Gold Nanoparticles Synthesis Using Stainless Steel as Solid Reductant: A Critical Overview
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- AISI 430 (Fe = 81%, Cr = 17%, Mn, Si, C, S, P as minor components)
- AISI 410 (Fe = 87.5%, Cr = 12.5%)
- AISI 304 (Fe = 72%, Cr = 18%, Ni = 10%)
2.2. AuNPs Synthesis Using Stainless Steel Wire
2.3. Gold Colloids Characterization
3. Results and Discussion
3.1. Effect of the Stainless Steel Composition
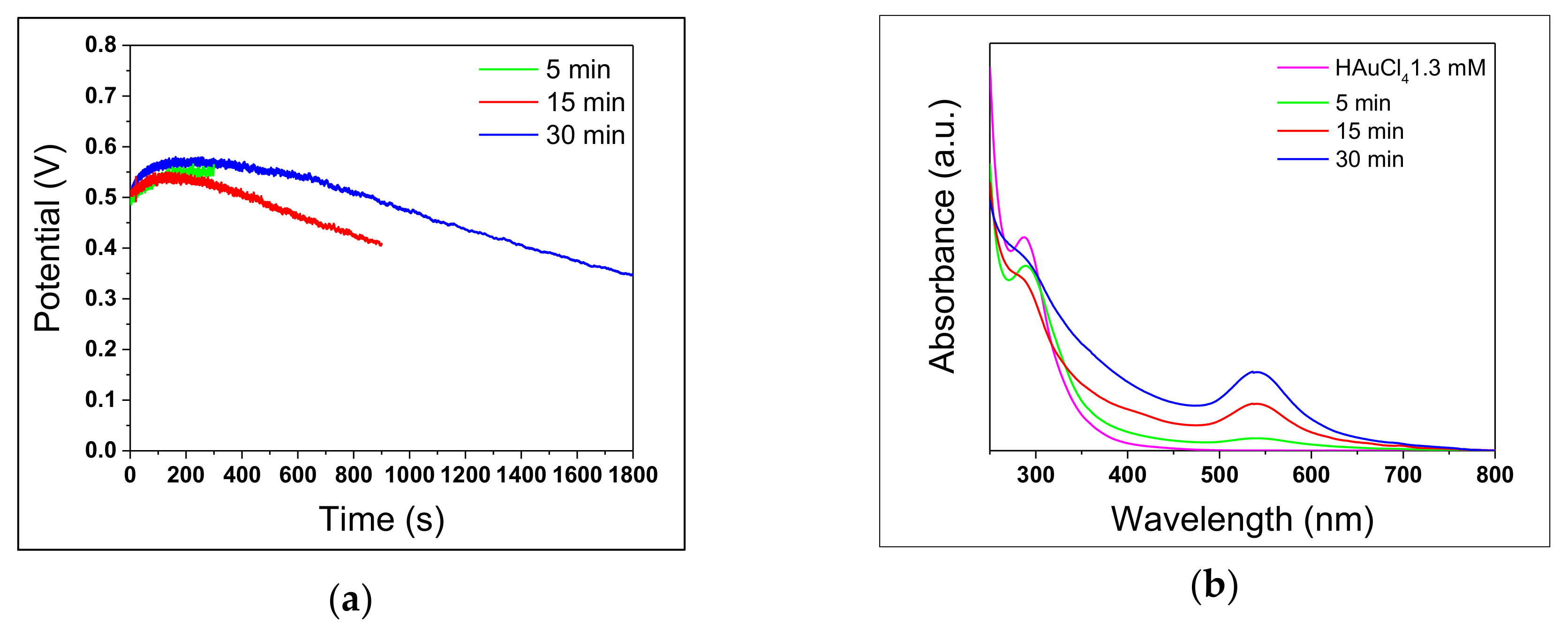
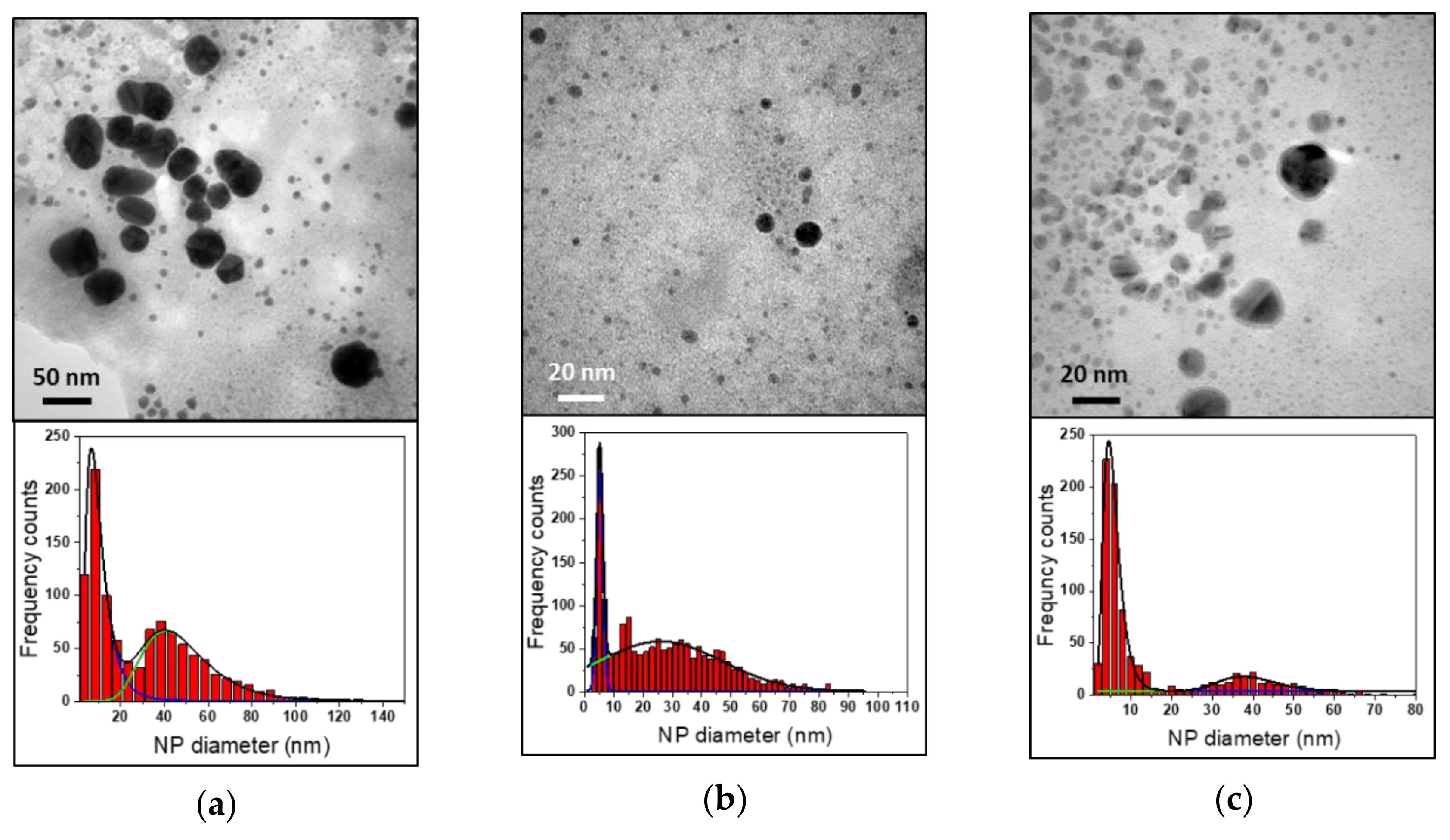
3.2. Reaction Time
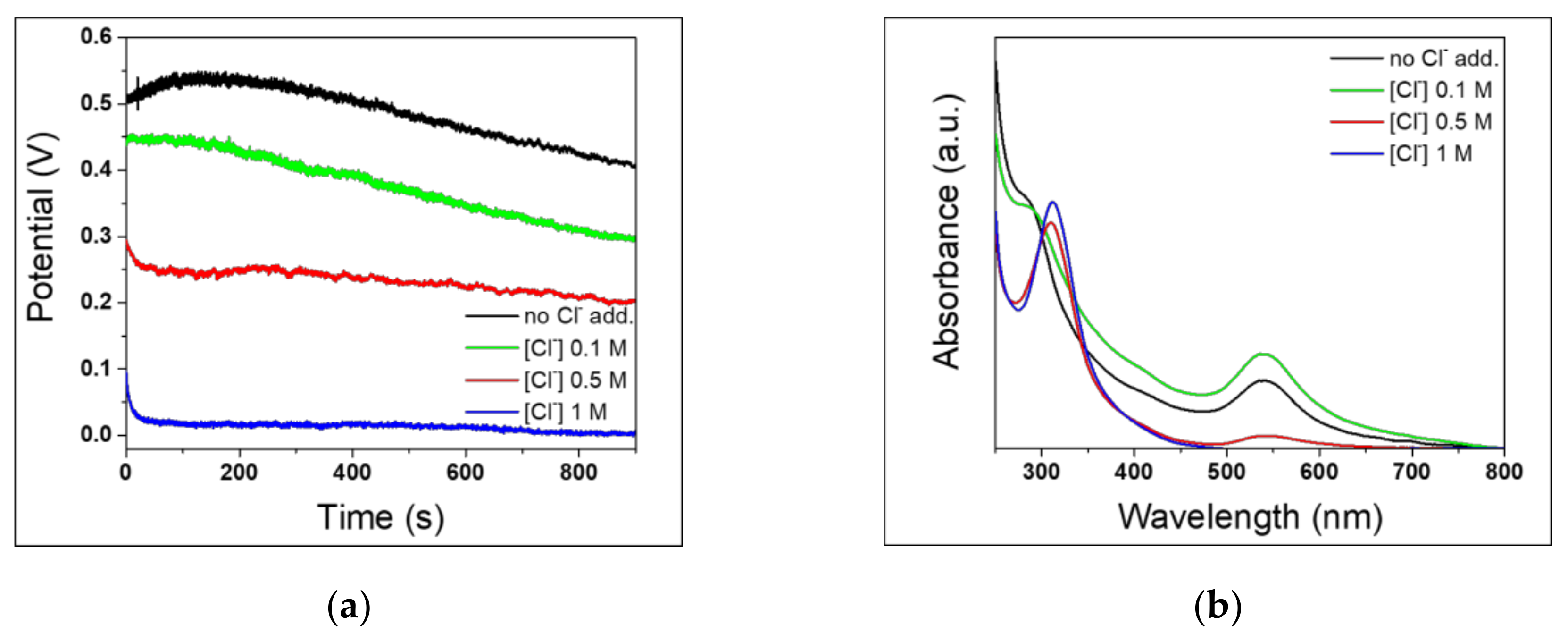
3.3. Effect of Cl− Concentration
3.4. Effect of pH
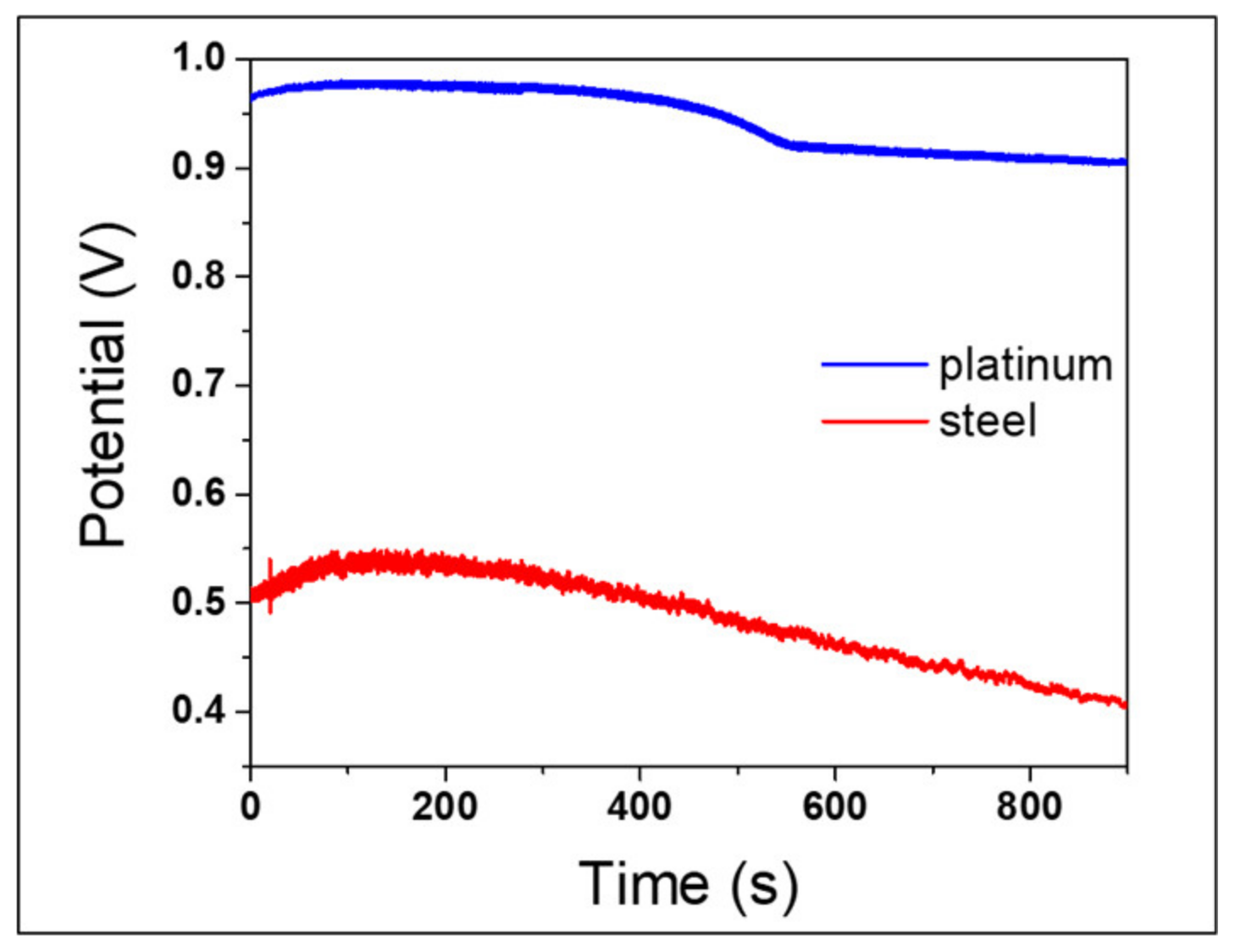
3.5. Decoupling the Effects related to the Electrode and the Solution in the OCP Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: a review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Johannsmeier, S.; Heeger, P.; Terakawa, M.; Kalies, S.; Heisterkamp, A.; Ripken, T.; Heinemann, D. Gold nanoparticle-mediated laser stimulation induces a complex stress response in neuronal cells. Sci. Rep. 2018, 8, 6533. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Baek, S.; Singh, R.K.; Kim, T.-H.; Seo, J.; Shin, U.S.; Chrzanowski, W.; Kim, H.-W. Triple Hit with Drug Carriers: pH- and Temperature-Responsive Theranostics for Multimodal Chemo- and Photothermal Therapy and Diagnostic Applications. ACS Appl. Mater. Interfaces 2016, 8, 8967–8979. [Google Scholar] [CrossRef]
- Kim, T.; Lee, N.; Arifin, D.R.; Shats, I.; Janowski, M.; Walczak, P.; Hyeon, T.; Bulte, J.W.M. In Vivo Micro-CT Imaging of Human Mesenchymal Stem Cells Labeled with Gold-Poly-l-Lysine Nanocomplexes. Adv. Funct. Mater. 2017, 27, 1604213. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Jang, B.; Oh, Y.-O.; Lim, I.G.; Oh, J. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int. J. Biol. Macromol. 2016, 91, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–Gold Nanoparticles Hybrid—Synthesis, Functionalization, and Application in a Electrochemical and Surface-Enhanced Raman Scattering Biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Han, L.; Wang, F.; Petrenko, V.A.; Liu, A. Gold nanoprobe functionalized with specific fusion protein selection from phage display and its application in rapid, selective and sensitive colorimetric biosensing of Staphylococcus aureus. Biosens. Bioelectron. 2016, 82, 195–203. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Izquierdo, J.; Kranz, C.; Mizaikoff, B. Boron-doped diamond modified with gold nanoparticles for the characterization of bovine serum albumin protein. Vib. Spectrosc. 2017, 91, 147–156. [Google Scholar] [CrossRef][Green Version]
- López-Lorente, Á.I.; Wang, P.; Mizaikoff, B. Towards label-free mid-infrared protein assays: in-situ formation of bare gold nanoparticles for surface enhanced infrared absorption spectroscopy of bovine serum albumin. Microchim Acta 2017, 184, 453–462. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 0, 801–802. [Google Scholar] [CrossRef]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef]
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis—A prospective review on drug delivery aspect. OpenNano 2017, 2, 37–46. [Google Scholar] [CrossRef]
- Freitas de Freitas, L.; Varca, G.H.C.; Dos Santos Batista, J.G.; Benévolo Lugão, A. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Lugarà, P.M.; Palazzo, G.; Cioffi, N. The Pros and Cons of the Use of Laser Ablation Synthesis for the Production of Silver Nano-Antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Izzi, M.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Innocenti, M.; Sabbatini, L.; Cioffi, N. Pros and Cons of Sacrificial Anode Electrolysis for the Preparation of Transition Metal Colloids: A Review. ChemElectroChem 2020, 7, 386–394. [Google Scholar] [CrossRef]
- Cioffi, N.; Colaianni, L.; Ieva, E.; Pilolli, R.; Ditaranto, N.; Angione, M.D.; Cotrone, S.; Buchholt, K.; Spetz, A.L.; Sabbatini, L.; et al. Electrosynthesis and characterization of gold nanoparticles for electronic capacitance sensing of pollutants. Electrochim. Acta 2011, 56, 3713–3720. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial synthesis of gold nanoparticles: Current status and future prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Patra, S.; Madhuri, R. Green Synthesis of Noble Metal Nanoparticles: A Step Forward to Economical and Sustainable Development. In Green Metal Nanoparticles: Synthesis, Characterization and Their aApplications; Kanchi, S., Ahmed, S., Eds.; Wiley-Scrivener: Hoboken, NJ, USA, 2018; pp. 553–602 . ISBN 978-1-119-41890-0. [Google Scholar]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Han, T.H.; Khan, M.M.; Kalathil, S.; Lee, J.; Cho, M.H. Synthesis of Positively Charged Gold Nanoparticles Using a Stainless-Steel Mesh. J. Nanosci. Nanotechnol. 2013, 13, 6140–6144. [Google Scholar] [CrossRef]
- López-Lorente, A.I.; Simonet, B.M.; Valcárcel, M.; Eppler, S.; Schindl, R.; Kranz, C.; Mizaikoff, B. Characterization of stainless steel assisted bare gold nanoparticles and their analytical potential. Talanta 2014, 118, 321–327. [Google Scholar] [CrossRef]
- Han, T.H.; Khan, M.M.; Lee, J.; Cho, M.H. Optimization of positively charged gold nanoparticles synthesized using a stainless-steel mesh and its application for colorimetric hydrogen peroxide detection. J. Ind. Eng. Chem. 2014, 20, 2003–2009. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 31 May 2019).
- Casiello, M.; Picca, R.A.; Fusco, C.; D’Accolti, L.; Leonardi, A.A.; Lo Faro, M.J.; Irrera, A.; Trusso, S.; Cotugno, P.; Sportelli, M.C.; et al. Catalytic Activity of Silicon Nanowires Decorated with Gold and Copper Nanoparticles Deposited by Pulsed Laser Ablation. Nanomaterials 2018, 8, 78. [Google Scholar] [CrossRef]
- Picca, R.A.; Calvano, C.D.; Faro, M.J.L.; Fazio, B.; Trusso, S.; Ossi, P.M.; Neri, F.; D’Andrea, C.; Irrera, A.; Cioffi, N. Functionalization of silicon nanowire arrays by silver nanoparticles for the laser desorption ionization mass spectrometry analysis of vegetable oils. J. Mass Spectrom. 2016, 51, 849–856. [Google Scholar] [CrossRef]
- McCafferty, E. Thermodynamics of Corrosion: Electrochemical Cells and Galvanic Corrosion. In Introduction to Corrosion Science; McCafferty, E., Ed.; Springer: New York, NY, USA, 2010; pp. 73–93. ISBN 978-1-4419-0455-3. [Google Scholar]
- Malik, A.U.; Mayan Kutty, P.C.; Siddiqi, N.A.; Andijani, I.N.; Ahmed, S. The influence of pH and chloride concentration on the corrosion behaviour of AISI 316L steel in aqueous solutions. Corros. Sci. 1992, 33, 1809–1827. [Google Scholar] [CrossRef]
- Loto, R.T.; Joseph, O.O.; Akanji, O. Electrochemical corrosion behaviour of austenitic stainless steel (type 304) in dilute hydrochloric acid solution. J. Mater. Environ. Sci. 2015, 6, 2409–2417. [Google Scholar]
- Liu, B.Y.; Xue, Y.J.; Yang, Z.H.; Fang, X.X. Corrosion Behavior of Austenitic Stainless Steel in Hydrochloric Acid Solution and Flow. Mater. Sci. Forum 2016, 850, 78–85. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Kim, S.-T.; Lee, J.-S.; Lee, I.-S.; Park, Y.-S. Effects of Sulfur Addition on the Formation of Inclusions and the Corrosion Behavior of Super Duplex Stainless Steels in Chloride Solutions of Different pH. Mater. Trans. 2012, 53, 1617–1626. [Google Scholar] [CrossRef]
- Gangopadhayay, A.K.; Chakravorty, A. Charge Transfer Spectra of some Gold(III) Complexes. J. Chem. Phys. 1961, 35, 2206–2209. [Google Scholar] [CrossRef]
- Gamlen, G.A.; Jordan, D.O. 295. A spectrophotometric study of the iron(III) chloro-complexes. J. Chem. Soc. 1953, 1435–1443. [Google Scholar] [CrossRef]
- Loures, C.C.A.; Alcântara, M.A.K.; Filho, H.J.I.; Teixeira, A.C.S.C.; Silva, F.T.; Paiva, T.C.B.; Samanamud, G.R.L. Advanced Oxidative Degradation Processes: Fundamentals and Applications. Int. Rev. of Chem. Eng. (IRECHE) 2013, 5, 102–120. [Google Scholar]
- Muramatsu, A.; Kanie, K. Mechanistic Study on Formation of Iron Hydroxides and Oxides with FT-IR and UV Photospectroscopy. In Characterization of Corrosion Products on Steel Surfaces; Waseda, Y., Suzuki, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 51–76. ISBN 978-3-540-35178-8. [Google Scholar]
- Mischler, S.; Vogel, A.; Mathieu, H.J.; Landolt, D. The chemical composition of the passive film on Fe-24Cr and Fe-24Cr-11Mo studied by AES, XPS and SIMS. Corros. Sci. 1991, 32, 925–944. [Google Scholar] [CrossRef]
- Lorang, G.; Belo, M.D.C.; Simões, A.M.P.; Ferreira, M.G.S. Chemical Composition of Passive Films on AISI 304 Stainless Steel. J. Electrochem. Soc. 1994, 141, 3347. [Google Scholar] [CrossRef]
- Suzuki, S.; Nakazawa, T.; Waseda, Y. Chromium and Nitrogen Segregation in Thin Oxide Layers Formed on the Surface of 17Cr-Ni-Mo-N Austenitic Steels Studied by Angle Resolved XPS. ISIJ Int. 1996, 36, 1273–1278. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1 (National Institute of Standards and Technology, Gaithersburg, 2012). Available online: http://srdata.nist.gov/xps/ (accessed on 10 December 2019).
- Clukay, C.J.; Grabill, C.N.; Hettinger, M.A.; Dutta, A.; Freppon, D.J.; Robledo, A.; Heinrich, H.; Bhattacharya, A.; Kuebler, S.M. Controlling formation of gold nanoparticles generated in situ at a polymeric surface. Appl. Surf. Sci. 2014, 292, 128–136. [Google Scholar] [CrossRef]
- Kitagawa, H.; Kojima, N.; Nakajima, T. Studies of mixed-valence states in three-dimensional halogen-bridged gold compounds, Cs2AuIAuIIIX6, (X = Cl, Br or I). Part 2. X-Ray photoelectron spectroscopic study. J. Chem. Soc. Dalton Trans. 1991, 11, 3121–3125. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Cárdenas, S.; González-Sánchez, Z.I. Effect of synthesis, purification and growth determination methods on the antibacterial and antifungal activity of gold nanoparticles. Mater. Sci. Eng., C 2019, 103, 109805. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. 3 - Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 43–58. ISBN 978-0-08-100557-6. [Google Scholar]
- Tang, J.; Gao, K.; Ou, Q.; Fu, X.; Man, S.-Q.; Guo, J.; Liu, Y. Calculation extinction cross sections and molar attenuation coefficient of small gold nanoparticles and experimental observation of their UV–vis spectral properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 513–520. [Google Scholar] [CrossRef]
- Roca, M.; Haes, A.J. Silica−Void−Gold Nanoparticles: Temporally Stable Surface-Enhanced Raman Scattering Substrates. J. Am. Chem. Soc. 2008, 130, 14273–14279. [Google Scholar] [CrossRef]
- Huang, D.; Niu, C.; Wang, X.; Lv, X.; Zeng, G. “Turn-On” Fluorescent Sensor for Hg2+ Based on Single-Stranded DNA Functionalized Mn:CdS/ZnS Quantum Dots and Gold Nanoparticles by Time-Gated Mode. Anal. Chem. 2013, 85, 1164–1170. [Google Scholar] [CrossRef]
- Zakaria, H.M.; Shah, A.; Konieczny, M.; Hoffmann, J.A.; Nijdam, A.J.; Reeves, M.E. Small Molecule- and Amino Acid-Induced Aggregation of Gold Nanoparticles. Langmuir 2013, 29, 7661–7673. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Valcárcel, M.; Mizaikoff, B. Continuous flow synthesis and characterization of tailor-made bare gold nanoparticles for use in SERS. Microchim. Acta 2014, 181, 1101–1108. [Google Scholar] [CrossRef]
- Reetz, M.T.; Helbig, W. Size-Selective Synthesis of Nanostructured Transition Metal Clusters. J. Am. Chem. Soc. 1994, 116, 7401–7402. [Google Scholar] [CrossRef]
- Hoar, T.P.; Jacob, W.R. Breakdown of Passivity of Stainless Steel by Halide Ions. Nature 1967, 216, 1299–1301. [Google Scholar] [CrossRef]
- Burstein, G.T.; Pistorius, P.C.; Mattin, S.P. The nucleation and growth of corrosion pits on stainless steel. Corros. Sci. 1993, 35, 57–62. [Google Scholar] [CrossRef]
- Wilde, B.E.; Williams, E. The use of current/voltage curves for the study of localized corrosion and passivity breakdown on stainless steels in chloride media. Electrochim. Acta 1971, 16, 1971–1985. [Google Scholar] [CrossRef]
- Landolt, D. Localized Corrosion Phenomena. In Corrosion and Surface Chemistry of Metals; CRC Press, Taylor and Francis Group LLC: Boca Raton, FL, USA, 2007; pp. 275–329. ISBN 978-0-8493-8233-8. [Google Scholar]
- Pacławski, K.; Sak, T. Kinetics and Mechanism of the Reaction of Gold(III) Chloride Complexes with Formic Acid. Available online: https://www.ingentaconnect.com/content/doaj/14505339/2015/00000051/00000002/art00004 (accessed on 3 June 2019).
- Wojnicki, M.; Rudnik, E.; Luty-Błocho, M.; Pacławski, K.; Fitzner, K. Kinetic studies of gold(III) chloride complex reduction and solid phase precipitation in acidic aqueous system using dimethylamine borane as reducing agent. Hydrometallurgy 2012, 127–128, 43–53. [Google Scholar] [CrossRef]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in New Robes: Key Questions Answered for the Most Common Gold Nanoparticle Synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef]
- McCafferty, E. Passivity. In Introduction to Corrosion Science; McCafferty, E., Ed.; Springer: New York, NY, USA, 2010; pp. 209–262 . ISBN 978-1-4419-0455-3. [Google Scholar]
- Biefer, G.J. Effects of alloying on polarization and corrosion of Type 430 stainless steel. Can. Metall. Q. 1970, 9, 537–550. [Google Scholar] [CrossRef]
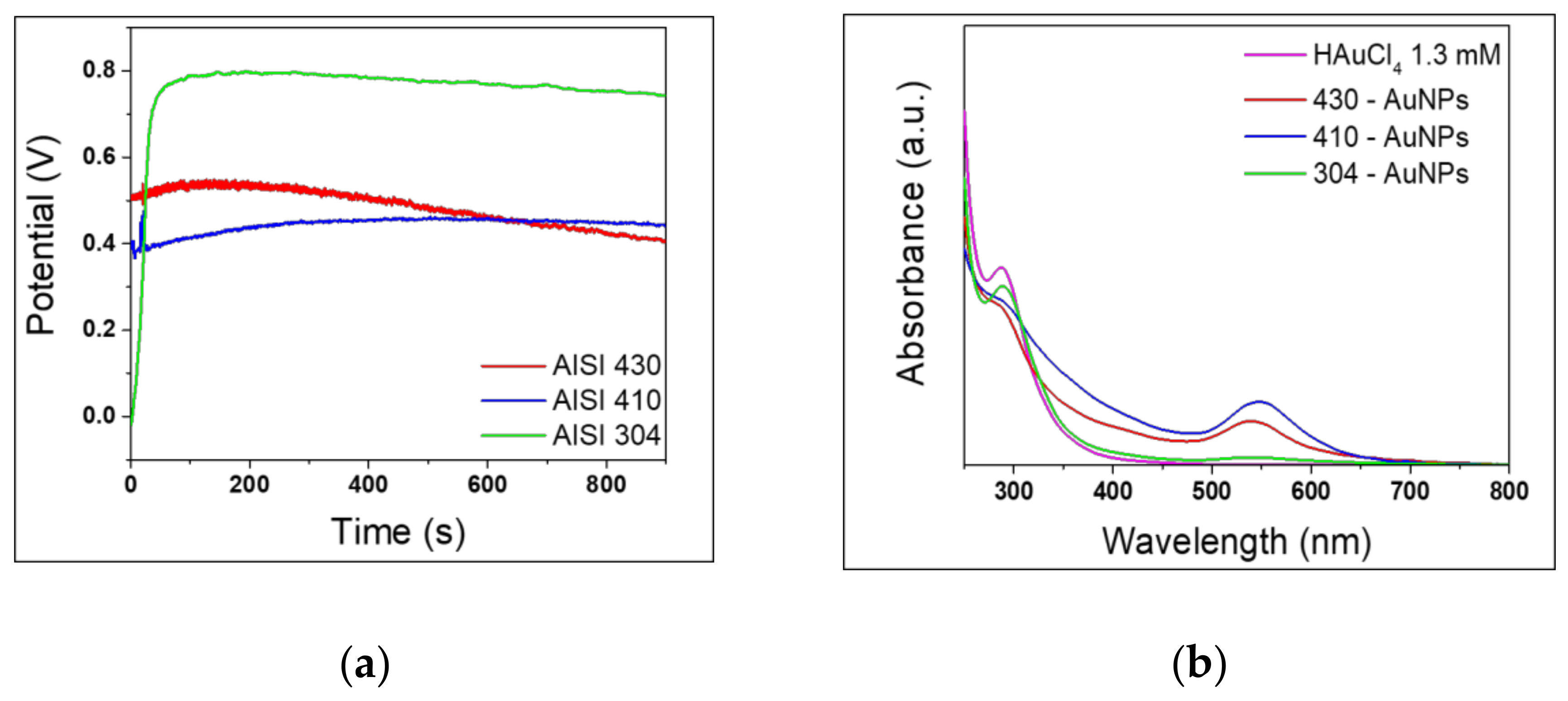
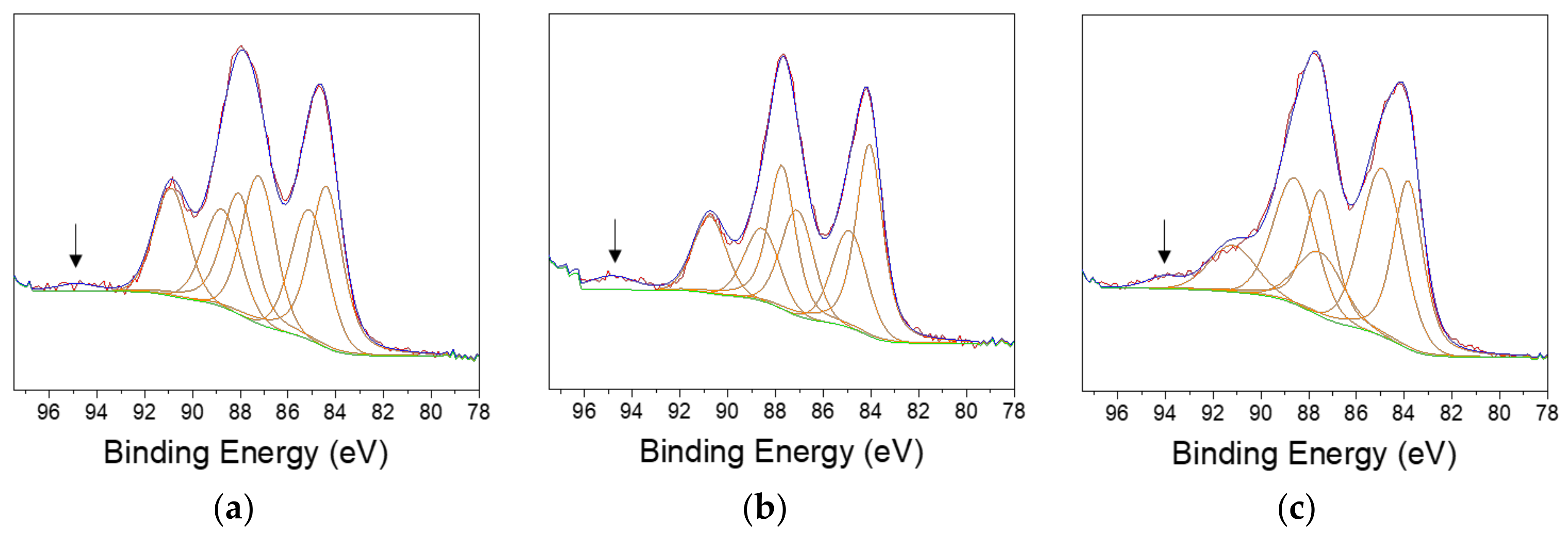



| 430 | 410 | 304 | ||||
|---|---|---|---|---|---|---|
| Before Use | After Use | Before Use | After Use | Before Use | After Use | |
| Au% | / | 17.4 | / | 30.1 | / | 5.5 |
| Fe% | 1.2 | 8.2 | 2.9 | 6 | 2.9 | 5.2 |
| Cr% | 1.3 | 4.2 | 1.1 | 2.4 | 1.3 | 2.2 |
| Ni% | / | / | / | / | 0.3 | < 0.2 |
| Cr/Fe | 1.1 ± 0.3 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Au/Fe | 2.1 ± 0.1 | 5.0 ± 0.2 | 1.1 ± 0.1 | |||
| Ni/Fe | ~ 0 | ~ 0 | ||||
| 430-AuNPs | 410-AuNPs | 304-AuNPs | |
|---|---|---|---|
| Au% | 1.4 | 0.6 | 1.0 |
| Fe% | 7.2 | 3.8 | 4.1 |
| Cr% | 1.8 | 1.1 | 1.0 |
| Ni% | 0.3 | ||
| Cr/Fe | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.1 |
| Au/Fe | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Ni/Fe | ~ 0 |
| rel. % | |||
|---|---|---|---|
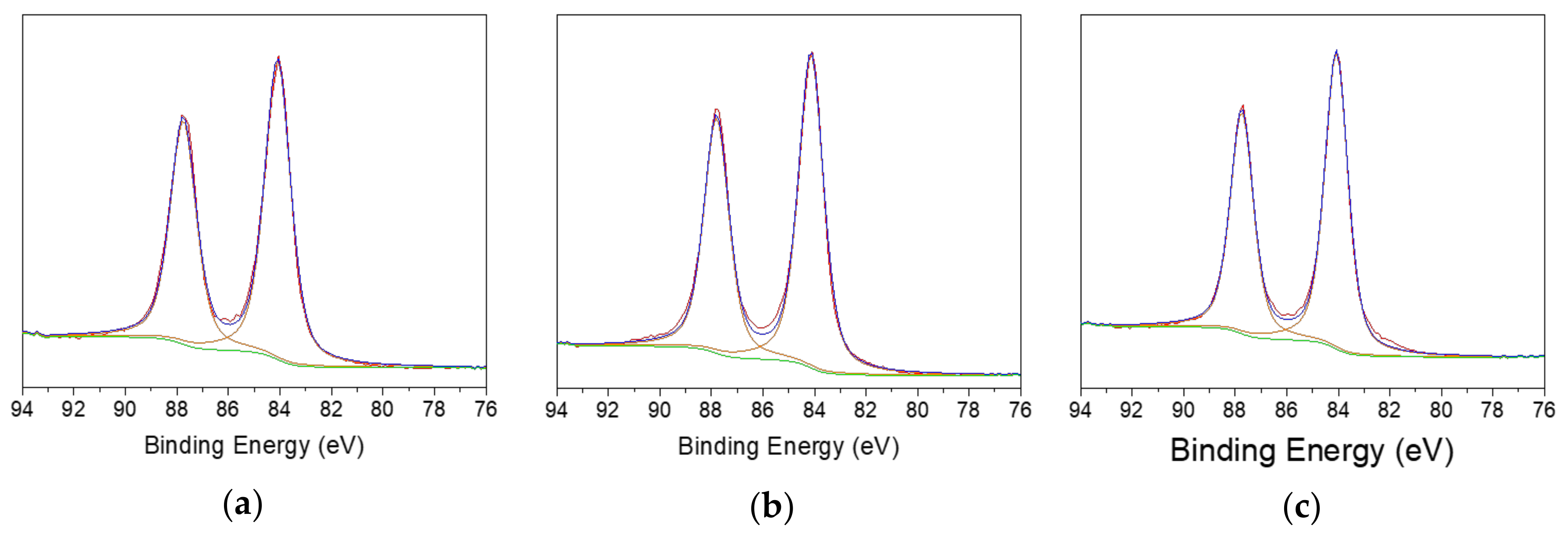
| Sample | Au(0) | Au(I) | Au(III) |
| 430-AuNPs | 39.5 | 29.5 | 31.0 |
| 410-AuNPs | 48.0 | 25.6 | 26.4 |
| 304-AuNPs | 37.9 | 43.8 | 18.3 |
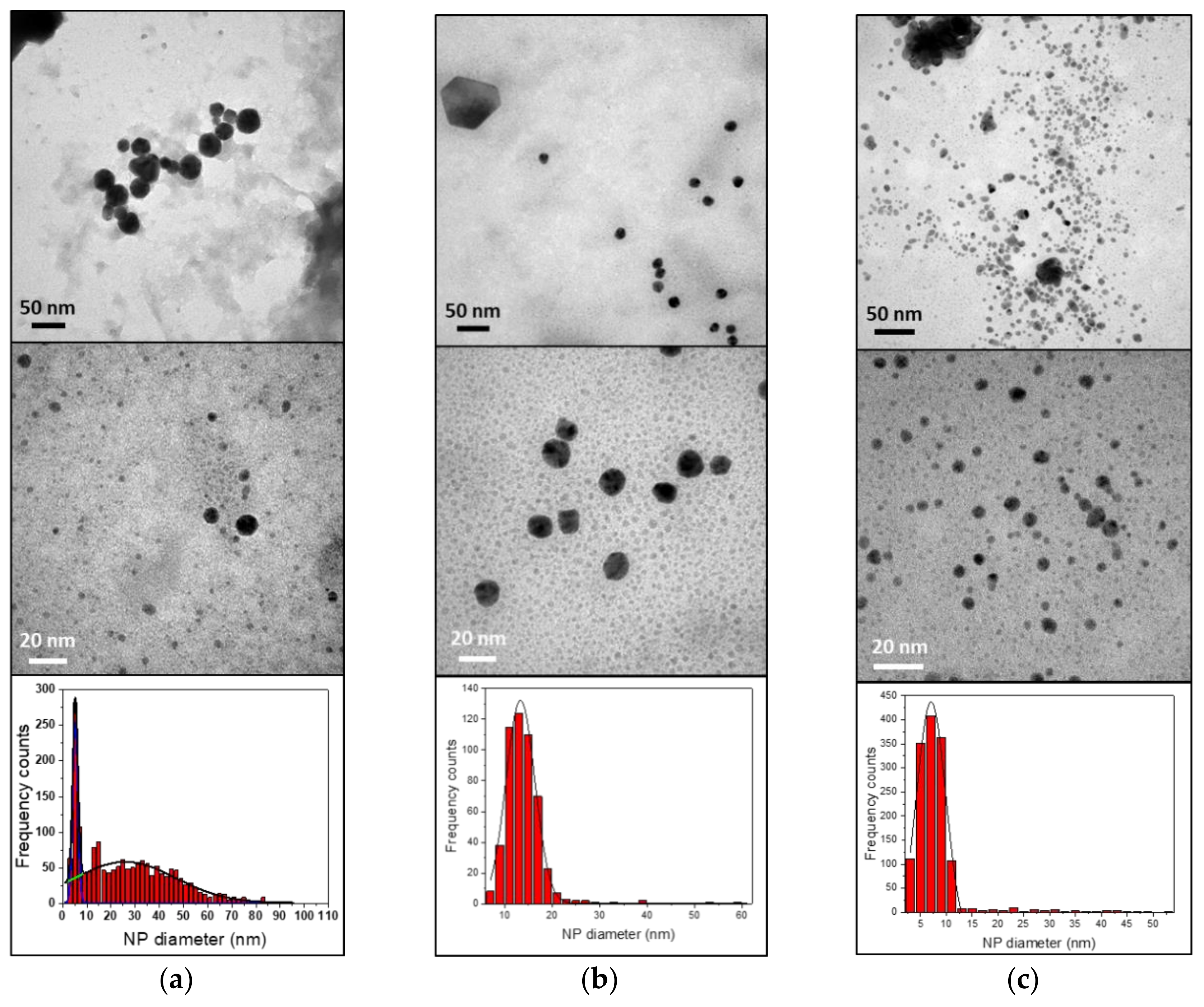
| Sample ID | λLSPR (nm) | Abs. (a.u.) | dTEM (nm) | ζ Potential (mV) |
|---|---|---|---|---|
| 430-AuNPs | 538 ± 3 | 0.690 ± 0.004 | 5 ± 1 30 ± 20 | 38.7 ± 0.7 |
| 410-AuNPs | 544 ± 4 | 1.004 ± 0.004 | 13 ± 3 | 40.6 ± 3.7 |
| 304-AuNPs | 538 ± 4 | 0.115 ± 0.004 | 7 ± 3 | 1.9 ± 0.7 |
| Reaction Time | LSPR Position (nm) | Abs. (a.u.) | dTEM (nm) | |
|---|---|---|---|---|
| 5’ | 541 ± 2 | 0.100 ± 0.004 | 9 ± 6 | 46 ± 24 |
| 15’ | 538 ± 3 | 0.500 ± 0.004 | 5 ± 1 | 26 ± 21 |
| 30’ | 541 ± 2 | 0.700 ± 0.004 | 5 ± 3 | 39 ± 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzi, M.; Sportelli, M.C.; Tursellino, L.; Palazzo, G.; Picca, R.A.; Cioffi, N.; López Lorente, Á.I. Gold Nanoparticles Synthesis Using Stainless Steel as Solid Reductant: A Critical Overview. Nanomaterials 2020, 10, 622. https://doi.org/10.3390/nano10040622
Izzi M, Sportelli MC, Tursellino L, Palazzo G, Picca RA, Cioffi N, López Lorente ÁI. Gold Nanoparticles Synthesis Using Stainless Steel as Solid Reductant: A Critical Overview. Nanomaterials. 2020; 10(4):622. https://doi.org/10.3390/nano10040622
Chicago/Turabian StyleIzzi, Margherita, Maria C. Sportelli, Luciana Tursellino, Gerardo Palazzo, Rosaria A. Picca, Nicola Cioffi, and Ángela I. López Lorente. 2020. "Gold Nanoparticles Synthesis Using Stainless Steel as Solid Reductant: A Critical Overview" Nanomaterials 10, no. 4: 622. https://doi.org/10.3390/nano10040622
APA StyleIzzi, M., Sportelli, M. C., Tursellino, L., Palazzo, G., Picca, R. A., Cioffi, N., & López Lorente, Á. I. (2020). Gold Nanoparticles Synthesis Using Stainless Steel as Solid Reductant: A Critical Overview. Nanomaterials, 10(4), 622. https://doi.org/10.3390/nano10040622











