Ordered Mesoporous Carbon with Chitosan for Disinfection of Water via Capacitive Deionization
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of CMK-3-chitosan
2.3. Characterization
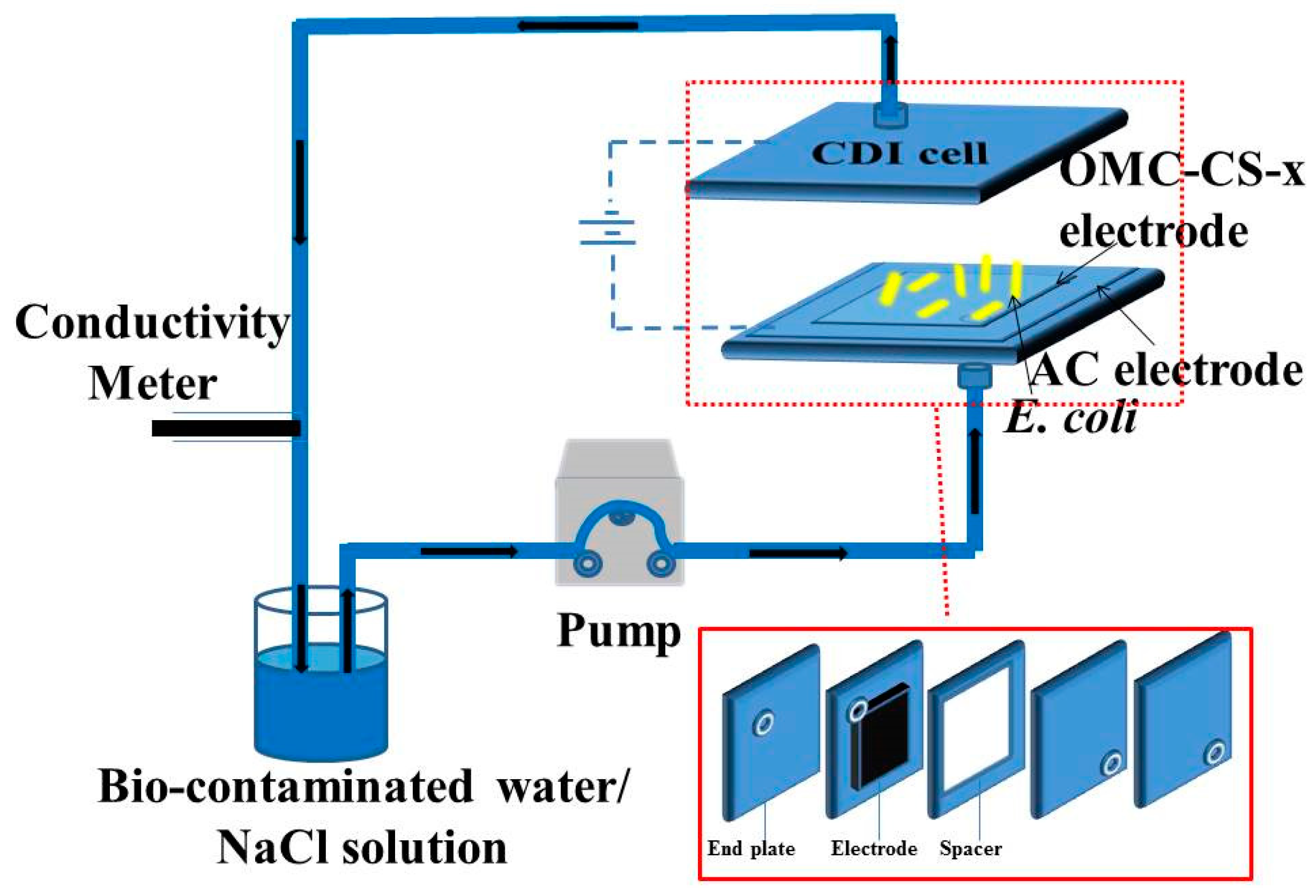
2.4. CDI Electrode Fabrication
2.5. CDI Experiments
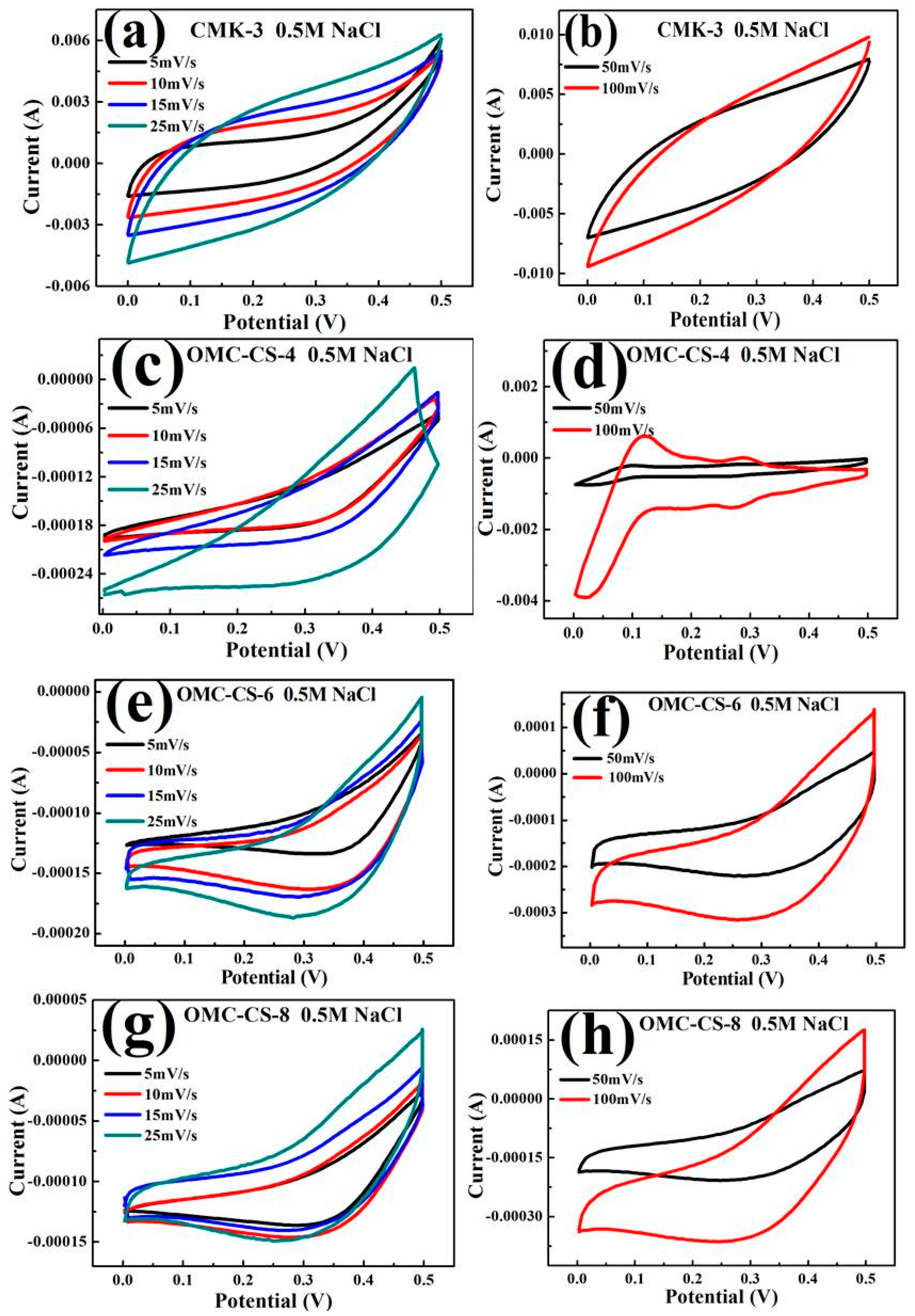
2.6. Electrochemical Measurements
2.7. Preparation of Microbial Cells
2.8. Vitro Culture
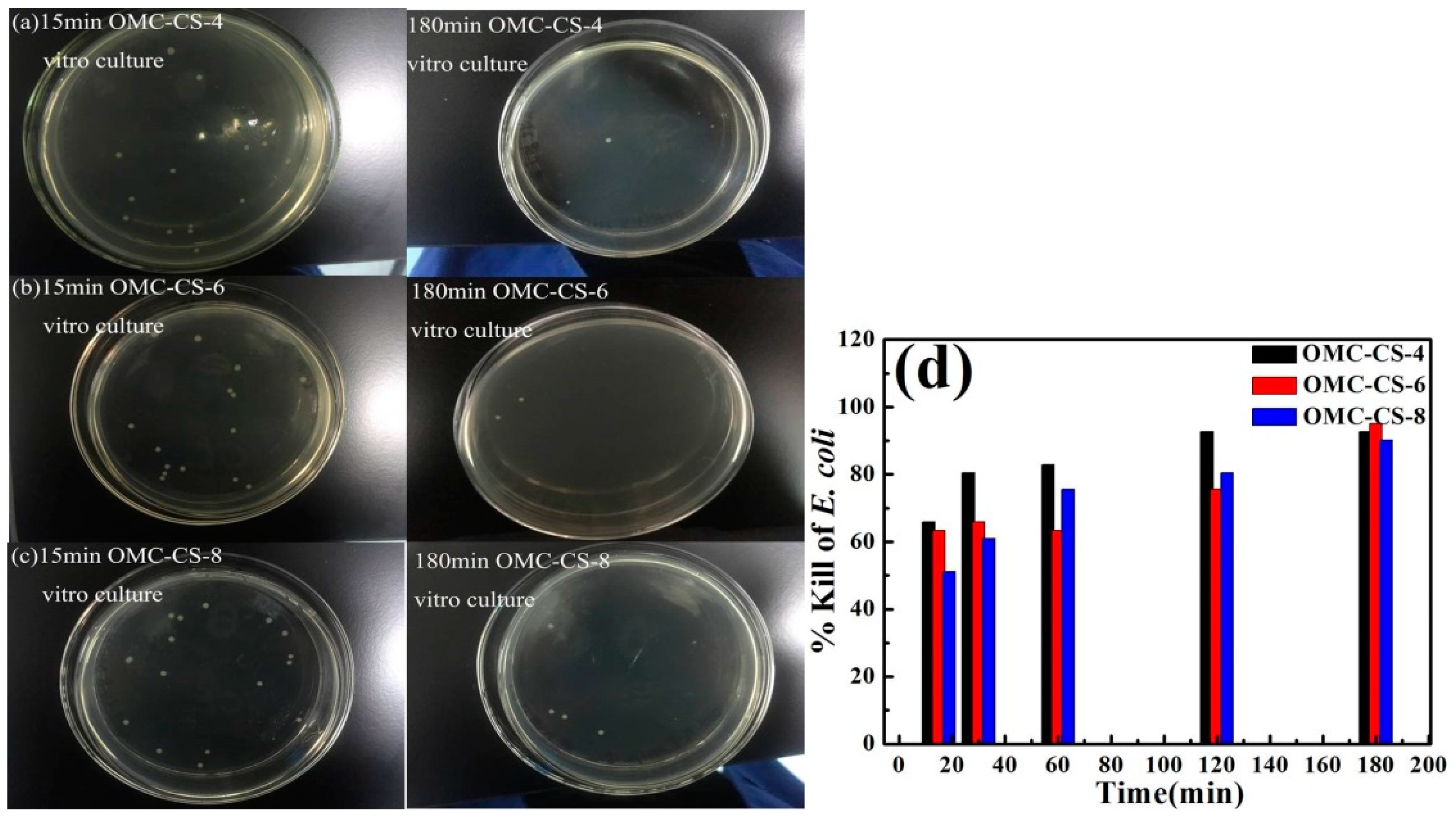
2.9. Percent Killing Calculation of CDI
3. Results and Discussion
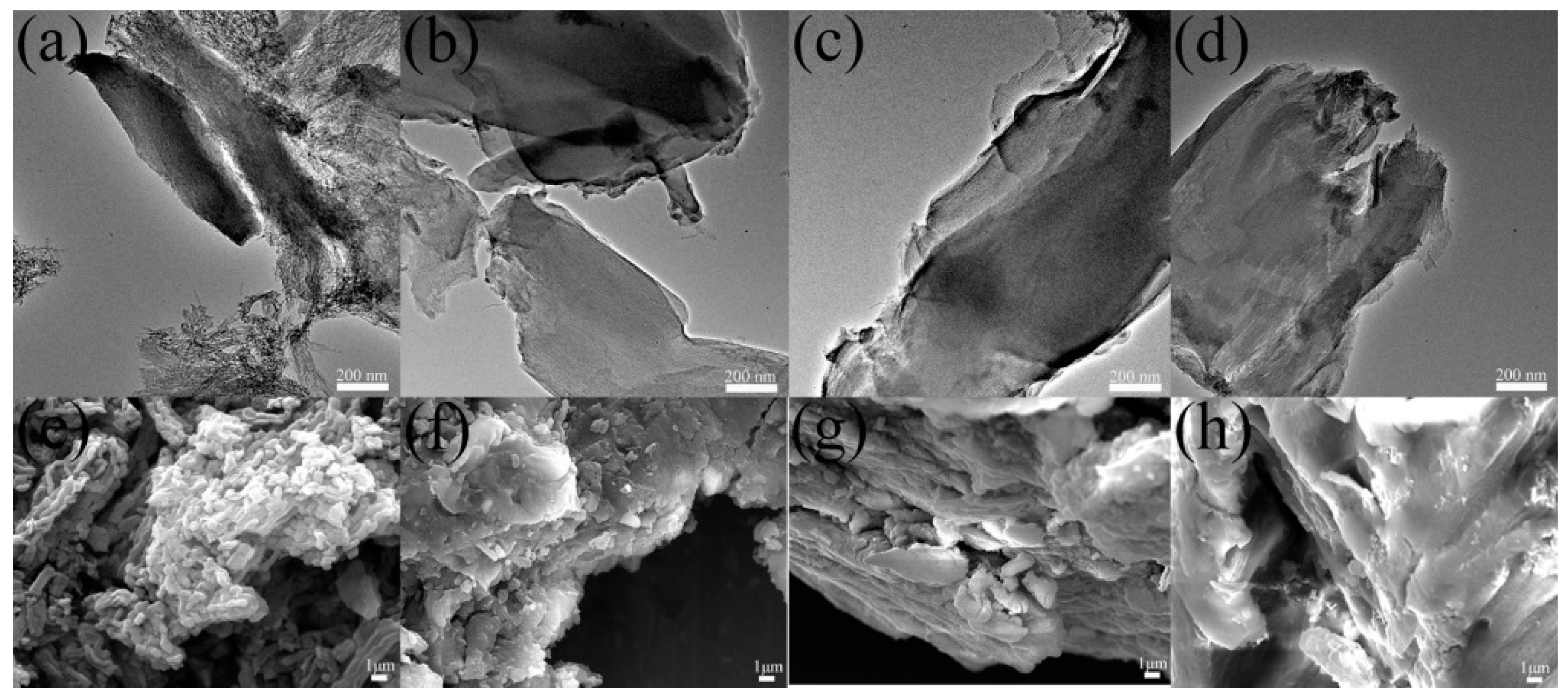
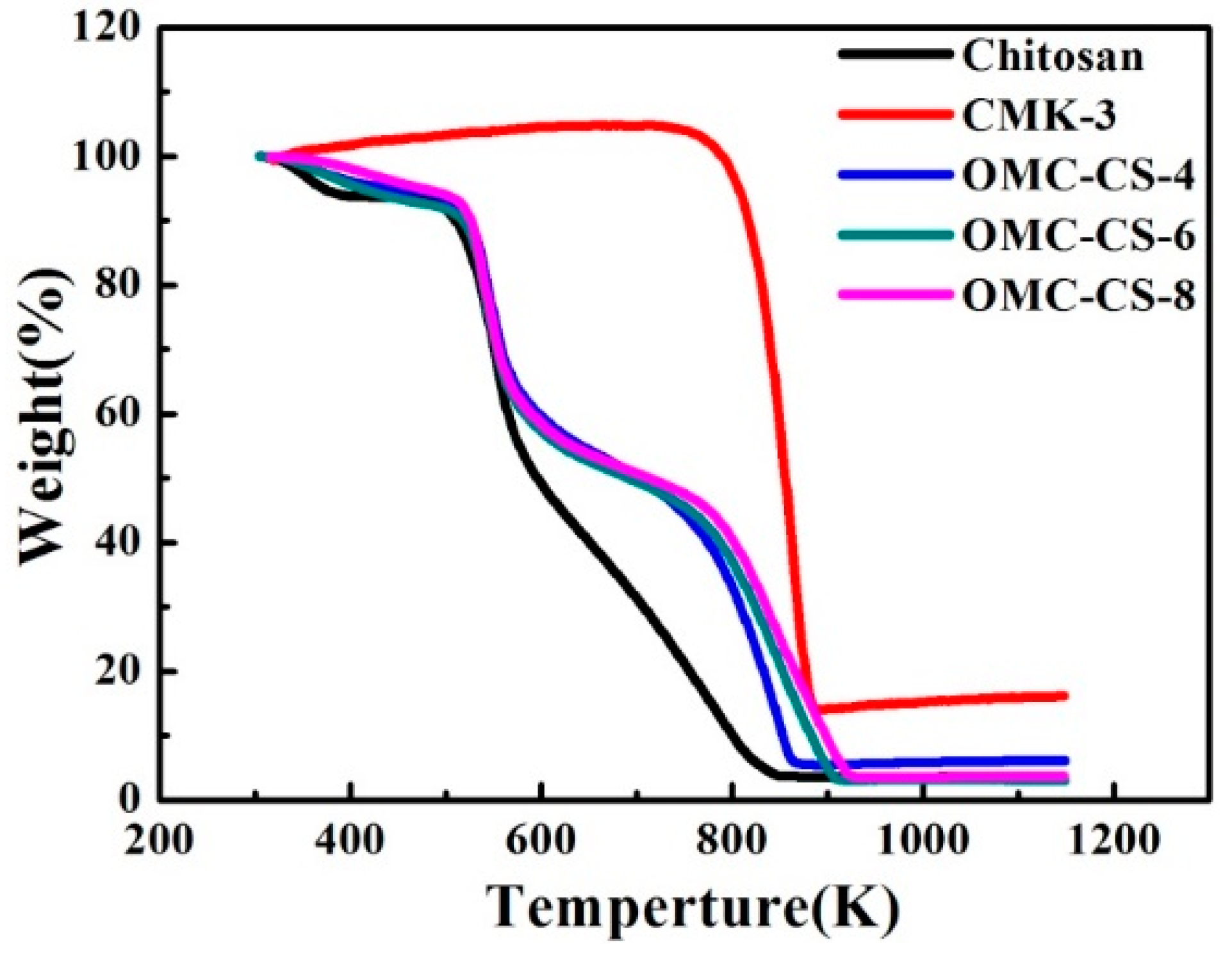
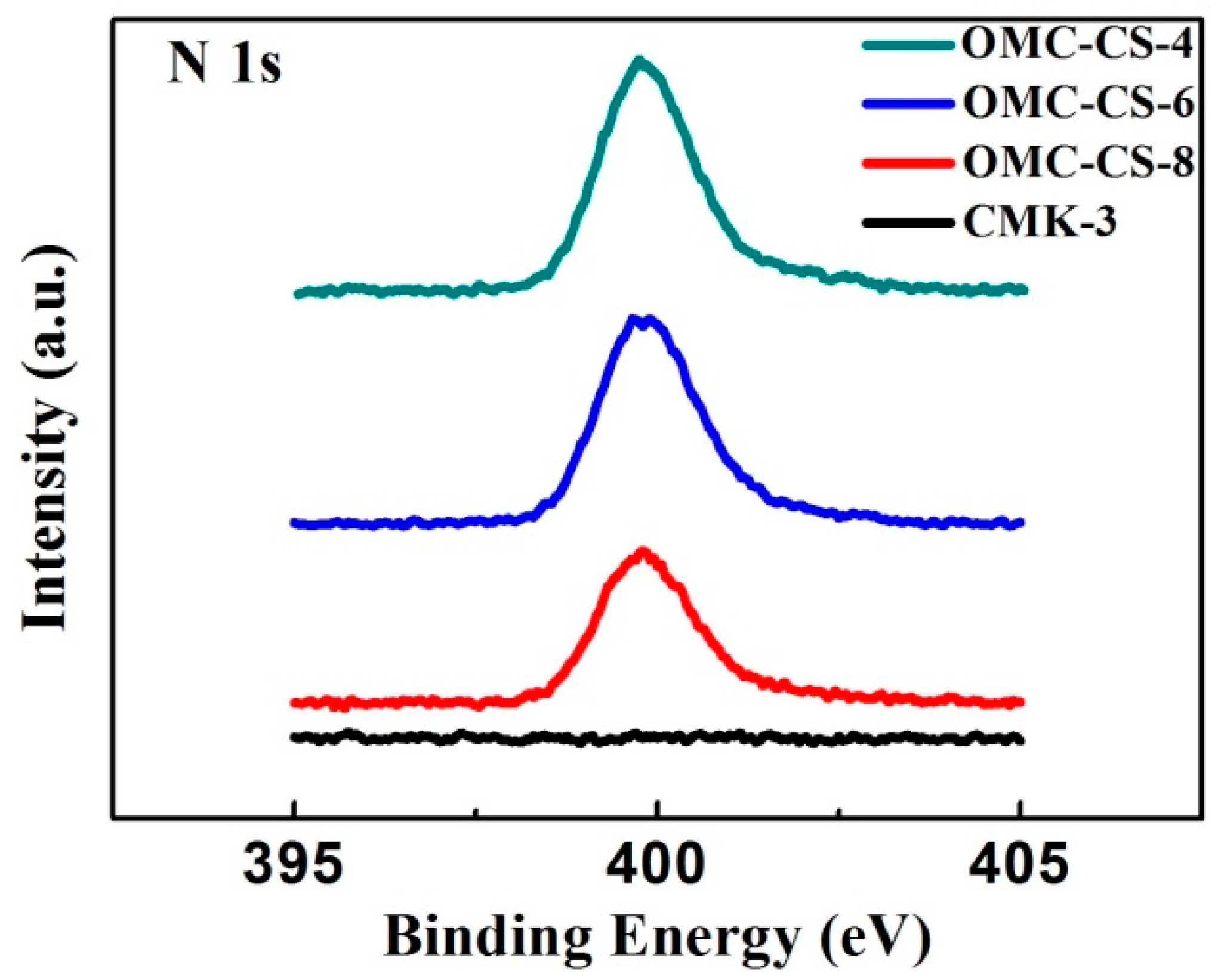
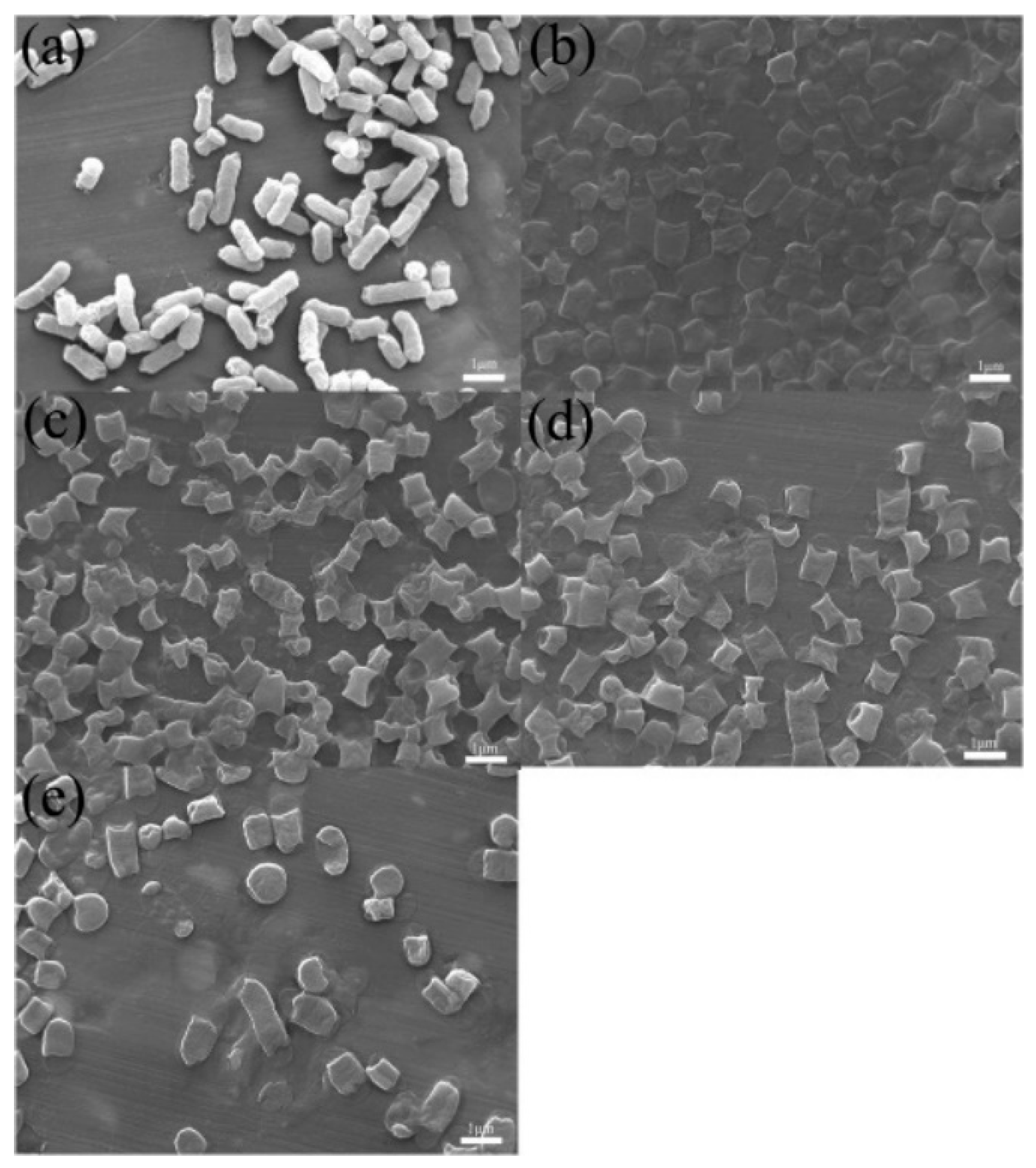
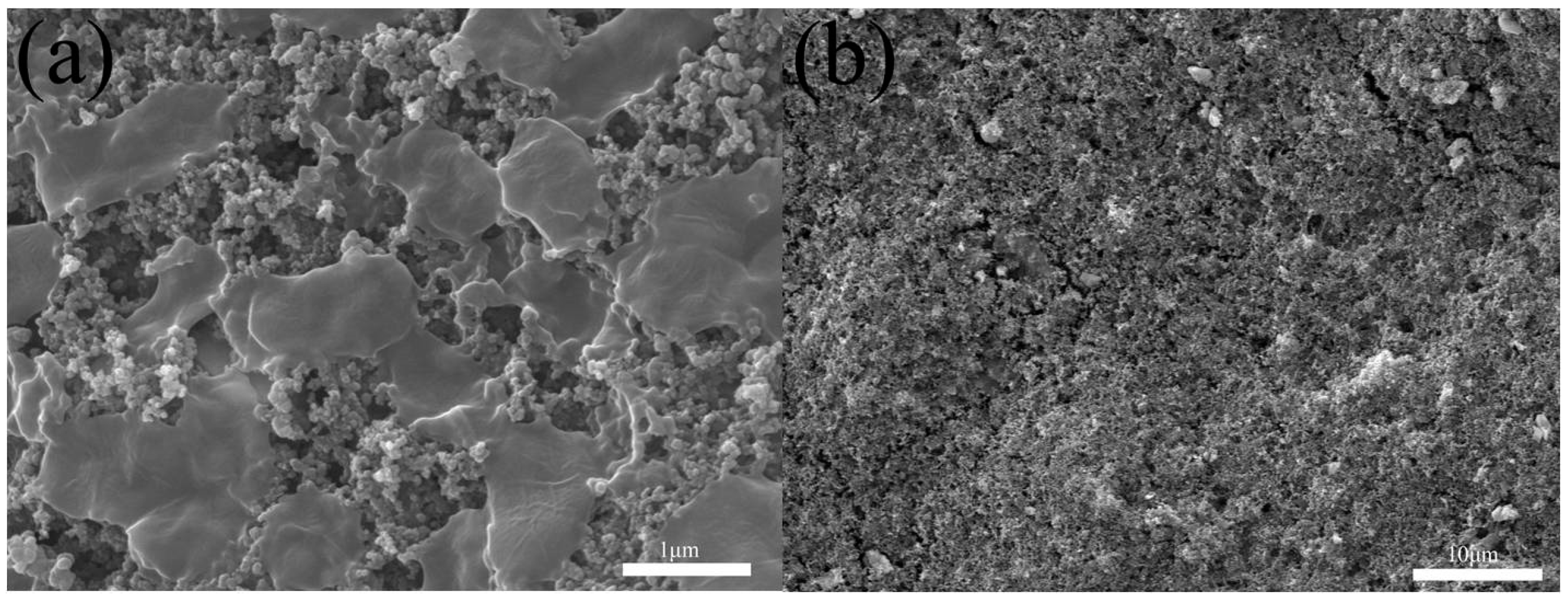
3.1. Characterization of CMK-3
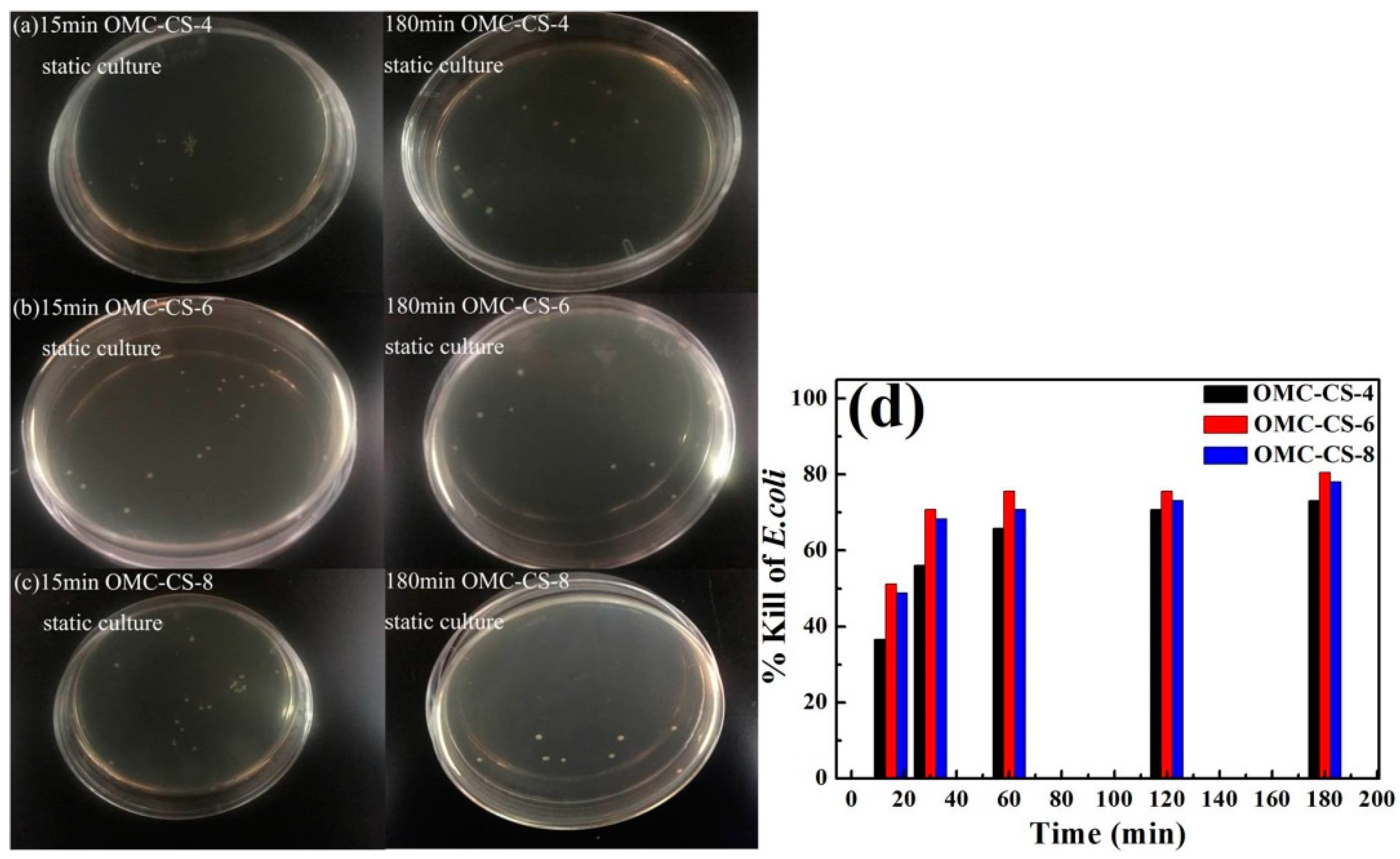
3.2. Antimicrobial Activity of OMC-CS-4,6,8
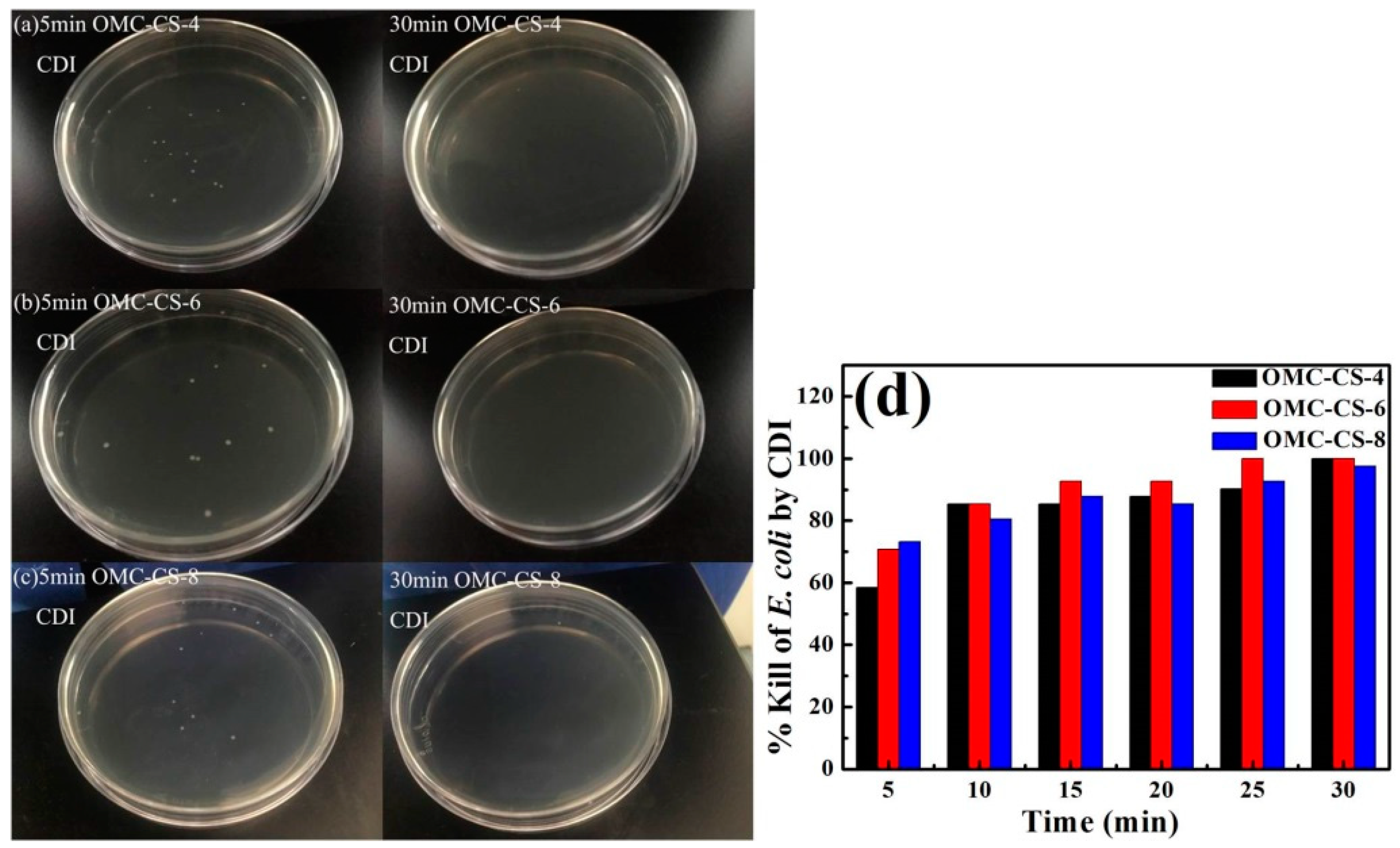
3.3. CDI Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.M.; Dilbaghi, N. Nanotechnology-based water treatment strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef]
- WHO/UNICEF, Progress on Sanitation and Drinking Water. Jt. Monit. Programme Water Supply Sanit. 2010, 4, 1.
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Hamidi, A.; Parham, K.; Atikol, U.; Shahbaz, A.H. A parametric performance analysis of single and multi-effect distillation systems integrated with open-cycle absorption heat transformers. Desalination 2015, 371, 37–45. [Google Scholar] [CrossRef]
- Gilbert, Y.S.; Chan, J.C.; Kurniawan, T.A.; Fu, C.-X.; Jiang, H.; Je, Y. Removal of nonbiodegradable compounds from stabilized leachate using VSEPRO membrane filtration. Desalination 2007, 202, 310–317. [Google Scholar]
- Kong, N.; Huang, X.; Cui, L.; Liu, J. Surface Modified Graphene for Heavy Metal Ions Adsorption. Sci. Adv. Mater. 2013, 5, 1083–1089. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.H.; Chan, G.Y. Degradation of recalcitrant compounds from stabilized landfill leachate using a combination of ozone-GAC adsorption treatment. J. Hazard. Mater. 2006, 137, 443–455. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Sillanpaa, M.E.T.; Sillanpaa, M. Nanoadsorbents for Remediation of Aquatic Environment: Local and Practical Solutions for Global Water Pollution Problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295. [Google Scholar] [CrossRef]
- Niu, H.; Volesky, B. Characteristics of gold biosorption from cyanide solution. J. Chem. Technol. Biotechnol. 1999, 74, 778–784. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Radzishevsky, I.S.; Rotem, S.; Bourdetsky, D.; Navon-Venezia, S.; Carmeli, Y.; Mor, A. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat Biotechnol 2007, 25, 657–659. [Google Scholar] [CrossRef]
- Fox, J.L. Antimicrobial peptides stage a comeback. Nat Biotechnol 2013, 31, 379–382. [Google Scholar] [CrossRef]
- Laxman, K.; Myint, M.T.Z.; Al Abri, M.; Sathe, P.; Dobretsov, S.; Dutta, J. Desalination and disinfection of inland brackish ground water in a capacitive deionization cell using nanoporous activated carbon cloth electrodes. Desalination 2015, 362, 126–132. [Google Scholar] [CrossRef]
- Eckert, R. Road to clinical efficacy: challenges and novel strategies for antimicrobial peptide development. Future Microbiol 2011, 6, 635–651. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.X.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Huang, C.C.; Shen, S.C. Adsorption of CO2 on chitosan modified CMK-3 at ambient temperature. J. Taiwan Inst. Chem. E. 2013, 44, 89–94. [Google Scholar] [CrossRef]
- Alhazmy, M.M. Economic and thermal feasibility of multi stage flash desalination plant with brine-feed mixing and cooling. Energy 2014, 76, 1029–1035. [Google Scholar] [CrossRef]
- Japas, M.S.; Rubinstein, N.A.; Gómez, A.L.R. Reverse Osmosis contributing to metal zoning in porphyry type deposits: A case study. Ore Geol. Rev. 2015, 71, 191–202. [Google Scholar] [CrossRef]
- Zhu, X.P.; He, W.H.; Logan, B.E. Influence of solution concentration and salt types on the performance of reverse electrodialysis cells. J. Membr. Sci. 2015, 494, 154–160. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; Wal, A.V. Energy consumption and constant current operation in membrane capacitive deionization. Energ. Environ. Sci. 2012, 5, 9520–9527. [Google Scholar] [CrossRef]
- Farmer, J.C. Method and apparatus for capacitive deionization and electrochemical purification and regeneration of electrodes. U.S. Patent US5954937A, 1995. [Google Scholar]
- Oren, Y. Capacitive Deionization (CDI) for Desalination and Water Treatment-Past, Present and Future. Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Jeon, S.I.; Park, H.R.; Yeo, J.G.; Yang, S.C.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energ. Environ. Sci. 2013, 6, 1471. [Google Scholar] [CrossRef]
- Ryoo, M.W.; Kim, J.H.; Seo, G. Role of titania incorporated on activated carbon cloth for capacitive deionization of NaCl solution. J. Colloid Interf. Sci. 2003, 264, 414–419. [Google Scholar] [CrossRef]
- Yang, K.L.; Ying, T.Y.; Yiacoumi, S.; Tsouris, C.; Vittoratos, E.S. Electrosorption of Ions from Aqueous Solutions by Carbon Aerogel An Electrical Double-Layer Model. Langmuir 2001, 17, 1961–1969. [Google Scholar] [CrossRef]
- Zou, L.; Morris, G.; Qi, D.D. Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination 2008, 225, 329–340. [Google Scholar] [CrossRef]
- Zhang, D.S.; Shi, L.Y.; Fang, J.H.; Dai, K. Influence of diameter of carbon nanotubes mounted in flow-through capacitors on removal of NaCl from salt water. J. Mater. Sci. 2007, 42, 2471–2475. [Google Scholar] [CrossRef]
- Yang, K.L.; Yiacoumi, S.; Tsouris, C. Electrosorption capacitance of nanostructured carbon aerogel obtained by cyclic voltammetry. J. Electroanal. Chem. 2003, 540, 159–167. [Google Scholar] [CrossRef]
- Mayes, R.T.; Tsouris, C.; Kiggans, J.O., Jr.; Mahurin, S.M.; DePaoli, D.W.; Dai, S. Hierarchical ordered mesoporous carbon from phloroglucinol-glyoxal and its application in capacitive deionization of brackish water. J. Mater. Chem. 2010, 20, 8674–8678. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.S.; Yan, T.T.; Wen, X.R.; Shi, L.Y.; Zhang, J.P. Graphene prepared via a novel pyridine–thermal strategy for capacitive deionization. J. Mater. Chem. 2012, 22, 23745–23748. [Google Scholar] [CrossRef]
- Yin, H.J.; Zhao, S.L.; Wan, J.W.; Tang, H.J.; Chang, L.; He, L.C.; Zhao, H.J.; Gao, Y.; Tang, Z.Y. Three-dimensional graphene/metal oxide nanoparticle hybrids for high-performance capacitive deionization of saline water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Zaviska, F.; Liang, S.; Li, J.; He, L.J.; Yang, H.Y. A high charge efficiency electrode by self-assembling sulphonated reduced graphene oxide onto carbon fibre: towards enhanced capacitive deionization. J. Mater. Chem. A 2014, 2, 3484–3491. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of Highly Ordered Carbon Molecular Sieves via Template-Mediated Structural Transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.Y.; Fierke, M.A. Functionalization of Porous Carbon Materials with Designed Pore Architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Pikus, S.; Jaroniec, M. Short-time synthesis of SBA-15 using various silica sources. J. Colloid Interface Sci. 2005, 287, 717–720. [Google Scholar] [CrossRef]
- Xia, K.S.; Gao, Q.M.; Wu, C.D.; Song, S.Q.; Ruan, M.L. Activation, characterization and hydrogen storage properties of the mesoporous carbon CMK-3. Carbon 2007, 45, 1989–1996. [Google Scholar] [CrossRef]
- Ge, H.; Wan, P.; Luo, D. Graft copolymerization of chitosan with acrylic acid under microwave irradiation and its water absorbency. Carbohydr. Polym. 2006, 66, 372–378. [Google Scholar]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. Acs Appl. Mater. Inter. 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Li, P.; Zhou, C.; Rayatpisheh, S.; Ye, K.; Poon, Y.F.; Hammond, P.T.; Duan, H.; Chan-Park, M.B. Cationic peptidopolysaccharides show excellent broad-spectrum antimicrobial activities and high selectivity. Adv. Mater. 2012, 24, 4130–4137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; El-Deen, A.G.; Li, P.; Oh, B.H.; Guo, Z.; Khin, M.M.; Vikhe, Y.S.; Wang, J.; Hu, R.G.; Boom, R.M.; et al. High-Performance Capacitive Deionization Disinfection of Water with Graphene Oxide-graft-Quaternized Chitosan Nanohybrid Electrode Coating. ACS Nano 2015, 9, 10142–10157. [Google Scholar] [CrossRef] [PubMed]
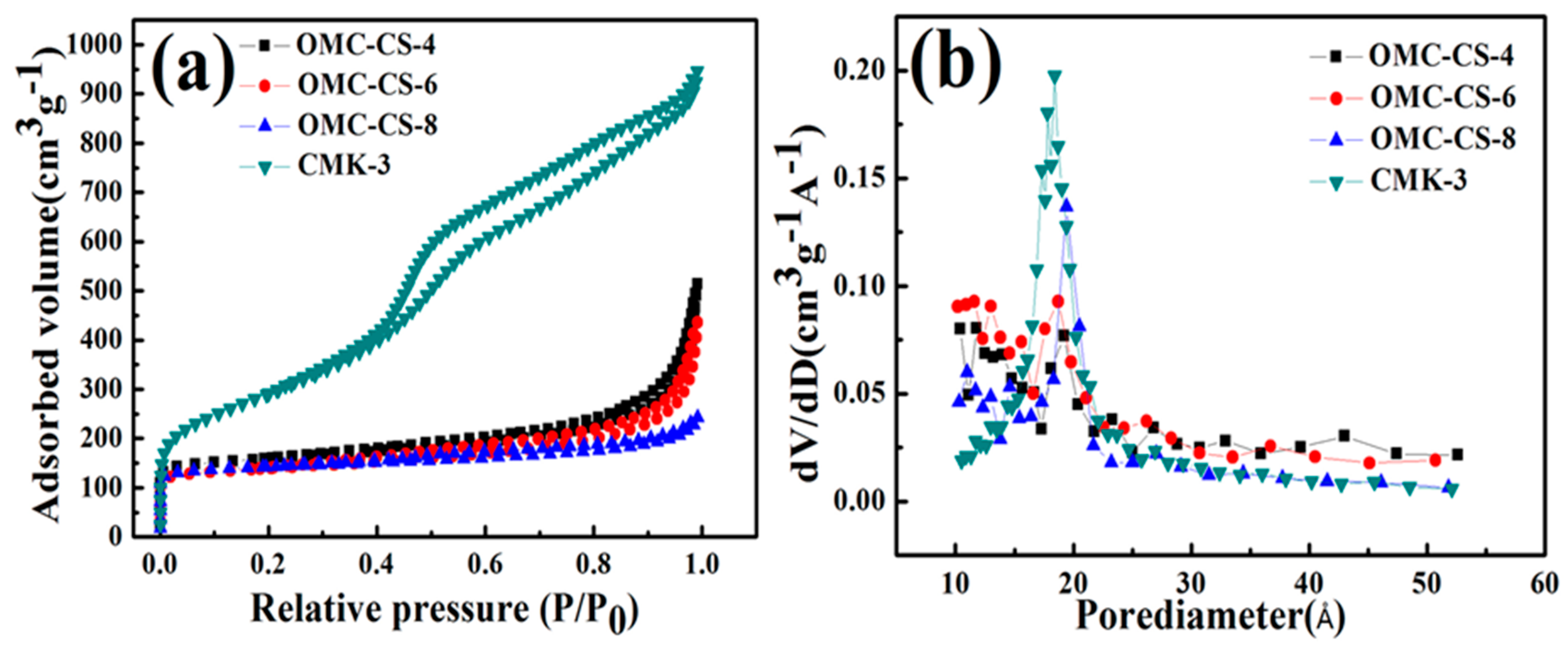

| Sample | SBET (m2/g) | Vtot (cm3/g) | Dpore (Å) |
|---|---|---|---|
| CMK-3 | 1291.35 | 1.49 | 23.2 |
| OMC-CS-4 | 193.87 | 0.59 | 61.9 |
| OMC-CS-6 | 193.39 | 0.50 | 51.8 |
| OMC-CS-8 | 119.09 | 0.19 | 31.2 |
| Sample | Atomic Concentration (%) | ||
|---|---|---|---|
| C | O | N | |
| CMK-3 | 94.91 | 5.09 | 0.00 |
| OMC-CS-4 | 65.62 | 28.70 | 5.68 |
| OMC-CS-6 | 59.88 | 33.08 | 7.04 |
| OMC-CS-8 | 58.68 | 34.08 | 7.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, C.; Wu, X.; Zheng, Y.; Zhang, D.; Chen, J.; Chen, Y. Ordered Mesoporous Carbon with Chitosan for Disinfection of Water via Capacitive Deionization. Nanomaterials 2020, 10, 489. https://doi.org/10.3390/nano10030489
Cao C, Wu X, Zheng Y, Zhang D, Chen J, Chen Y. Ordered Mesoporous Carbon with Chitosan for Disinfection of Water via Capacitive Deionization. Nanomaterials. 2020; 10(3):489. https://doi.org/10.3390/nano10030489
Chicago/Turabian StyleCao, Cuihui, Xiaofeng Wu, Yuming Zheng, Donghai Zhang, Jianhua Chen, and Yunfa Chen. 2020. "Ordered Mesoporous Carbon with Chitosan for Disinfection of Water via Capacitive Deionization" Nanomaterials 10, no. 3: 489. https://doi.org/10.3390/nano10030489
APA StyleCao, C., Wu, X., Zheng, Y., Zhang, D., Chen, J., & Chen, Y. (2020). Ordered Mesoporous Carbon with Chitosan for Disinfection of Water via Capacitive Deionization. Nanomaterials, 10(3), 489. https://doi.org/10.3390/nano10030489






