Nanocellulose-Based Patches Loaded with Hyaluronic Acid and Diclofenac towards Aphthous Stomatitis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Materials and Cells
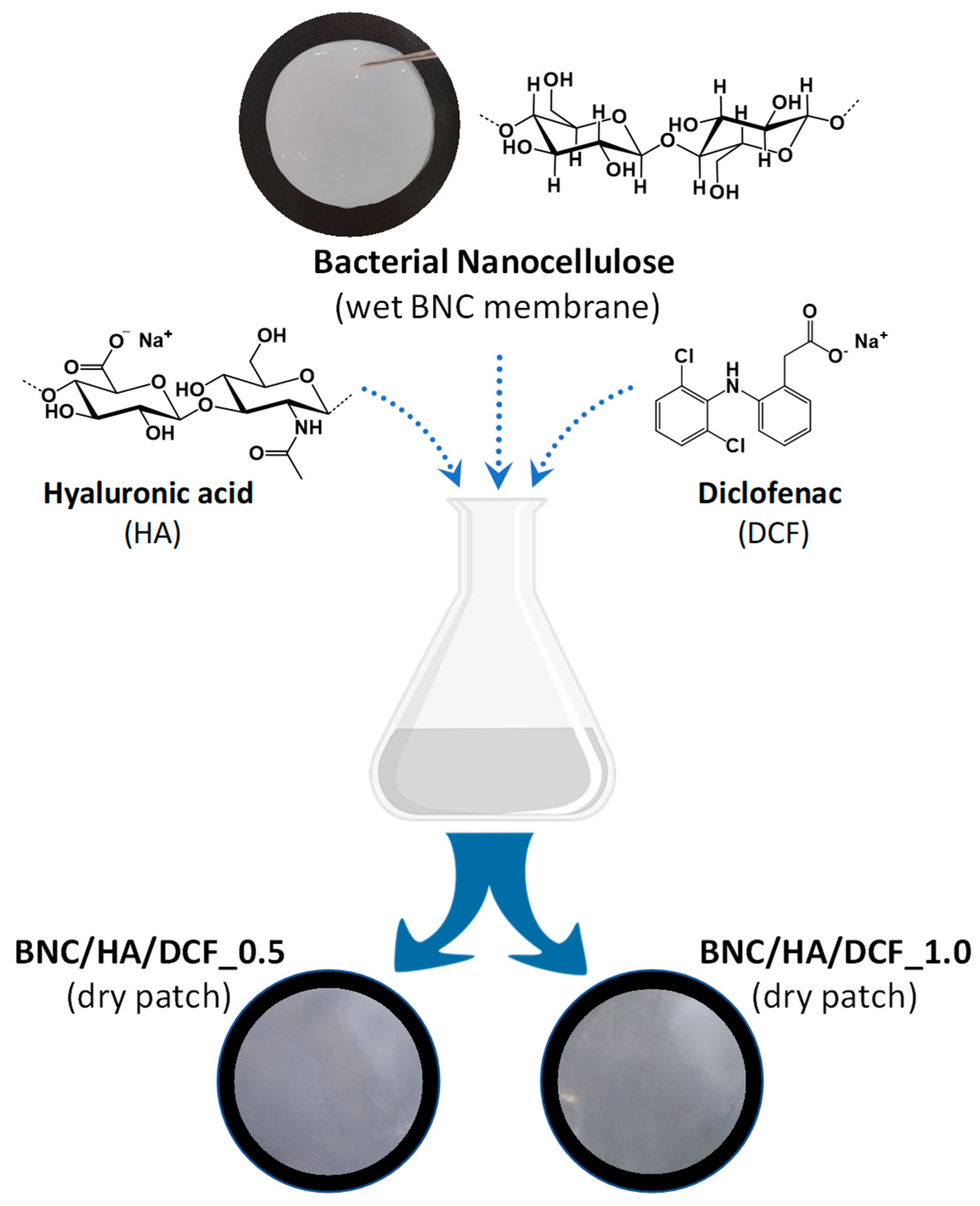
2.2. Preparation of Nanocellulose-Based Patches
2.3. Characterization Methods
2.4. Moisture- and Water-Uptake Capacity
2.5. In Vitro Cytotoxicity Assay
2.6. In Vitro Diclofenac Release Assay
2.7. Statistical Analysis
3. Results and Discussion
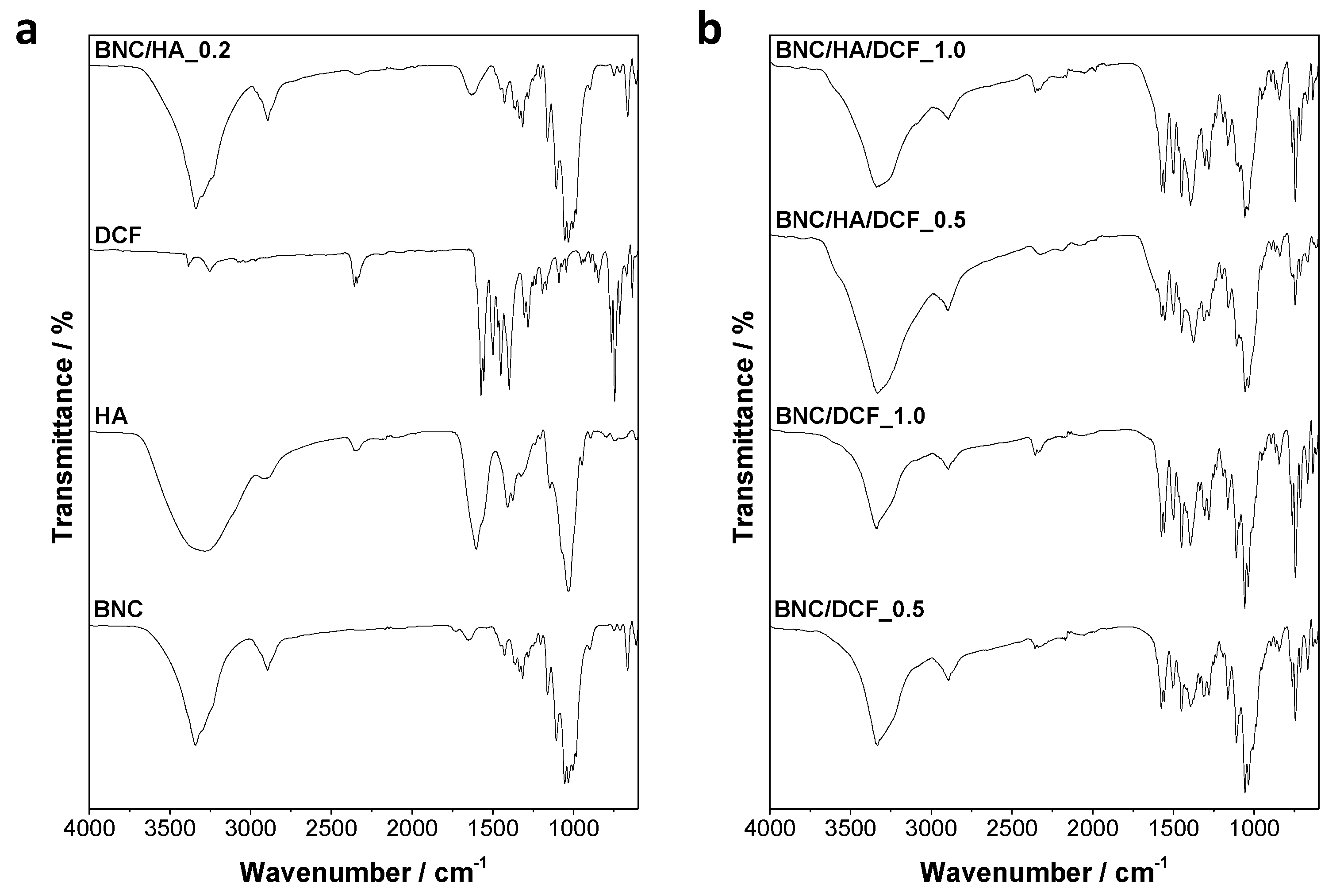
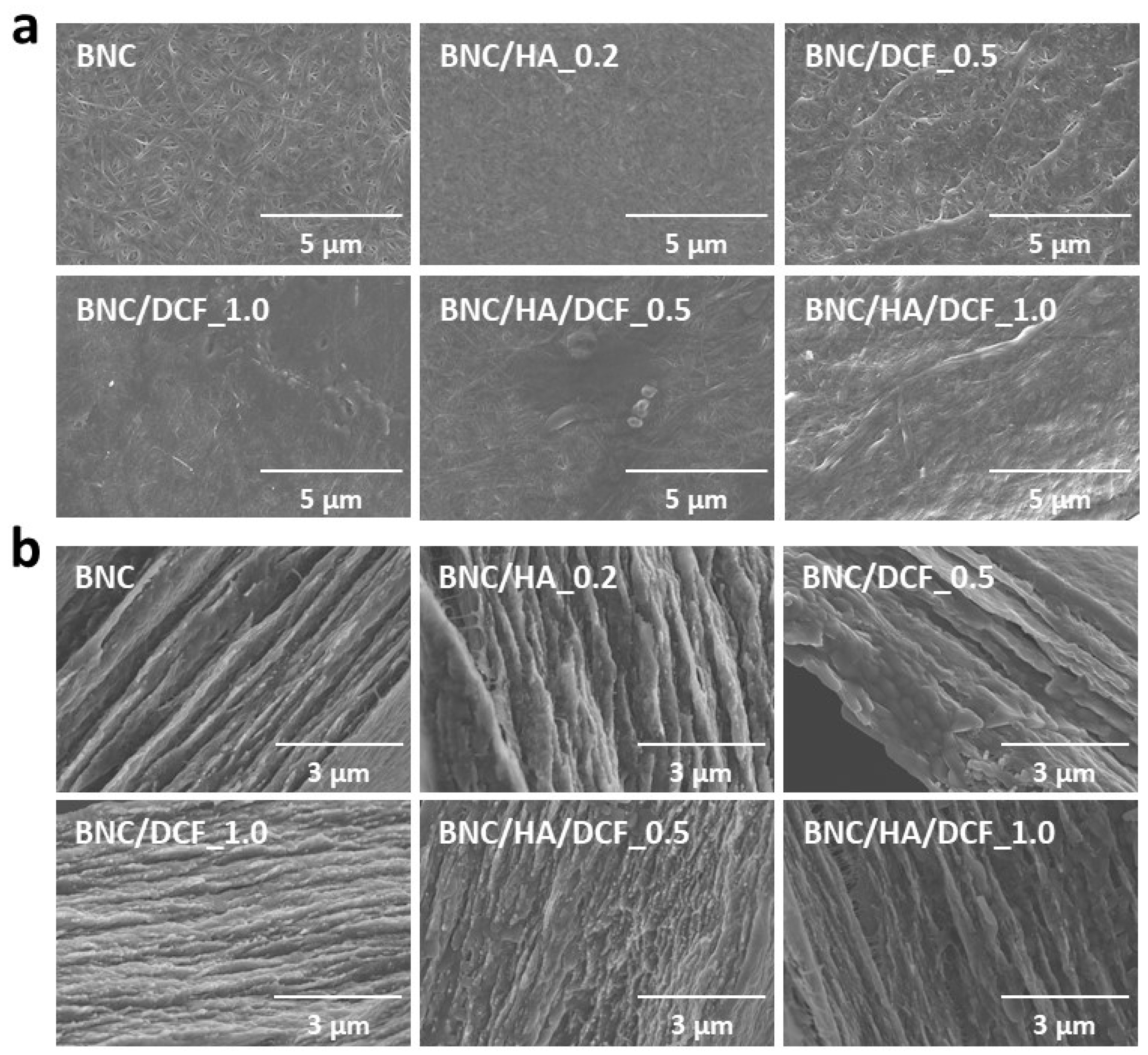
3.1. Structure and Morphology
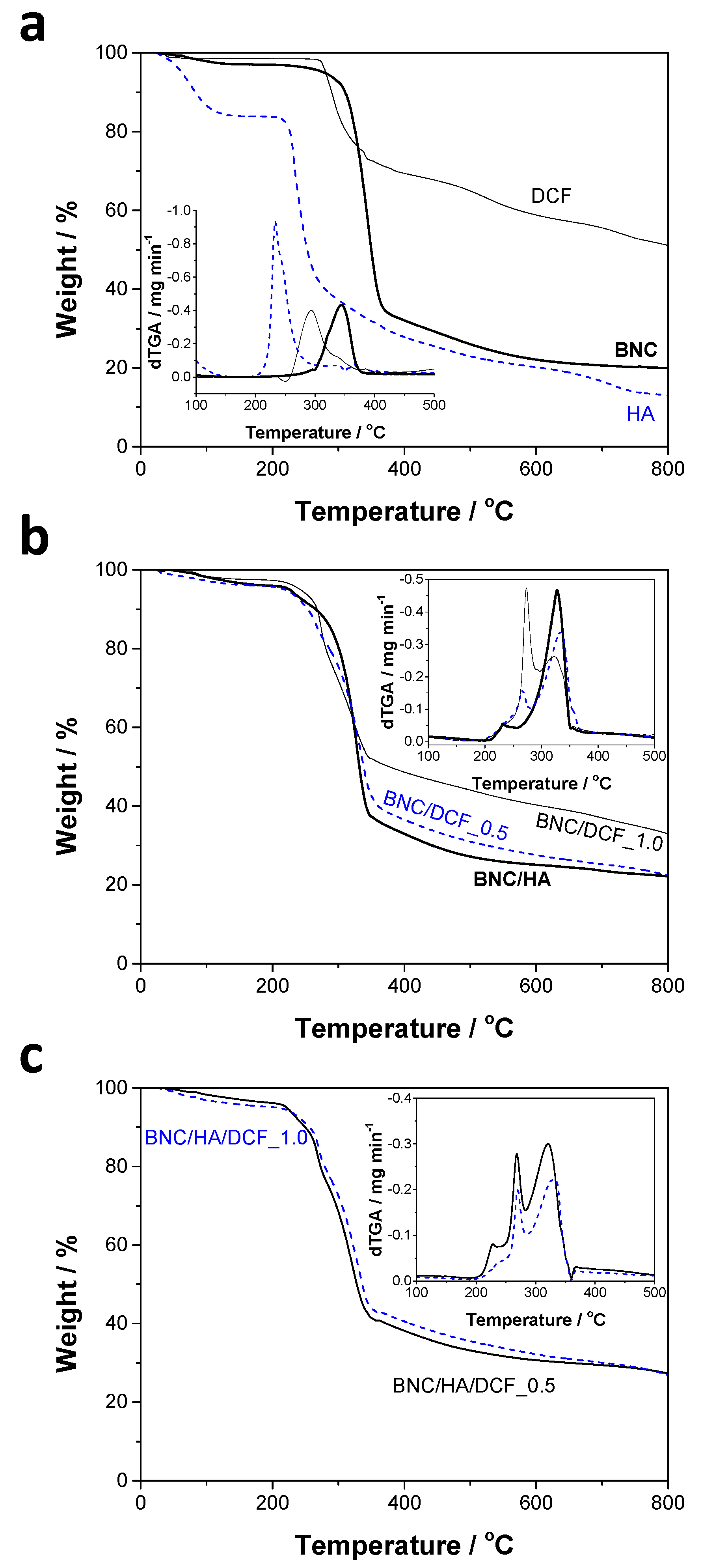
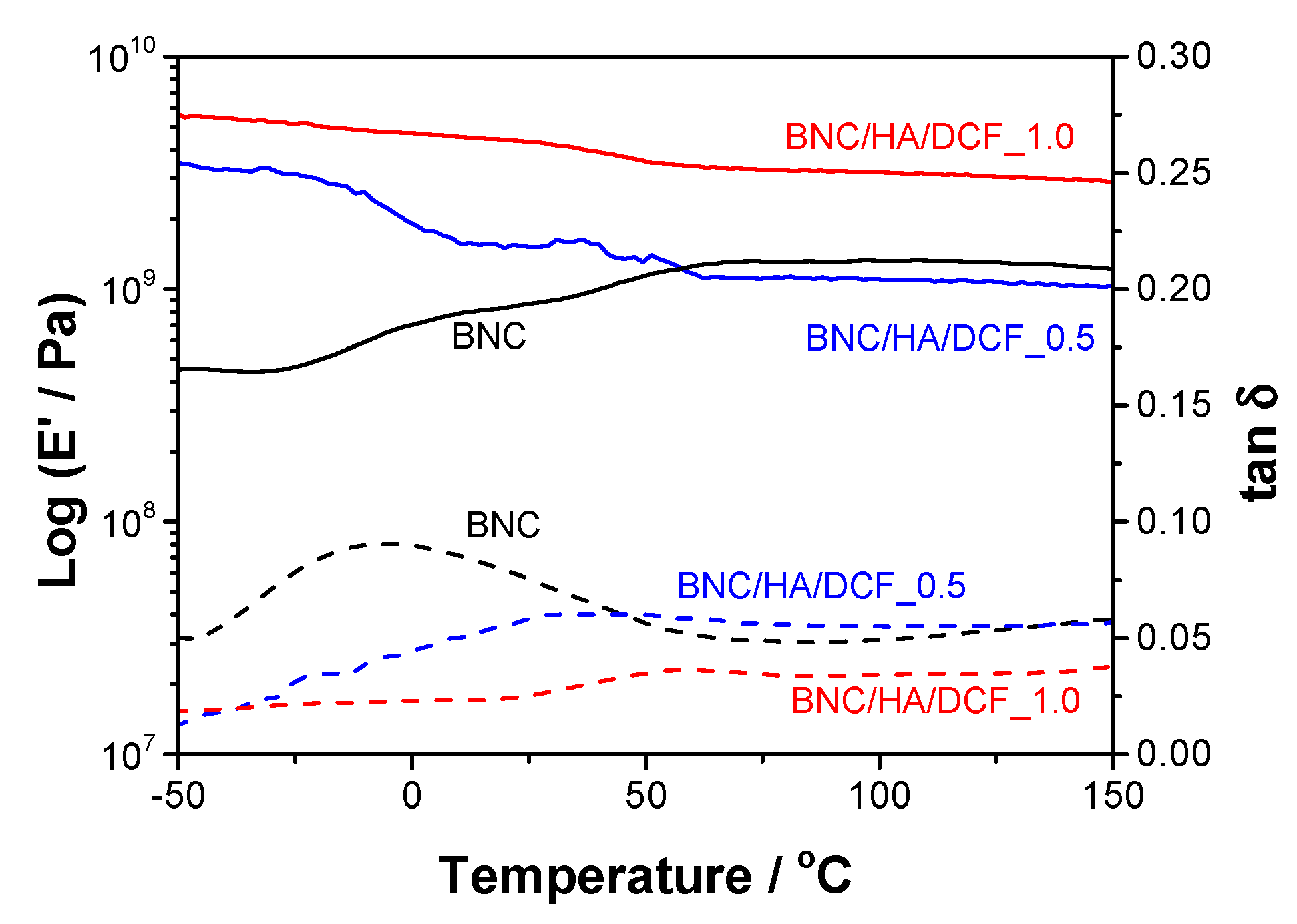
3.2. Thermal and Mechanical Properties
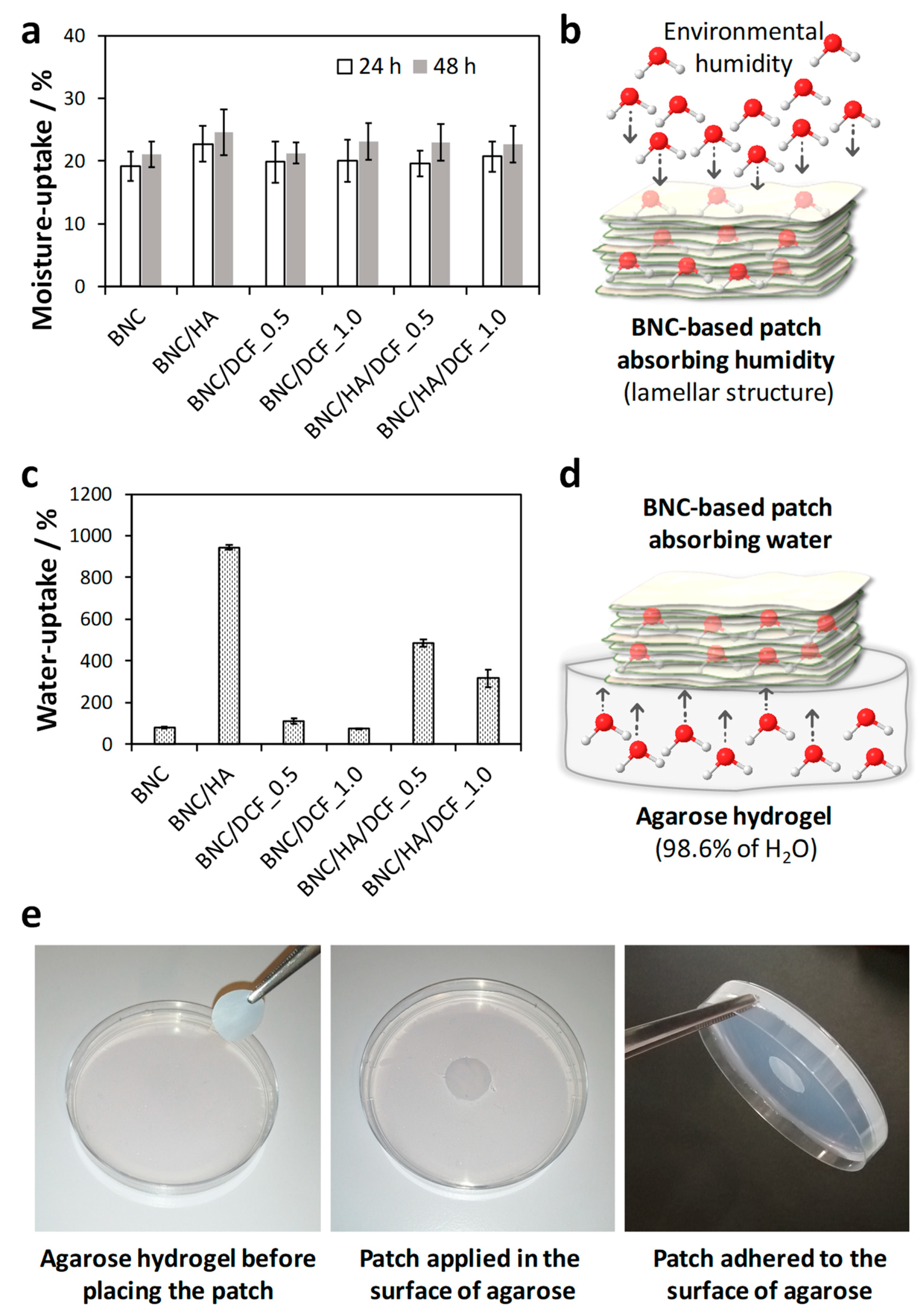
3.3. Moisture- and Water-Uptake Capacity
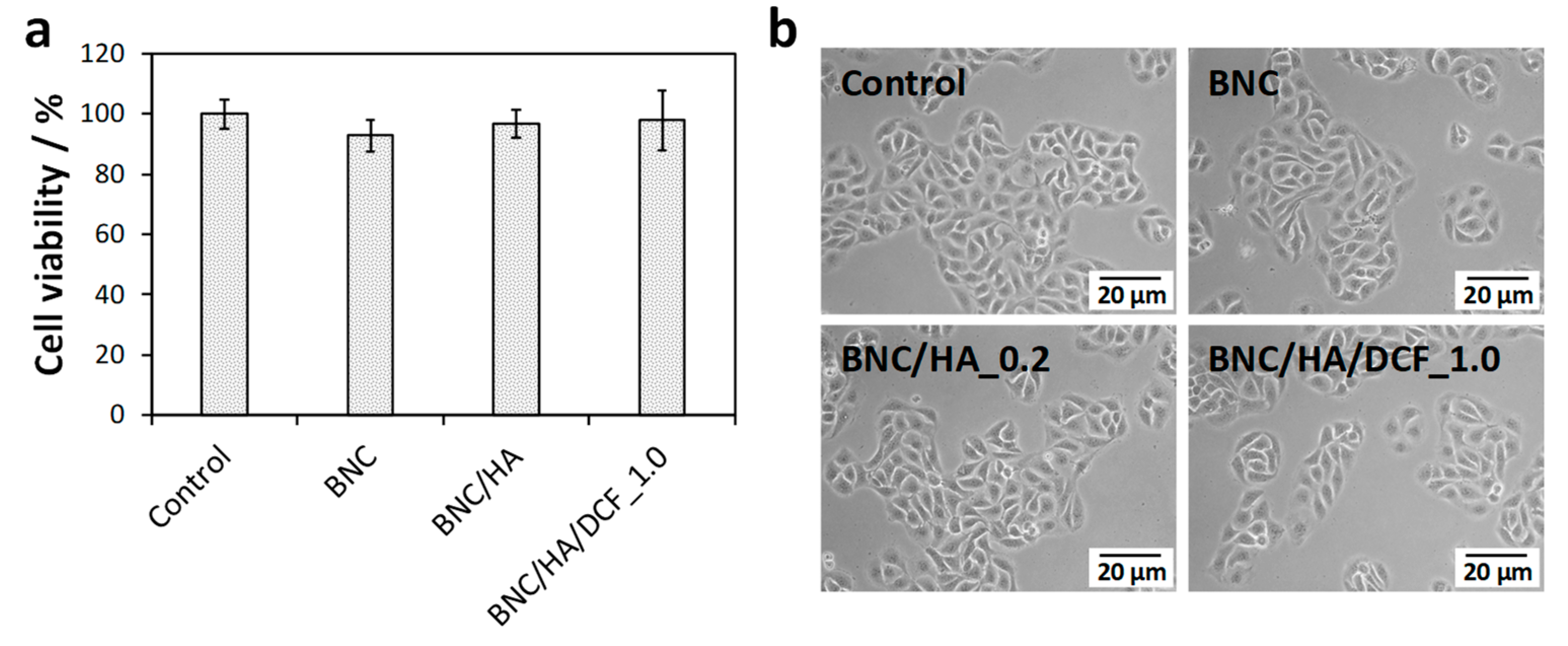
3.4. In Vitro Cytotoxicity
3.5. In Vitro Drug Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chiang, C.P.; Chang, J.Y.-F.; Wang, Y.P.; Wu, Y.H.; Wu, Y.C.; Sun, A. Recurrent aphthous stomatitis – Etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2019, 118, 1279–1289. [Google Scholar] [CrossRef]
- Sharma, D.; Garg, R. A comprehensive review on aphthous stomatitis, its types, management and treatment available. J. Dev. Drugs 2018, 7, 1000188. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of recurrent aphthous stomatitis. Biomed. Rep. 2019, 11, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Dalessandri, D.; Zotti, F.; Laffranchi, L.; Migliorati, M.; Isola, G.; Bonetti, S.; Visconti, L. Treatment of recurrent aphthous stomatitis (RAS; Aphthae; canker sores) with a barrier forming mouth rinse or topical gel formulation containing hyaluronic acid: A retrospective clinical study. BMC Oral Health 2019, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, A.; El-Haj, N.; Micheli, C.; Puttkammer, M.; Abdel-Naser, M.B.; Zouboulis, C.C. The treatment of chronic recurrent oral aphthous ulcers. Dtsch. Arztebl. Int. 2014, 111, 665–673. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Wolf, K.J.; Kumar, S. Hyaluronic acid: Incorporating the bio into the material. ACS Biomater. Sci. Eng. 2019, 5, 3753–3765. [Google Scholar] [CrossRef]
- Nolan, A.; Baillie, C.; Badminton, J.; Rudralingham, M.; Seymour, R.A. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J. Oral Pathol. Med. 2006, 35, 461–465. [Google Scholar] [CrossRef]
- Laffleur, F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 591–598. [Google Scholar] [CrossRef]
- Saxen, M.A.; Ambrosius, W.T.; Rehemtula, A.K.F.; Russell, A.L.; Eckert, G.J. Sustained relief of oral aphthous ulcer pain from topical diclofenac in hyaluronan: A randomized, double-blind clinical trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 356–361. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Pakes, G.E.; Speight, T.M.; Avery, G.S. Diclofenac sodium: A review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs 1980, 20, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Taylor-Gjevre, R. A review of topical diclofenac use in musculoskeletal disease. Pharmaceuticals 2010, 3, 1892–1908. [Google Scholar] [CrossRef] [PubMed]
- Kürklü-Gürleyen, E.; Öğüt-Erişen, M.; Çakır, O.; Uysal, Ö.; Ak, G. Quality of life in patients with recurrent aphthous stomatitis treated with a mucoadhesive patch containing citrus essential oil. Patient Pref. Adherence 2016, 10, 967–973. [Google Scholar] [CrossRef]
- Colley, H.E.; Said, Z.; Santocildes-Romero, M.E.; Baker, S.R.; D’Apice, K.; Hansen, J.; Madsen, L.S.; Thornhill, M.H.; Hatton, P.V.; Murdoch, C. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials 2018, 178, 134–146. [Google Scholar] [CrossRef]
- Laffleur, F.; Küppers, P. Adhesive alginate for buccal delivery in aphthous stomatitis. Carbohydr. Res. 2019, 477, 51–57. [Google Scholar] [CrossRef]
- Daněk, Z.; Gajdziok, J.; Doležel, P.; Landová, H.; Vetchý, D.; Štembírek, J. Buccal films as a dressing for the treatment of aphthous lesions. J. Oral Pathol. Med. 2017, 46, 301–306. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods – A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular aspects of bacterial nanocellulose biosynthesis. Microb. Biotechnol. 2019, 12, 633–649. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Freire, C.S.R.; Neto, C.P. Do bacterial cellulose membranes have potential in drug-delivery systems? Expert Opin. Drug Deliv. 2014, 11, 1113–1124. [Google Scholar] [CrossRef]
- Moniri, M.; Moghaddam, A.B.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef]
- Chang, W.-S.; Chen, H.-H. Physical properties of bacterial cellulose composites for wound dressings. Food Hydrocoll. 2016, 53, 75–83. [Google Scholar] [CrossRef]
- Wichai, S.; Chuysinuan, P.; Chaiarwut, S.; Ekabutr, P.; Supaphol, P. Development of bacterial cellulose/alginate/chitosan composites incorporating copper (II) sulfate as an antibacterial wound dressing. J. Drug Delivery Sci. Technol. 2019, 51, 662–671. [Google Scholar] [CrossRef]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, F.P.; Silvestre, A.J.D.; Freire, C.S.R.; Sousa Lobo, J.M.; Costa, P.C. Bacterial cellulose membranes as drug delivery systems: An in vivo skin compatibility study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose membranes as supports for dermal release of lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Pascoal Neto, C.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Rodrigues, A.F.; Almeida, I.F.; Costa, P.C.; Rosado, C.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial cellulose membranes as transdermal delivery systems for diclofenac: In vitro dissolution and permeation studies. Carbohydr. Polym. 2014, 106, 264–269. [Google Scholar] [CrossRef]
- Saïdi, L.; Vilela, C.; Oliveira, H.; Silvestre, A.J.D.; Freire, C.S.R. Poly(N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohydr. Polym. 2017, 169, 357–365. [Google Scholar] [CrossRef]
- Pavaloiu, R.D.; Stoica, A.; Stroescu, M.; Dobre, T. Controlled release of amoxicillin from bacterial cellulose membranes. Cent. Eur. J. Chem. 2014, 12, 962–967. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Islan, G.A.; Drachemberg, M.F.; Alvarez, V.A.; Bartel, L.C.; Bolzán, A.D.; Castro, G.R. Hybrid bacterial cellulose-pectin films for delivery of bioactive molecules. New J. Chem. 2018, 42, 7457–7467. [Google Scholar] [CrossRef]
- Vilela, C.; Oliveira, H.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Nanocellulose-based antifungal nanocomposites against the polymorphic fungus Candida albicans. Carbohydr. Polym. 2019, 217, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.M.; Veiga, M.; Antunes, F.; Pintado, M.M.; Sèbe, G.; Coma, V.; et al. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]
- Lopes, T.D.; Riegel-Vidotti, I.C.; Grein, A.; Tischer, C.A.; Faria-Tischer, P.C.d.S. Bacterial cellulose and hyaluronic acid hybrid membranes: Production and characterization. Int. J. Biol. Macromol. 2014, 67, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Gluconacetobacter sacchari: An efficient bacterial cellulose cell-factory. Carbohydr. Polym. 2011, 86, 1417–1420. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Nat. Bur. Stand. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Dabrowska, A.K.; Rotaru, G.M.; Derler, S.; Spano, F.; Camenzind, M.; Annaheim, S.; Stämpfli, R.; Schmid, M.; Rossi, R.M. Materials used to simulate physical properties of human skin. Ski. Res. Technol. 2016, 22, 3–14. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Hammad, H.M.; Hammad, M.M.; Abdelhadi, I.N.; Khalifeh, M.S. Effects of topically applied agents on intra-oral wound healing in a rat model: A clinical and histomorphometric study. Int. J. Dent. Hyg. 2011, 9, 9–16. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, J.Y.; Bang, D. The efficacy of topical 0.2% hyaluronic acid gel on recurrent oral ulcers: Comparison between recurrent aphthous ulcers and the oral ulcers of Behçet’s disease. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 590–595. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wu, B.; Xu, Z.; Gu, Q. Determination of hyaluronan by spectroscopic methods. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2006, 21, 32–34. [Google Scholar] [CrossRef]
- Carneiro, J.; Döll-Boscardin, M.; Fiorin, B.C.; Nadal, J.M.; Farago, P.V.; De Paula, J.P. Development and characterization of hyaluronic acid-lysine nanoparticles with potential as innovative dermal filling. Braz. J. Pharm. Sci. 2016, 52, 645–651. [Google Scholar] [CrossRef]
- Ramachandran, E.; Ramukutty, S. Growth, morphology, spectral and thermal studies of gel grown diclofenac acid crystals. J. Cryst. Growth 2014, 389, 78–82. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Vilela, C.; Silva, A.C.Q.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: the case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef]
- Pa’e, N.; Salehudin, M.H.; Hassan, N.D.; Marsin, A.M.; Muhamad, I.I. Thermal behavior of bacterial cellulose-based hydrogels with other composites and related instrumental analysis. In Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A Reference Series; Mondal, M.I.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 763–787. ISBN 9783319778303. [Google Scholar]
- Hussain, Z.; Thu, H.E.; Katas, H.; Bukhari, S.N.A. Hyaluronic acid-based biomaterials: A versatile and smart approach to tissue regeneration and treating traumatic, surgical, and chronic wounds. Polym. Rev. 2017, 57, 594–630. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B. Surface and thermal properties of collagen/hyaluronic acid blends containing chitosan. Int. J. Biol. Macromol. 2016, 92, 371–376. [Google Scholar] [CrossRef]
- Lacerda, P.S.S.; Barros-Timmons, A.M.M.V.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Nanostructured composites obtained by ATRP sleeving of bacterial cellulose nanofibers with acrylate polymers. Biomacromolecules 2013, 14, 2063–2073. [Google Scholar] [CrossRef]
- Moore, R.J.; Watts, J.T.F.; Hood, J.A.A.; Burritt, D.J. Intra-oral temperature variation over 24 h. Eur. J. Orthod. 1999, 21, 249–261. [Google Scholar] [CrossRef]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and conductive nanocellulose-based films for active and intelligent food packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Z.; Chen, A.; He, H.; He, C.; Shuai, X.; Li, X.; Chen, S.; Zhang, Y.; Ren, B.; et al. Sulfated zwitterionic poly(sulfobetaine methacrylate) hydrogels promote complete skin regeneration. Acta Biomater. 2018, 71, 293–305. [Google Scholar] [CrossRef]
- Chen, P.; Mancini, M.; Sonis, S.T.; Fernandez-Martinez, J.; Liu, J.; Cohen, E.E.W.; Toback, F.G. A novel peptide for simultaneously enhanced treatment of head and neck cancer and mitigation of oral mucositis. PLoS ONE 2016, 11, e0152995. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Neves, B.M.; Sèbe, G.; Coma, V.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Design of nonsteroidal anti-inflammatory drug-based ionic liquids with improved water solubility and drug delivery. ACS Sustain. Chem. Eng. 2019, 7, 14126–14134. [Google Scholar] [CrossRef]
- Figueiredo, A.G.P.R.; Figueiredo, A.R.P.; Alonso-Varona, A.; Fernandes, S.C.M.; Palomares, T.; Rubio-Azpeitia, E.; Barros-Timmons, A.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. Biocompatible bacterial cellulose-poly(2-hydroxyethyl methacrylate) nanocomposite films. BioMed Res. Int. 2013, 698141, 1–14. [Google Scholar] [CrossRef][Green Version]
- Sharma, M.; Sahu, K.; Singh, S.P.; Jain, B. Wound healing activity of curcumin conjugated to hyaluronic acid: in vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1009–1017. [Google Scholar] [CrossRef]
- Boeckel, D.G.; Shinkai, R.S.A.; Grossi, M.L.; Teixeira, E.R. In vitro evaluation of cytotoxicity of hyaluronic acid as an extracellular matrix on OFCOL II cells by the MTT assay. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e423–e428. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(E) Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009.
- Silva, N.H.C.S.; Mota, J.P.; Almeida, T.S.D.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical drug delivery systems based on bacterial nanocellulose: Accelerated stability testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profile. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Dubey, D.; Malviya, R.; Sharma, P.K. Mathematical Modelling and Release Behaviour of Drug. Drug Deliv. Lett. 2014, 4, 254–268. [Google Scholar] [CrossRef]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef]

| Membrane Patch | HA/% a | DCF/% a | Thickness/μm |
|---|---|---|---|
| BNC | – | – | 39 ± 8 |
| BNC/HA_0.2 | 0.2 | – | 45 ± 9 |
| BNC/DCF_0.5 | – | 0.5 | 51 ± 4 |
| BNC/DCF_1.0 | – | 1.0 | 79 ± 15 |
| BNC/HA/DCF_0.5 | 0.2 | 0.5 | 79 ± 12 |
| BNC/HA/DCF_1.0 | 0.2 | 1.0 | 83 ± 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, J.P.F.; Silva, A.C.Q.; Bastos, V.; Oliveira, H.; Pinto, R.J.B.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Nanocellulose-Based Patches Loaded with Hyaluronic Acid and Diclofenac towards Aphthous Stomatitis Treatment. Nanomaterials 2020, 10, 628. https://doi.org/10.3390/nano10040628
Carvalho JPF, Silva ACQ, Bastos V, Oliveira H, Pinto RJB, Silvestre AJD, Vilela C, Freire CSR. Nanocellulose-Based Patches Loaded with Hyaluronic Acid and Diclofenac towards Aphthous Stomatitis Treatment. Nanomaterials. 2020; 10(4):628. https://doi.org/10.3390/nano10040628
Chicago/Turabian StyleCarvalho, João P. F., Ana C. Q. Silva, Verónica Bastos, Helena Oliveira, Ricardo J. B. Pinto, Armando J. D. Silvestre, Carla Vilela, and Carmen S. R. Freire. 2020. "Nanocellulose-Based Patches Loaded with Hyaluronic Acid and Diclofenac towards Aphthous Stomatitis Treatment" Nanomaterials 10, no. 4: 628. https://doi.org/10.3390/nano10040628
APA StyleCarvalho, J. P. F., Silva, A. C. Q., Bastos, V., Oliveira, H., Pinto, R. J. B., Silvestre, A. J. D., Vilela, C., & Freire, C. S. R. (2020). Nanocellulose-Based Patches Loaded with Hyaluronic Acid and Diclofenac towards Aphthous Stomatitis Treatment. Nanomaterials, 10(4), 628. https://doi.org/10.3390/nano10040628












