Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Membranes
2.3. Characterization of Membranes
2.3.1. Attenuated Total Reflectance–Fourier Transform Infrared (ATR–FTIR) Spectroscopy
2.3.2. Potentiometric Titration
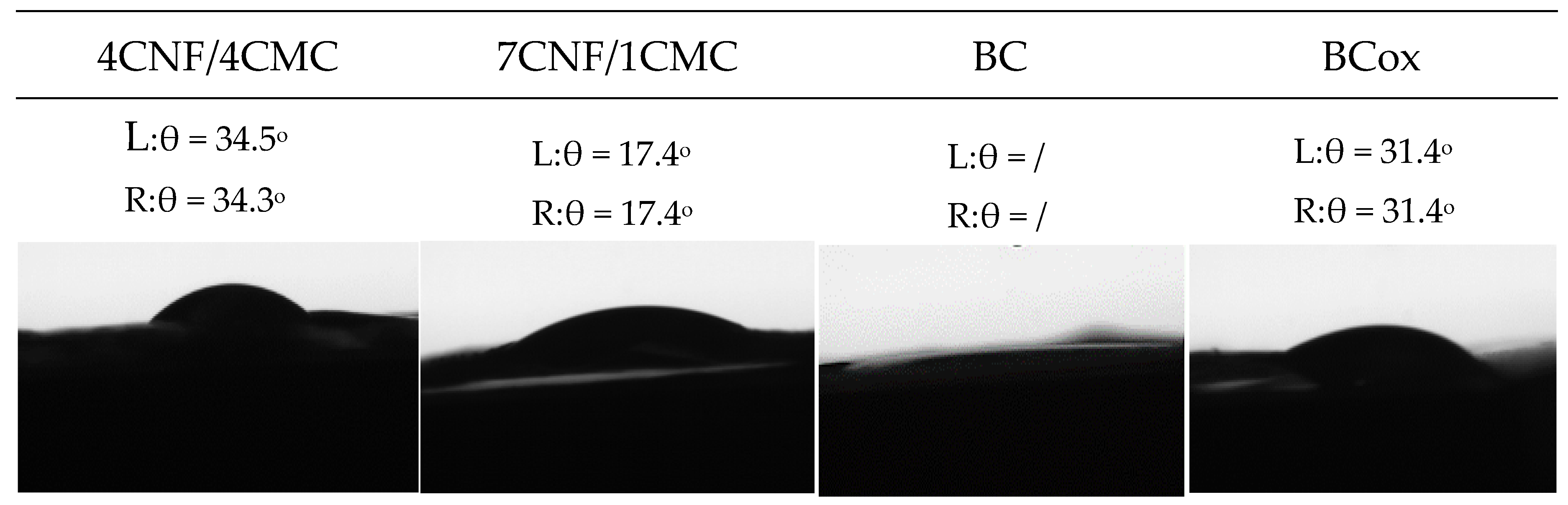
2.3.3. Contact Angle Goniometry
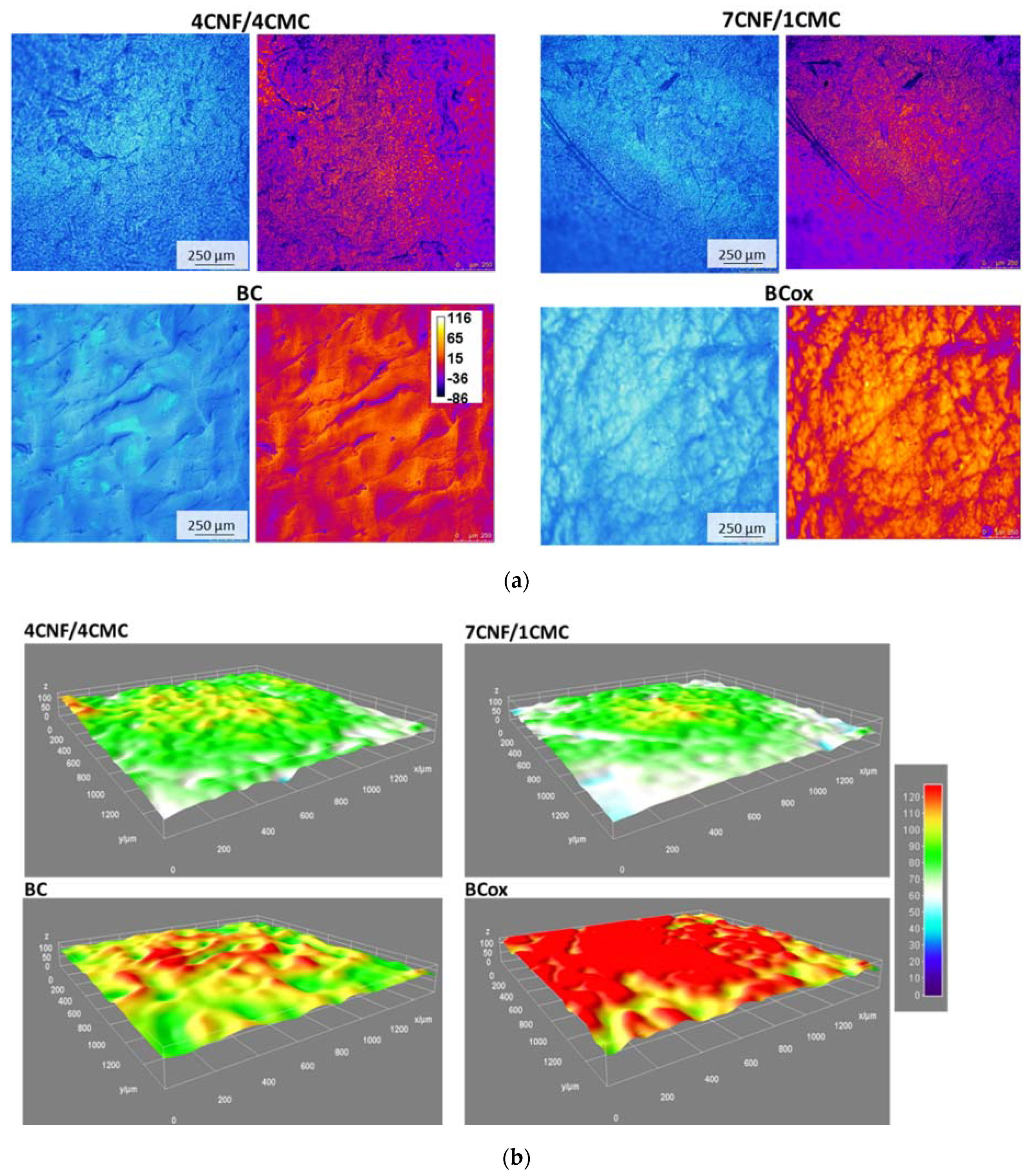
2.3.4. Confocal Fluorescent Microscopy (CFM) Imaging
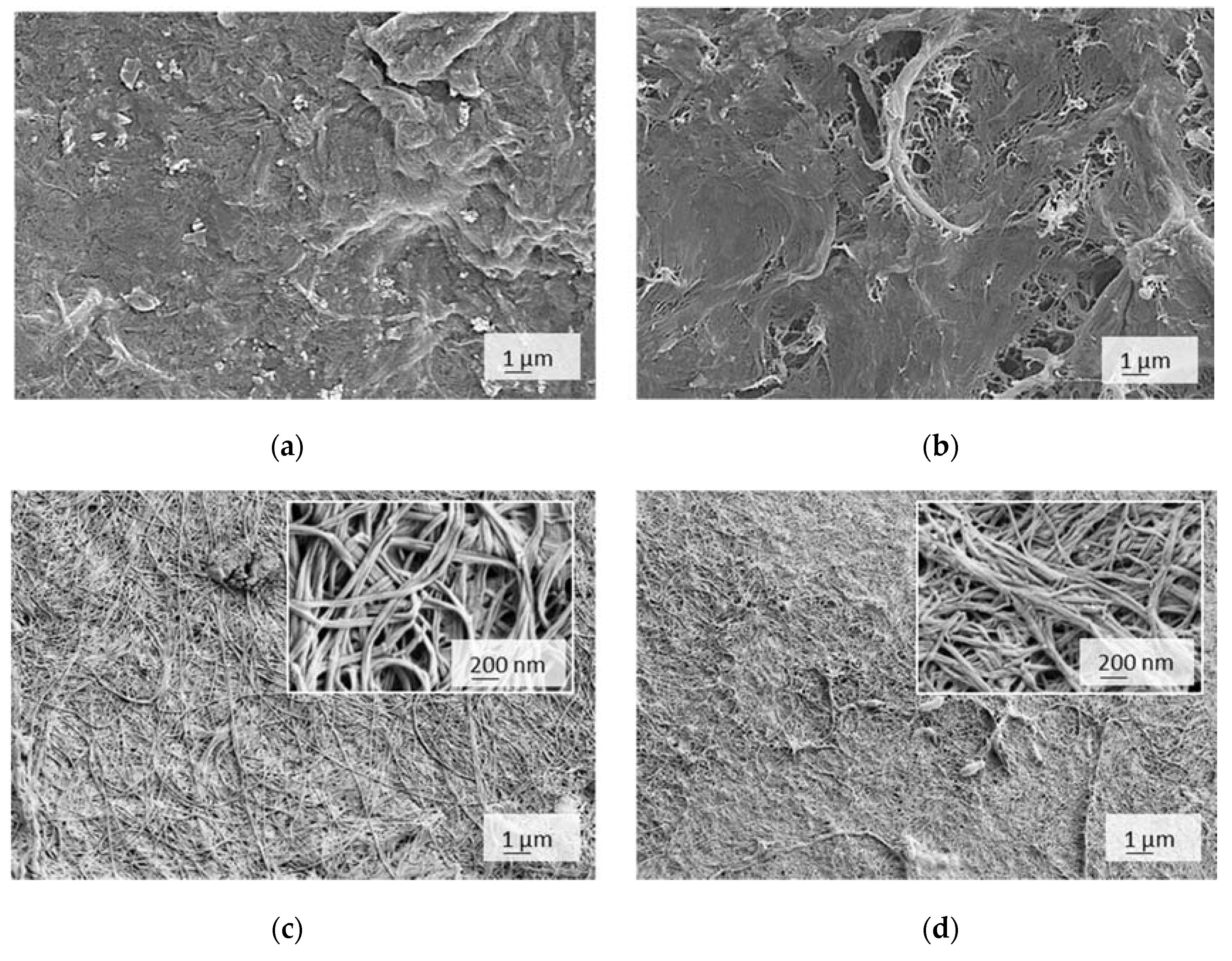
2.3.5. Scanning Electron Microscopy (SEM) Imaging
2.3.6. UV–VIS Spectroscopy
3. Results and Discussion
3.1. Esterification and Oxidation within Cellulose-Based Membranes
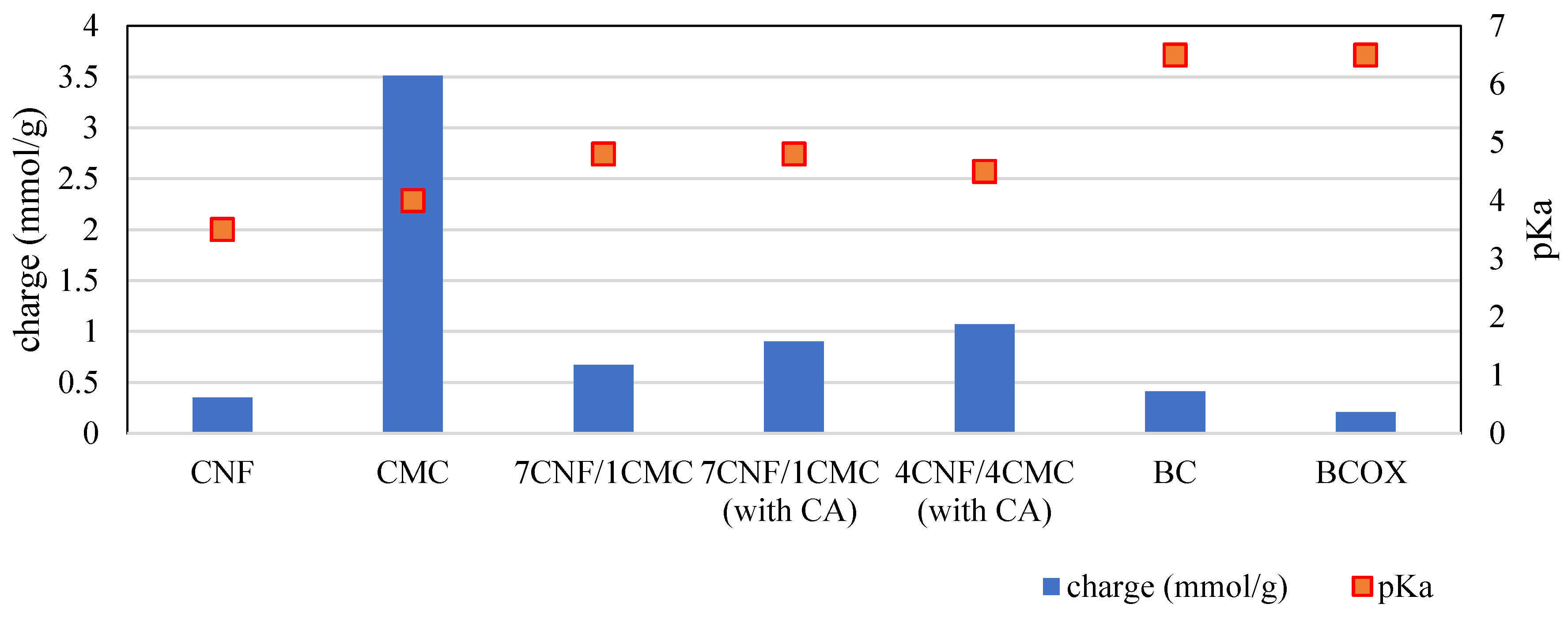
3.2. Charge, Hydrophilicity, and Morphology of Membranes
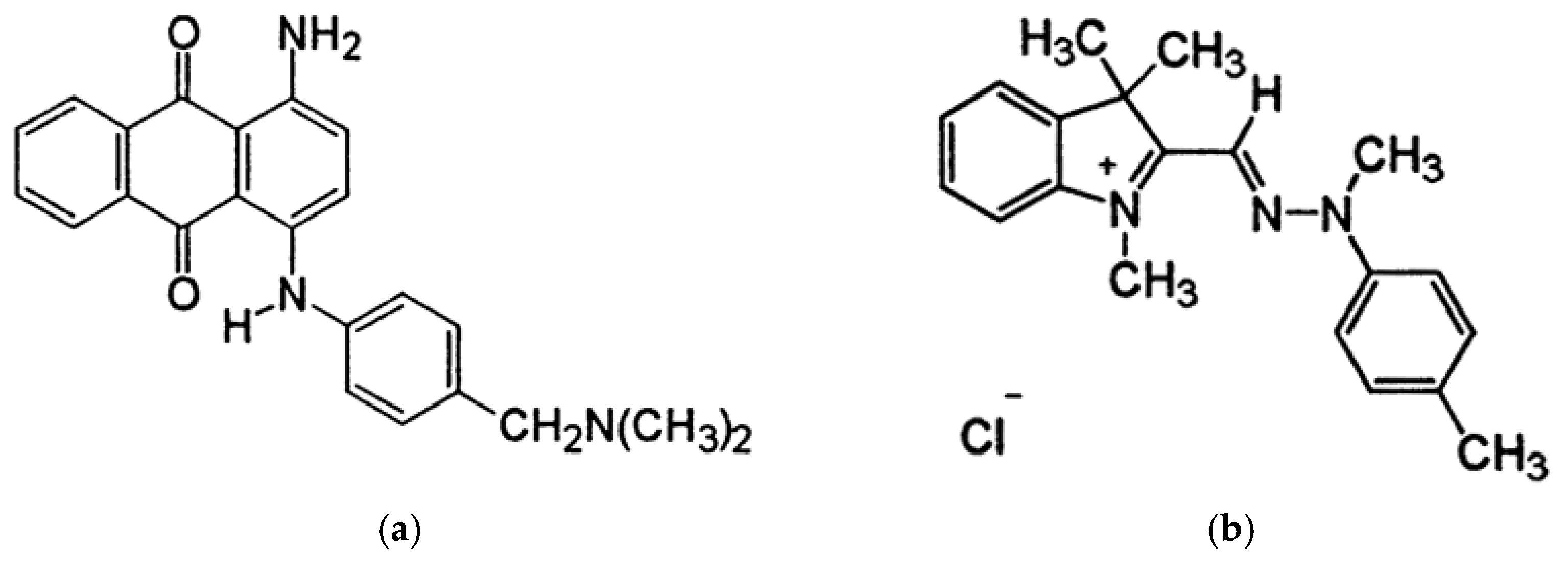
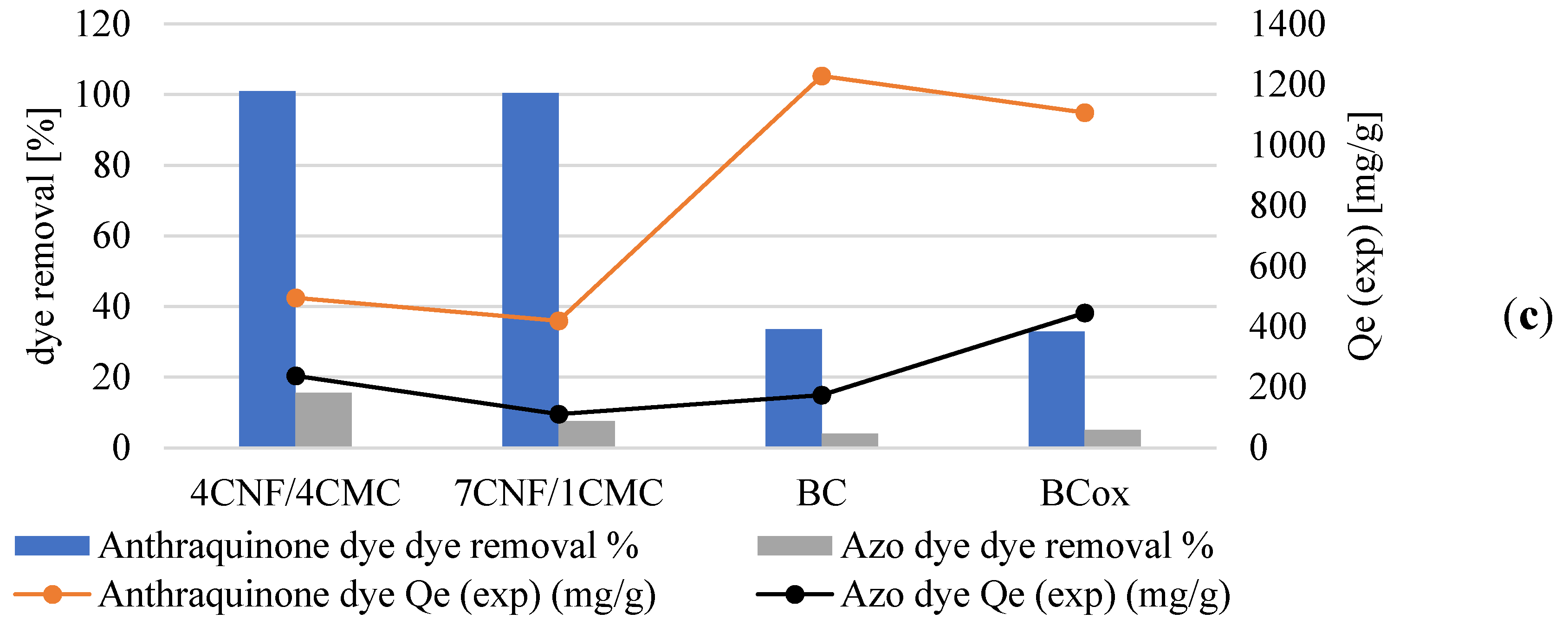
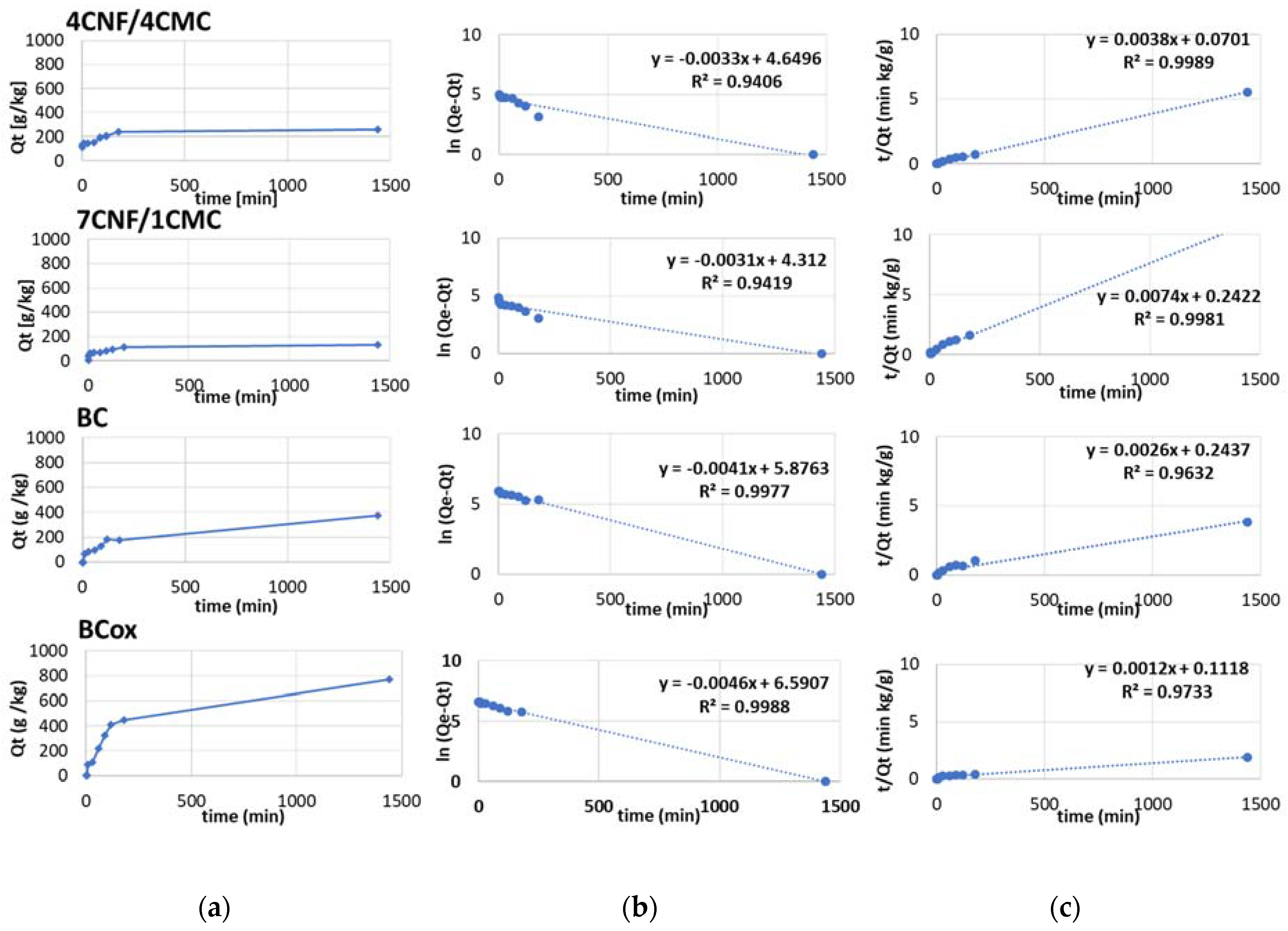
3.3. Adsorption Efficiency and Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rafii, F.; Hall, J.D.; Cerniglia, C.E. Mutagenicity of azo dyes used in foods, drugs and cosmetics before and after reduction by clostridium species from the human intestinal tract. Food Chem. Toxicol. 1997, 35, 897–901. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Noel, M. Membrane processes for dye wastewater treatment: Recent progress in fouling control. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1007–1040. [Google Scholar] [CrossRef]
- Sadri Moghaddam, S.; Alavi Moghaddam, M.R.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-DÍaz, J.; Pavón-Silva, T.; Gutiérrez-Segura, E.; Colín-Cruz, A. Electrocoagulation-Adsorption to Remove Anionic and Cationic Dyes from Aqueous Solution by PV-Energy. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Zhu, M.X.; Lee, L.; Wang, H.H.; Wang, Z. Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J. Hazard. Mater. 2007, 149, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Wijannarong, S.; Aroonsrimorakot, S.; Thavipoke, P.; Kumsopa, C.; Sangjan, S. Removal of Reactive Dyes from Textile Dyeing Industrial Effluent by Ozonation Process. APCBEE Procedia 2013, 5, 279–282. [Google Scholar] [CrossRef]
- Gorgieva, S.; Vogrinčič, R.; Kokol, V. The Effect of Membrane Structure Prepared from Carboxymethyl Cellulose and Cellulose Nanofibrils for Cationic Dye Removal. J. Polym. Environ. 2019, 27, 318–332. [Google Scholar] [CrossRef]
- Han, R.; Zhang, L.; Song, C.; Zhang, M.; Zhu, H.; Zhang, L.J. Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr. Polym. 2010, 79, 1140–1149. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, T.; Zeng, Y.; Zhou, Q.; Peng, Y. Effective adsorption of cationic dyes by lignin sulfonate polymer based on simple emulsion polymerization: Isotherm and kinetic studies. RSC Adv. 2015, 5, 3757–3766. [Google Scholar] [CrossRef]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Chan, C.H.; Chia, C.H.; Zakaria, S.; Sajab, M.S.; Chin, S.X. Cellulose nanofibrils: A rapid adsorbent for the removal of methylene blue. RSC Adv. 2015, 5, 18204–18212. [Google Scholar] [CrossRef]
- Fardim, P.; Moreno, T.; Holmbom, B. Anionic groups on cellulosic fiber surfaces investigated by XPS, FTIR-ATR, and different sorption methods. J. Colloid Interface Sci. 2005, 290, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Carvalho, J.; De Sousa Bezerra, R.D.; Silva, M.S.; Ferreira, F.J.L.; Osajima, J.A.; Da Silva Filho, E.C. Potential of cellulose functionalized with carboxylic acid as biosorbent for the removal of cationic dyes in aqueous solution. Molecules 2018, 23. [Google Scholar]
- Liu, L.; Gao, Z.Y.; Su, X.P.; Chen, X.; Jiang, L.; Yao, J.M. Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustain. Chem. Eng. 2015, 3, 432–442. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Zhang, G.; Yi, L.; Deng, H.; Sun, P. Dyes adsorption using a synthetic carboxymethyl cellulose-acrylic acid adsorbent. J. Environ. Sci. (China) 2014, 26, 1203–1211. [Google Scholar] [CrossRef]
- Wang, M.M.; Wang, L. Synthesis and characterization of carboxymethyl cellulose/organic montmorillonite nanocomposites and its adsorption behavior for Congo Red dye. Water Sci. Eng. 2013, 6, 272–282. [Google Scholar]
- Jin, H.-X.; Xu, H.; Wang, N.; Yang, L.-Y.; Wang, Y.-G.; Yu, D.; Ouyang, X.-K. Fabrication of Carboxymethylcellulose/Metal-Organic Framework Beads for Removal of Pb(II) from Aqueous Solution. Materials 2019, 12, 942. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lü, X.; Xu, X.; Guan, X. Thermodynamics and kinetics of bacterial cellulose adsorbing persistent pollutant from aqueous solutions. Chem. Res. Chin. Univ. 2015, 31, 298–302. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Jongsomjit, B.; Robison, J.; Phisalaphong, M. Activated carbon from bacterial cellulose as an effective adsorbent for removing dye from aqueous solution. Sep. Sci. Technol. 2019, 54, 2180–2193. [Google Scholar] [CrossRef]
- Jamal, F.; Qidwai, T.; Pandey, P.K.; Singh, R.; Singh, S. Azo and anthraquinone dye decolorization in relation to its molecular structure using soluble Trichosanthes dioica peroxidase supplemented with redox mediator. Catal. Commun. 2011, 12, 1218–1223. [Google Scholar] [CrossRef]
- Gorgieva, S.; Hribernik, S. Microstructured and Degradable Bacterial Cellulose–Gelatin Composite Membranes: Mineralization Aspects and Biomedical Relevance. Nanomaterials 2019, 9, 303. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Čakara, D.; Michaelis, N.; Heinze, T.; Kleinschek, K.S. Protonation behavior of 6-deoxy-6-(2-aminoethyl)amino cellulose: A potentiometric titration study. Cellulose 2011, 18, 33–43. [Google Scholar] [CrossRef]
- Karasu, F.; Müller, L.; Ridaoui, H.; Ibn ElHaj, M.; Flodberg, G.; Aulin, C.; Axrup, L.; Leterrier, Y. Organic-Inorganic Hybrid Planarization and Water Vapor Barrier Coatings on Cellulose Nanofibrils Substrates. Front. Chem. 2018, 6, 571. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zimmermann, T.; Tingaut, P. Hydrophobic cellulose nanopaper through a mild esterification procedure. Cellulose 2013, 21, 367–382. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, Z.; Sui, S.; Wang, Q.; Xie, Y. Esterification of wood with citric acid: The catalytic effects of sodium hypophosphite (SHP). Holzforschung 2014, 68. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J.; Mali, K.K.; Pargaonkar, S.S.; Shinde, P.V.; Dhane, N.S. Citric acid crosslinked carboxymethylcellulose-poly(ethylene glycol) hydrogel films for delivery of poorly soluble drugs. Int. J. Biol. Macromol. 2018, 118, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Menzel, C.; Johansson, C.; Andersson, R.; Koch, K.; Järnström, L. The effect of pH on hydrolysis, cross-linking and barrier properties of starch barriers containing citric acid. Carbohydr. Polym. 2013, 98, 1505–1513. [Google Scholar] [CrossRef]
- Raucci, M.G.; Alvarez-Perez, M.A.; Demitri, C.; Giugliano, D.; De Benedictis, V.; Sannino, A.; Ambrosio, L. Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. J. Biomed. Mater. Res. Part A 2015, 103, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Pantze, A. Ph.D. Thesis. Studies of ester formation on a cellulose matrix 2006.
- Fan, Q.G.; Lewis, D.M.; Tapley, K.N. Characterization of Cellulose Aldehyde Using Fourier Transform Infrared Spectroscopy. J. Appl. Polym. Sci. 2001, 82, 1195–1202. [Google Scholar] [CrossRef]
- Khalid, A.; Zhang, L.; Tetienne, J.-P.; Abraham, A.N.; Poddar, A.; Shukla, R.; Shen, W.; Tomljenovic-Hanic, S. Intrinsic fluorescence from cellulose nanofibers and nanoparticles at cell friendly wavelengths. APL Photonics 2019, 4, 020803. [Google Scholar] [CrossRef]
- Khalil, S.; El-Badri, N.; El-Mokhtaar, M.; Al-Mofty, S.; Farghaly, M.; Ayman, R.; Habib, D.; Mousa, N. A Cost-Effective Method to Assemble Biomimetic 3D Cell Culture Platforms. PLoS ONE 2016, 11, e0167116. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D.J.; Han, Y. Drying cellulose nanofibrils: in search of a suitable method. Cellulose 2011, 19, 91–102. [Google Scholar] [CrossRef]
- Kanti Sen, T.; Afroze, S.; Ang, H.M.; Sen, T.K.; Afroze, S. Equilibrium, Kinetics and Mechanism of Removal of Methylene Blue from Aqueous Solution by Adsorption onto Pine Cone Biomass of Pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar]
- Broadbent, A.D. Basic Principles of Textile Coloration 2001 Society of Dyers and Colourists; Society of Dyers & Colourists: Bradford, UK, 2001. [Google Scholar]
- Upadhye, G.C.; Yamgar, R.S. Analytical study of agricultural waste as non-conventional low cost adsorbent removal of dyes from aqueous solutions. Int. J. Chem. Stud. 2016, 4, 128–133. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleš, L.; Fakin, D.; Bračič, M.; Gorgieva, S. Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents. Nanomaterials 2020, 10, 642. https://doi.org/10.3390/nano10040642
Maleš L, Fakin D, Bračič M, Gorgieva S. Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents. Nanomaterials. 2020; 10(4):642. https://doi.org/10.3390/nano10040642
Chicago/Turabian StyleMaleš, Laura, Darinka Fakin, Matej Bračič, and Selestina Gorgieva. 2020. "Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents" Nanomaterials 10, no. 4: 642. https://doi.org/10.3390/nano10040642
APA StyleMaleš, L., Fakin, D., Bračič, M., & Gorgieva, S. (2020). Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents. Nanomaterials, 10(4), 642. https://doi.org/10.3390/nano10040642






