Evaluation of Ecotoxicology Assessment Methods of Nanomaterials and Their Effects
Abstract
1. Introduction
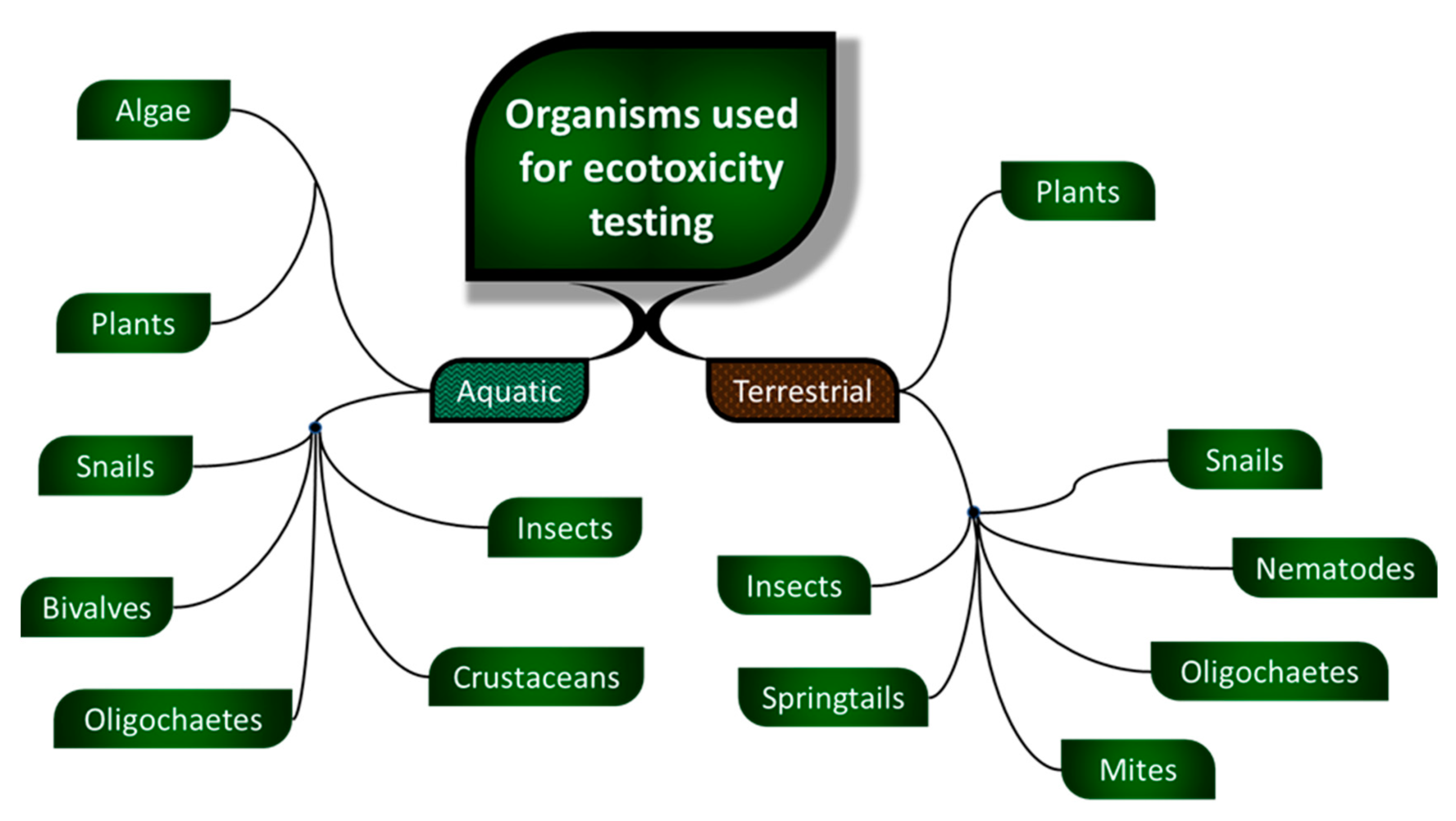
1.1. Ecotoxicity
- aquatic organisms
- ○
- algae
- ○
- plants
- ○
- invertebrates
- ○
- vertebrates
- terrestrial organisms
- ○
- algae
- ○
- plants
- ○
- invertebrates
- ○
- vertebrates
1.2. Nanomaterials
- by dimension
- ○
- zero-dimensional (0D)
- ○
- one-dimensional (1D)
- ○
- two-dimensional (2D)
- ○
- three-dimensional (3D)
- by composition
- ○
- carbon based NMs
- ○
- organic based NMs
- ○
- inorganic-based NMs
- ▪
- metal-based NMs
- ▪
- metal oxide NMs
- ○
- composite-based NMs [6]
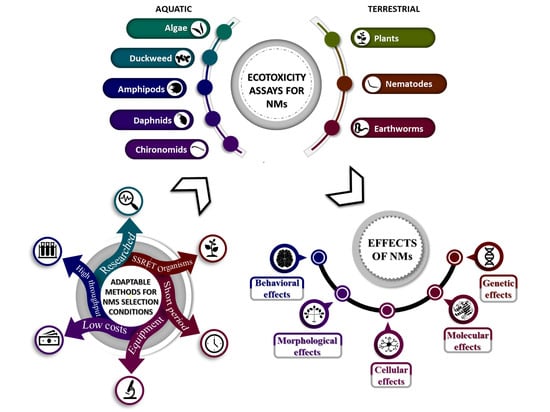
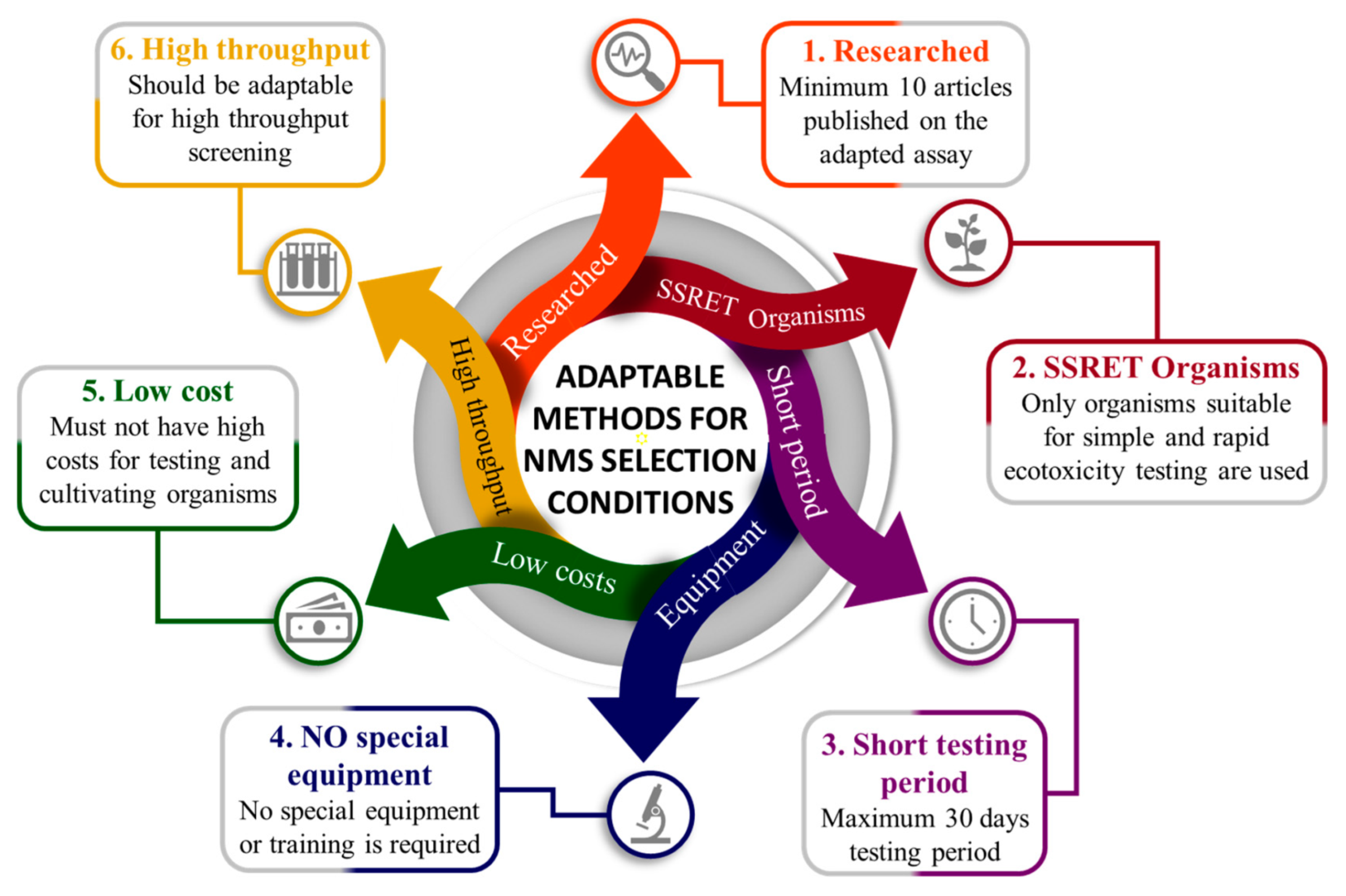
2. Ecotoxicity Assessment of Nanomaterials on SSRET Organisms
- agglomeration in test media during the procedures of test suspensions preparations;
- agglomeration, dissolution, and association with dissolved chemical species and colloidal/particulate matter already present in the natural waters;
- sedimentation due to agglomeration in the water column;
- chemical transformation processes such as sulphidation, hydroxylation;
- adhesion/deposition of nanomaterial onto soil minerals [10].
- minimum 10 articles have already been published with the assay applied and adapted to NMs;
- only SSRET organisms are used;
- assessment period not longer than 30 days;
- no special apparatus or training needed;
- low testing costs;
- high-throughput methods (if possible).
- the watermilfoil assays (sediment free toxicity assay and water-sediment toxicity assay) were eliminated due to the restricted number of published articles; in the published articles there are presented only the modification for the preparation of NM suspension, but not for the assay itself;
- the snail assays (on mud snail and on pond snail) were eliminated due to the restricted number of published articles, because bioconcentration was primarily assessed which takes longer than 30 days and because it involves the use of special apparatus like a flame atomic absorption spectrometer or MC-ICP-MS (Multicollector-Inductively Coupled Plasma Mass Spectrometer);
- the bivalve, aquatic oligochaetes, mysid, penaeid, enchytraeid, mite, springtail and the bumblebee assays were eliminated due to the restricted number of published articles; the published articles present only the modification for the preparation of NM suspension, but not for the assay itself;
- the fly assay was eliminated because only a few articles were found for ecotoxicity assessment of NMs; even if there are numerous ecotoxicity articles for NMs using as test organism Drosophila sp., these assays were not included in the description due to a lack of standardization.
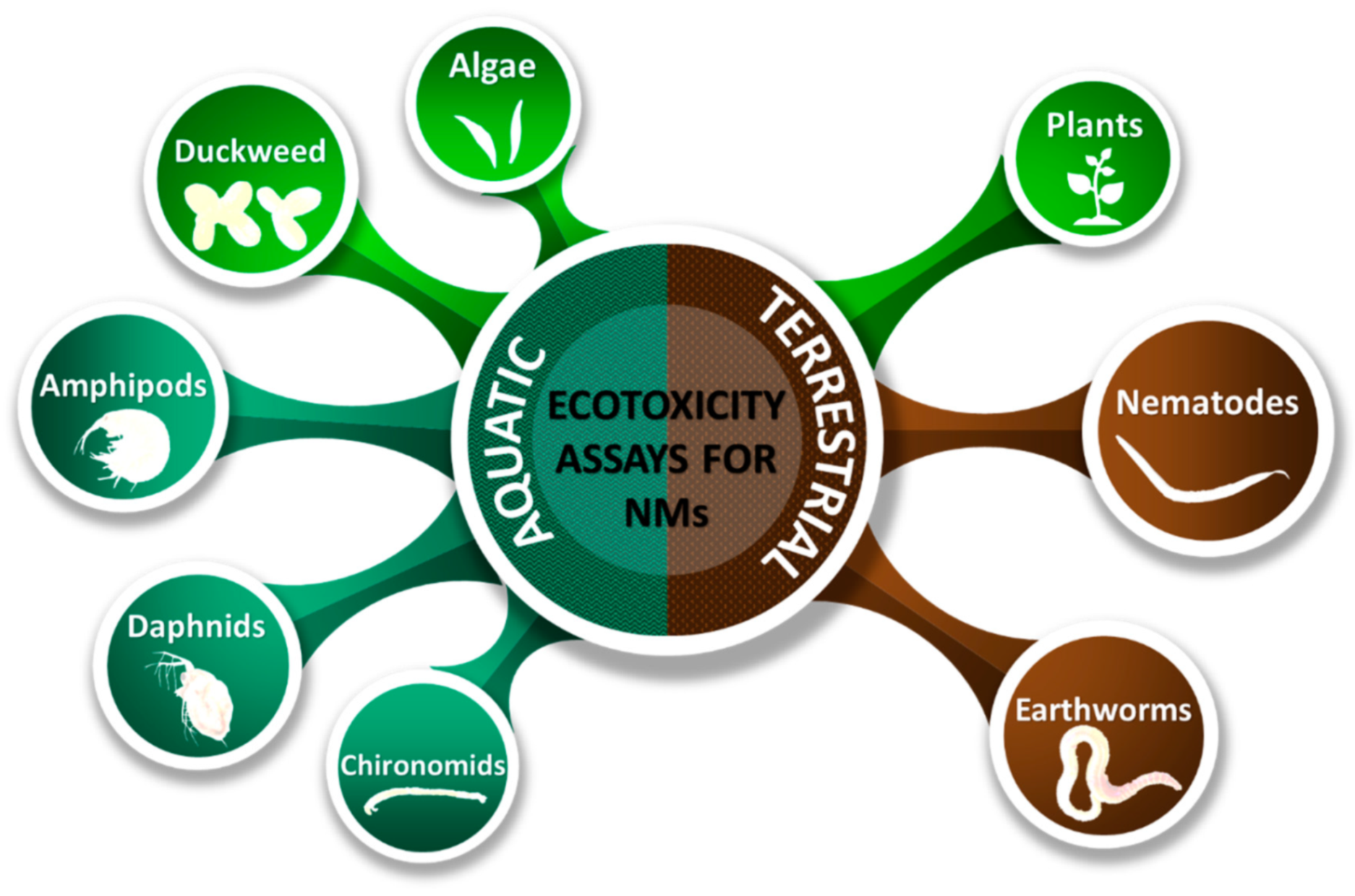
3. Description of Ecotoxicity Assays Suitable for Nanomaterials and the Necessary Adaptations
3.1. General Adaptations and Considerations
3.1.1. Physico-Chemical Adaptations and Considerations
3.1.2. Biological Adaptations and Considerations
3.2. Aquatic Plants Used for Ecotoxicity Testing of Nanomaterials
3.2.1. Algae
- Adaptations
- ○
- an EDTA-free version of the OECD medium for Raphidocelis subcapitata is recommended for metallic NMs [12];
- ○
- by supplying iron as FeSO4 instead of FeCl3, the OECD medium permits a better growth for the algae. With this medium the amount of phosphorus is also increased, as mono and dibasic salts [12];
- ○
- a shacking procedure is recommended during the tests [21];
- ○
- the determination of algal biomass by cell counting with a hemocytometer is very laborious, has a large variance and it may not reflect the true biomass if there are changes in the mean size of the cells as a response to a toxicant or other condition. The interference caused by NMs could be avoided by the determination of biomass by fluorometry followed by Chl a extraction [12];
- ○
- the determination of in vivo chlorophyll content could be realized in microtiter plates but NMs might interfere with the measurements [21];
- ○
- the in vivo determination of fluorescence was found to be an unstable parameter; it depends on the prior light exposure of the culture, since different results are obtained by the repeated measurement of chlorophyll on the same sample [12];
- ○
- the most reliable quantification method of algal biomass for testing the effects of NMs has been found to be the measurement of fluorescence of chlorophyll extracts. However the background fluorescence of the NMs should be reduced as much as possible [21].
- Uptake
- ○
- The uptake of NMs in bacteria or algae can’t take place without the attraction of the NMs to a biofilm or their absorption to a substrate. The attachment of NMs to the cell membrane is assumed to take place via electrostatic attraction, but there are other forces that could be involved such as random collision. If no uptake is observed in microalgae it is possible that the concentration of NMs was too low for attachment by collision to occur or that the electrostatic attraction was too weak for adsorption to have taken place [22].
- ○
- Released metal ions from metallic NMs may diffuse across cell membranes. For some cyanobacteria (e.g., Anabaena flos-aquae), however, by the production of extracellular polysaccharides the internalization of NMs could be avoided due to the electrostatic interaction of these substances with NMs, which are entrapped outside the cell [22].
- Advantages
- ○
- short testing period of 72–96 hours;
- ○
- high-throughput assay microplates can be used;
- ○
- simple culture method;
- ○
- simple quantification method: chlorophyll extraction;
- ○
- one of the most sensitive ecotoxicological assays [23].
- Disadvantages
- ○
- fluorescence reader is necessary for optimal chlorophyll quantification;
- ○
- NM–algae aggregates may form: heteroaggregation of NM-algae was reported [21];
- ○
- NMs may cause shading by scattering the light from reaching algal cells and thereby reduce their growth rate, rather than or in addition to any toxic effect. Even if the algae can temporarily overcome the shading from distant NMs, the adhesion of NMs to the algal cells can result in permanent shading and limitation of nutrient availability [24];
- ○
- if optical density measurements are used, there could be interferences with the NMs [23].
3.2.2. Duckweed
- Adaptations
- Advantages
- ○
- simple cultivation method;
- ○
- can be grown indefinitely as genetically homogeneous clonal colonies due to their predominantly vegetative reproduction [31];
- ○
- ready contact with substances dissolved in the culture medium is ensured by their high surface-to-volume ratio and lack of cuticle on their surface in contact with water [31];
- ○
- some species of duckweed have a wide pH tolerance such as Spirodela polyrhiza [32].
- Disadvantages
- ○
- medium testing period: seven days;
- ○
- relatively large laboratory space necessary for testing of multiple experiments at once;
- ○
- the actual environmental conditions, under which the test organisms live in nature, are not accurately reflected by the standard experimental conditions employed in any standardized duckweed toxicity test [31].
3.3. Aquatic Invertebrates Used for Ecotoxicity Testing of Nanomaterials
3.3.1. Amphipods
- Adaptations
- ○
- only NM suspension preparation adaptations are specified.
- Advantages
- ○
- short testing period for the acute toxicity assay: 96 hours.
- Disadvantages
- ○
- relatively large laboratory space necessary for testing of multiple experiments at once.
3.3.2. Daphnia
- Adaptations
- ○
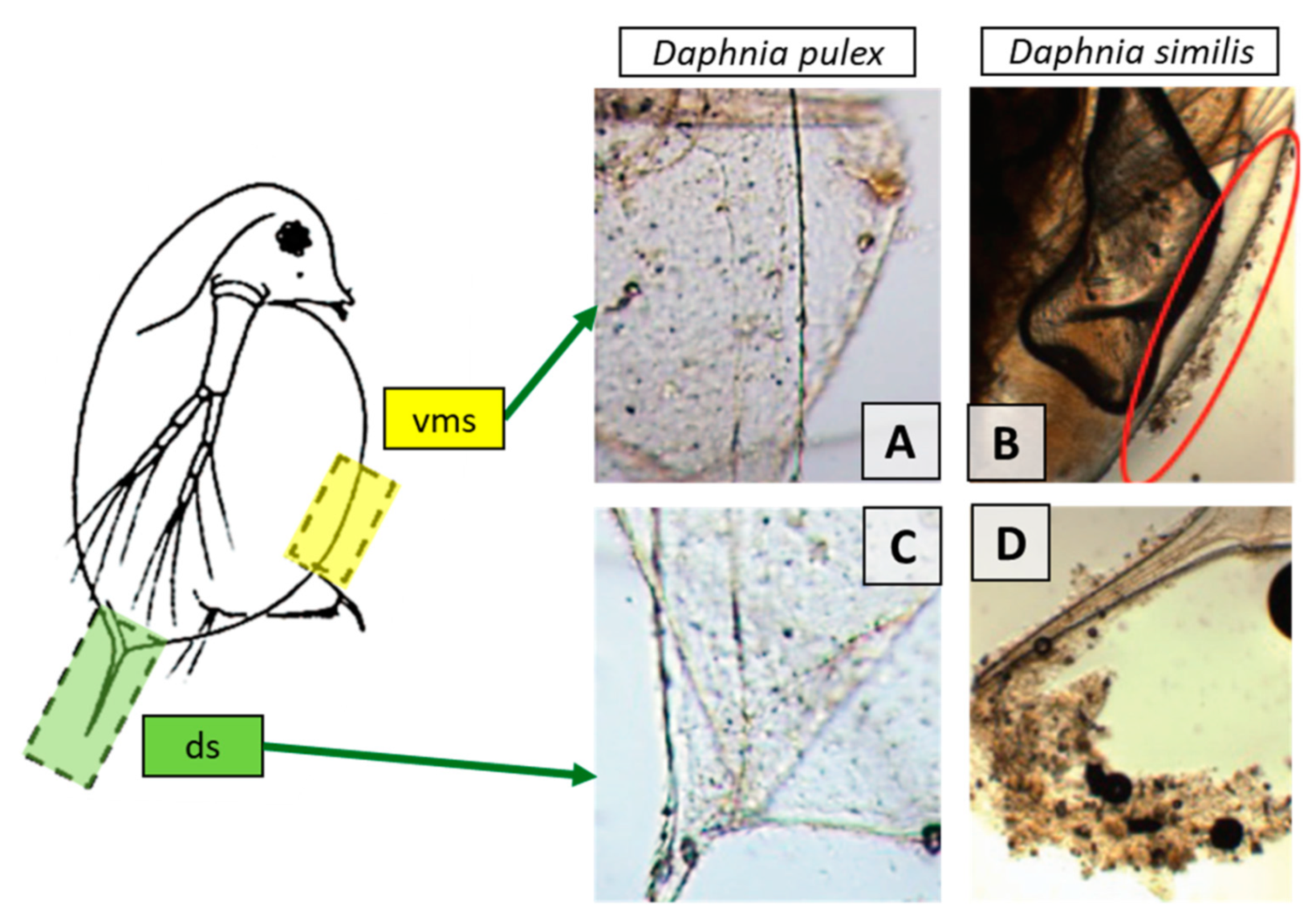
- the use of a medium with a very low ionic strength, under which daphnids can grow and reproduce normally, and which has a pH value where more stable dispersions can be obtained is recommended [12];
- ○
- NMs can be absorbed on the exoskeleton, cuticle and antenna of crustaceans like daphnids. This influences the mobility, molting and swimming velocity of the tested crustaceans. Thus, the inclusion of both lethality and immobilization assays is recommended. The use of only immobility as endpoint, such as the OECD assay, may be problematic in cases where immobility reflects physical impairment rather than toxicity [24];
- ○
- to prevent the mechanical impairment of daphnids by absorbed NMs, a mesh could be inserted at the bottom of the test vessel to prevent the contact of the daphnids with larger clusters of NMs that accumulate at the bottom of the beaker [24];
- ○
- greater water hardness is used for Daphnia magna growth and reproduction assays which leads to a greater agglomeration rate of NMs for charge-stabilized NMs, resulting in less consistent exposure. Thus, using daphnid species that are adapted for softer waters is recommended (such as Daphnia pulex) [13];
- ○
- to increase the contact between the daphnids and the NMs the use of shallow test vessels or semi-static and flow-through systems is recommended [21];
- ○
- the feeding of daphnids during the reproduction assay is not recommended. It was observed that the outcome of the test is food quantity dependent, as the addition of higher food levels resulted in higher animal survival, growth and reproduction compared to tests with lower food levels. Also, the uptake of NMs is influenced by the presence of food. This may influence the chronic effects found in long-term exposure tests. Furthermore, the presence of food (algae) in daphnia reproduction test may affect the observed toxicity, due to the interaction of NMs with algal exudates which affect the bioavailability of the NMs [24].
- Advantages
- Disadvantages
3.3.3. Chironomids
- Adaptations
- ○
- an additional parameter is recommended for the assessment of the toxic effects. This parameter is represented by morphological malfunctions revealed by the analysis of the mouthpart structures [49].
- Advantages
- ○
- short testing period for acute tests: 48 hours;
- ○
- chironomids have a widespread distribution. The aquatic sediment environment is strongly influenced by them through processes such as sediment ingestion, digestion, resuspension, excretion, and secretion, bioirrigation and bioturbation [50].
- Disadvantages
- ○
- long testing periods for the sediment–water system tests: 10–28 days.
3.4. Terrestrial Plants Used for Ecotoxicity Testing of Nanomaterials
- Adaptations
- ○
- to avoid NMs precipitation, which are poorly soluble in water, and to distribute NMs evenly, the use of plant agar tests is recommended. In the preparation of the agar solutions, to avoid the possible precipitation of NMs, the test plates were immediately hardened after pouring in a freezer [61].
- Advantages
- Disadvantages
- ○
- long testing periods: 14–28 days;
- ○
- the analysis of NM content of plants may imply the use of special apparatus like ICP-MS [61];
- ○
- special techniques like the scanning electron microscopy–cathodoluminescence technique may be used for the analysis of NM content of agar paste [61];
- ○
- the biological effects determined could depend on the species selected for the assay [64].
3.5. Terrestrial Invertebrates Used for Ecotoxicity Testing of Nanomaterials
3.5.1. Nematodes
- Adaptations
- Advantages
- ○
- short testing period: 96 hours;
- ○
- can be applied in microplates, thus it is a high throughput assay [68];
- ○
- a major change in the abundance of soil invertebrates such as nematodes, which are key organisms in soil, could have serious adverse effects on the entire terrestrial system [66];
- ○
- due to its ability to grow and reproduce in both soil and aqueous environments, Caenorhabditis elegans is a well suited organism for toxicity assessment [69].
- Disadvantages
3.5.2. Earthworms
- Adaptations
- ○
- the reduction of organic matter content of the standard OECD soil is recommended, due to the reduction of bioavailability of NMs by artificial soil (the NMs are absorbed to soil organic matter) [12];
- ○
- it is recommended to dose the NMs by directly adding the dry nanopowder to the soil, because it was observed that NMs agglomerate in water at the concentration needed for the dosing of the soil [73];
- ○
- to ensure a continuous exposure even if the worms would attempt to escape into the added food mixture, the food should also be dosed with NMs [73].
- Advantages
- ○
- Eisenia fetida is a recommended species for ecotoxicity assays as it can be easily cultured in the laboratory [74];
- ○
- earthworms represent 60–80% of the total soil biomass and have a wide range distribution of soil, thus being an ideal organism for use in ecotoxicity assays, as these are also sensitive organisms that are readily available. Furthermore, historical data are available for their use in ecotoxicity assessment [74].
- Disadvantages
- ○
- long testing period: 14–28 days.
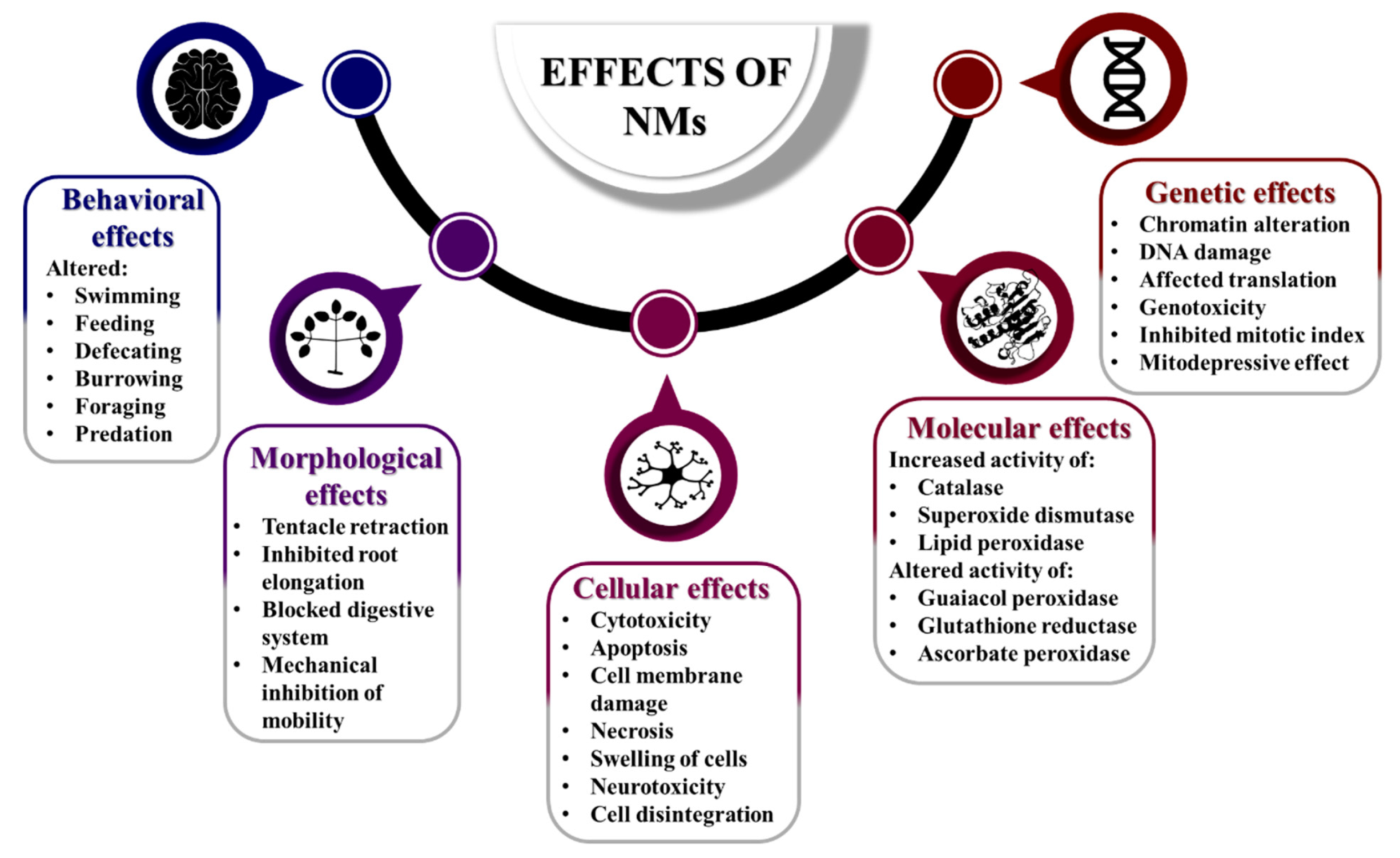
4. Ecotoxicity of Nanomaterials
- Surface of NMs
- ○
- control the distribution of materials in tissue;
- ○
- NMs undergo adsorption on macromolecules of the tested organism;
- ○
- ionic crystal NMs are observed to be accumulated in cytoplasm or body fluid through circulation.
- Size of NMs
- ○
- controls the distribution and penetration of tissue by NMs;
- ○
- reduction in size to nanoscale level leads to an increase of surface-to-volume ratio, thereby increasing the number of chemical molecules on surface, leading to an increase in intrinsic toxicity;
- ○
- the NMs size can control the dose–response relationship in relation to its solubility and toxicity.
- Shape of NMs
- ○
- plays an important role in determining the toxic nature of NMs, as high aspect ratio NMs (with only one or two dimensions in nanoscale) like nanofibers (that are longer than 10–20 µm and thinner than 3 µm) may remain in the pleural cavity, causing lung inflammation and even cancer (for example asbestos and carbon nanotubes).
- Aggregation of NMs
- ○
- NMs tend to form aggregates;
- ○
- the size of aggregates/agglomerates influences the residence time and reduces the potential for a NM to be inhaled;
- ○
- the aggregation/agglomeration is controlled by external environment like air and dispersion media;
- ○
- NMs may undergo disaggregation and disagglomeration within respiratory system, thereby penetrating lung cells [76].
4.1. Ecotoxicity of Bionanomolecules
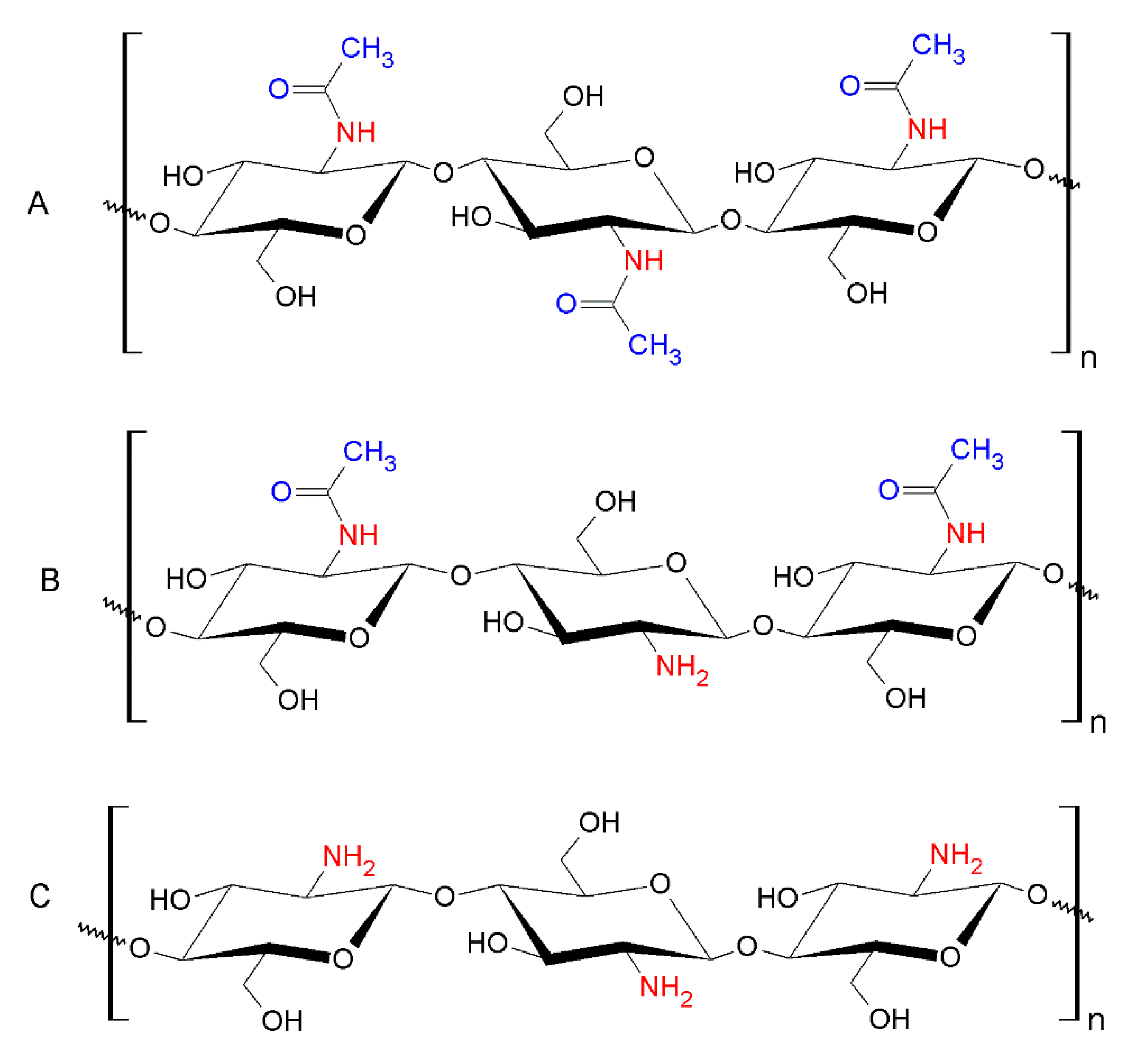
4.1.1. Nanochitosan
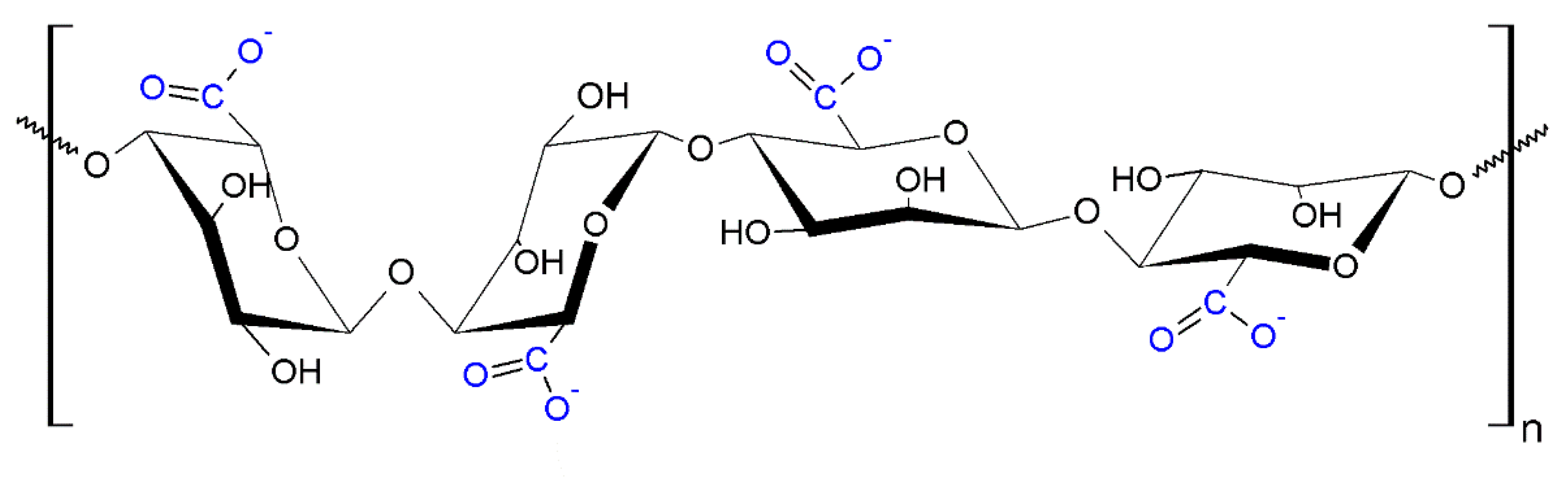
4.1.2. Nanoalginic acid
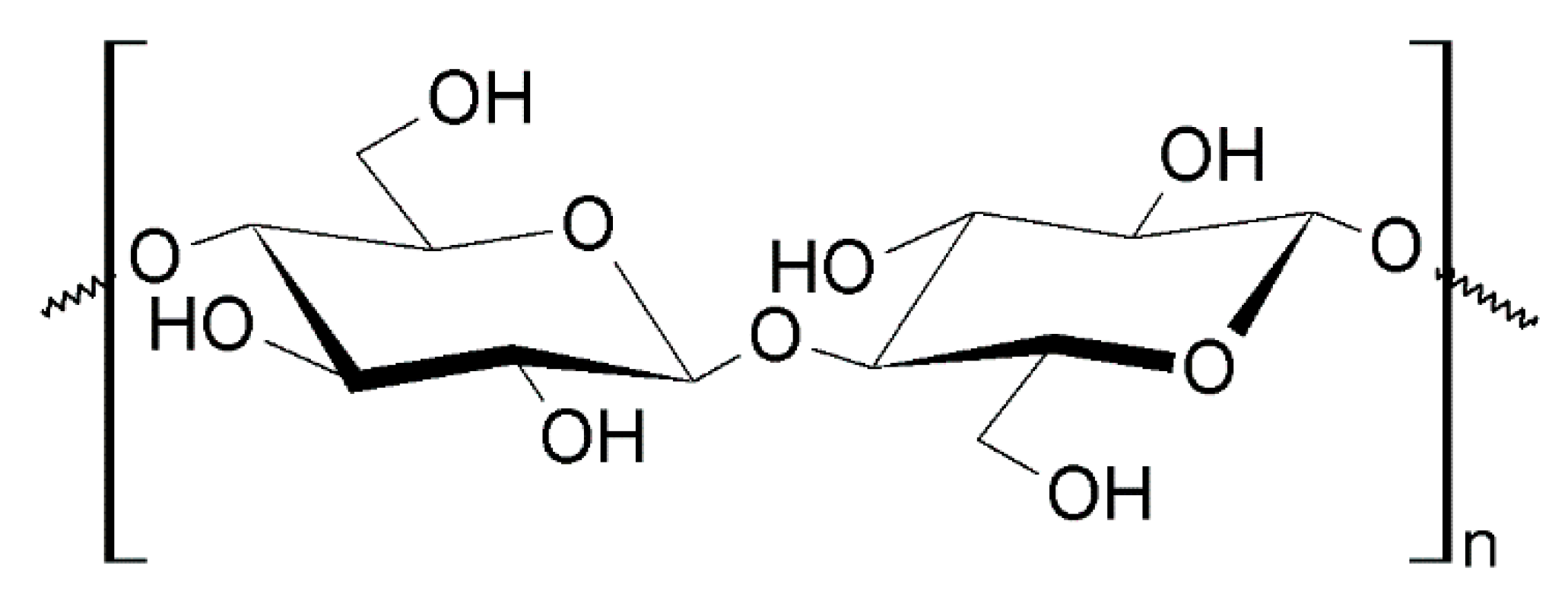
4.1.3. Nanocellulose
4.1.4. Nano polyhydroxyalkanoates (PHA)
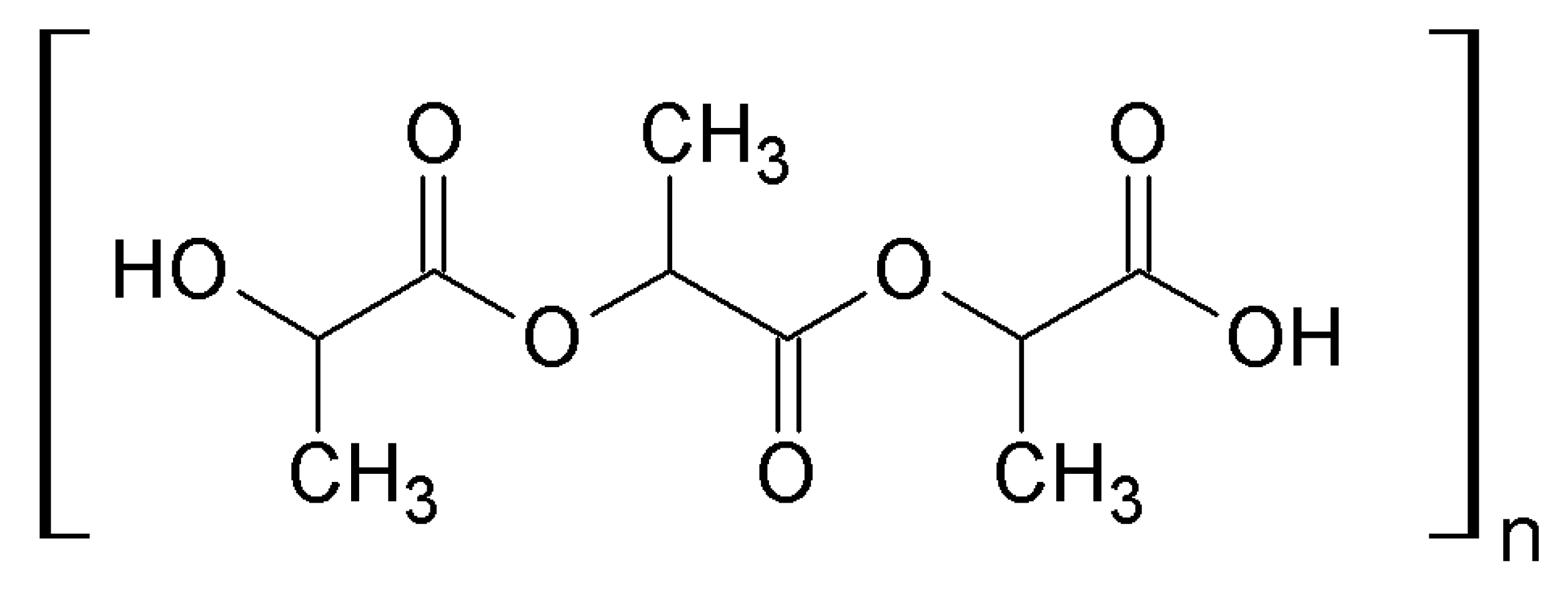
4.1.5. Nano polylactic acid (PLA)
4.2. Ecotoxicity of Carbon-Based Nanomaterials
4.2.1. Carbon Nanotubes
4.2.2. Fullerenes
4.2.3. Graphene
4.3. Ecotoxicity of Metallic Nanomaterials
4.3.1. Aluminum Nanomaterials
4.3.2. Cerium Nanomaterials
4.3.3. Cadmium Nanomaterials
4.3.4. Cobalt Nanomaterials
4.3.5. Copper Nanomaterials
4.3.6. Gold Nanomaterials
4.3.7. Iron Nanomaterials
4.3.8. Platinum Nanomaterials
4.3.9. Silver Nanomaterials
4.3.10. Titanium Nanomaterials
4.3.11. Zinc Nanomaterials
4.4. Comparison of Ecotoxicity of Described NMs Based on Their Half Maximal Effective Concentration Values
- Aquatic tests
- ○
- algae assay—Raphidocelis subcapitata
- ○
- duckweed assay—Lemna minor
- ○
- daphnid assay—Daphnia magna
- Terrestrial tests
- ○
- plant assay—Allium cepa
- ○
- nematode assay—Caenorhabditis elegans
- ○
- earthworm assay—Eisenia foetida
4.4.1. Comparison of Ecotoxicity of NMs in the Aquatic Environment
4.4.2. Comparison of Ecotoxicity of NMs in the Terrestrial Environment
5. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandermann, H. Molecular ecotoxicology of plants. Trends Plant Sci. 2004, 9, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Dubourguier, H.C. From ecotoxicology to nanoecotoxicology. Toxicology 2010, 269, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.; Handy, R.D.; Garrod, J.; Owen, R. Ecotoxicity test methods and environmental hazard assessment for engineered nanoparticles. Ecotoxicology (Lond. Engl.) 2008, 17, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.H.; Greig-Smith, P.W.; Crossland, N.O.; Brown, R. Ecotoxicology. In Animals and Alternatives in Toxicology: Present Status and Future Prospects; Balls, M., Bridges, J., Southee, J., Eds.; Macmillan Education UK: London, UK, 1991; pp. 223–251. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature. The IUCN Red List of Threatened Species. Version 2019-3. Available online: http://www.iucnredlist.org (accessed on 28 January 2020).
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Chapter 12—Nanomaterials history, classification, unique properties, production and market. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Barhoum, A., Makhlouf, A.S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–384. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Chapter 4—Synthesis, classification, and properties of nanomaterials. In Nanomaterial and Polymer Membranes; Saleh, T.A., Gupta, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 83–133. [Google Scholar] [CrossRef]
- Dhawan, A.; Sharma, V. Toxicity assessment of nanomaterials: Methods and challenges. Anal. Bioanal. Chem. 2010, 398, 589–605. [Google Scholar] [CrossRef]
- Naqvi, S.; Kumar, V.; Gopinath, P. Chapter 11—Nanomaterial toxicity: A challenge to end users. In Applications of Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 315–343. [Google Scholar] [CrossRef]
- Baun, A.; Sayre, P.; Steinhauser, K.G.; Rose, J. Regulatory relevant and reliable methods and data for determining the environmental fate of manufactured nanomaterials. NanoImpact 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Cattaneo, A.G.; Gornati, R.; Chiriva-Internati, M.; Bernardini, G. Ecotoxicology of nanomaterials: The role of invertebrate testing. ISJ-Invert. Surviv. J. 2009, 6, 78–97. [Google Scholar]
- Hund-Rinke, K.; Baun, A.; Cupi, D.; Fernandes, T.F.; Handy, R.; Kinross, J.H.; Navas, J.M.; Peijnenburg, W.; Schlich, K.; Shaw, B.J.; et al. Regulatory ecotoxicity testing of nanomaterials—Proposed modifications of OECD test guidelines based on laboratory experience with silver and titanium dioxide nanoparticles. Nanotoxicology 2016, 10, 1442–1447. [Google Scholar] [CrossRef]
- Petersen, E.J.; Diamond, S.A.; Kennedy, A.J.; Goss, G.G.; Ho, K.; Lead, J.; Hanna, S.K.; Hartmann, N.B.; Hund-Rinke, K.; Mader, B.; et al. Adapting OECD aquatic toxicity tests for use with manufactured nanomaterials: Key issues and consensus recommendations. Environ. Sci. Technol. 2015, 49, 9532–9547. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. ENV/JM/MONO1: Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines. Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology; OECD: Paris, France, 2014. [Google Scholar]
- Bour, A.; Mouchet, F.; Silvestre, J.; Gauthier, L.; Pinelli, E. Environmentally relevant approaches to assess nanoparticles ecotoxicity: A review. J. Hazard. Mater. 2015, 283, 764–777. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Freshwater Alga and Cyanobacteria, Growth Inhibition Test. OECD Guidelines for the Testing of Chemicals. 2; Test No. 201; OECD: Paris, France, 2011; Available online: https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-2-effects-on-biotic-systems_20745761 (accessed on 13 November 2019).
- U.S. Environmental Protection Agency. Algal Toxicity; OCSPP 850.4500; EPA: Washington, DC, USA, 2012. Available online: www.epa.gov (accessed on 12 November 2019).
- U.S. Environmental Protection Agency. Cyanobacteria (Anabaena flos-aquae) Toxicity; OCSPP 850.4550; EPA: Washington, DC, USA, 2012. Available online: www.epa.gov (accessed on 12 November 2019).
- International Organization for Standardization. Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae; ISO Standard No. 8692; ISO: Geneva, Switzerland, 2012; Available online: www.iso.org (accessed on 3 December 2019).
- International Organization for Standardization. Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum Tricornutum; ISO Standard No. 10253; ISO: Geneva, Switzerland, 2016; Available online: www.iso.org (accessed on 3 December 2019).
- Organisation for Economic Co-operation and Development. ENV/JM/MONO(1). Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines; OECD: Paris, France, 2014. [Google Scholar]
- Wong, S.W.Y.; Leung, K.M.Y.; Djurisic, A.B. A Comprehensive review on the aquatic toxicity of engineered nanomaterials. Rev. Nanosci. Nanotechnol. 2013, 2, 79–105. [Google Scholar] [CrossRef]
- Bondarenko, O.M.; Heinlaan, M.; Sihtmae, M.; Ivask, A.; Kurvet, I.; Joonas, E.; Jemec, A.; Mannerstrom, M.; Heinonen, T.; Rekulapelly, R.; et al. Multilaboratory evaluation of 15 bioassays for (eco)toxicity screening and hazard ranking of engineered nanomaterials: FP7 project NANOVALID. Nanotoxicology 2016, 10, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, R.; Skjolding, L.M.; Sørensen, S.N.; Baun, A. Regulatory adequacy of aquatic ecotoxicity testing of nanomaterials. NanoImpact 2017, 8, 28–37. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development: Lemna sp. Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals. 2; Test No. 221; OECD: Paris, France, 2006. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Aquatic Plant Toxicity Test Using Lemna spp; OCSPP 850.4400; EPA: Washington, DC, USA, 2012. Available online: www.epa.gov (accessed on 12 November 2019).
- International Organization for Standardization. Determination of the Toxic Effect of Water Constituents and Waste Water on Duckweed (Lemna Minor)—Duckweed Growth Inhibition Test; ISO Standard No. 20079; ISO: Geneva, Switzerland, 2005; Available online: www.iso.org (accessed on 3 December 2019).
- International Organization for Standardization. Determination of the Growth Inhibition Effects of Waste Waters, Natural Waters and Chemicals on the Duckweed Spirodela Polyrhiza—Method Using a Stock Culture Independent Microbiotest; ISO Standard No. 20227; ISO: Geneva, Switzerland, 2017; Available online: www.iso.org (accessed on 3 December 2019).
- Dolenc Koce, J. Effects of exposure to nano and bulk sized TiO2 and CuO in Lemna minor. Plant Physiol. Biochem. 2017, 119, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Zhao, J.; Yu, X.; Lv, K.; Wang, Z.; Xing, B. Interaction of CuO nanoparticles with duckweed (Lemna minor. L): Uptake, distribution and ROS production sites. Environ. Pollut. 2018, 243, 543–552. [Google Scholar] [CrossRef]
- Ziegler, P.; Sree, K.S.; Appenroth, K.J. Duckweeds for water remediation and toxicity testing. Toxicol. Environ. Chem. 2016, 98, 1127–1154. [Google Scholar] [CrossRef]
- Sallenave, R.; Fomin, A. Some advantages of the duckweed test to assess the toxicity of environmental samples. Acta Hydrochim. Hydrobiol. 1997, 25, 135–140. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Gammarid Amphipod Acute Toxicity Test; OCSPP 850.1020; EPA: Washington, DC, USA, 2016. Available online: www.epa.gov (accessed on 12 November 2019).
- U.S. Environmental Protection Agency. Spiked Whole Sediment 10-Day Toxicity Test, Freshwater Invertebrates; OCSPP 850.1735; EPA: Washington, DC, USA, 2016. Available online: www.epa.gov (accessed on 12 November 2019).
- U.S. Environmental Protection Agency. Spiked Whole Sediment 10-Day Toxicity Test, Saltwater Invertebrates; OCSPP 850.1740; EPA: Washington, DC, USA, 2016. Available online: www.epa.gov (accessed on 18 November 2019).
- International Organization for Standardization. Determination of Acute Toxicity of Marine or Estuarine Sediment to Amphipods; ISO Standard No. 16712; ISO: Geneva, Switzerland, 2005; Available online: www.iso.org (accessed on 3 December 2019).
- Organisation for Economic Co-operation and Development. Daphnia sp. Acute Immobilisation Test; OECD Guidelines for the Testing of Chemicals. 2; Test No. 202; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Daphnia Magna Reproduction Test; OECD Guidelines for the Testing of Chemicals. 2; Test No. 211; OECD: Paris, France, 2012. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Aquatic Invertebrate Acute Toxicity Test, Freshwater Daphnids; OCSPP 850.1010; EPA: Washington, DC, USA, 2016. Available online: www.epa.gov (accessed on 18 November 2019).
- U.S. Environmental Protection Agency. Daphnid Chronic Toxicity Test; OCSPP 850.1300; EPA: Washington, DC, USA, 2016. Available online: www.epa.gov (accessed on 18 November 2019).
- International Organization for Standardization. Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)—Acute Toxicity Test; ISO Standard No. 6341; ISO: Geneva, Switzerland, 2012; Available online: www.iso.org (accessed on 3 December 2019).
- International Organization for Standardization. Determination of Chronic Toxicity to Ceriodaphnia Dubia; ISO Standard No. 20665; ISO: Geneva, Switzerland, 2008; Available online: www.iso.org (accessed on 3 December 2019).
- International Organization for Standardization. Determination of Long Term Toxicity of Substances to Daphnia Magna Straus (Cladocera, Crustacea); ISO Standard No. 10706; ISO: Geneva, Switzerland, 2000; Available online: www.iso.org (accessed on 3 December 2019).
- Wiench, K.; Wohlleben, W.; Hisgen, V.; Radke, K.; Salinas, E.; Zok, S.; Landsiedel, R. Acute and chronic effects of nano- and non-nano-scale TiO2 and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere 2009, 76, 1356–1365. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Chironomus sp., Acute Immobilisation Test; OECD Guidelines for the Testing of Chemicals. 2; Test No. 235; OECD: Paris, France, 2011. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment; OECD Guidelines for the Testing of Chemicals. 2; Test No. 233; OECD: Paris, France, 2010. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Sediment-Water Chironomid Toxicity Using Spiked Sediment; OECD Guidelines for the Testing of Chemicals. 2; Test No. 218; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Sediment-Water Chironomid Toxicity Using Spiked Water; OECD Guidelines for the Testing of Chemicals. 2; Test No. 219; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- Tomilina, I.I.; Gremyachikh, V.A.; Grebenyuk, L.P.; Klevleeva, T.R. The effect of zinc oxide nano- and microparticles and zinc ions on freshwater organisms of different trophic levels. Inland Water Biol. 2014, 7, 88–96. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Musee, N.; Botha, A.M.; Chelule, P.K.; Focke, W.W.; Ashton, P.J. Assessment of the effect of nanomaterials on sediment-dwelling invertebrate Chironomus tentans larvae. Ecotoxicol. Environ. Saf. 2011, 74, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; OECD Guidelines for the Testing of Chemicals. 2; Test No. 208; OECD: Paris, France, 2006. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Terrestrial Plant Test: Vegetative Vigour Test; OECD Guidelines for the Testing of Chemicals. 2; Test No. 227; OECD: Paris, France, 2006. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Seedling Emergence and Seedling Growth; OCSPP 850.4100; EPA: Washington, DC, USA, 2012. Available online: www.epa.gov (accessed on 12 November 2019).
- U.S. Environmental Protection Agency. Vegetative Vigor; OCSPP 850.4150; EPA: Washington, DC, USA, 2012. Available online: www.epa.gov (accessed on 18 November 2019).
- U.S. Environmental Protection Agency. Early Seedling Growth Toxicity Test; OCSPP 850.4230; EPA: Washington, DC, USA, 2012. Available online: www.epa.gov (accessed on 12 November 2019).
- International Organization for Standardization. Determination of the Effects of Pollutants on Soil Flora—Part 1: Method for the Measurement of Inhibition of Root Growth; ISO Standard No. 11269-1; ISO: Geneva, Switzerland, 2012; Available online: www.iso.org (accessed on 3 December 2019).
- International Organization for Standardization. Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants; ISO Standard No. 11269-2; ISO: Geneva, Switzerland, 2012; Available online: www.iso.org (accessed on 9 December 2019).
- International Organization for Standardization. Determination of the Effects of Pollutants on Soil Flora—Screening Test for Emergence of Lettuce Seedlings (Lactuca sativa L.); ISO Standard No. 17126; ISO: Geneva, Switzerland, 2005; Available online: www.iso.org (accessed on 9 December 2019).
- International Organization for Standardization. Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants; ISO Standard No. 18763; ISO: Geneva, Switzerland, 2016; Available online: www.iso.org (accessed on 9 December 2019).
- International Organization for Standardization ISO-22030. Biological Methods—Chronic Toxicity in Higher Plants; ISO: Geneva, Switzerland, 2005; Available online: www.iso.org (accessed on 9 December 2019).
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Costa Devoti, A.; Zanella, M.; Sabbioni, E.; Mičetić, I.; Manodori, L.; Pigozzo, A.; Manenti, S.; Groppi, F.; Volpi Ghirardini, A. Phytotoxicity of ionic, micro- and nano-sized iron in three plant species. Ecotoxicol. Environ. Saf. 2016, 123, 81–88. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Determination of the Toxic Effect of Sediment and Soil Samples on Growth, Fertility and Reproduction of Caenorhabditis Elegans (Nematoda); ISO Standard No. 10872; ISO: Geneva, Switzerland, 2010; Available online: www.iso.org (accessed on 9 December 2019).
- American Society for Testing and Materials-. Standard Guide for Conducting Laboratory Soil Toxicity Tests with the Nematode Caenorhabditis Elegans; Standard No. E2172-01; ASTM: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Hoss, S.; Ahlf, W.; Bergtold, M.; Bluebaum-Gronau, E.; Brinke, M.; Donnevert, G.; Menzel, R.; Mohlenkamp, C.; Ratte, H.T.; Traunspurger, W.; et al. Interlaboratory comparison of a standardized toxicity test using the nematode Caenorhabditis elegans (ISO 10872). Environ. Toxicol. Chem. 2012, 31, 1525–1535. [Google Scholar] [CrossRef]
- Jung, S.K.; Qu, X.; Aleman-Meza, B.; Wang, T.; Riepe, C.; Liu, Z.; Li, Q.; Zhong, W. Multi-endpoint, high-throughput study of nanomaterial toxicity in Caenorhabditis elegans. Environ. Sci. Technol. 2015, 49, 2477–2485. [Google Scholar] [CrossRef]
- Hanna, S.K.; Cooksey, G.A.; Dong, S.; Nelson, B.C.; Mao, L.; Elliott, J.T.; Petersen, E.J. Feasibility of using a standardized Caenorhabditis elegans toxicity test to assess nanomaterial toxicity. Environ. Sci. Nano 2016, 3, 1080–1089. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Earthworm, Acute Toxicity Tests; OECD Guidelines for the Testing of Chemicals. 2; Test No. 207; OECD: Paris, France, 1984. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Earthworm Subchronic Toxicity Test; OCSPP 850.3100; EPA: Washington, DC, USA, 2012. Available online: www.epa.gov (accessed on 18 November 2019).
- International Organization for Standardization. Effects of Pollutants on Earthworms—Part 1: Determination of Acute Toxicity to Eisenia Fetida/Eisenia Andrei; ISO Standard No. 11268-1; ISO: Geneva, Switzerland, 2012; Available online: www.iso.org (accessed on 9 December 2019).
- Hooper, H.L.; Jurkschat, K.; Morgan, A.J.; Bailey, J.; Lawlor, A.J.; Spurgeon, D.J.; Svendsen, C. Comparative chronic toxicity of nanoparticulate and ionic zinc to the earthworm Eisenia veneta in a soil matrix. Environ. Int. 2011, 37, 1111–1117. [Google Scholar] [CrossRef]
- Hu, C.W.; Li, M.; Cui, Y.B.; Li, D.S.; Chen, J.; Yang, L.Y. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Chen, Y.; Tian, S. Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. J. Nanopart. Res. 2009, 11, 67–75. [Google Scholar] [CrossRef]
- Ghosh, S. Nanomaterials Safety: Toxicity and Health Hazards; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018; p. 288. [Google Scholar]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Zhao, L.-M.; Shi, L.-E.; Zhang, Z.-L.; Chen, J.-M.; Shi, D.-D.; Yang, J.; Tang, Z.-X. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011, 28, 353–362. [Google Scholar] [CrossRef]
- Andersen, S.O. Chapter 64—Cuticle. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 245–246. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Dananjaya, S.H.S.; Erandani, W.K.C.U.; Kim, C.-H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef]
- Murugan, K.; Anitha, J.; Dinesh, D.; Suresh, U.; Rajaganesh, R.; Chandramohan, B.; Subramaniam, J.; Paulpandi, M.; Vadivalagan, C.; Amuthavalli, P.; et al. Fabrication of nano-mosquitocides using chitosan from crab shells: Impact on non-target organisms in the aquatic environment. Ecotoxicol. Environ. Saf. 2016, 132, 318–328. [Google Scholar] [CrossRef]
- Hu, C.; Liu, L.; Li, X.; Xu, Y.; Ge, Z.; Zhao, Y. Effect of graphene oxide on copper stress in Lemna minor L.: Evaluating growth, biochemical responses, and nutrient uptake. J. Hazard. Mater. 2018, 341, 168–176. [Google Scholar] [CrossRef]
- Souza, P.M.S.; Corroqué, N.A.; Morales, A.R.; Marin-Morales, M.A.; Mei, L.H.I. PLA and organoclays nanocomposites: Degradation process and evaluation of ecotoxicity using Allium cepa as test organism. J. Polym. Environ. 2013, 21, 1052–1063. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef]
- Mosleh, Y.Y.; Paris-Palacios, S.; Ahmed, M.T.; Mahmoud, F.M.; Osman, M.A.; Biagianti-Risbourg, S. Effects of chitosan on oxidative stress and metallothioneins in aquatic worm Tubifex tubifex (Oligochaeta, Tubificidae). Chemosphere 2007, 67, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Sowjanya, J.A.; Singh, J.; Mohita, T.; Sarvanan, S.; Moorthi, A.; Srinivasan, N.; Selvamurugan, N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf. B Biointerfaces 2013, 109, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Cui, F.Z.; Liao, S.S.; Zhu, Y.; Han, L. Synthesis and biocompatibility of porous nano-hydroxyapatite/collagen/alginate composite. J. Mater. Sci. Mater. Med. 2003, 14, 641–645. [Google Scholar] [CrossRef]
- Kim, H.L.; Jung, G.Y.; Yoon, J.H.; Han, J.S.; Park, Y.J.; Kim, D.G.; Zhang, M.; Kim, D.J. Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 54, 20–25. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Seo, J. Nano zinc oxide–sodium alginate antibacterial cellulose fibres. Carbohydr. Polym. 2016, 135, 349–355. [Google Scholar] [CrossRef]
- Fourest, E.; Volesky, B. Contribution of sulfonate groups and alginate to heavy metal biosorption by the dry biomass of Sargassum fluitans. Environ. Sci. Technol. 1996, 30, 277–282. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus. J. Hazard. Mater. 2009, 163, 555–562. [Google Scholar] [CrossRef]
- Fourest, E.; Volesky, B. Alginate properties and heavy metal biosorption by marine algae. Appl. Biochem. Biotechnol. 1997, 67, 215–226. [Google Scholar] [CrossRef]
- Lau, P.S.; Tam, N.F.Y.; Wong, Y.S. Wastewater nutrients (N and P) removal by carrageenan and alginate immobilized Chlorella vulgaris. Environ. Technol. 1997, 18, 945–951. [Google Scholar] [CrossRef]
- Shih, Y.J.; Su, C.C.; Chen, C.W.; Dong, C.D.; Liu, W.S.; Huang, C.P. Adsorption characteristics of nano-TiO2 onto zebrafish embryos and its impacts on egg hatching. Chemosphere 2016, 154, 109–117. [Google Scholar] [CrossRef]
- Callegaro, S.; Minetto, D.; Pojana, G.; Bilanicová, D.; Libralato, G.; Volpi Ghirardini, A.; Hassellöv, M.; Marcomini, A. Effects of alginate on stability and ecotoxicity of nano-TiO2 in artificial seawater. Ecotoxicol. Environ. Saf. 2015, 117, 107–114. [Google Scholar] [CrossRef]
- Oliveira, C.R.d.; Fraceto, L.F.; Rizzi, G.M.; Salla, R.F.; Abdalla, F.C.; Costa, M.J.; Silva-Zacarin, E.C.M. Hepatic effects of the clomazone herbicide in both its free form and associated with chitosan-alginate nanoparticles in bullfrog tadpoles. Chemosphere 2016, 149, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Abi Nassif, L.; Rioual, S.; Trepos, R.; Fauchon, M.; Farah, W.; Hellio, C.; Abboud, M.; Lescop, B. Development of alginate hydrogels active against adhesion of microalgae. Mater. Lett. 2019, 239, 180–183. [Google Scholar] [CrossRef]
- Storebakken, T. Binders in fish feeds: I. Effect of alginate and guar gum on growth, digestibility, feed intake and passage through the gastrointestinal tract of rainbow trout. Aquaculture 1985, 47, 11–26. [Google Scholar] [CrossRef]
- Chenoweth, M.B. The Toxicity of sodium alginate in cats. Ann. Surg. 1948, 127, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Malaikozhundan, B.; Ramasamy, P.; Vaseeharan, B. Assessment of biopolymer stabilized silver nanoparticle for their ecotoxicity on Ceriodaphnia cornuta and antibiofilm activity. J. Environ. Chem. Eng. 2016, 4, 2076–2083. [Google Scholar] [CrossRef]
- Savadekar, N.R.; Mhaske, S.T. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Mincea, M.; Negrulescu, A.; Ostafe, V. Preparation, modification, and applications of chitin nanowhiskers: A review. Rev. Adv. Mater. Sci. 2012, 30, 225–242. [Google Scholar]
- Pitkänen, M.; Kangas, H.; Laitinen, O.; Sneck, A.; Lahtinen, P.; Peresin, M.S.; Niinimäki, J. Characteristics and safety of nano-sized cellulose fibrils. Cellulose 2014, 21, 3871–3886. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Kovacs, T.; Naish, V.; O’Connor, B.; Blaise, C.; Gagné, F.; Hall, L.; Trudeau, V.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Harper, B.J.; Clendaniel, A.; Sinche, F.; Way, D.; Hughes, M.; Schardt, J.; Simonsen, J.; Stefaniak, A.B.; Harper, S.L. Impacts of chemical modification on the toxicity of diverse nanocellulose materials to developing zebrafish. Cellulose 2016, 23, 1763–1775. [Google Scholar] [CrossRef]
- VanGinkel, C.G.; Gayton, S. The biodegradability and nontoxicity of carboxymethyl cellulose (DS 0.7) and intermediates. Environ. Toxicol. Chem. 1996, 15, 270–274. [Google Scholar] [CrossRef]
- Chada, H.L. Toxicity of cellulose acetate and vinyl plastic cages to barley plants and greenbugs1. J. Econ. Entomol. 1962, 55, 970–972. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Kalia, V.C. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Li, X.T.; Zhang, Y.; Chen, G.Q. Nanofibrous polyhydroxyalkanoate matrices as cell growth supporting materials. Biomaterials 2008, 29, 3720–3728. [Google Scholar] [CrossRef]
- Dinjaski, N.; Prieto, M.A. Smart polyhydroxyalkanoate nanobeads by protein based functionalization. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 885–899. [Google Scholar] [CrossRef]
- Ran, G.; Tan, D.; Dai, W.; Zhu, X.; Zhao, J.; Ma, Q.; Lu, X. Immobilization of alkaline polygalacturonate lyase from Bacillus subtilis on the surface of bacterial polyhydroxyalkanoate nano-granules. Appl. Microbiol. Biotechnol. 2017, 101, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.; Li, G.; Nowack, B. Environmental hazard assessment for polymeric and inorganic nanobiomaterials used in drug delivery. J. Nanobiotechnol. 2019, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Li, X.T.; Peng, S.W.; Xiao, J.F.; Liu, C.; Fang, G.; Chen, K.C.; Chen, G.Q. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials 2010, 31, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Tomita, K.; Juni, K.; Ooba, K. In vivo and In Vitro degradation of poly(3-hydroxybutyrate) in pat. Biomaterials 1991, 12, 309–312. [Google Scholar] [CrossRef]
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743. [Google Scholar] [CrossRef]
- Avérous, L. Chapter 21—Polylactic acid: Synthesis, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 433–450. [Google Scholar] [CrossRef]
- Hartmann, M.H. High Molecular Weight Polylactic Acid Polymers. In Biopolymers from Renewable Resources; Kaplan, D.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 367–411. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.L.; Filho, R.M. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Smith, A.; Hunneyball, L.M. Evaluation of poly(lactic acid) as a biodegradable drug delivery system for parenteral administration. Int. J. Pharm. 1986, 30, 215–220. [Google Scholar] [CrossRef]
- Ignatius, A.A.; Claes, L.E. In vitro biocompatibility of bioresorbable polymers: Poly(L, DL-lactide) and poly(L-lactide-co-glycolide). Biomaterials 1996, 17, 831–839. [Google Scholar] [CrossRef]
- Bos, R.R.M.; Rozema, F.B.; Boering, G.; Nijenhius, A.J.; Pennings, A.J.; Verwey, A.B.; Nieuwenhuis, P.; Jansen, H.W.B. Degradation of and tissue reaction to biodegradable poly(L-lactide) for use as internal fixation of fractures: A study in rats. Biomaterials 1991, 12, 32–36. [Google Scholar] [CrossRef]
- Monthioux, M.; Flahaut, E.; Laurent, C.; Escoffier, W.; Raquet, B.; Bacsa, W.; Puech, P.; Machado, B.; Serp, P. Properties of carbon nanotubes. In Handbook of Nanomaterials Properties; Bhushan, B., Luo, D., Schricker, S.R., Sigmund, W., Zauscher, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–49. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Jackson, P.; Jacobsen, N.R.; Baun, A.; Birkedal, R.; Kühnel, D.; Jensen, K.A.; Vogel, U.; Wallin, H. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem. Cent. J. 2013, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Imahori, H.; Umeyama, T. Chapter 13 Fullerene-modified electrodes and solar cells. In Fullerenes: Principles and Applications (2); Langa, F., Nierengarten, J.F., Eds.; Royal Society of Chemistry: London, UK, 2011. [Google Scholar] [CrossRef]
- Bianco, A.; Da Ros, T. Chapter 14 Biological applications of fullerenes. In Fullerenes: Principles and Applications (2); Langa, F., Nierengarten, J.F., Eds.; Royal Society of Chemistry: London, UK, 2011. [Google Scholar] [CrossRef]
- Tao, X.; Fortner, J.D.; Zhang, B.; He, Y.; Chen, Y.; Hughes, J.B. Effects of aqueous stable fullerene nanocrystals (nC60) on Daphnia magna: Evaluation of sub-lethal reproductive responses and accumulation. Chemosphere 2009, 77, 1482–1487. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Rizoli, C.; Ávila, D.S.; Amorim, M.J.B.; de Jesus, M.B. nanomaterials in the environment: perspectives on in vivo terrestrial toxicity testing. Front. Environ. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Cao, C.; Wu, X.; Xi, X.; Filleter, T.; Sun, Y. Mechanical characterization of graphene. In Handbook of Nanomaterials Properties; Bhushan, B., Luo, D., Schricker, S.R., Sigmund, W., Zauscher, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 121–135. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Q.; Zhao, H.; Wang, L.; Guo, S.; Li, X. Ecotoxicological effects of graphene oxide on the protozoan Euglena gracilis. Chemosphere 2015, 128, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, P.F.M.; Nakabayashi, D.; Zucolotto, V. The effects of graphene oxide on green algae Raphidocelis subcapitata. Aquat. Toxicol. 2015, 166, 29–35. [Google Scholar] [CrossRef]
- Pretti, C.; Oliva, M.; Pietro, R.D.; Monni, G.; Cevasco, G.; Chiellini, F.; Pomelli, C.; Chiappe, C. Ecotoxicity of pristine graphene to marine organisms. Ecotoxicol. Environ. Saf. 2014, 101, 138–145. [Google Scholar] [CrossRef]
- Svartz, G.; Papa, M.; Gosatti, M.; Jordán, M.; Soldati, A.; Samter, P.; Guraya, M.M.; Pérez Coll, C.; Perez Catán, S. Monitoring the ecotoxicity of γ-Al2O3 and Ni/γ-Al2O3 nanomaterials by means of a battery of bioassays. Ecotoxicol. Environ. Saf. 2017, 144, 200–207. [Google Scholar] [CrossRef]
- Kumari, M.; Khan, S.S.; Pakrashi, S.; Mukherjee, A.; Chandrasekaran, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J. Hazard. Mater. 2011, 190, 613–621. [Google Scholar] [CrossRef]
- Arnold, M.C.; Badireddy, A.R.; Wiesner, M.R.; Di Giulio, R.T.; Meyer, J.N. Cerium oxide nanoparticles are more toxic than equimolar bulk cerium oxide in Caenorhabditis elegans. Arch. Environ. Contam. Toxicol. 2013, 65, 224–233. [Google Scholar] [CrossRef]
- Taylor, N.S.; Merrifield, R.; Williams, T.D.; Chipman, J.K.; Lead, J.R.; Viant, M.R. Molecular toxicity of cerium oxide nanoparticles to the freshwater alga Chlamydomonas reinhardtii is associated with supra-environmental exposure concentrations. Nanotoxicology 2016, 10, 32–41. [Google Scholar] [CrossRef]
- Artells, E.; Issartel, J.; Auffan, M.; Borschneck, D.; Thill, A.; Tella, M.; Brousset, L.; Rose, J.; Bottero, J.-Y.; Thiéry, A. Exposure to cerium dioxide nanoparticles differently affect swimming performance and survival in two daphnid species. PLoS ONE 2013, 8, e71260. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst 2013, 138, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.S.; Kumar, A.; Senapati, V.A.; Pandey, A.K.; Shanker, R.; Dhawan, A. Laboratory scale microbial food chain to study bioaccumulation, biomagnification, and ecotoxicity of cadmium telluride quantum dots. Environ. Sci. Technol. 2017, 51, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Movafeghi, A.; Nazari, F.; Vafaei, F.; Dadpour, M.R.; Hanifehpour, Y.; Joo, S.W. The toxic effects of l-Cysteine-capped cadmium sulfide nanoparticles on the aquatic plant Spirodela polyrrhiza. J. Nanoparticle Res. 2014, 16, 2774. [Google Scholar] [CrossRef]
- Contreras, E.Q.; Cho, M.; Zhu, H.; Puppala, H.L.; Escalera, G.; Zhong, W.; Colvin, V.L. Toxicity of quantum dots and cadmium salt to Caenorhabditis elegans after multigenerational exposure. Environ. Sci. Technol. 2013, 47, 1148–1154. [Google Scholar] [CrossRef]
- Petrarca, C.; Perrone, A.; Verna, N.; Verginelli, F.; Ponti, J.; Sabbioni, E.; Di Giampaolo, L.; Dadorante, V.; Schiavone, C.; Boscolo, P.; et al. Cobalt nano-particles modulate cytokine in vitro release by human mononuclear cells mimicking autoimmune disease. Int. J. Immunopathol. Pharm. 2006, 19, 11–14. [Google Scholar]
- Anusha, L.; Devi, C.S.; Sibi, G. Inhibition effects of cobalt nano particles against fresh water algal blooms caused by Microcystis and Oscillatoria. Am. J. Appl. Sci. Res. 2017, 3, 26–32. [Google Scholar] [CrossRef][Green Version]
- Ghodake, G.; Seo, Y.D.; Lee, D.S. Hazardous phytotoxic nature of cobalt and zinc oxide nanoparticles assessed using Allium cepa. J. Hazard. Mater. 2011, 186, 952–955. [Google Scholar] [CrossRef]
- Rubilar, O.; Rai, M.; Tortella, G.; Diez, M.C.; Seabra, A.B.; Durán, N. Biogenic nanoparticles: Copper, copper oxides, copper sulphides, complex copper nanostructures and their applications. Biotechnol. Lett. 2013, 35, 1365–1375. [Google Scholar] [CrossRef]
- Perreault, F.; Oukarroum, A.; Melegari, S.P.; Matias, W.G.; Popovic, R. Polymer coating of copper oxide nanoparticles increases nanoparticles uptake and toxicity in the green alga Chlamydomonas reinhardtii. Chemosphere 2012, 87, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.F.; Buffet, P.E.; Poirier, L.; Amiard-Triquet, C.; Gilliland, D.; Joubert, Y.; Pilet, P.; Guibbolini, M.; Risso de Faverney, C.; Roméo, M.; et al. Size dependent bioaccumulation and ecotoxicity of gold nanoparticles in an endobenthic invertebrate: The Tellinid clam Scrobicularia plana. Environ. Pollut. 2012, 168, 37–43. [Google Scholar] [CrossRef]
- Zhai, G.; Walters, K.S.; Peate, D.W.; Alvarez, P.J.J.; Schnoor, J.L. Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ. Sci. Technol. Lett. 2014, 1, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Holt-Hindle, P. platinum-based nanostructured materials: synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, M.; Shimizu, T.; Shirasawa, T.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech. Ageing Dev. 2008, 129, 322–331. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika Rajasree, S.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Analysis Phase: Ecological Effects Characterization. In Technical Overview of Ecological Risk Assessment; EPA: Washington, DC, USA, 2017. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-0 (accessed on 28 November 2019).
- United Nations. Part 4 Environmental Hazards. In Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 5th ed.; United Nations: New York, NY, USA; Geneva, Switzerland, 2013; pp. 219–243. [Google Scholar]
- Sohn, E.K.; Chung, Y.S.; Johari, S.A.; Kim, T.G.; Kim, J.K.; Lee, J.H.; Lee, Y.H.; Kang, S.W.; Yu, I.J. Acute toxicity comparison of single-walled carbon nanotubes in various freshwater organisms. BioMed Res. Int. 2015, 2015, 323090. [Google Scholar] [CrossRef]
- Sanchís, J.À. Occurrence and Toxicity of Nanomaterials and Nanostructures in the Environment. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, July 2015. Available online: http://www.tdx.cat/handle/10803/305366 (accessed on 7 November 2019).
- Alam, B.; Philippe, A.; Rosenfeldt, R.R.; Seitz, F.; Dey, S.; Bundschuh, M.; Schaumann, G.E.; Brenner, S.A. Synthesis, characterization, and ecotoxicity of CeO2 nanoparticles with differing properties. J. Nanopart. Res. 2016, 18, 303. [Google Scholar] [CrossRef]
- Brown, D.M.; Johnston, H.J.; Gaiser, B.; Pinna, N.; Caputo, G.; Culha, M.; Kelestemur, S.; Altunbek, M.; Stone, V.; Roy, J.C.; et al. A cross-species and model comparison of the acute toxicity of nanoparticles used in the pigment and ink industries. NanoImpact 2018, 11, 20–32. [Google Scholar] [CrossRef]
- Modlitbova, P.; Novotny, K.; Porizka, P.; Klus, J.; Lubal, P.; Zlamalova-Gargosova, H.; Kaiser, J. Comparative investigation of toxicity and bioaccumulation of Cd-based quantum dots and Cd salt in freshwater plant Lemna minor L. Ecotox Environ. Saf. 2018, 147, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Vijver, M.G.; Peijnenburg, W. Comparative toxicity of copper nanoparticles across three Lemnaceae species. Sci. Total Environ. 2015, 518, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Daniel-da-Silva, A.L.; Venancio, C.; Soares, S.F.; Soares, A.; Trindade, T.; Lopes, I. Effects of long-term exposure to colloidal gold nanorods on freshwater microalgae. Sci. Total Environ. 2019, 682, 70–79. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Dringen, R.; Petters, C.; Rastedt, W.; Koser, J.; Filser, J.; Stolte, S. Toxicity of dimercaptosuccinate-coated and un-functionalized magnetic iron oxide nanoparticles towards aquatic organisms. Environ. Sci. Nano 2016, 3, 754–767. [Google Scholar] [CrossRef]
- Hlavkova, D.; Beklova, M.; Kopel, P.; Havelkova, B. Evaluation of platinum nanoparticles ecotoxicity using representatives of distinct trophic levels of aquatic biocenosis. Neuroendocr. Lett. 2018, 39, 465–472. [Google Scholar]
- Pereira, S.P.P.; Jesus, F.; Aguiar, S.; de Oliveira, R.; Fernandes, M.; Ranville, J.; Nogueira, A.J.A. Phytotoxicity of silver nanoparticles to Lemna minor: Surface coating and exposure period-related effects. Sci. Total Environ. 2018, 618, 1389–1399. [Google Scholar] [CrossRef]
- Picado, A.; Paixao, S.M.; Moita, L.; Silva, L.; Diniz, M.; Lourenco, J.; Peres, I.; Castro, L.; Correia, J.B.; Pereira, J.; et al. A multi-integrated approach on toxicity effects of engineered TiO2 nanoparticles. Front. Environ. Sci. Eng. 2015, 9, 793–803. [Google Scholar] [CrossRef][Green Version]
- Hartmann, N.B.; Gottardo, S.; Sokull-Klüttgen, B. Part II: Toxicity assessment. In Review of Available Criteria for Non-Aquatic Organisms within PBT/vPvB Frameworks; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Lahive, E.; Jurkschat, K.; Shaw, B.J.; Handy, R.D.; Spurgeon, D.J.; Svendsen, C. Toxicity of cerium oxide nanoparticles to the earthworm Eisenia fetida: Subtle effects. Environ. Chem. 2014, 11, 268–278. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Amorim, M.J.B.; Scott-Fordsmand, J.J. Cell in vitro testing with soil invertebrates-challenges and opportunities toward modeling the effect of nanomaterials: a surface-modified cuo case study. Nanomaterials 2019, 9, 9. [Google Scholar] [CrossRef]
- Yekeen, T.A.; Azeez, M.A.; Akinboro, A.; Lateef, A.; Asafa, T.B.; Oladipo, I.C.; Oladokun, S.O.; Ajibola, A.A. Safety evaluation of green synthesized Cola nitida pod, seed and seed shell extract-mediated silver nanoparticles (AgNPs) using an Allium cepa assay. J. Taibah Univ. Sci. 2017, 11, 895–909. [Google Scholar] [CrossRef]
- Kleiven, M.; Rossbach, L.M.; Gallego-Urrea, J.A.; Brede, D.A.; Oughton, D.H.; Coutris, C. Characterizing the behavior, uptake, and toxicity of NM300K silver nanoparticles in Caenorhabditis elegans. Environ. Toxicol. Chem. 2018, 37, 1799–1810. [Google Scholar] [CrossRef]
- Mariyadas, J.; Amorim, M.J.B.; Jensen, J.; Scott-Fordsmand, J.J. Earthworm avoidance of silver nanomaterials over time. Environ. Pollut 2018, 239, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Rocheleau, S.; Arbour, M.; Elias, M.; Sunahara, G.I.; Masson, L. Toxicogenomic effects of nano- and bulk-TiO2 particles in the soil nematode Caenorhabditis elegans. Nanotoxicology 2015, 9, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomez, C.; Babin, M.; Garcia, S.; Almendros, P.; Perez, R.A.; Fernandez, M.D. Joint effects of zinc oxide nanoparticles and chlorpyrifos on the reproduction and cellular stress responses of the earthworm Eisenia andrei. Sci. Total Environ. 2019, 688, 199–207. [Google Scholar] [CrossRef] [PubMed]
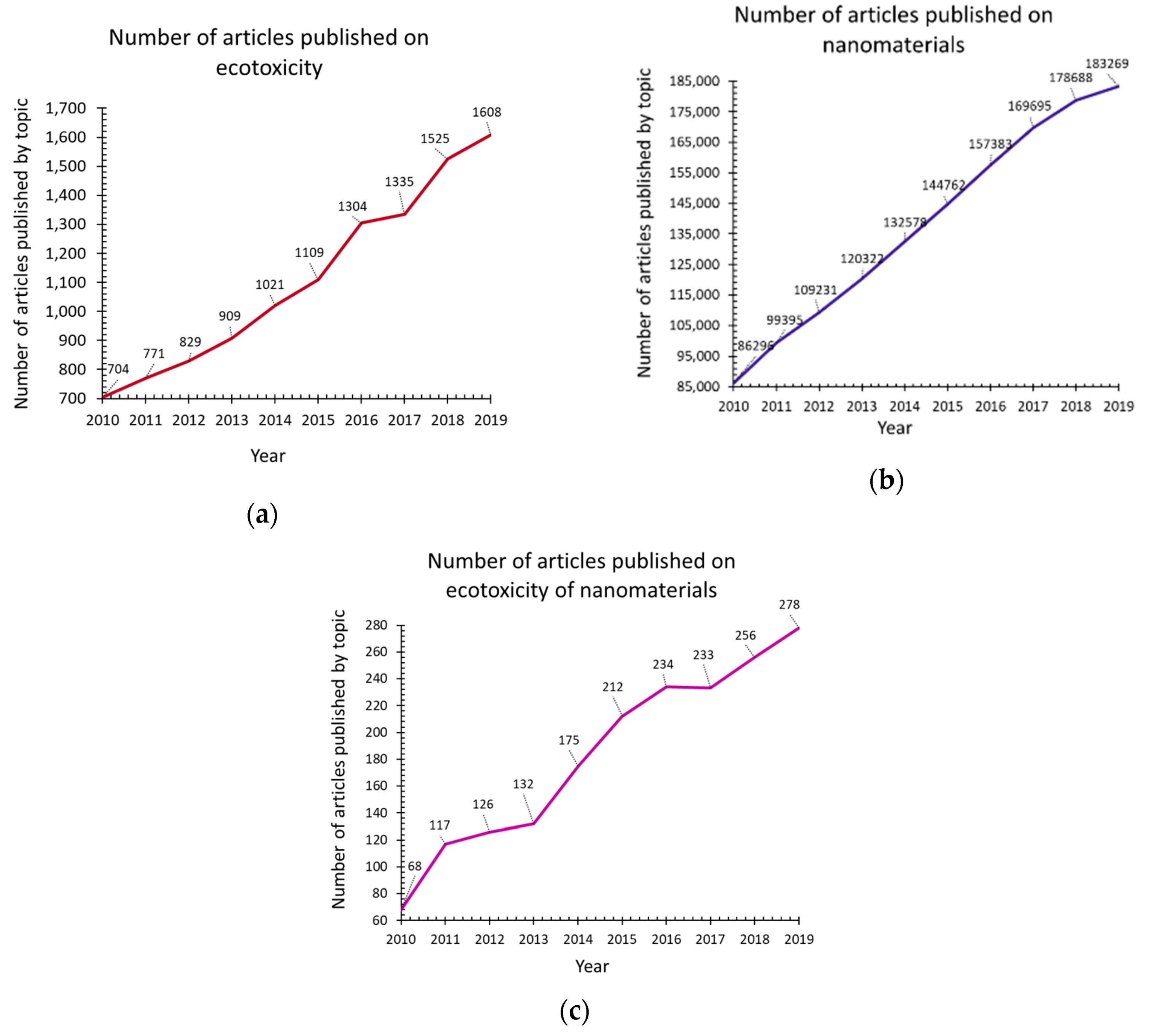
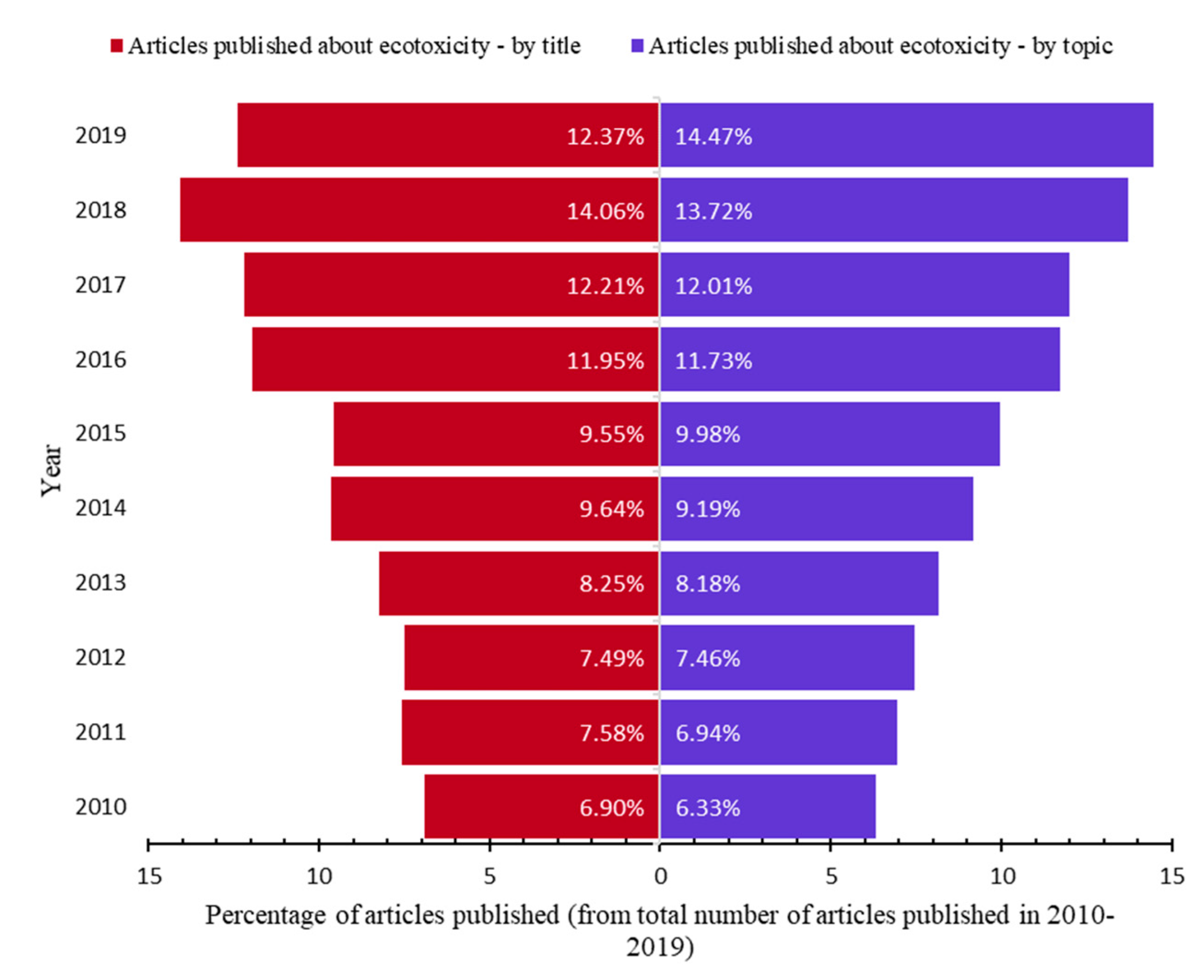
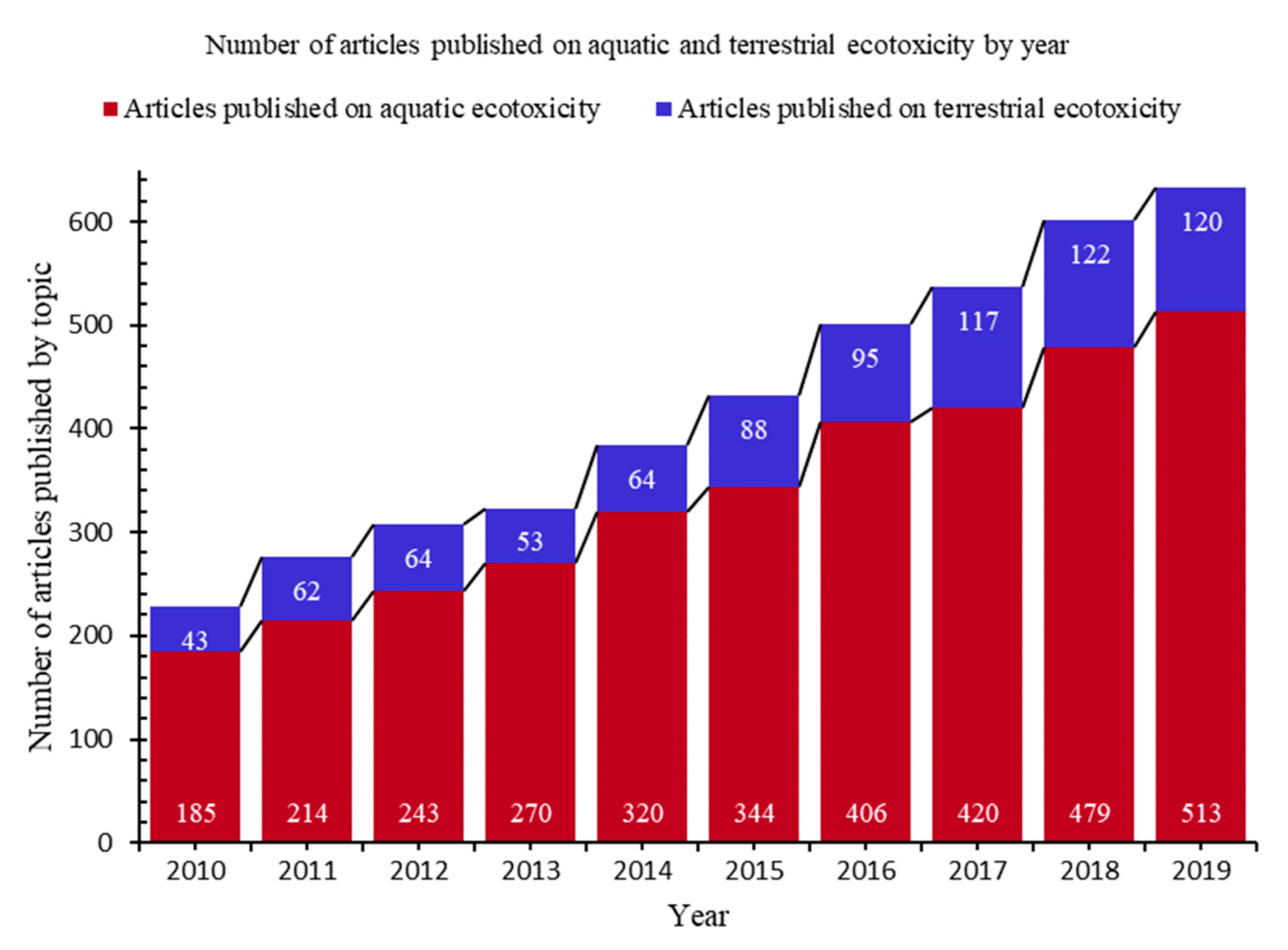
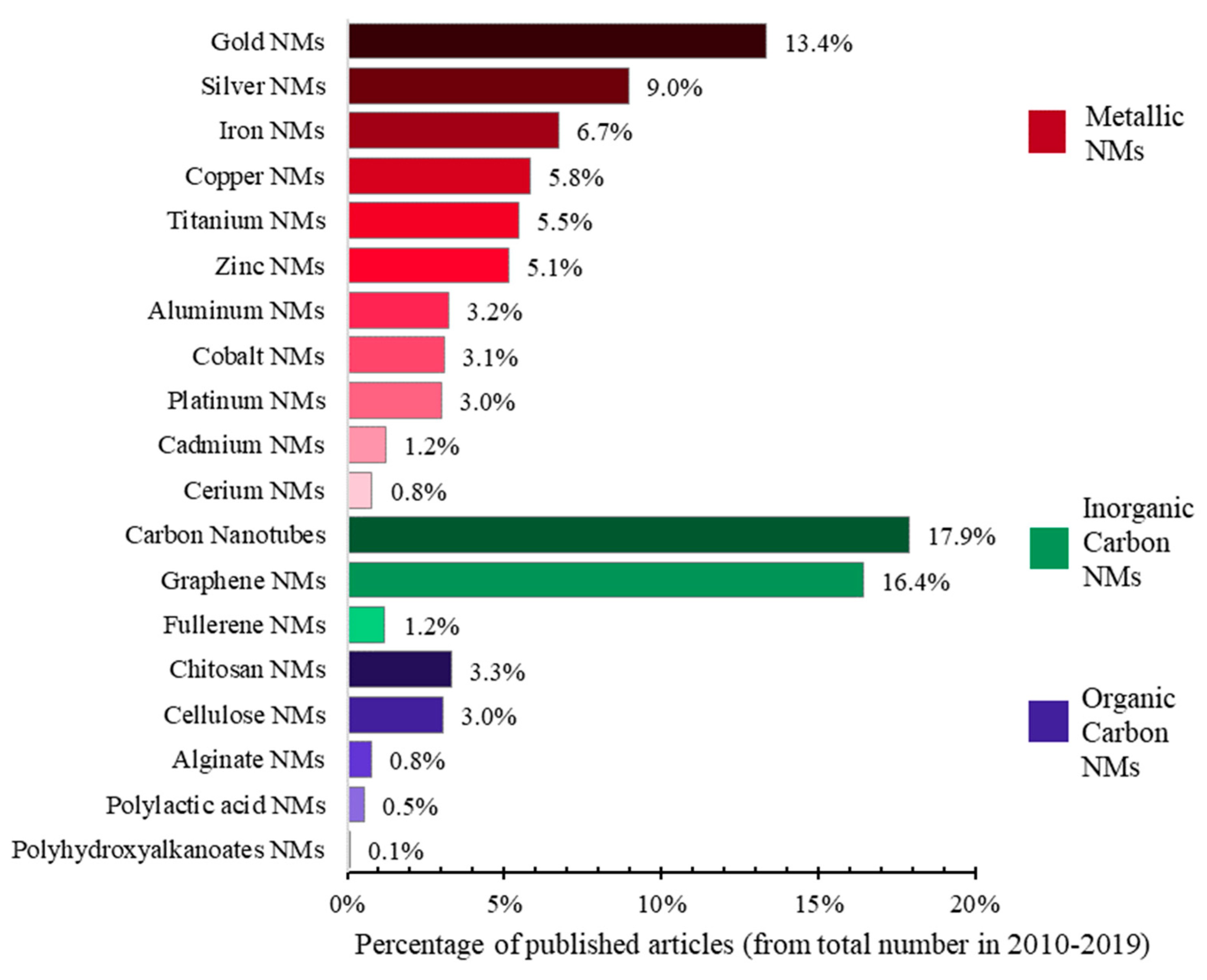
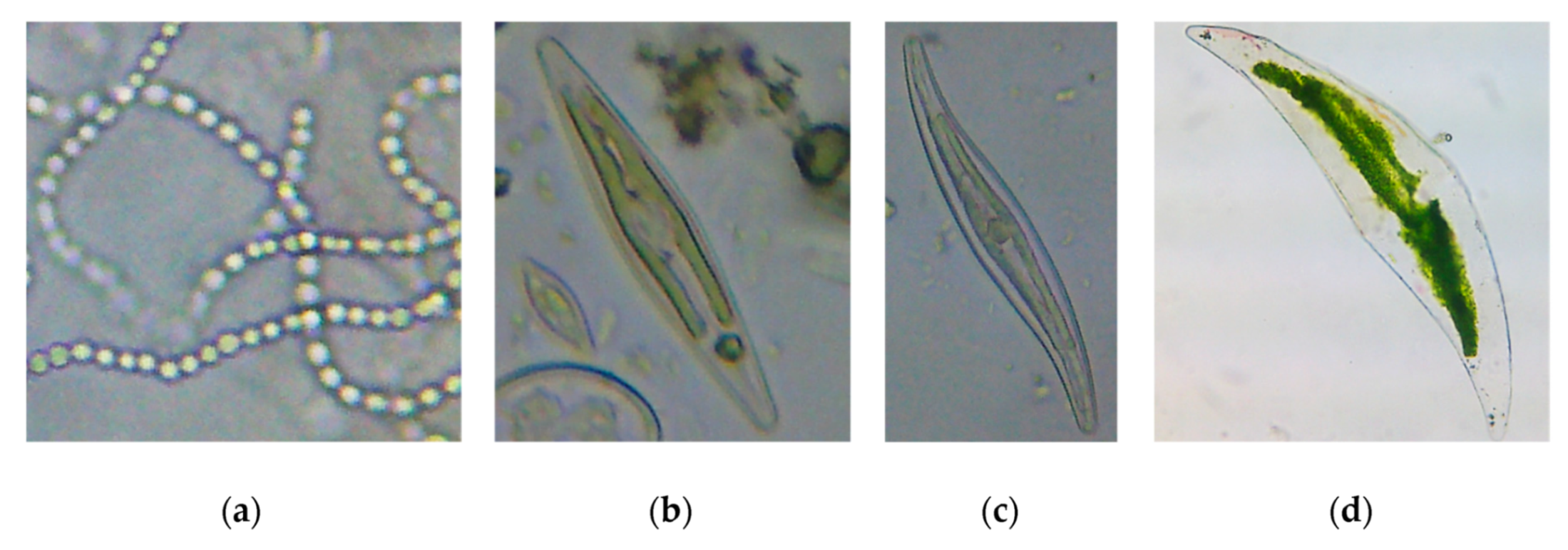


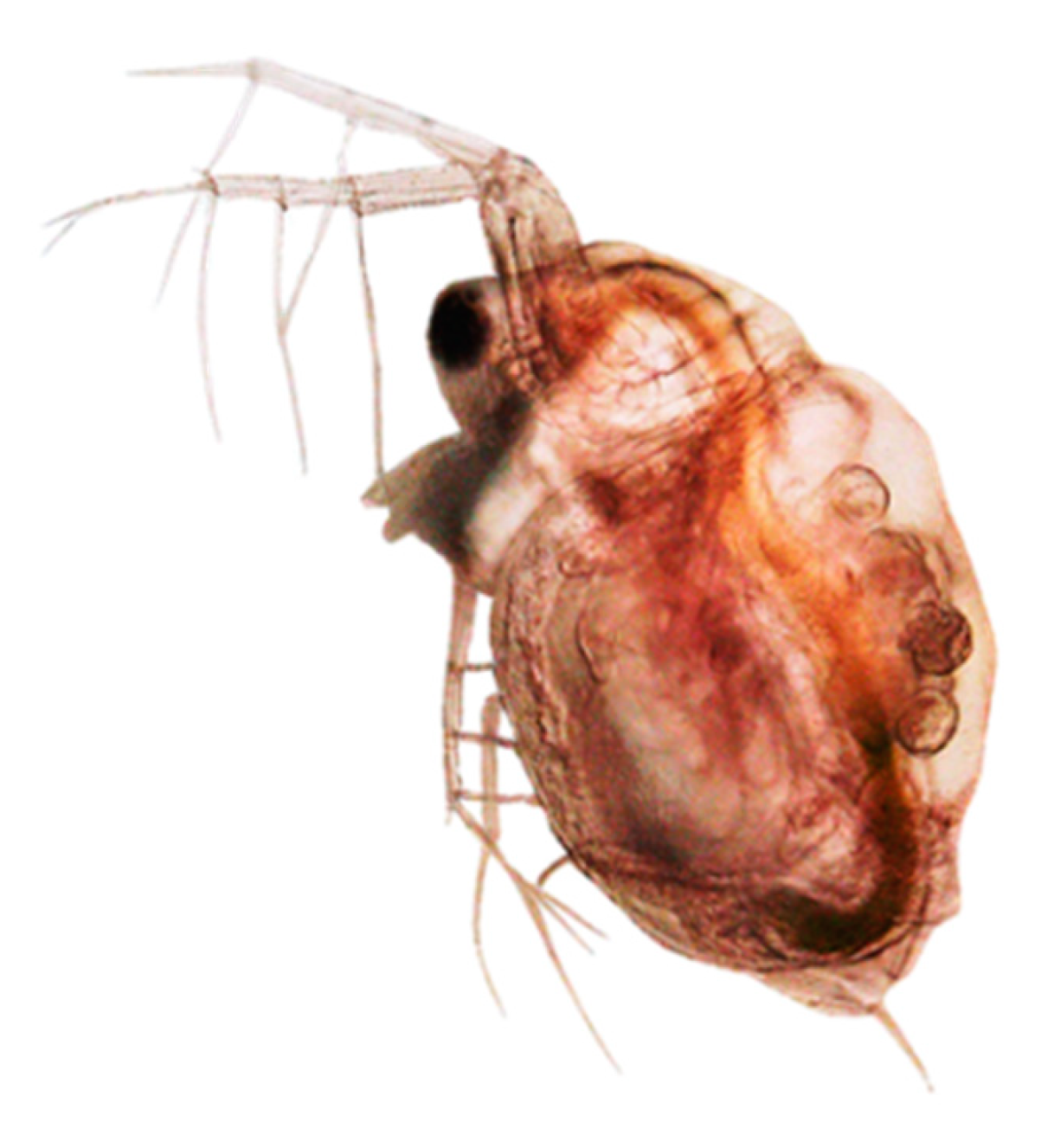

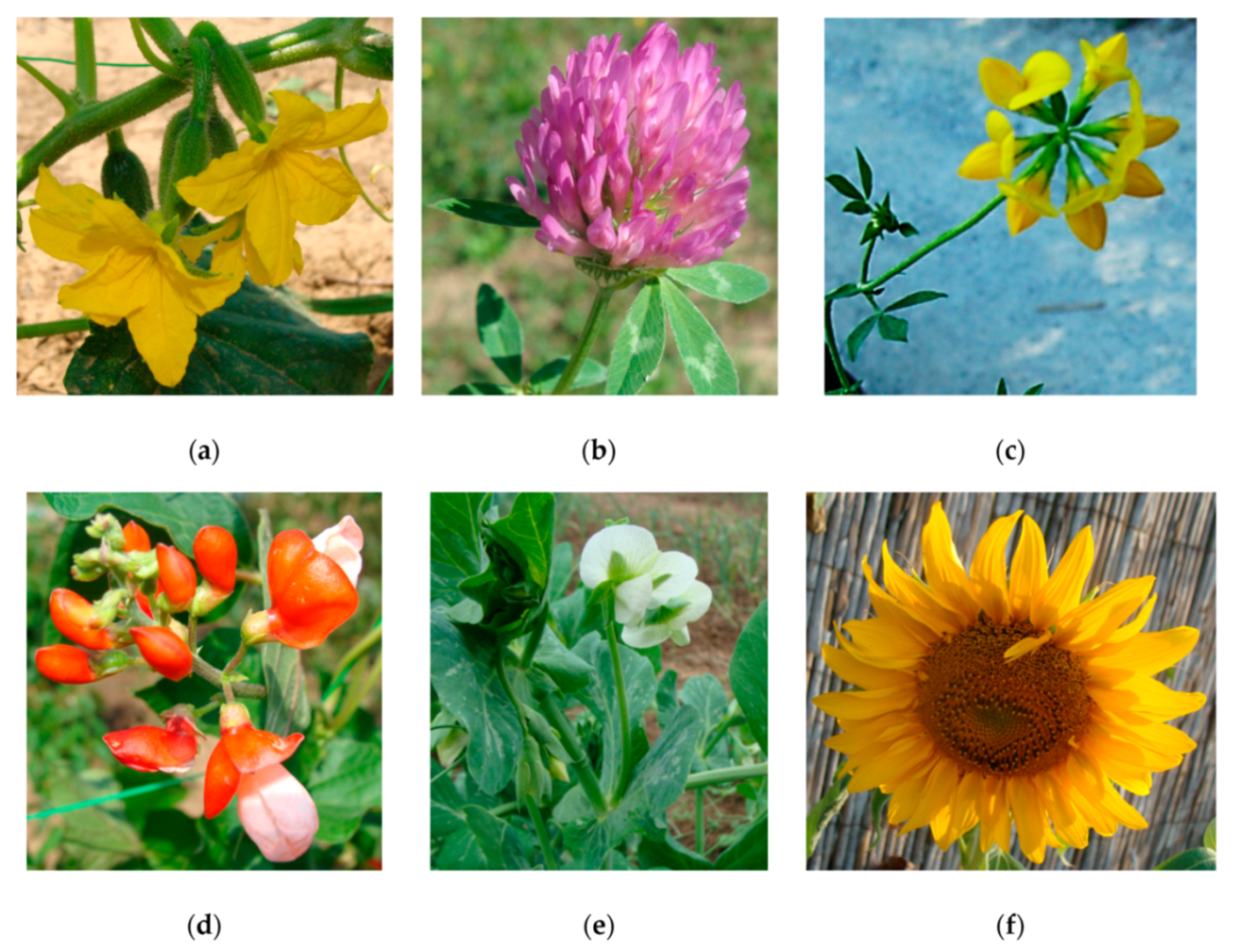
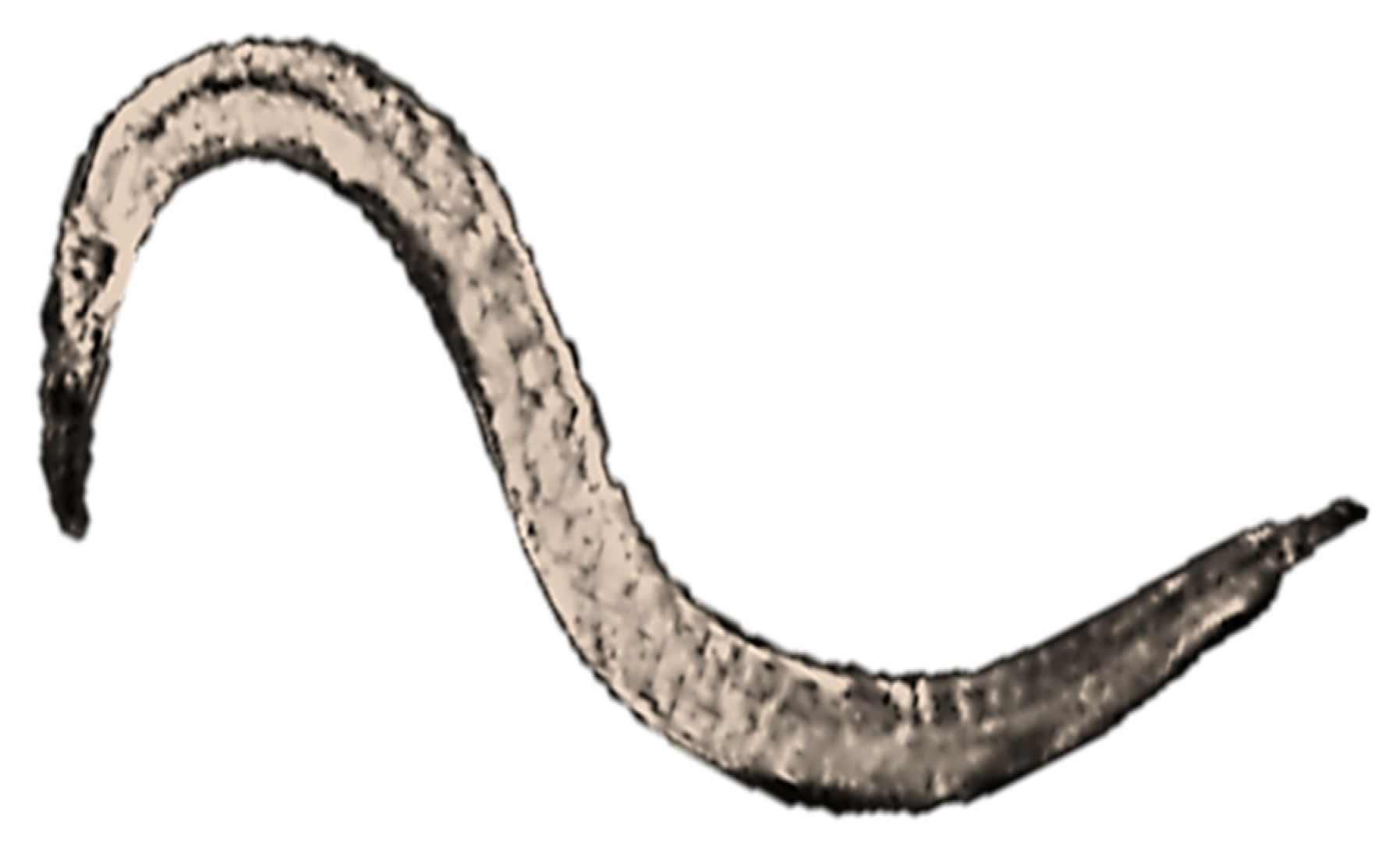

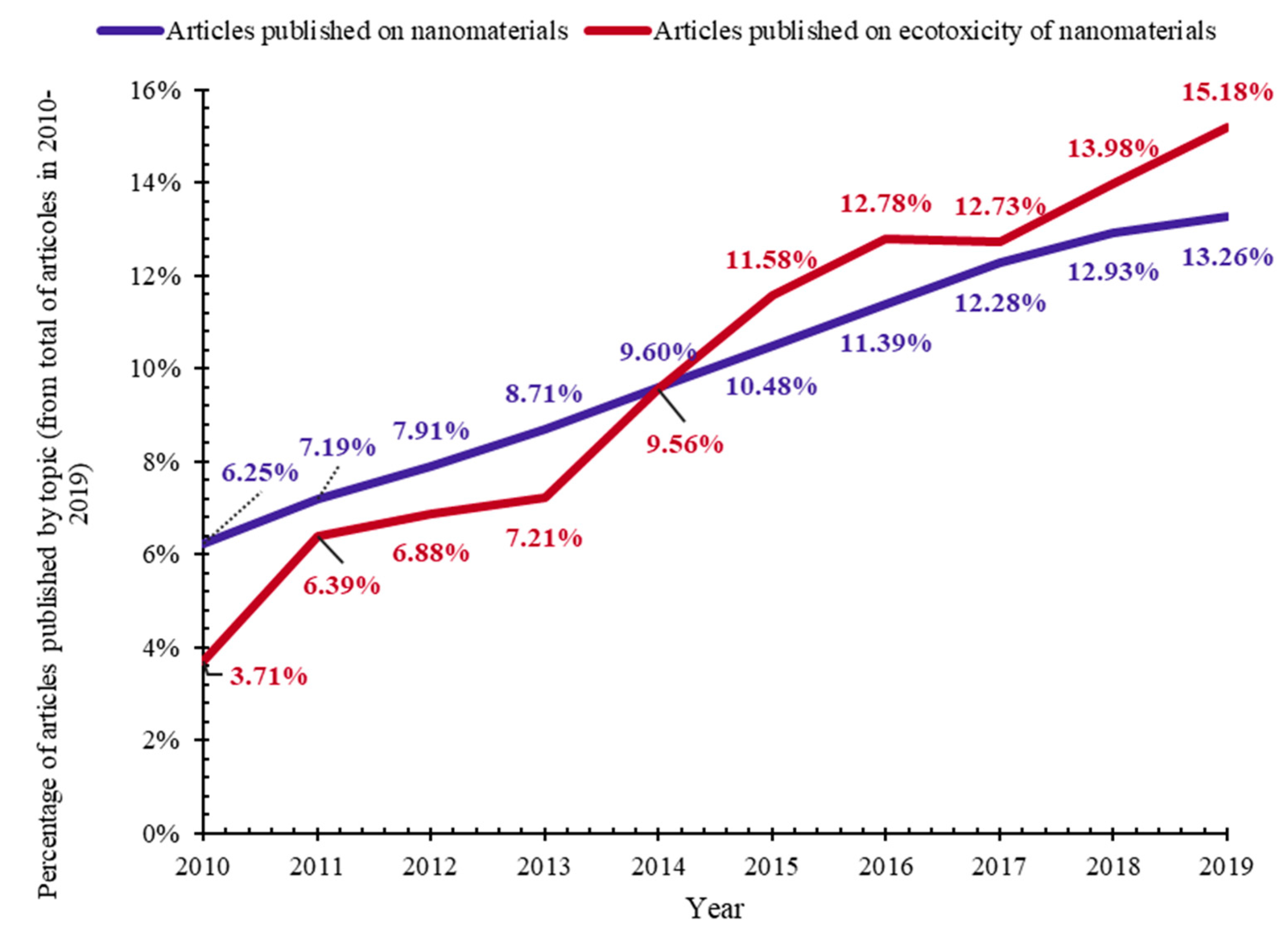
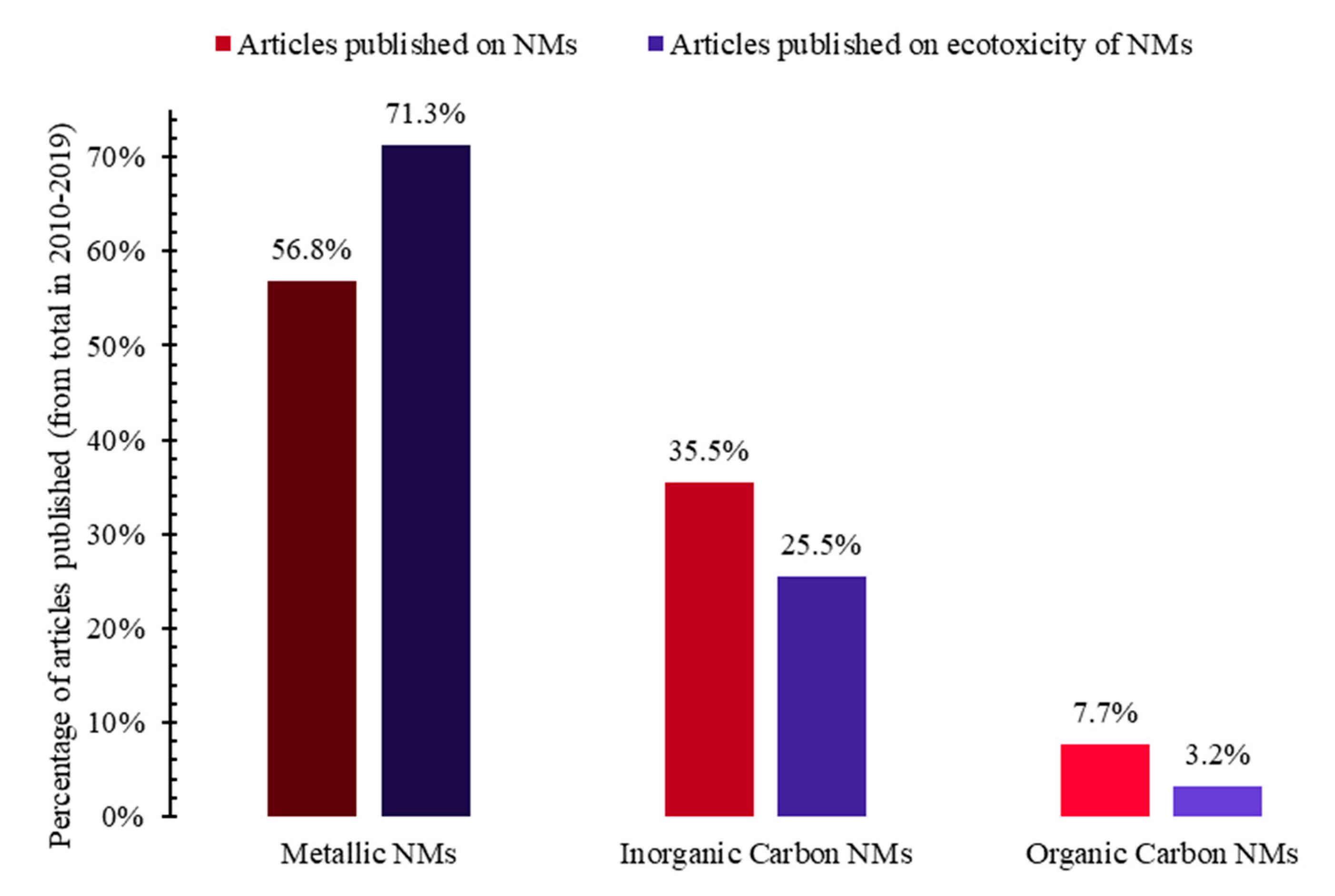
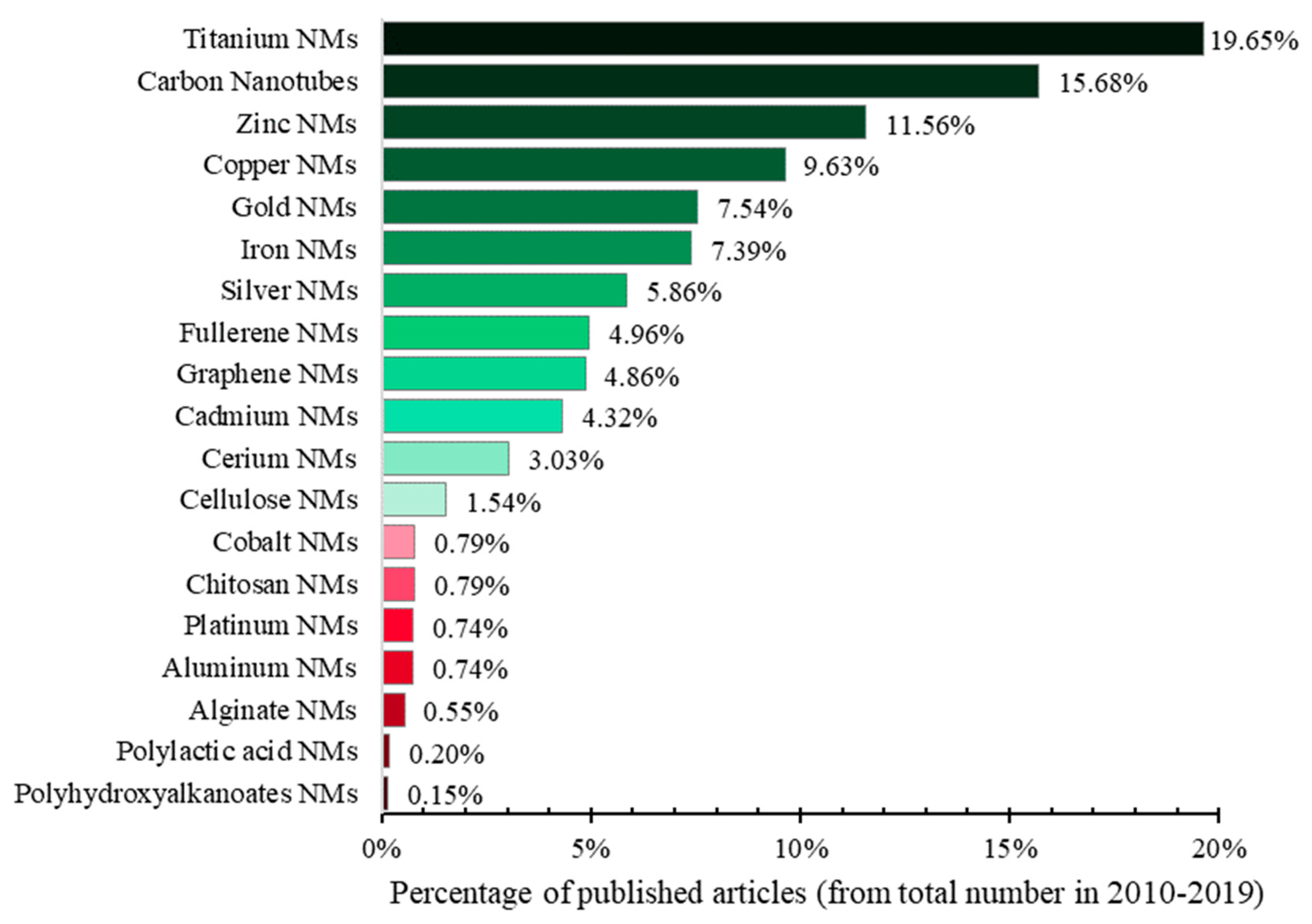



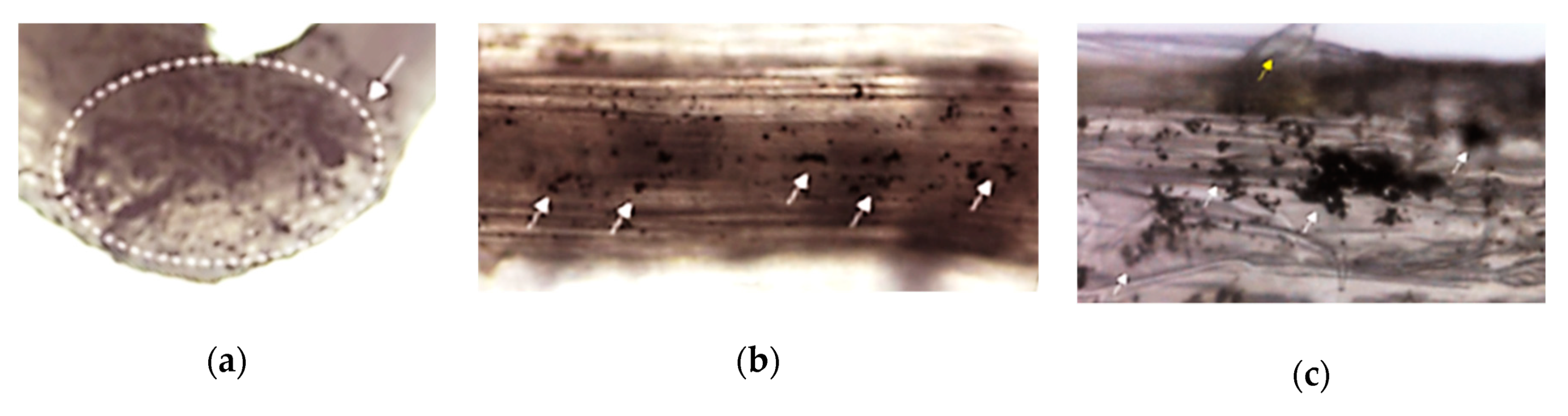
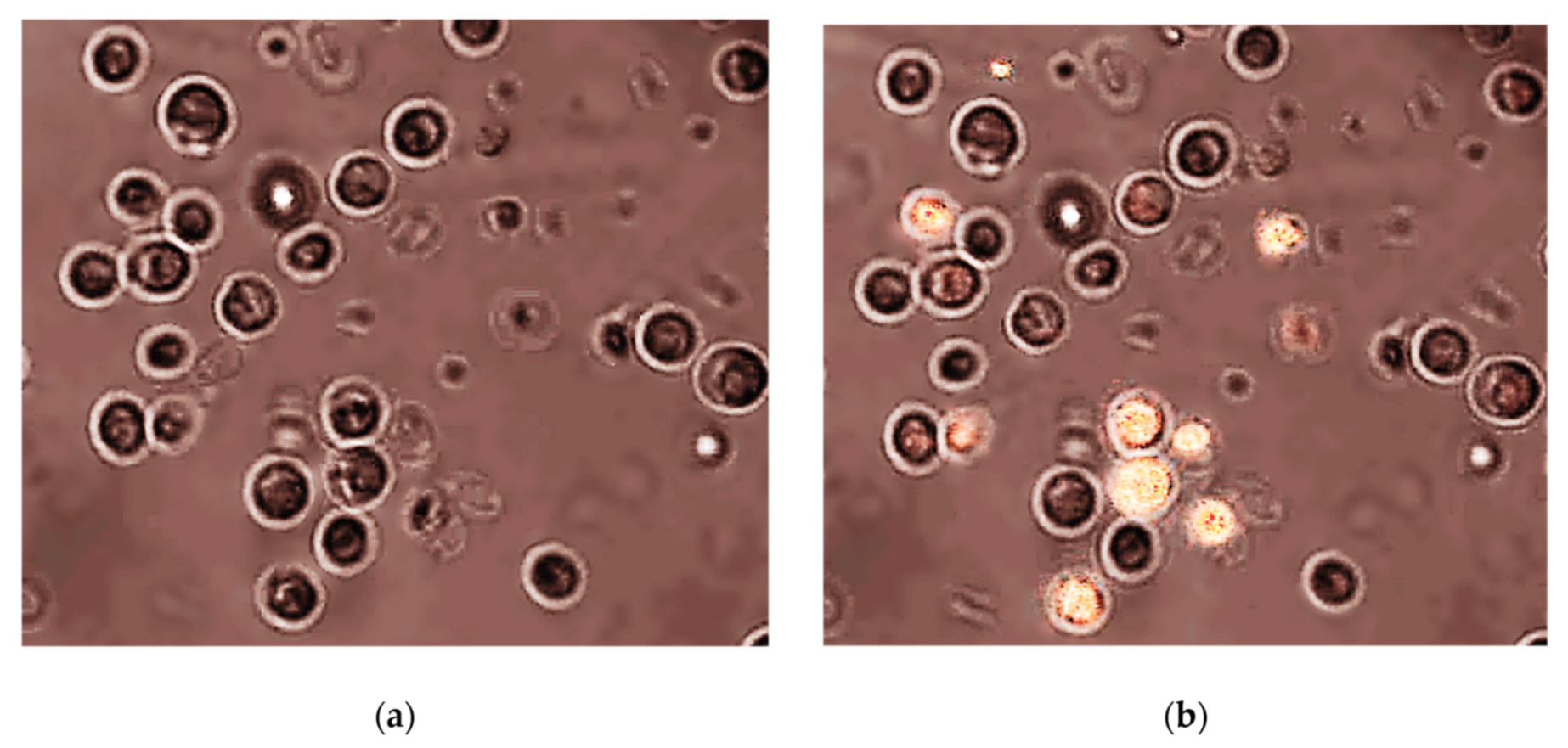
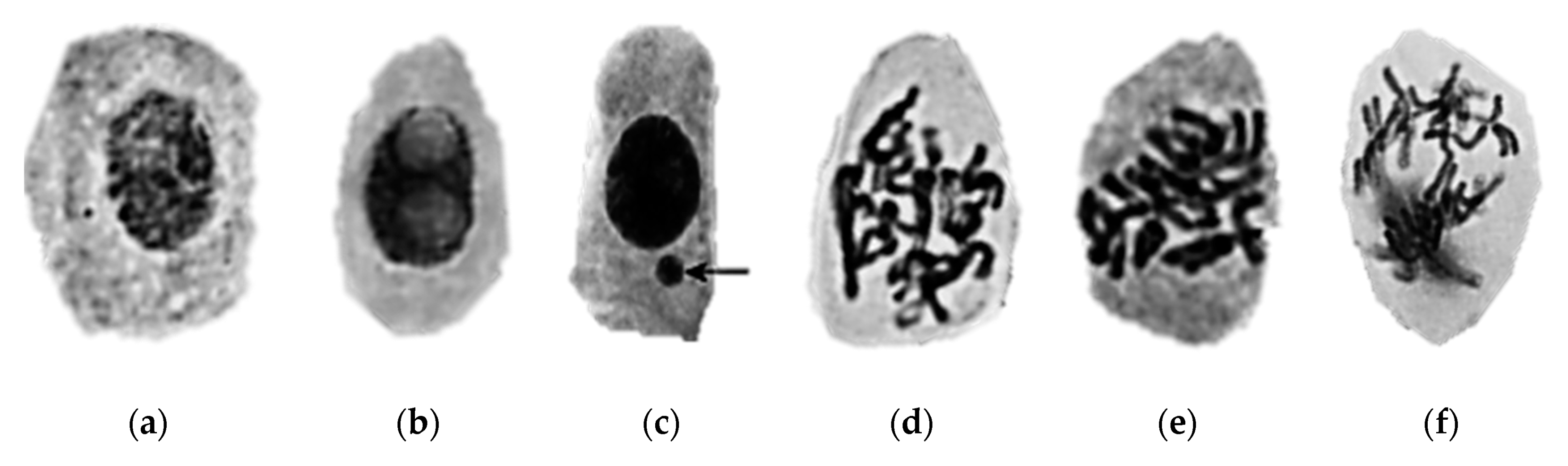
| Ecotoxicity | Nanomaterials | Ecotoxicity of NMs | Ecotoxicity of NMs by Type |
|---|---|---|---|
| 1. Ecotoxicity by topic (Figure 1a) | 4. Nanomaterials by topic (Figure 1b) | 6. NMs vs. ecotoxicity of NMs by topic (Figure 1c and Figure 15) | 7. Ecotoxicity of types of NMs by topic (Figure 17) |
| 2. Ecotoxicity by title vs. by topic (Figure 2) | 5. Types of NMs by topic (Figure 5) | 8. Categories of NMs and ecotoxicity of NMs by topic (Figure 16) | |
| 3. Aquatic vs. terrestrial ecotoxicity by topic (Figure 3) |
| LE 1 | OT 2 | Organism Category | Common Name of Group | Species | Standard Guidelines |
|---|---|---|---|---|---|
| Aquatic | Plants | Algae | Cyanobacteria | Anabaena flos-aquae Synechococcus leopoliensis Microcystis aeruginosa | OECD 201; EPA 850.4500; 850.4550; ISO 8692; 10253; 11044; ASTM E1218-04; GC EPS1/RM/25; |
| Diatoms | Navicula pelliculosa Skeletonema costatum Thalassiosira pseudonana Phaeodactylum tricornutum | ||||
| Green algae | Raphidocelis subcapitata (Pseudokirchneriella subcapitata; Selenastrum capricornutum) Desmodesmus subspicatus (Scenedesmus subspicatus) Dunaliella tertiolecta | ||||
| Red algae | Ceramium tenuicorne | ISO 10710; | |||
| Angiosperms | Duckweed | Lemna minor Lemna gibba Spirodela polyrhiza | OECD 221; EPA 850.4400; ISO 20079; 20227; ASTM E1415-91; GC EPS1/RM/37; | ||
| Great Manna grass | Glyceria maxima | OECD 239; | |||
| Watermilfoil | Myriophyllum spicatum Myriophyllum aquaticum | OECD 238; 239; ISO 16191; | |||
| Animals | Snails | Mud snail | Potamopyrgus antipodarum | OECD 242; | |
| Pond snail | Lymnaea stagnalis | OECD 243; | |||
| Bivalves | Clams | Mercenaria mercenaria | EPA 850.1025; 850.1055; 850.1710; ISO 17244; ASTM E2455-06; E724-98; | ||
| Mussel | Mytilus edulis Mytilus galloprovincialis | ||||
| Oyster | Crassostrea virginica Crassostrea gigas | ||||
| Oligochaetes | Blackworms | Lumbriculus variegatus | OECD 225; 315; | ||
| Tubificid worms | Tubifex tubifex Branchiura sowerbyi | ||||
| Crustaceans | Amphipods | Gammarus fasciatus Gammarus pseudolimnaeus Gammarus lacustris Ampelisca abdita Eohaustorius estuaries Rhepoxynius abronius Leptocheirus plumulosus Hyalella azteca | EPA 850.1020; 850.1735; 850.1740; ISO 16712; GC EPS1/RM/26; EPS1/RM/33; EPS1/RM/35; | ||
| Copepods | Acartia tonsa Nitocra spinipes | ISO 14669; 16778; 18220; ASTM E2317-04; | |||
| Mysids | Americamysis bahia (Mysidopsis bahia) | EPA 850.1035; ASTM E1191-03a; E1463-92; | |||
| Ostracods | Heterocypris incongruens | ISO 14371; | |||
| Penaeids | Farfantepenaeus aztecus (Penaeus aztecus) Farfantepenaeus duorarum (Penaeus duorarum) Litopenaeus setiferus (Penaeus setiferus) | EPA 850.1045; | |||
| Water fleas | Daphnia magna Daphnia pulex Ceriodaphnia dubia | OECD 202; 211; EPA 850.1010; 850.1300; ISO 6341; 10706; 20665; ASTM E1193-97; E1295-01; GC EPS1/RM/11; EPS1/RM/14; EPS1/RM/21; | |||
| Insects | Chironomids | Chironomus riparius Chironomus yohimatsui Chironomus tenans Chironomus dilutus | OECD 218; 219; 233; 235; EPA 850.1735; GC EPS1/RM/32; | ||
| Terrestrial | Plants | Angiosperms | Monocots | Allium cepa Avena sativa Hordeum vulgare Lolium perenne Oryza sativa Secale cereal Sorghum bicolor Triticum aestivum Zea mays | OECD 208; 227; EPA 850.4100; 850.4150; 850.4230; 850.4300; 850.4800; ISO 11269-1; 11269-2; 17126; 18763; 21479; 22030; ASTM E1963-09; GC EPS1/RM/45; EPS1/RM/56; |
| Dicots | Beta vulgaris Brassica campestris var. chinensis Brassica napus Brassica oleracea var. capitate Brassica rapa Cucumis sativus Daucus carota Fagopyrum esculentum Glycine max (Glycine. soja) Gossypium sp. Helianthus annuus Lactuca sativa Lepidium sativum Linum usitatissimum Lotus corniculatus Phaseolus aureus Phaseolus vulgaris Pisum sativum Raphanus sativus Sinapis alba Solanum lycopersicum (Lycopersicon esculentum) Trifolium pratense Trigonella foenum-graecum Vicia sativa | ||||
| Animals | Snails | Helicidae | Helix aspersa aspersa | ISO 15952; | |
| Nematodes | Caenorhabditis elegans | ISO 10872; ASTM E2172-01; | |||
| Oligochaetes | Earthworms | Eisenia foetida Eisenia andrei | OECD 207; 222; 317; EPA 850.3100; ISO 11268-1; 11268-2; 11268-3; 17512-1; 23611-1; ASTM E1676-12; GC EPS1/RM/43; | ||
| Potworms | Enchytraeus buchholzi Enchytraeus albidus Enchytraeus crypticus Enchytraeus luxuriosus | OECD 220; 222; 317; ISO 16387; 23611-3; ASTM E1676-12; | |||
| Mites | Hypoaspis aculeifer (Geolaelaps aculeifer) | OECD 226; | |||
| Springtails | Folsomia candida Folsomia fimetaria | OECD 232; ISO 11267; 17512-2; GC EPS1/RM/47; | |||
| Insects | Beetles | Oxythyrea funesta | ISO 20963; | ||
| Bumble bees | Bombus sp. | OECD 246; 247; | |||
| Honeybees | Apis mellifera | OECD 213; 214; 237; 245; EPA 850.3020; 850.3030; 850.3040; | |||
| Leaf cutter bees | Megachile rotundata | EPA 850.3040; | |||
| Sweat bees | Nomia melanderi | EPA 850.3040; | |||
| Flies | Musca autumnalis Scathophaga stercoraria | OECD 228; | |||
| Organism Type | Life Environment | Common Name | Standard Guidelines |
|---|---|---|---|
| Vertebrates | Aquatic | fish | OECD 203; 204; 210; 212; 215; 229; 230; 234; 236; 240; 305; EPA 850.1075; 850.1400; 850.1730; ISO 10229; 22082; 7346-1; 7346-2; 7346-3; 12890; 15088; 23893-1; 23893-2; 23893-3; ASTM E1192-97; E729-96; E1241-05; E1711-12; GC EPS1/RM/09; EPS1/RM/10; EPS1/RM/13; EPS1/RM/22; EPS1/RM/28; |
| amphibians | OECD 231; 241; ISO 21427-1; ASTM E1192-97; E2591-07; E729-96; | ||
| Terrestrial | birds | OECD 205; 206; 223; EPA 850.2100; 850.2200; 850.2300; ASTM E857-05; | |
| mammals | EPA 850.2400; ASTM E1163-10; E1619-11; |
| LE 1 | OT 2 | Organism | Test | NMAP 3 | SAT 4 | TD 5 |
|---|---|---|---|---|---|---|
| Aquatic | Plants | Algae | Growth inhibition | ✓ | ✗ | 72 h |
| Toxicity | ✓ | ✗ | 96 h | |||
| Duckweed | Growth inhibition | ✓ | ✗ | 7 days | ||
| Toxicity | ✓ | ✗ | ||||
| Watermilfoil | Sediment free toxicity | ✗ | ✗ | 14 days | ||
| Water-sediment toxicity | ✗ | ✗ | ||||
| Animals | Mud snails | Reproduction | ✗ | ✗ | 28 days | |
| Pond snails | Reproduction | ✗ | ✗ | 28 days | ||
| Bivalves | Acute toxicity | ✗ | ✗ | 48–96 h | ||
| Oligochaetes | Sediment-water toxicity | ✗ | ✗ | 28 days | ||
| Amphipods | Acute toxicity | ✓ | ✗ | 96 h | ||
| Spiked whole sediment toxicity | ✓ | ✗ | 10 days | |||
| Mysids | Acute toxicity | ✗ | ✗ | 96 h | ||
| Penaeids | Acute toxicity | ✗ | ✗ | 96 h | ||
| Water fleas | Acute immobilization | ✓ | ✗ | 48 h | ||
| Acute toxicity | ✓ | ✗ | 48 h | |||
| Chronic toxicity | ✓ | ✗ | 21 days | |||
| Reproduction | ✓ | ✗ | 21 days | |||
| Chironomids | Acute immobilization | ✓ | ✗ | 48 h | ||
| Sediment-water toxicity with spiked sediment | ✓ | ✗ | 20–28 days | |||
| Sediment-water toxicity with spiked water | ✓ | ✗ | 20–28 days | |||
| Spiked whole sediment toxicity | ✓ | ✗ | 10 days | |||
| Terrestrial | Plants | Angiosperms | Early seedling growth toxicity | ✓ | ✗ | 14 days |
| Seedling emergence/Seedling growth | ✓ | ✗ | 14–21 days | |||
| Vegetative vigor | ✓ | ✗ | 21–28 days | |||
| Animals | Nematodes | Toxicity | ✓ | ✗ | 96 h | |
| Earthworms | Acute toxicity (contact and soil) | ✓ | ✗ | 14 days | ||
| Subchronic toxicity | ✓ | ✗ | 28 days | |||
| Mites | Reproduction | ✗ | ✗ | 14 days | ||
| Springtails | Reproduction | ✗ | ✗ | 21–28 days | ||
| Bumblebees | Acute contact toxicity | ✗ | ✗ | 48–96 h | ||
| Acute oral toxicity | ✗ | ✗ | 48–96 h | |||
| Honeybees | Acute contact toxicity | ✗ | ✗ | 48–96 h | ||
| Acute oral toxicity | ✗ | ✗ | 48–96 h | |||
| Chronic oral toxicity | ✗ | ✗ | 10 days | |||
| Larval toxicity | ✗ | ✗ | 7 days | |||
| Toxicity of residue on foliage | ✗ | ✗ | 24 h | |||
| Flies | Developmental toxicity | ✗ | ✗ | 18–23 days |
| Categories According to U.S. EPA [164] | Categories According to U.N. [165] | EC50 (mg/L) |
|---|---|---|
| Very highly toxic (VHT) | Acute 1.1 (A1.1) | < 0.1 |
| Highly toxic (HT) | Acute 1.2 (A1.2) | 0.1–1 |
| Moderately toxic (MT) | Acute 2 (A2) | > 1–10 |
| Slightly toxic (ST) | Acute 3 (A3) | > 10–100 |
| Practically nontoxic (PNT) | Acute 4 (A4) | > 100 |
| NMs Based on: | EC50 Value (mg/L) for Aquatic Organisms | Tox. Cat. for Mean EC50 for All Assays | ||
|---|---|---|---|---|
| Algae 72 h Test | Duckweed 168 h (7 d) Test | Daphnid 48 h Test | ||
| Carbon NT | 29.9 [166] | NDA | >100 [23] | N/A |
| Fullerene | NDA | NDA | 11 [167] | N/A |
| Graphene | 20 [140] | NDA | 20 [167] | N/A |
| Cerium | NDA | NDA | 52.42 ** [168] | N/A |
| Cadmium | 3.5 * [169] | 0,45 [170] | 0.33 * [169] | 1.427 |
| Copper | 0.7 [23] | 0,84 [171] | 0.9 [23] | 0.813 |
| Gold | 0.048 [172] | NDA | >30 [23] | N/A |
| Iron | 0.07 [173] | >100 [173] | 43.41 [173] | 48.16 |
| Platinum | NDA | 0.213 [174] | 0.444 [174] | N/A |
| Silver | 0.003 [23] | 0,03 [175] | 0.003 [23] | 0.012 |
| Titanium | 6.8 [23] | >90 [176] | 29.5 [176] | 42.433 |
| Zinc | 0.14 [23] | NDA | 1.87 [23] | N/A |
| AVERAGE EC50 | 6.795 | 32.255 | 24.323 | |
| Toxicity Category | EC50 (mg/L) | Algae Assay | Duckweed Assay | Daphnid Assay |
|---|---|---|---|---|
| Very highly toxic | < 0.1 | Ag, Au, Fe NMs | Ag NMs | Ag NMs |
| Highly toxic | 0.1–1 | Zn, Cu NMs | Pt, Cd, Cu NMs | Cd, Pt, Cu NMs |
| Moderately toxic | > 1–10 | Cd, Ti NMs | - | Zn NMs |
| Slightly toxic | > 10–100 | Graphene, Carbon NT | Ti NMs | Fullerene, graphene, Ti, Au, Fe, Ce NMs |
| Practically nontoxic | > 100 | - | Fe NMs | Carbon NT |
| Toxicity Categories | EC50 (mg/kg Soil Dry Weight) |
|---|---|
| Very toxic (VT)/Acute 1 (A1) | ≤ 10 |
| Toxic (T)/Acute 2 (A2) | > 10 – ≤ 100 |
| Harmful (H)/Acute 3 (A3) | > 100 – ≤ 1000 |
| NMs Based on: | EC50 Value (mg/kg Soil Dry Weight) for Terrestrial Organisms | Tox. Cat. for Mean EC50 for All Assays | ||
|---|---|---|---|---|
| Plants 72 h Test | Nematodes 24 h Test | Earthworms 28 d Test | ||
| Cerium | NDA | NDA | 294.6 [178] | N/A |
| Copper | NDA | NDA | 197 [179] | N/A |
| Silver | 12.973 [180] | 2.553 [181] | 31 ** [182] | 15.508 |
| Titanium | NDA | 18 [183] | NDA | N/A |
| Zinc | NDA | NDA | 179 *** [184] | N/A |
| Toxicity Categories. | EC50 (mg/kg Soil Dw) | Plant Assay | Nematode Assay | Earthworm Assay |
|---|---|---|---|---|
| Very toxic | ≤ 10 | - | Ag NMs | - |
| Toxic | > 10 – ≤ 100 | Ag NMs | Ti NMs | Ag NMs |
| Harmful | > 100 – ≤ 1000 | - | - | Zn, Cu, Ce NMs |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boros, B.-V.; Ostafe, V. Evaluation of Ecotoxicology Assessment Methods of Nanomaterials and Their Effects. Nanomaterials 2020, 10, 610. https://doi.org/10.3390/nano10040610
Boros B-V, Ostafe V. Evaluation of Ecotoxicology Assessment Methods of Nanomaterials and Their Effects. Nanomaterials. 2020; 10(4):610. https://doi.org/10.3390/nano10040610
Chicago/Turabian StyleBoros, Bianca-Vanesa, and Vasile Ostafe. 2020. "Evaluation of Ecotoxicology Assessment Methods of Nanomaterials and Their Effects" Nanomaterials 10, no. 4: 610. https://doi.org/10.3390/nano10040610
APA StyleBoros, B.-V., & Ostafe, V. (2020). Evaluation of Ecotoxicology Assessment Methods of Nanomaterials and Their Effects. Nanomaterials, 10(4), 610. https://doi.org/10.3390/nano10040610