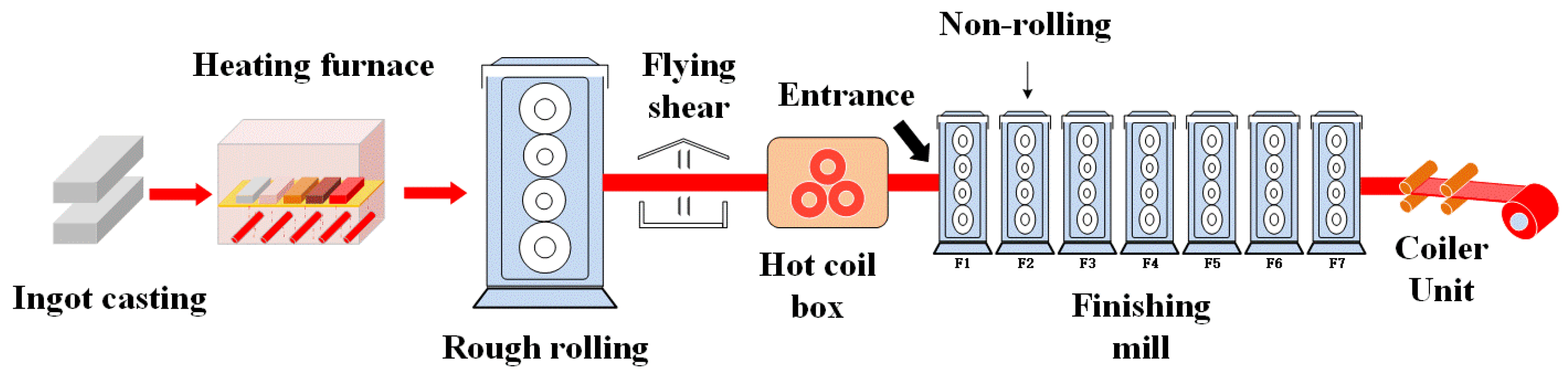
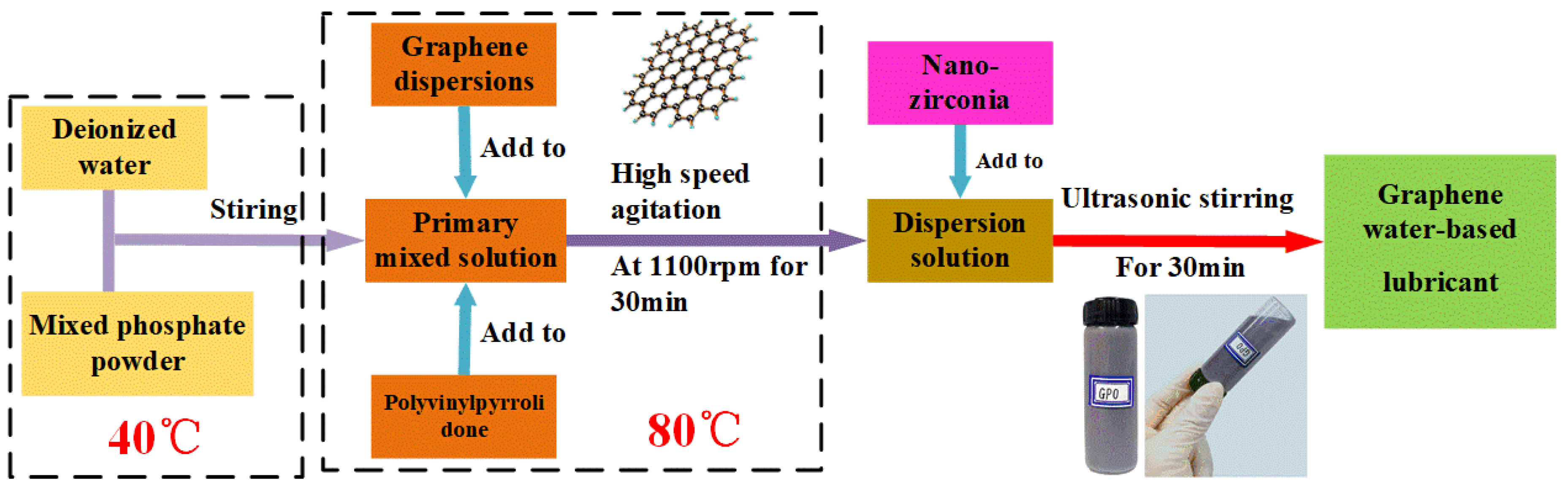
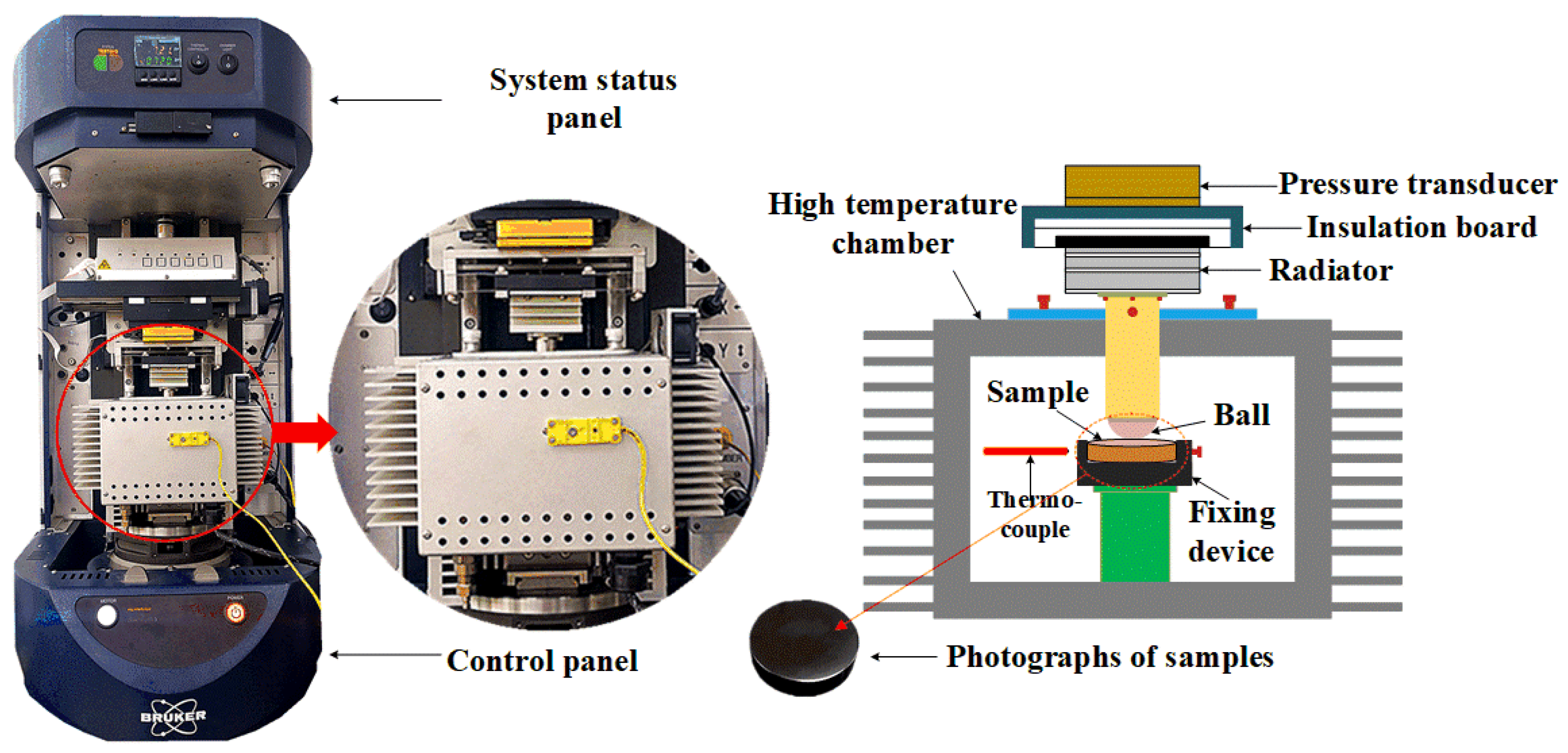
3.1. Coefficient of Friction
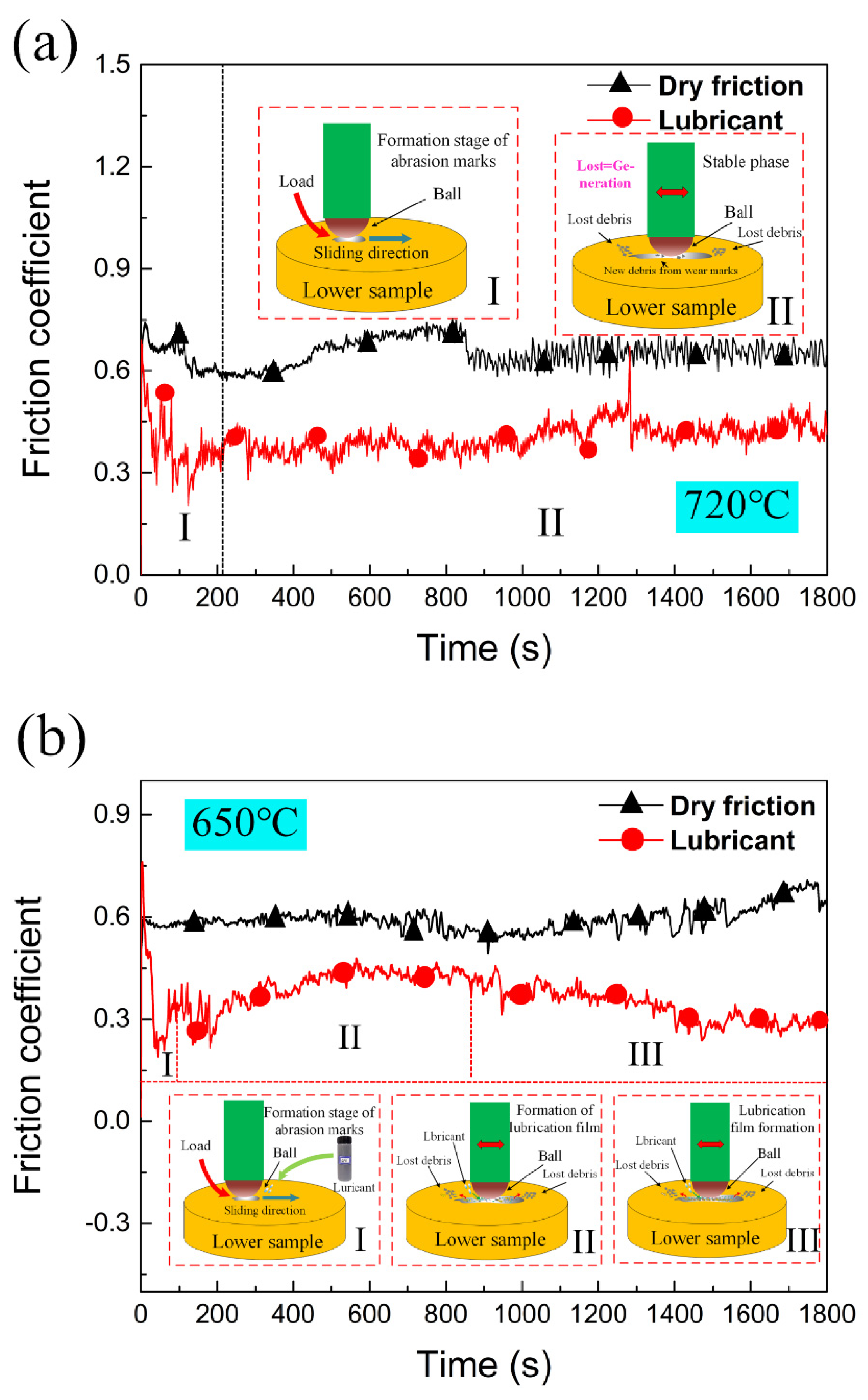
Figure 4 shows the coefficient of friction (COF) curves under dry and lubricated friction conditions with different contact parameters.
Figure 4a,b show the experimental results under condition 1 and condition 2 respectively. The abscissa represents time and the ordinate represents friction coefficient. The friction coefficient decreases markedly after using lubricant. In addition, a schematic diagram of the friction process under dry friction and lubricated conditions are shown in
Figure 4a,b. The average COF under an unlubricated condition ranges from 0.57 to 0.75 under condition 1, compared with 0.3–0.45 after using lubricant. In condition 2, the average COF under unlubricated condition ranges from 0.55 to 0.70, while the COF under lubricated condition is stable at 0.25–0.45. The COF under condition 1 is generally higher than that of condition 2, and the COF measured after using lubricant is lower than that of measured unlubricated. The fluctuation of each curve obviously shows different contact states on the surface of the friction pair during the tribological process.
The trend of whole friction process can be analyzed by the schematic diagram of friction coefficient evolution obtained from experimental condition 1. The evolution process of COF obtained by the tribological tests follows a similar tendency with two stages. The first stage is the initial stage of friction. This is the stage with large variation of COF, which is characterized by the sudden increase of COF and slow decrease of COF in the beginning of the experiment. The measured COF reaches about 1.2 in a short time, and then slowly decreases to around 0.6. After that, the friction coefficient shows a relatively stable trend in the second stage and fluctuates slightly until the end of the friction experiment. The same evolution trend of friction coefficient can be seen from the curve of COF obtained under experimental condition 2 in
Figure 4b. After a number of tribological tests and the SEM investigation in the following section, the reason for the first phase occurrence is that the upper sample adheres to the lower sample during the high-temperature loading process. Before the sliding track of the lower specimen is formed, the friction force is responsible for the plastic deformation. With the formation of wear track, the plastic deformation rate decreases, which leads to a downward trend of the friction coefficient. The average friction coefficient in this stage is higher than that in the next stage [
37]. In the second stage, due to the generation of debris and the stable friction process, the change of friction coefficient in this stage presents a relatively stable trend. At the same time, the movement of wear debris in the wear scar forms an isolation layer, which transforms part of the sliding friction into rolling friction. It is able to reduce the friction coefficient.
Under lubricated conditions, the evolution of friction coefficient curve can be divided into three stages under a lubricated condition in
Figure 4b. The first stage is generally consistent with that of dry friction. The plastic deformation force at the initial stage is provided by friction force, the friction coefficient increases to 0.9 firstly and then decreases slowly. The difference is in the second and third stages. After using lubricant, the friction coefficient of the second stage increases slightly. The reason is that the addition of lubricant leads to an increase of debris on the surface of friction pairs. In the third stage, the friction coefficient shows a downward trend. The friction state is stable at this time, and a relatively stable and compact isolation layer is formed on the surface of the friction pair. This trend continues until the end of the experiment, which shows that the isolation layer formed by this lubricant is not easily destroyed. It also proves that there is a synergistic effect among the components in the proposed lubricant. By comparing the dry friction condition, the friction coefficient after applying the mixed graphene-incorporating lubricant is always lower with a more stable tendency. This shows that the use of lubricant is helpful to improving the anti-friction and anti-wear performance in the whole tribological process.
Table 5 shows the average friction coefficients of both dry and lubrication conditions under the experimental conditions of 1 and 2. After applying lubrication, the average friction coefficient decreases by 30.27% (condition 1) and 35.22% (condition 2), respectively. The wear volumes of titanium discs are 0.23 mm
3 and 0.14 mm
3 in dry and lubricated tests under condition 1. While the wear volumes are 0.18 mm
3 and 0.09 mm
3 in dry and lubricated tests under condition 2. This proves that the proposed lubricant plays a critical role in reducing the friction coefficient and wear volume during the hot rolling of titanium alloy sheets.
3.2. Surface Morphology
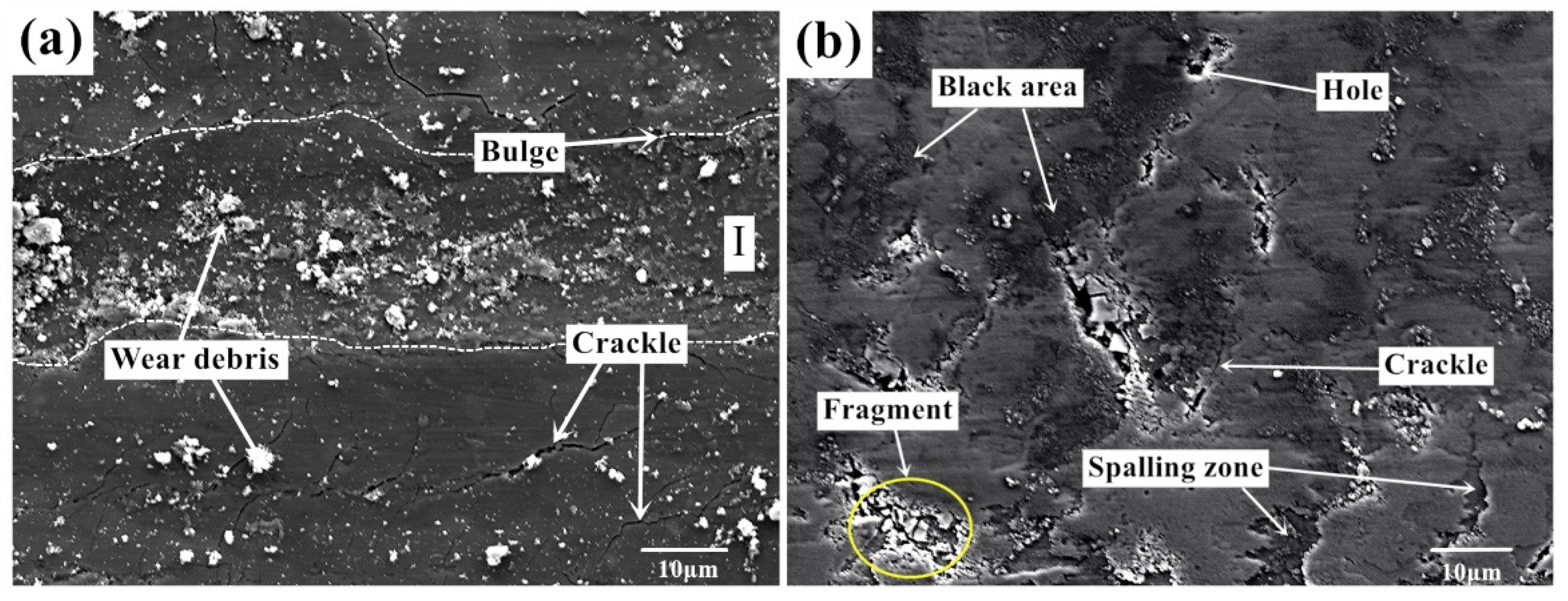
Figure 5 shows the surface morphology of Ti-0.3mo-0.8Ni alloy with two experimental processes under a dry friction condition. The wear surface of Ti-0.3Mo-0.8Ni titanium alloy presents plastic deformation, tear marks and plough marks parallel to the sliding direction under the action of elevated temperature and high contact stress, which are typically defined as adhesion wear and abrasive wear. A number of large fragments can be seen on the worn surface as well. In the adhesion and sliding processes, strong ion bonds and other covalent bonds form between the micro-convex bodies of friction pairs, which are sheared under the action of contact load. Thus, larger size debris form in this process. Large debris is easily detached from the surface, which is not conducive to the formation of stable friction layer. A large amount of granular substances and debris accumulate on the surface of wear marks. It can be inferred that the debris comes from the oxide scale of titanium. There are some holes on the worn surface as well. It can be judged comprehensively that the plough formed in the sliding process of the titanium alloy introduces the metal plastic deformation. As the friction continues, the accumulated part is flattened again. After repeated plastic deformation, cracks begin to appear and extend into the alloy substrate. The flake peeling and fatigue damage then occur on the surface. According to the characteristics on the worn surface, the wear mechanism of Ti-0.3mo-0.8Ni alloy during the hot-rolling process is concluded to the combination effect of abrasive wear, adhesive wear and oxidation wear.
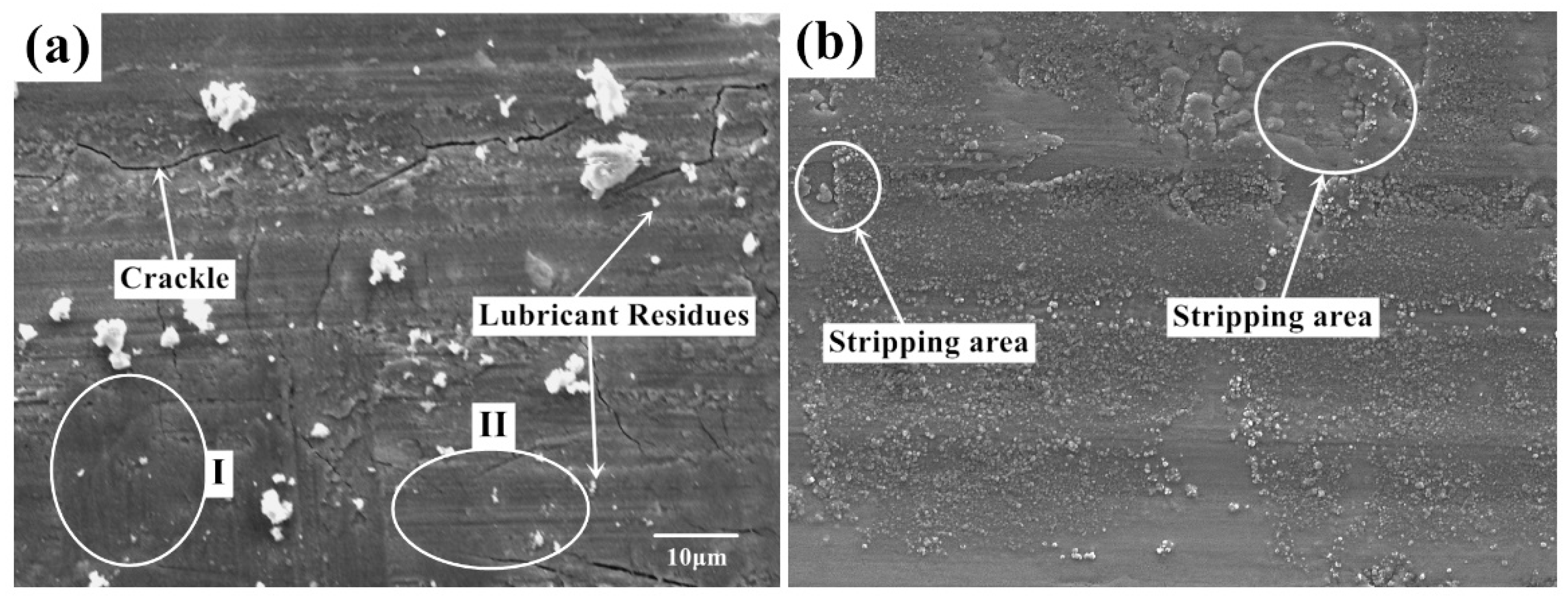
Figure 6 shows the micro-morphology of the worn surface with lubrication under two experimental conditions.
Figure 6a,b are the surface morphology in condition 1 (720 °C /11.42 N/4.50 Hz) and 2 (650 °C /9.69 N/12.62 Hz). The surface quality of the sample has been greatly improved after using lubricant. Compared with the samples obtained from dry friction, the lubricated surface is smoother and the surface defects are greatly reduced. The surface cracks, holes, and peeling debris are significantly decreased. The plough on the worn surface is no longer obvious. In
Figure 6a, there are still some small particles with different sizes on the specimen surface. Some cracks can also be observed on the worn surface. Furthermore, there are almost no wear marks parallel to the friction direction at Mark I compared with the morphology at Mark II, and the structure is relatively uniform and dense. In
Figure 6b, the surface obtained with lubricant also shows a typical characteristics of abrasive wear-strip wear marks. There are also different sizes of debris mounted on the surface without obvious tears and raised marks. The wear form of the wear marks are abrasive wear and oxidation wear under this contact condition. This shows that the use of lubricants greatly improves the severe wear between friction pairs. In
Figure 6a, under the action of friction movement, the lubricant materials and contact pairs form a protective film near position I. The lubrication film in this area is continuous and compact with deformation patterns. In
Figure 6b, a relatively large area of uniform coverage of lubrication film can be found. The protective film forms an isolation layer at the friction interface, which prevents the uneven contact between friction pairs. The leading wear-type transforms to the abrasive wear and oxidation wear.
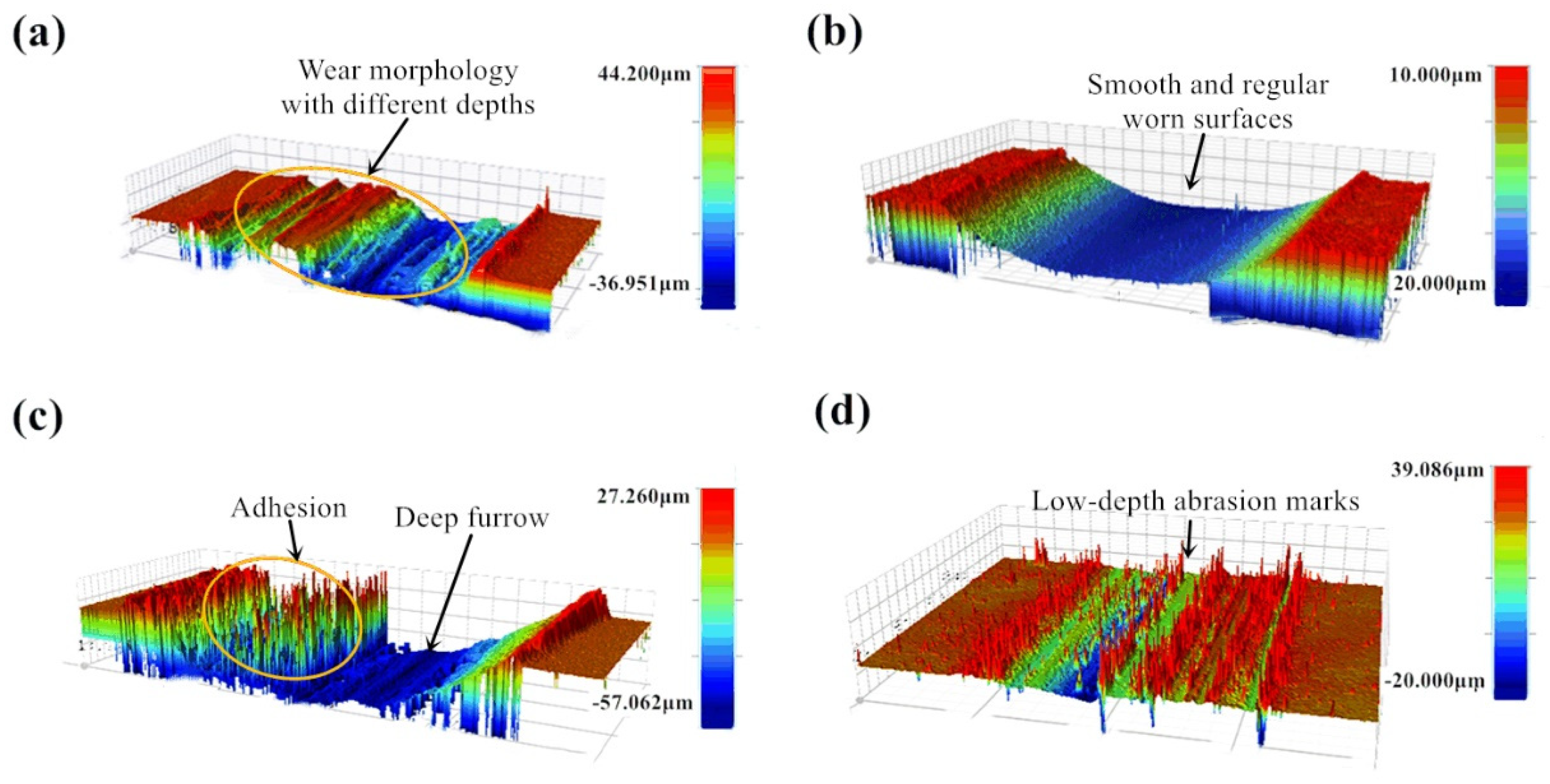
In order to verify the effect of proposed lubricant on the worn track from a macroscopic point of view, three-dimensional morphologies were measured under dry friction and lubricated conditions.
Figure 7 a–d show the three-dimensional topography of the tests. The morphology and surface quality of the friction interface of the sample were greatly improved after using lubricant.
The width of wear marks with and without using lubricant does not change much, but the wear depth is quite different. The wear depth of the sample with lubricant is lower than that of without lubricant. From
Figure 7a,c, the wear marks produced under dry friction condition are irregular with different distribution and depths. There are large distribution variation through the direction perpendicular to the friction direction. From
Figure 7b,d, wear marks are uniform and the wear mark surface is relatively regular. This reflects that the use of lubricant not only improves the wear scar morphology, but also reduces the wear amount. For condition 1 (720 °C/11.42 N/4.50 Hz), after using lubrication the specimen worn surface becomes smoother and more uniform than it is under dry conditions. By contrast, for condition 2 (650 °C/9.69 N/12.62 Hz), there are still many small peaks in the friction interface after using lubricant, but the depth of the wear marks is greatly reduced and more uniform than that under dry friction conditions. The reason for the occurrence of the small peak may be that lubricant particles are mounted in the lubrication film at the friction interface under the experimental conditions.
3.3. Chemical and Component Analysis of the Contact Interface
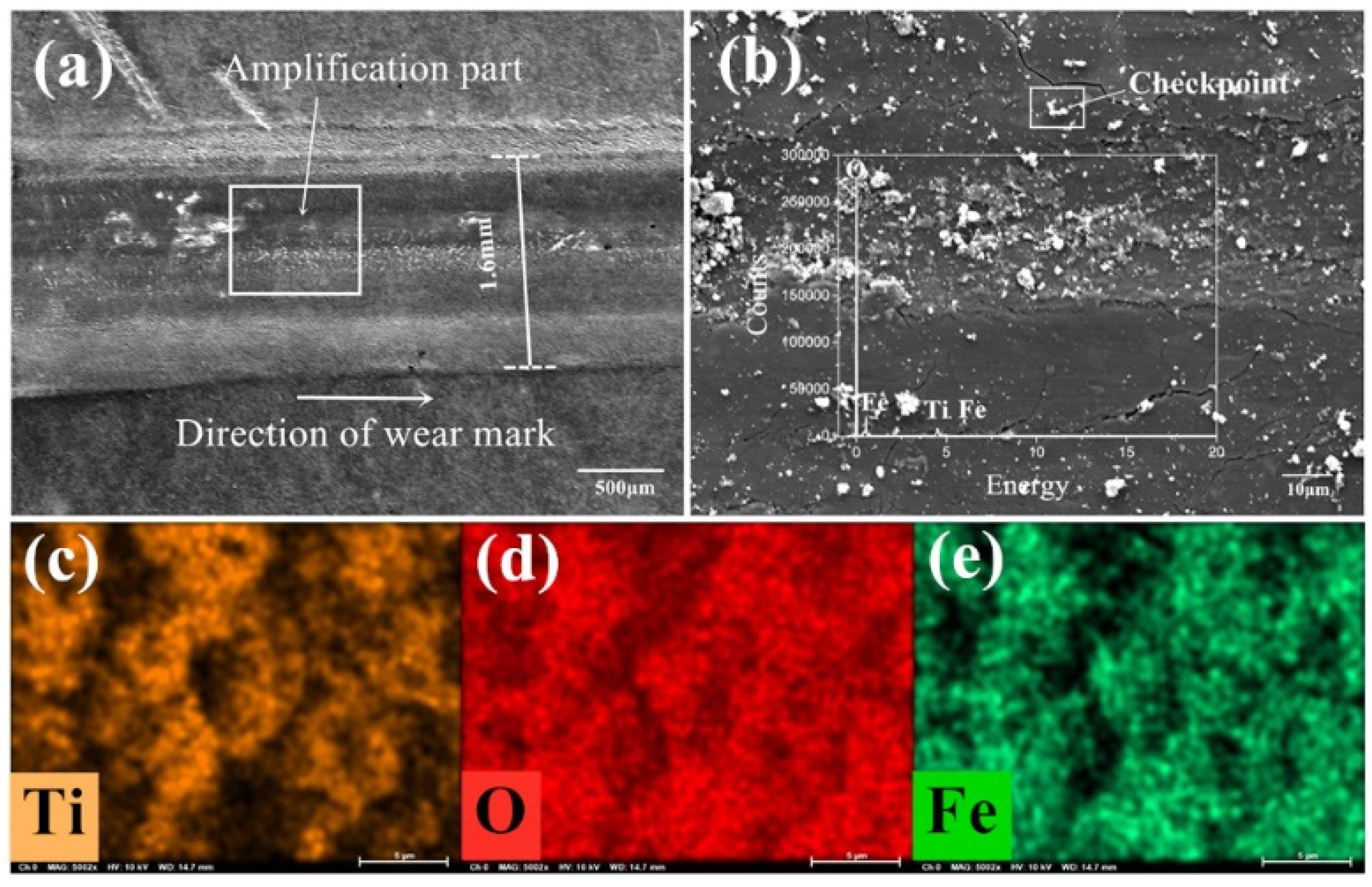
Figure 8a–e is the SEM image and element distribution under the condition of 720 °C/11.42 N/4.50 Hz without lubrication.
Figure 8a,b are the appearance of the wear marks in micromorphology with a magnification until 5000 times.
Figure 8c–e are the distribution of elements on the detected area of wear marks. Ti, O and Fe elements can be found on the worn marks after the tribological test from the elemental distribution diagram and the peak position distribution of each element in
Figure 8b. There are iron oxide and titanium oxide forms on the surface of the wear marks. The titanium oxide scale is covered on the wear scar, which prevent the direct contact of the friction pair and reduces the friction and wear to a certain extent. The iron oxide in some certain form can be considered as a kind of high-quality wear-resistant material or abrasive particles, and it is possible to improve or destroy the surface contact state.
From
Figure 8a, the width of the wear mark is about 1.6 mm, which can be divided into a plastic deformation area and main worn area in the direction perpendicular to the wear mark. Both sides of the wear mark are plastic deformation areas located on both sides of the wear scar, while the main worn area is in the middle of the wear track. No obvious furrow and scratch can be seen, instead, a relatively shallow row of fishbone-like stripes can be found in the upper left corner of the wear mark. It is the crack-produced tearing area during the friction process. The O element is distributed over the whole interface, but the distribution intensity is quite different, which is due to the elements distribution of Ti and Fe in the forms of oxide with Ti
4+ and Fe
3+ ions. Part of the Ti element from the contact surface is replaced by the Fe element. The iron oxide may accelerate the deformation of titanium oxide scale. The distribution of Ti and Fe is approximately misaligned. However, in some regions the distribution of the two elements are overlapped. Under the proposed experimental condition, there is no uniform and protective covering film formed at the abrasion mark of the lower sample. Some iron oxide is pressed into the sample surface. The products from the tribological and thermal actions on the surface peel off and expose the base metal. Only some titanium and iron oxide are overlapped and covered on the surface of wear mark to perform as a protective film.
Figure 9a–e is the SEM image and element distribution mapping detected under conditions of 650 °C/9.69 N/12.62 Hz. Similar to the results detected under condition 1 above, Ti, O and Fe elements have been also detected on the worn surface. In
Figure 9a, the width of the wear mark is about 1.4 mm, which is similar to the result of condition 1 above. The wear mark area also presents both plastic deformation area and worn area. Several striations can be seen on the contact surface. Compared with the elements’ distribution in
Figure 8, the Ti element covers a wider distribution range. Ti element is distributed in the whole detection area, while O element is weak in the detected area. The distribution of Fe locates on the plastic deformation area, while some Ti element is overlapped on these areas. This indicates the iron oxide is compressed in a thin film covered on the titanium substrate with less titanium oxide scale [
38,
39]. Meanwhile, the surface presents less oxide particle spread on the wear track with a compacted deformation area. In a lower rolling temperature, less oxide scale performs as a solid lubrication in the hot interfaces, which also contributes to a lower coefficient of friction.
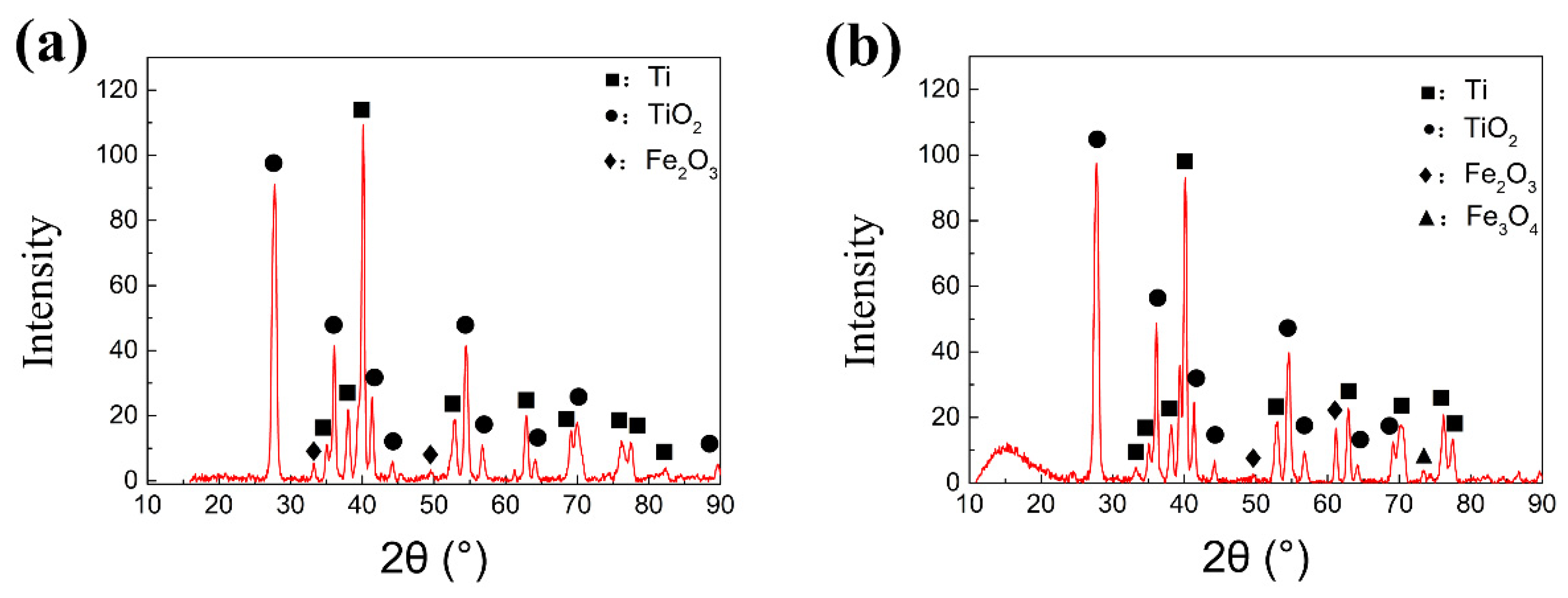
In order to further confirm the compound on the wear track, X-ray diffraction analysis has been performed on the worn surface, as shown in
Figure 10. Oxides of titanium and iron can be found on the surface of the wear marks in both conditions 1 and 2 under dry condition. Clear diffraction peak of Fe
3O
4 can be only viewed in condition 2 at 650 °C. From the oxidation theory [
40], the iron oxide scale above 600 °C is composed of FeO, Fe
3O
4 and Fe
2O
3 from the inner to the outer layer. Fe
3O
4 is the most compact layer with a good mechanical property compared with another two kinds of iron oxide. Only Fe
2O
3 was detected on the worn surface under the condition 1, which indicates that the thickness of the Fe
2O
3 layer at the outermost layer became thicker with increasing temperature. The brittle Fe
2O
3 scale peels off from the upper steel testing ball to form wear debris during the friction process. In condition 2, the thickness of Fe
2O
3 scale is thinner. In the friction process, the Fe
3O
4 peels off to the wear mark under the action of friction, and then forms a discontinuous protective film [
41], which also causes a lower COF at 650 °C.
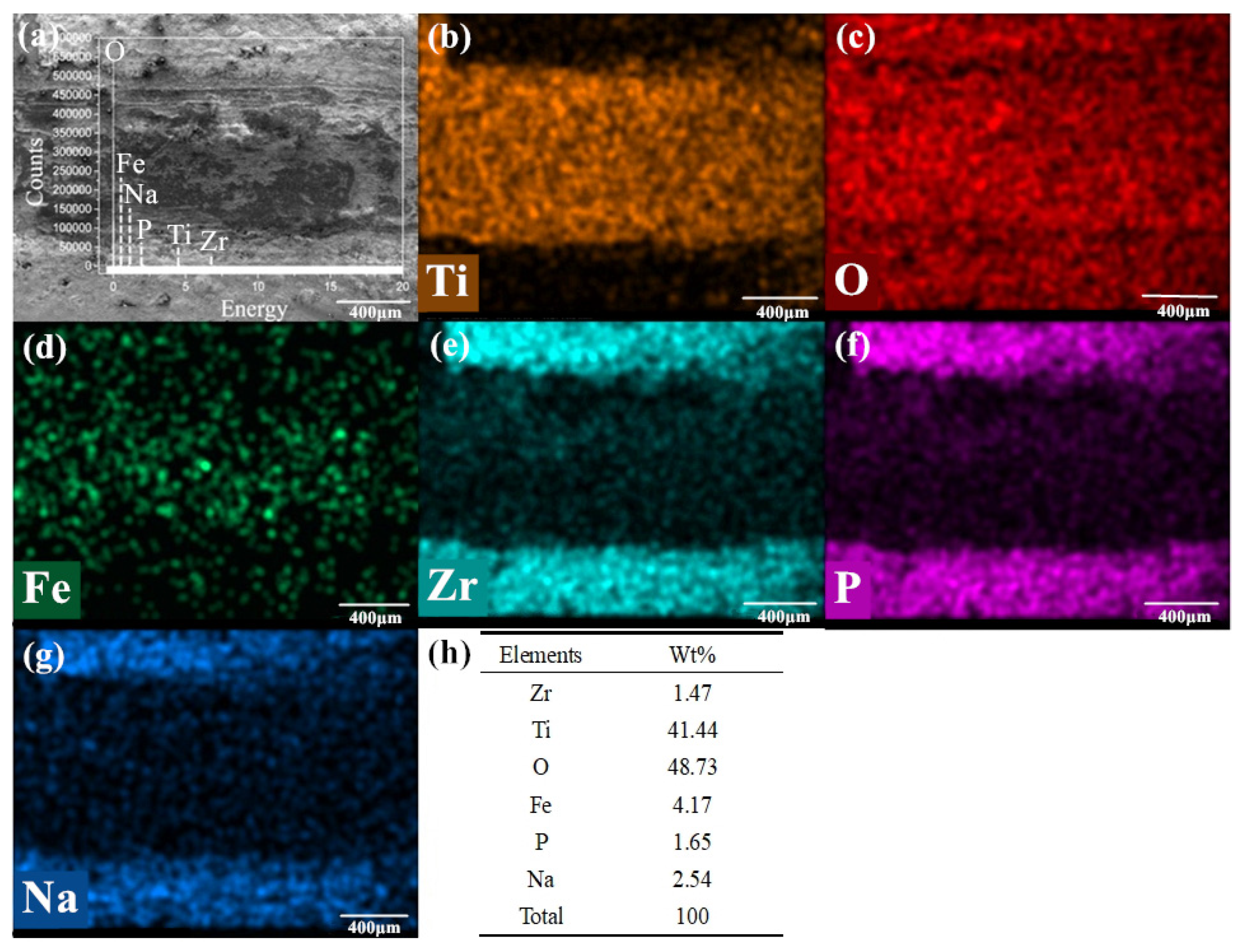
Figure 11a–e is the SEM image and element distribution under the condition of 720 °C/11.42 N/4.50 Hz with mixed graphene-incorporating lubricant.
Figure 11a are the appearance of the wear marks.
Figure 11b–h are the distribution of elements and their mass percentages on the detected area of the wear marks. Ti, O, Fe, Zr, P and Na elements can be found on the worn marks after the tribological test from the elemental distribution diagram and the peak position distribution of each element in
Figure 11a. There are some residual lubricating elements such as P, Na and Zr that can be found on the worn surface. But the surface is mainly covered by oxides of iron and titanium. Little C element can be found on the worn track. The O element is distributed over the whole interface. P, Na and Zr elements are mainly distributed both outside and near the edges of the wear mark. This means that most of the lubricants are extruded outside the wear mark during tribological process. The iron oxide scale locates mainly inside the wear marks, but there are still some iron oxide scales distribute outside the wear mark, which are the wear debris produced from the upper testing ball during friction. In the wear marks, the Zr element also presents similar distribution tendency with P and Na, which shows that ZrO
2 nano particles possess some lubricating performance during hot tribological process [
42,
43]. The composition of graphene is not detected, which means that the graphene is exhausted at this temperature. It also shows that the lubricant composition on the wear mark surface is not covered by a continuous lubricant film. The lubricant film formed on the friction interface has been broken. At 720 °C, the friction-reduction and anti-wear components on the friction interface are mainly ZrO
2 nano particles with a part of the phosphate lubricant composition.
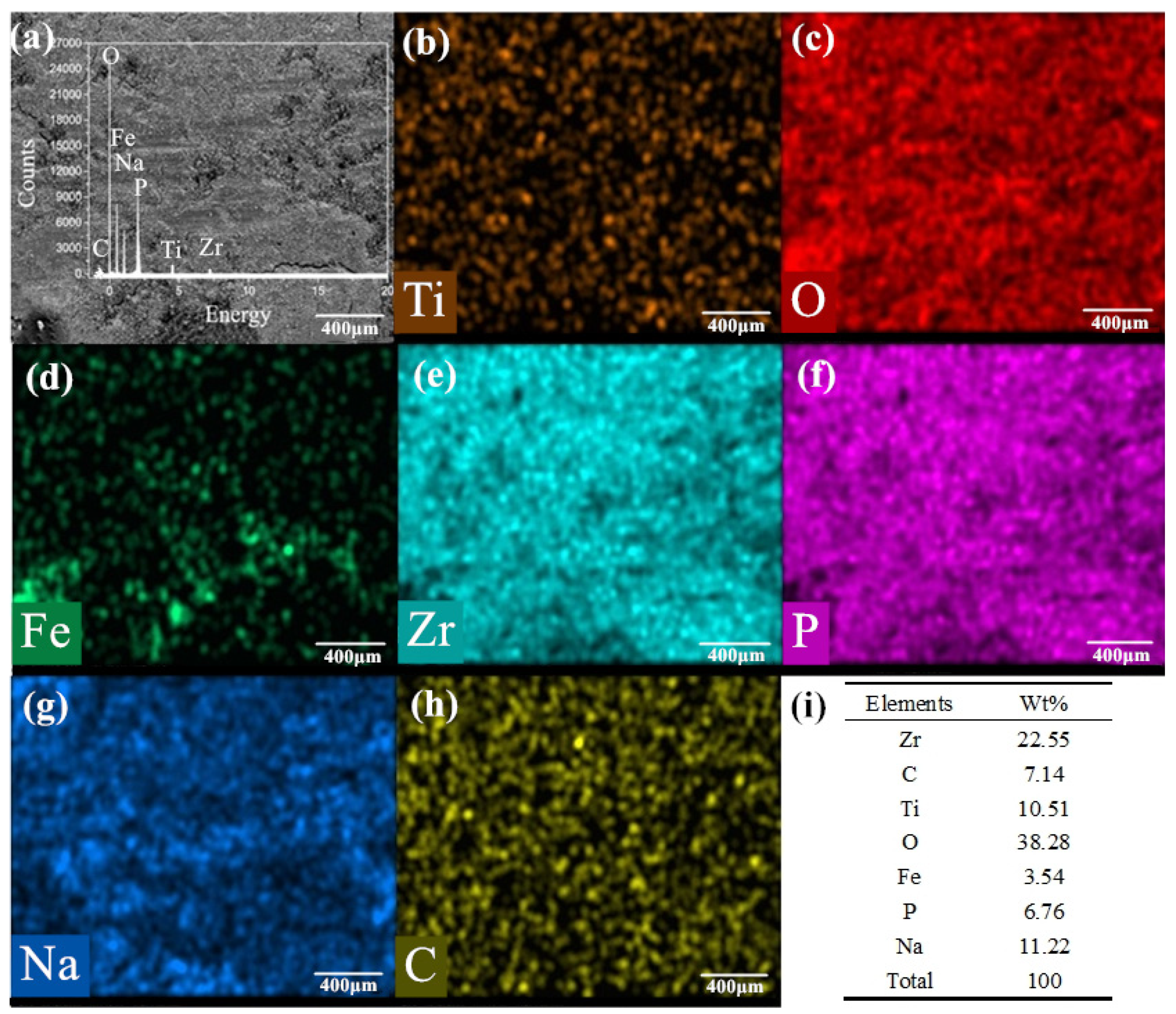
Figure 12a–h is the SEM image, element distribution and their mass percentages under the condition of 650 °C/9.69 N/12.62 Hz with mixed graphene-incorporating lubricant. Compared with the test results at 720 °C, the lubrication film is uniform on the surface of the wear marks. It can be seen clearly that the elements of P, Na and Zr which is from the lubricating material are uniformly distributed on the worn surface. From the distribution mapping of the Ti element, the lubricating film isolates the base metal from the upper sample. The iron and oxygen elements are overlapped in the wear track, which indicates that iron oxide is formed during the friction process. The C element is detected in the elemental mapping. This shows the possibility for the existence of graphene in the wear track after the tribotest. It can also be found from the table in
Figure 12h that the proportion of elements in lubricants is relatively obvious. It certifies that the lubricant composition on the surface of the wear marks still exists after 30 min of friction. Compared with the test results under experimental condition 1 with lubrication above, the lubrication film is uniform and continuous covered on the surface of the wear marks. Under the synergistic action of the lubricants, an effective barrier layer is formed on the surface of the friction pairs [
44,
45]. It effectively reduces the friction coefficient. The friction coefficient and wear rate are greatly reduced at the friction interface because of the excellent friction reducing materials at high temperatures.
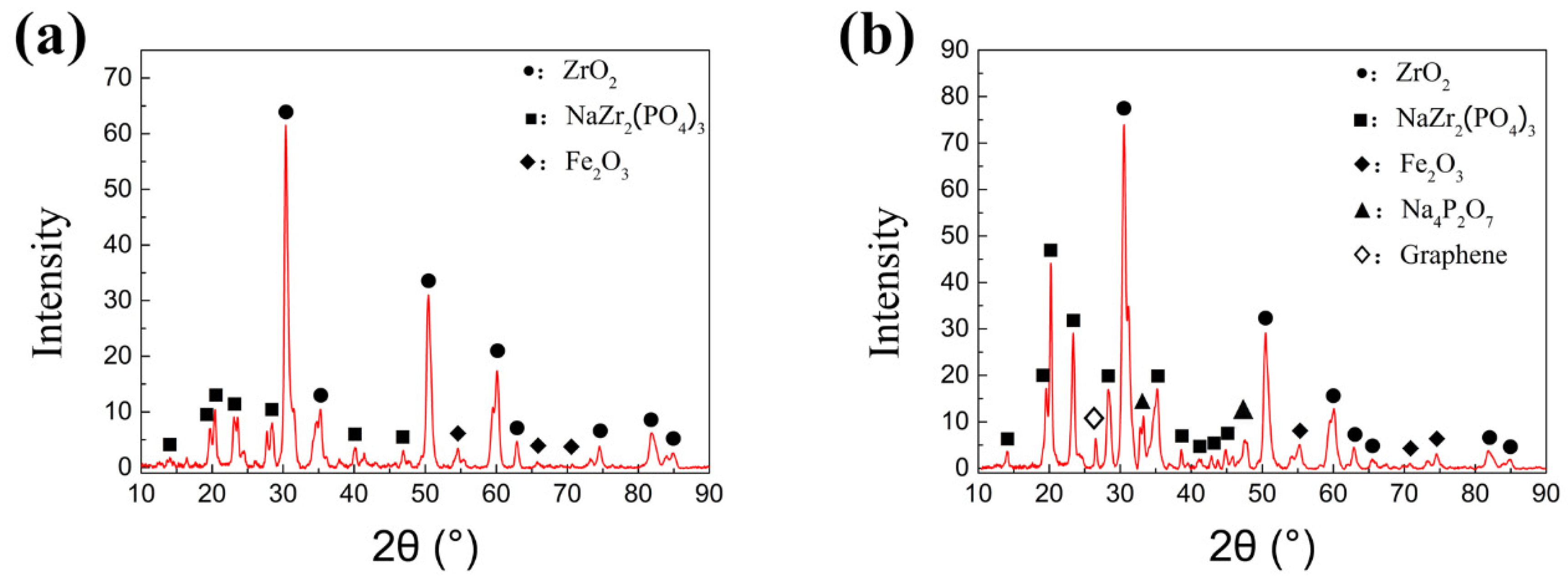
X-ray diffraction analysis was carried out for the phase composition on the worn surface of the specimens obtained under two experimental conditions with lubrication, as shown in
Figure 13. A fine step size is set as 0.02° for the test accuracy. The film information can be obtained from the equipment although the basement may also be detected if some part of the film is too thin. It can be seen from
Figure 13a that under the condition 1(720 °C/11.42 N/4.50 Hz), the lubricant component ZrO
2 and the lubricant product NaZr
2(PO
4)
3 are detected on the wear mark surface. This indicates that in the lubricant components chemical reactions occur at the wear mark interface. The phosphate melts at high temperature with increasing ion transfer rate and diffusion rate. When the phosphate melts, the phosphate and zirconia undergo atom rearrangement and electron transfer, so as to form sodium zirconium phosphate which is advanced for the metal forming process. However, the graphene is not detected in this condition, which may be due to the combustion loss of graphene itself at 720 °C.
From
Figure 13b, it can be found that there are five identical lubricant product components including graphene from the wear mark interface at 650 °C. The sodium zirconate phosphate shows a unique skeleton structure with high thermal and chemical stability. The structure of NaZr
2(PO
4)
3 also shows high isomorphic ability, which is conducive to combining the ions with different ionic radius, valence state and electronic shell structure. It is beneficial to form uniform and adhesion chemical adsorption film on the surface of the base metal. The phosphate not only provides the oxidation resistance, but also a foundation base for the connection of iron oxide particles, ZrO
2 nano particles and graphene. Meanwhile, the graphene and nano particles are able to strengthen the tribofilm, which prompts higher wear resistance and friction reduction performance during tribological process. Owing to the excellent synergistic effect of the lubricating materials, a stable lubrication film with high bonding strength is formed in the friction process so as to greatly reduce the COF and wear volume.
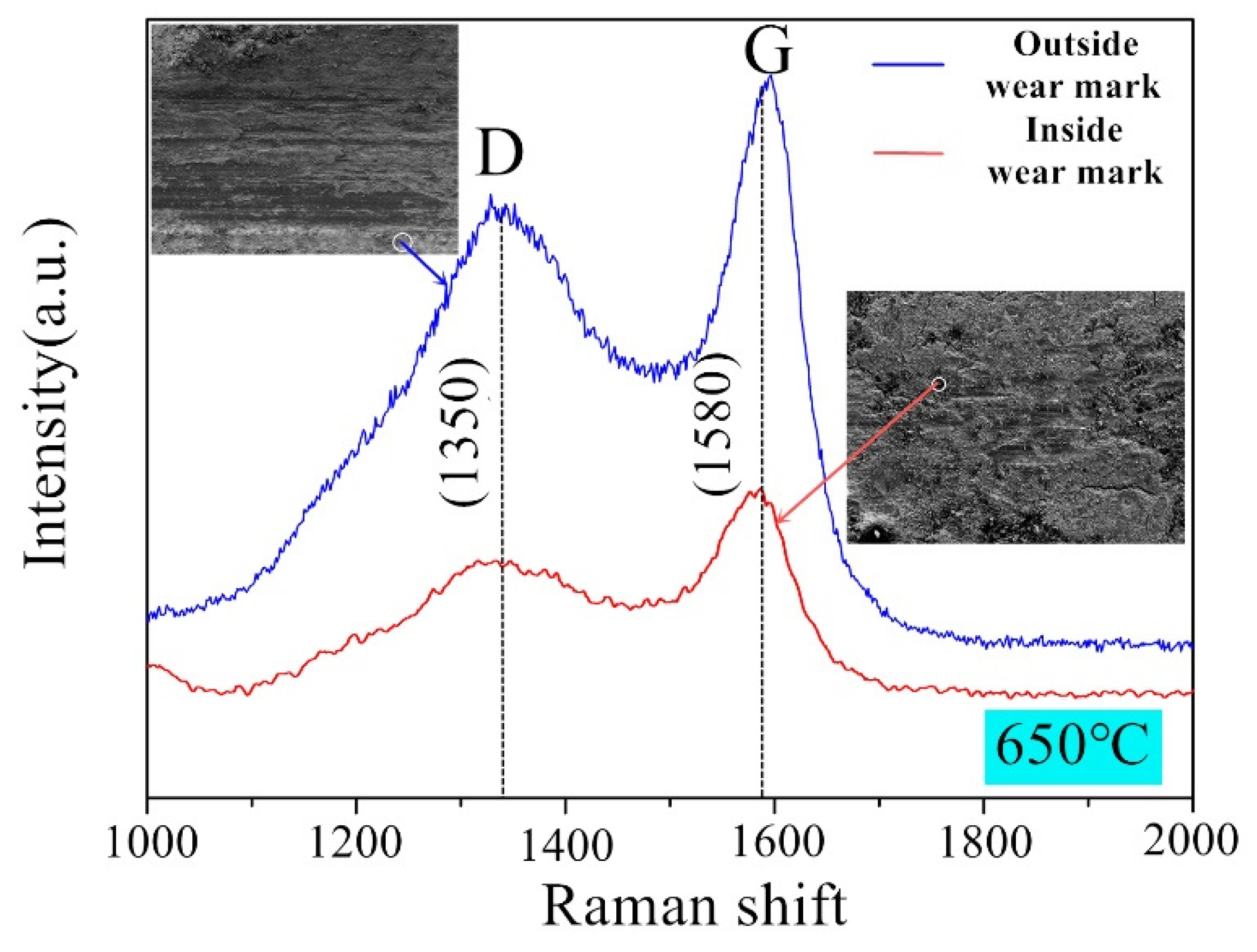
The Raman spectra of the worn surface of the sample have been measured at targeted points to confirm the existence of graphene under condition 2 with lubrication. The Raman results are obtained by detecting both inside and outside of the wear marks, as shown in
Figure 14. It is generally believed that the characteristic peak of graphene exists the D peak at 1350 cm
−1 and the G peak at 1580 cm
−1 [
46,
47,
48]. The diffraction peaks have been detected at 1350 cm
−1 and 1580 cm
−1 in both curves with a similar tendency. The results of the two detection sites confirm the existence of graphene. Although most of the graphene can be found in the wear marks which act as an excellent lubricating material, there are still some graphene may be extruded from the wear marks to the external area or consumed at high temperature.
3.4. Chemical- and Mechanical-Induced Lubrication Mechanism
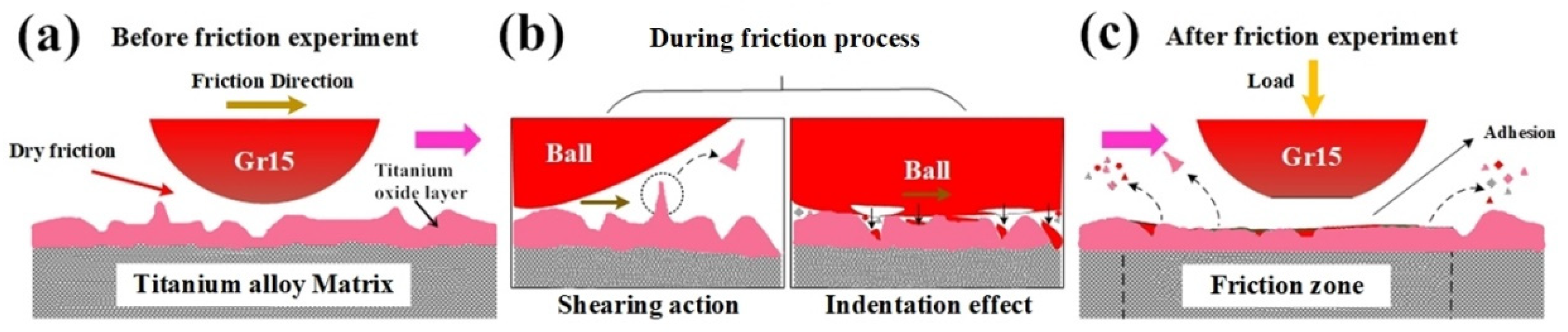
Figure 15 below is a schematic diagram of the tribological mechanism at the tribo-interface under dry friction conditions. Before the friction experiment, the surface micro morphology of the upper and lower samples are rough due to the oxidation of both steel ball and titanium alloy disc. During the contact process, at the contact interface there mainly occur two processes as shown in
Figure 15b. One process is the convex part of the surface on the lower sample is cut under the action of the shear force generated by the sliding with upper steel sample. The cut convex material forms the wear debris, which is peeled off from the contact interfaces. Another process is the indentation and adhesion phenomenon under high temperature and high pressure condition. The hard material (steel ball) of the upper sample forms a bonding layer of oxide steel on the surface. The soft materials (titanium alloy disc) is under the action of high temperature and pressure with the contact pair. Part of the upper iron oxide scale is compressed into the titanium oxide layer of the lower sample or adhered to the lower sample surface. In this stage, the rough points on the titanium oxide layer are mainly attrited by the shear force from upper test ball, so the wear of both upper steel ball and lower titanium disc are serious and the friction coefficient increases accordingly. Under the action of friction, the movement of debris formed during friction process between contact pairs leads to a plough groove, which is a typical feature of abrasive wear. Some parts of the titanium oxide layer are deformed during the friction process with the iron oxide particles. After the friction experimental process, a discontinuous and irregular oxide layer forms on the titanium alloy surface of the sample, which also accelerates the friction and wear process, as shown in
Figure 15c.
On the consideration of the hot-rolling process of titanium alloy sheets, the absence of cooling water and lubrication during the hot-rolling process goes against the surface quality of titanium alloy, as well as the life of the work rolls. The tribological tests under dry friction conditions indicate the main wear types are adhesive and abrasive wear. During the hot-rolling process, the deformation speed may reach 5 m/s. The steel work roll material is much harder than the titanium alloy sheets. Even small wear particles at the interfaces may lead to serious scratches on the sheet surface, which is not qualified for the production requirement.
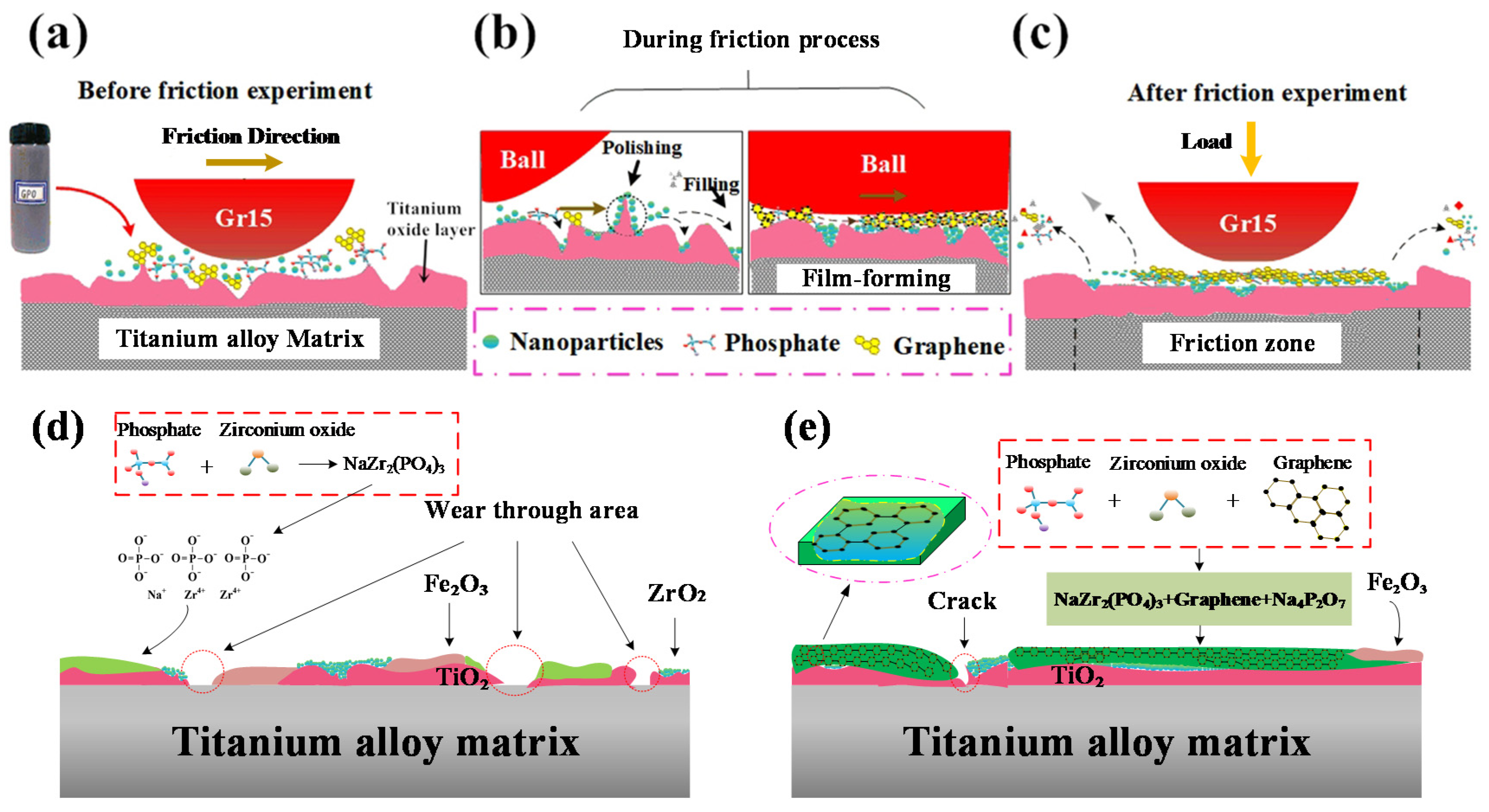
Figure 16 shows a schematic diagram of the tribological process and its chemical- and mechanical-induced lubrication mechanism after conducting mixed graphene-incorporating lubricant. From the previous work [
49], this kind of modified layer normally is about 100–950 nm in thickness from 600–800 °C. The thickness increases with the increased temperatures. When the temperature reaches 700 °C, the layer thickness increases sharply due to the intense chemical reactions on the tribo-interfaces at elevated temperatures. From
Figure 16a, the contact surface is uneven before applying the proposed mixed graphene-incorporating lubricant due to the metal oxidation at high temperatures. When the lubricant is added, the lubricant components which are mixed in a disorderly way start a molding process under the effect of high temperature and high pressure. When the mixed lubricant enters into the wear mark, the water vapor firstly boils and vaporizes. The phosphate, ZrO
2 nano particles and graphene are left between the contact interfaces. During the vaporization, the mixed lubricant components are uniformly mixed. Molten phosphate flows over the whole wear tracks and it can be firmly attached to the contact surface. Under the action of relative sliding and high-temperature conditions, the phosphate between the contact steel-titanium pairs acts as the carrier and forms a phosphate-graphene lubrication film. This lubrication film prevents direct contact between friction pairs. The presence of graphene plays the role of a skeleton which can be used for strengthening the molten phosphate film. It improves the strength of the lubricating film so as to avoid cracks after the friction process. The graphene which is embedded into the phosphate film can be effectively applied as a lubricating material with less combustion loss at such a high temperature. Nano ZrO
2 plays different roles because of their size advantages. Some nanoparticles play a role of bearing pressure on the contact surface. Some parts enter the voids and cracks of the interface during the friction process to make the contact surface flatter. The ZrO
2 nano particles also work on repairing surface defects. This reflects the self-repairing function of nano particles. Some nanoparticles present a polishing function to grind off the protruding parts of the surface, which reduce the roughness of the contact surface. This can be considered a mechanical-induced lubrication mechanism of the mixed graphene-incorporating lubricant. Therefore, the COF of the tribological surface is greatly reduced and the wear volume is also decreased under the synergistic action of the proposed mixed graphene-incorporating lubricant.
Figure 16d,e shows the chemical- and mechanical-induced lubrication mechanism under two experimental conditions (similar conditions from the hot rolling of titanium at stand 1 and 6 on finishing mills) respectively. Under condition 1 (720 °C/11.42 N/4.50 Hz), it can be seen from
Figure 16d that the formation of the lubrication interface is mainly composed of TiO
2 oxide layer and fragmented lubrication layer. During the tribological process, the high temperature environment causes the formation of a core-rim structure of the TiC particles [
50]. The TiC particle presents a much higher hardness compared to the matrix alloy. This indicates the TiC particle enhances the resistance to mechanical damage and wear [
51]. The Fe-TiC composite may also be produced under this condition with iron oxide particles from the ball. The wear properties of the Fe-TiC composite under different conditions have been described as hard carbide particles for the increase of wear resistance [
52]. After applying lubricant, phosphate reacts chemically with zirconia to form NaZr
2(PO
4)
3 lubrication component. The sodium zirconate phosphate presents a high isomorphic ability owing to its unique skeleton structure with high thermal and chemical stability. It is chemically welcomed in order to combine the ions with different ionic radius, valence state and electronic shell structure. The new generated lubrication film shows well wear reduction and friction resistance performance at the first 15 min. However, the COF tends to be increased after a certain friction process. It indicates the lubrication life is reasonable but still need to be improved during the hot rolling of titanium alloy at stand 1 of a hot finishing mill. In some worn areas, the lubricating layer and oxide layer are worn through to expose the base metal. There are also iron oxide scales in the lubricating films. This is the reason why the COF increases with the friction time. Under condition 2 (650 °C/9.69 N/12.62 Hz), owing to the existence of graphene during the friction process, the lubrication film is continuous with both original lubrication materials and tribo-chemical products. The graphene improves the strength of the lubrication film, which extends the life of the tribofilm. The chemical- and mechanical-induced lubrication film shows excellent lubricating performance due to the synergistic lubrication mechanism of the graphene, phosphate and ZrO
2 nano particles at high temperatures with load.
From the industrial production experience of titanium alloy sheets by hot rolling, the surface defects normally happen at the exit of the stand 1 and stand 6 of a hot-finishing mill. Under both hot rolling conditions, the mixed graphene-incorporating lubricant shows good lubrication characteristics, especially in the condition of stand 6 at 650 °C with a synergistic action of graphene and other lubrication materials. It provides the possibility for the proposed mixed graphene-incorporating lubricant to be applied for the hot rolling of titanium alloy sheets so as to improve the surface quality and roll life. These results may also provide guidelines for optimizing the lubrication performance with other synergistic lubrication mechanism which are subjected to the hot metal forming.