Colorectal Cancer Study with Nanostructured Sensors: Tumor Marker Screening of Patient Biopsies
Abstract
1. Introduction
- Vascular endothelial growth factors;
- Wastes from circulating tumor cells;
- Molecules produced by the cellular metabolism;
1.1. Metal-Oxide Chemoresistive Sensors
1.2. Prototypes
2. Materials and Methods
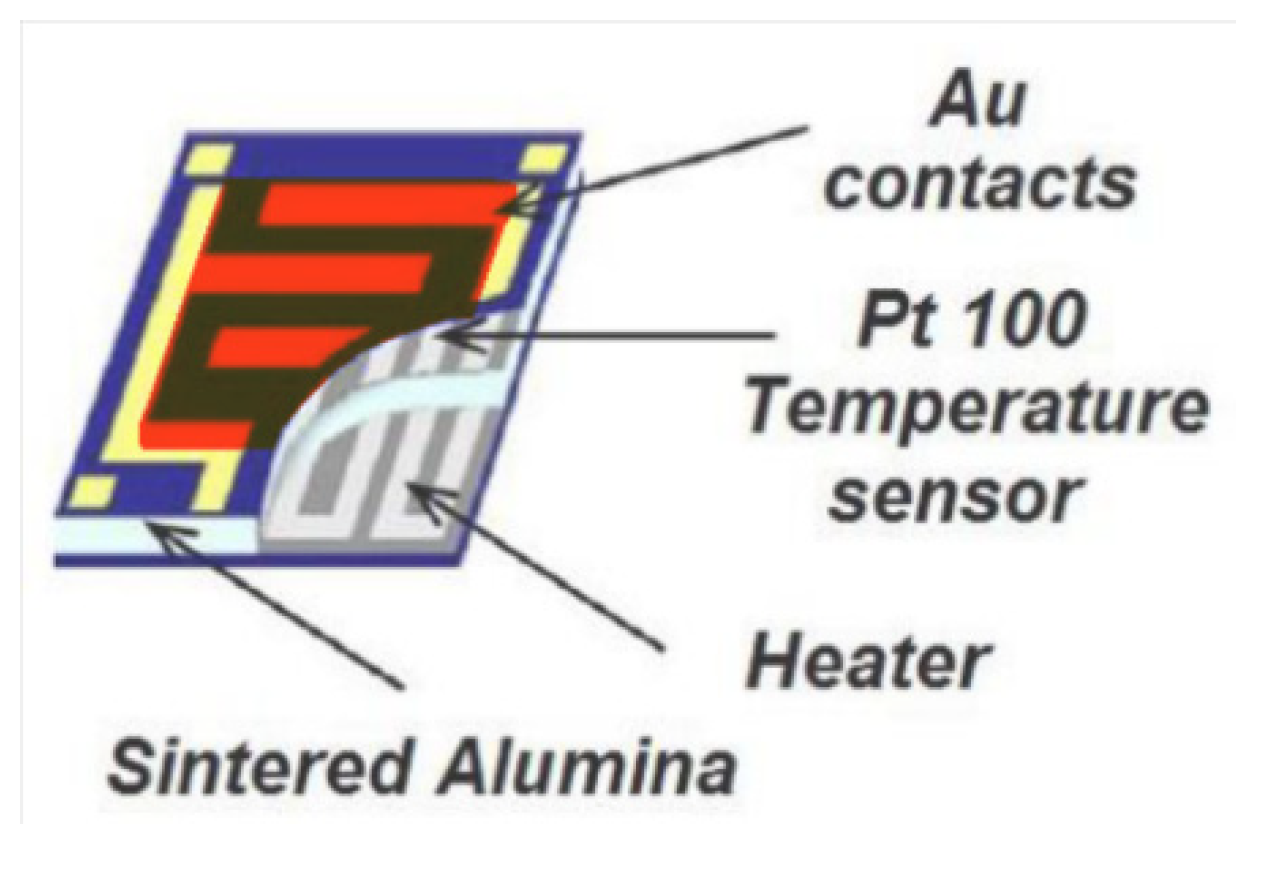
2.1. SCENT B1 Device
2.2. Patient Recruitment and Sample Collection
2.3. Sample Handling
2.4. Ethics Approval and Informed Consent
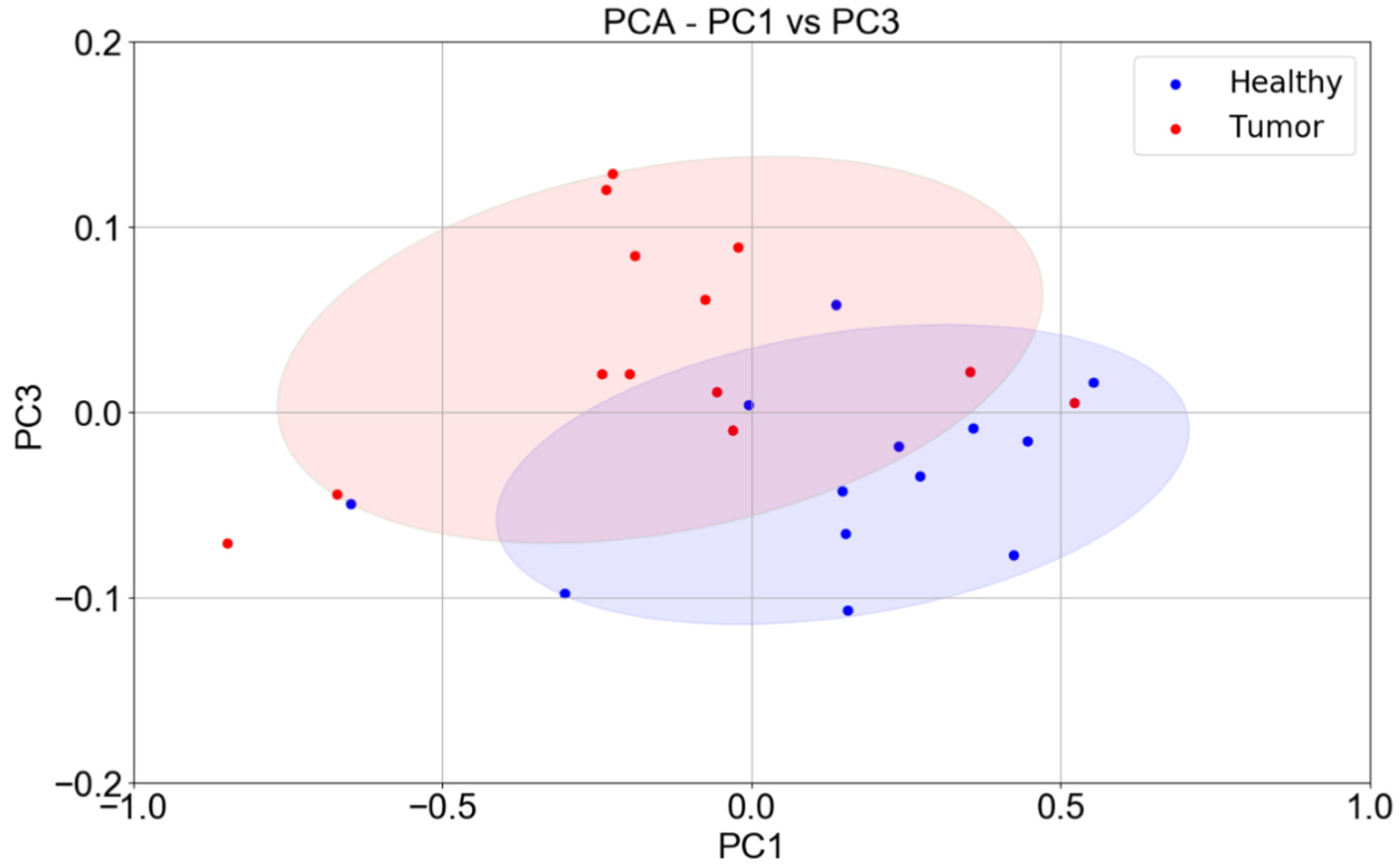
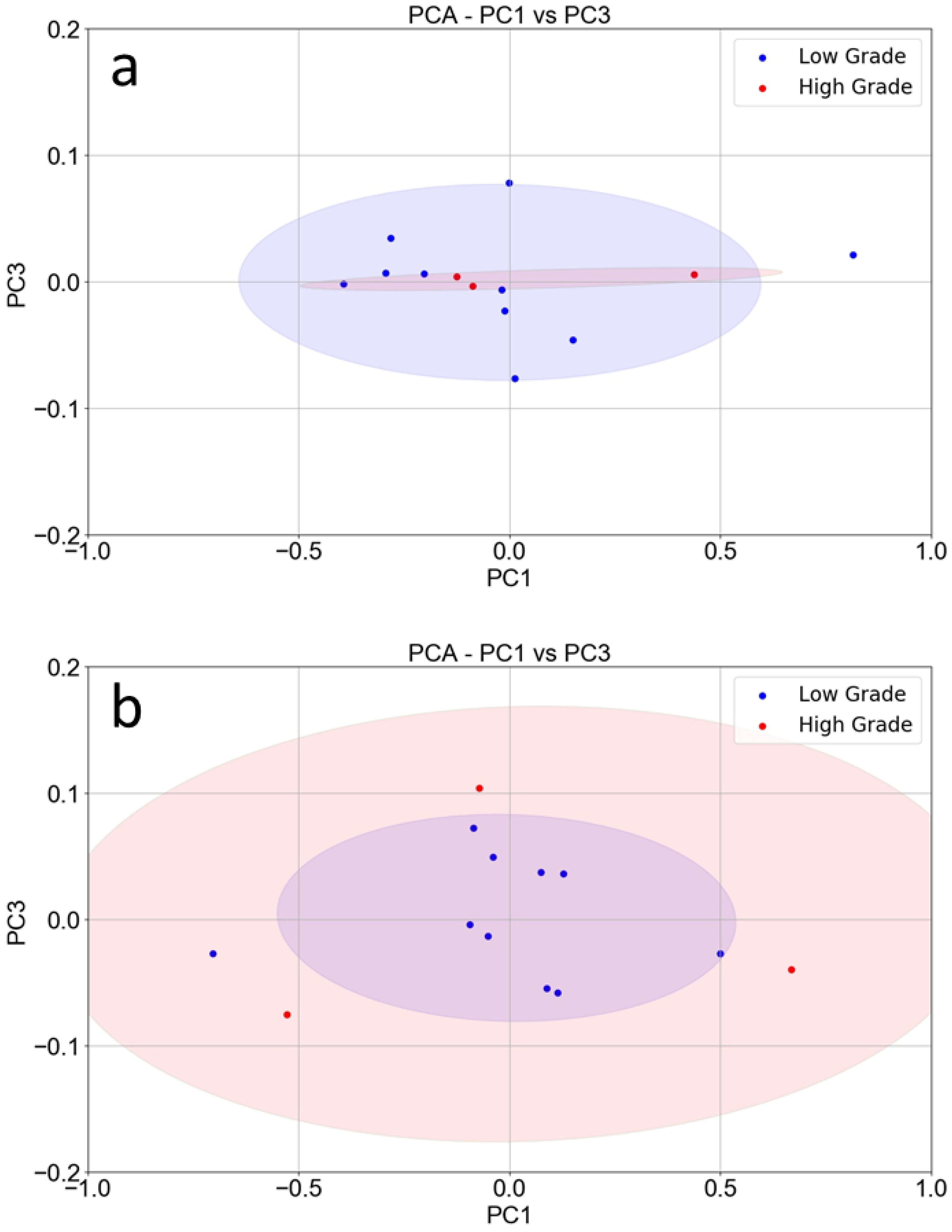
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 6, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Probert, C.S.; Khalid, T.; Ahmed, I.; Johnson, E.; Smith, S.; Ratcliffe, N.M. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J. Gastrointest. Liver Dis. 2009, 18, 337–343. [Google Scholar]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; De Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zonta, G.; Anania, G.; Feo, C.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Palmonari, C.; Ricci, L.; et al. Use of gas sensors and FOBT for the early detection of colorectal cancer. Sens. Actuators B 2018, 262, 884–891. [Google Scholar] [CrossRef]
- Ponzoni, A.; Comini, E.; Concina, I.; Ferroni, M.; Falasconi, M.; Gobbi, E.; Sberveglieri, V.; Sberveglieri, G. Nanostructured Metal Oxide Gas Sensors, a Survey of Applications. Sensors 2012, 12, 17023–17045. [Google Scholar] [CrossRef] [PubMed]
- Malagù, C.; Benetti, M.; Carotta, M.C.; Giberti, A.; Guidi, V.; Milano, L.; Martinelli, G. Investigation of the humidity effects on SNO2-based sensors in CO detection. Mater. Res. Soc. Symp. Proc. 2006, 915, 0915-R07-05. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Landini, N.; Anania, G.; Fabbri, B.; Gaiardo, A.; Gherardi, S.; Guidi, V.; Rispoli, G.; Scagliarini, L.; Zonta, G.; Malagù, C. Neoplasms and metastasis detection in human blood exhalations with a device composed by nanostructured sensors. Sens. Actuators B 2018, 271, 203–214. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Lian, A.; Sun, B.; Wang, X.; Guo, L.; Chi, C.; Liu, S.; Zhao, W.; Luo, S.; et al. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol. Ther. 2014, 15, 200–2016. [Google Scholar] [CrossRef] [PubMed]
- Malagù, C.; Gherardi, S.; Zonta, G.; Landini, N.; Giberti, A.; Giberti, A.; Fabbri, B.; Anania, G.; Rispoli, G.; Scagliarini, L. SCENT B1. Italian Patent No. 102015000057717, 2 October 2015. [Google Scholar]
- Giberti, A.; Benetti, M.; Carotta, M.C.; Guidi, V.; Malagu, C.; Martinelli, G. Heat exchange and temperature calculation in thick-film semiconductor gas sensor systems. Sens. Actuators B 2008, 130, 277–280. [Google Scholar] [CrossRef]
- National Cancer Institute. Tumor Grade, 2013. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/prognosis/tumor-grade-fact-sheet (accessed on 25 November 2019).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astolfi, M.; Rispoli, G.; Anania, G.; Nevoso, V.; Artioli, E.; Landini, N.; Benedusi, M.; Melloni, E.; Secchiero, P.; Tisato, V.; et al. Colorectal Cancer Study with Nanostructured Sensors: Tumor Marker Screening of Patient Biopsies. Nanomaterials 2020, 10, 606. https://doi.org/10.3390/nano10040606
Astolfi M, Rispoli G, Anania G, Nevoso V, Artioli E, Landini N, Benedusi M, Melloni E, Secchiero P, Tisato V, et al. Colorectal Cancer Study with Nanostructured Sensors: Tumor Marker Screening of Patient Biopsies. Nanomaterials. 2020; 10(4):606. https://doi.org/10.3390/nano10040606
Chicago/Turabian StyleAstolfi, Michele, Giorgio Rispoli, Gabriele Anania, Veronica Nevoso, Elena Artioli, Nicolò Landini, Mascia Benedusi, Elisabetta Melloni, Paola Secchiero, Veronica Tisato, and et al. 2020. "Colorectal Cancer Study with Nanostructured Sensors: Tumor Marker Screening of Patient Biopsies" Nanomaterials 10, no. 4: 606. https://doi.org/10.3390/nano10040606
APA StyleAstolfi, M., Rispoli, G., Anania, G., Nevoso, V., Artioli, E., Landini, N., Benedusi, M., Melloni, E., Secchiero, P., Tisato, V., Zonta, G., & Malagù, C. (2020). Colorectal Cancer Study with Nanostructured Sensors: Tumor Marker Screening of Patient Biopsies. Nanomaterials, 10(4), 606. https://doi.org/10.3390/nano10040606








