Controllable Synthesis of Mn3O4 Nanowires and Application in the Treatment of Phenol at Room Temperature
Abstract
:1. Introduction
2. Experimental
2.1. Preparation and Characterization of Mn3O4
2.2. Treatment of Phenol Waste Water at Room Temperature
3. Results and Discussion
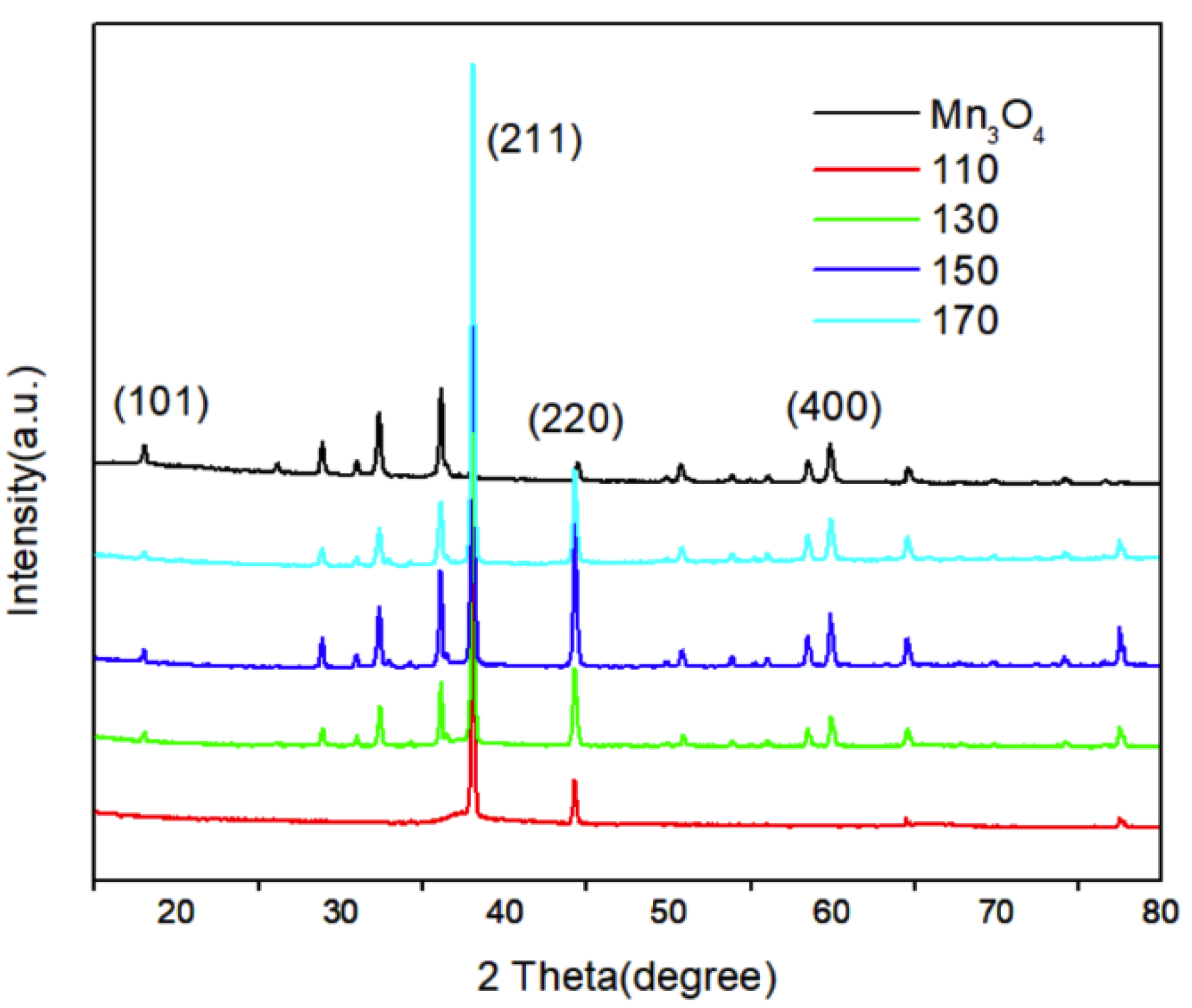
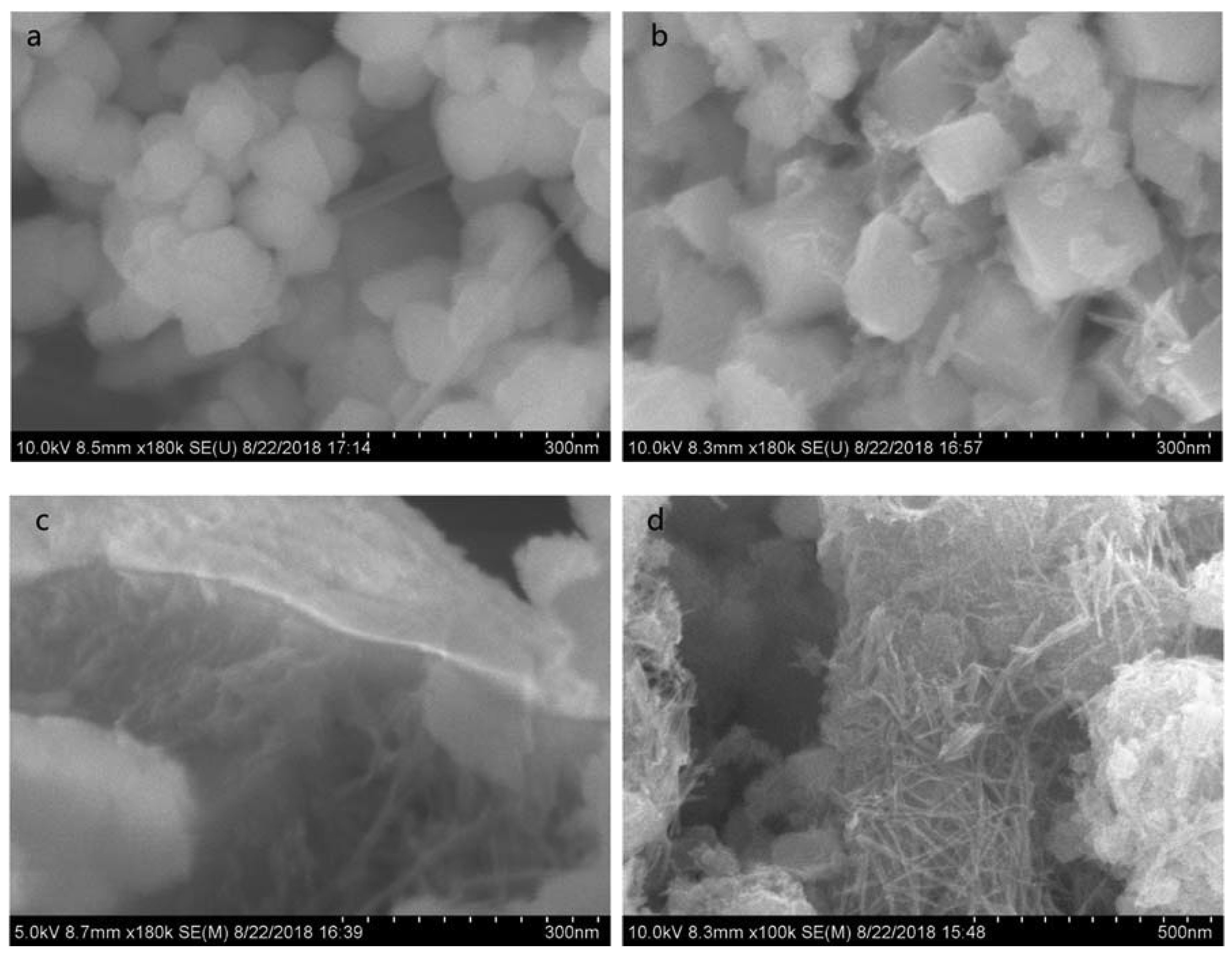
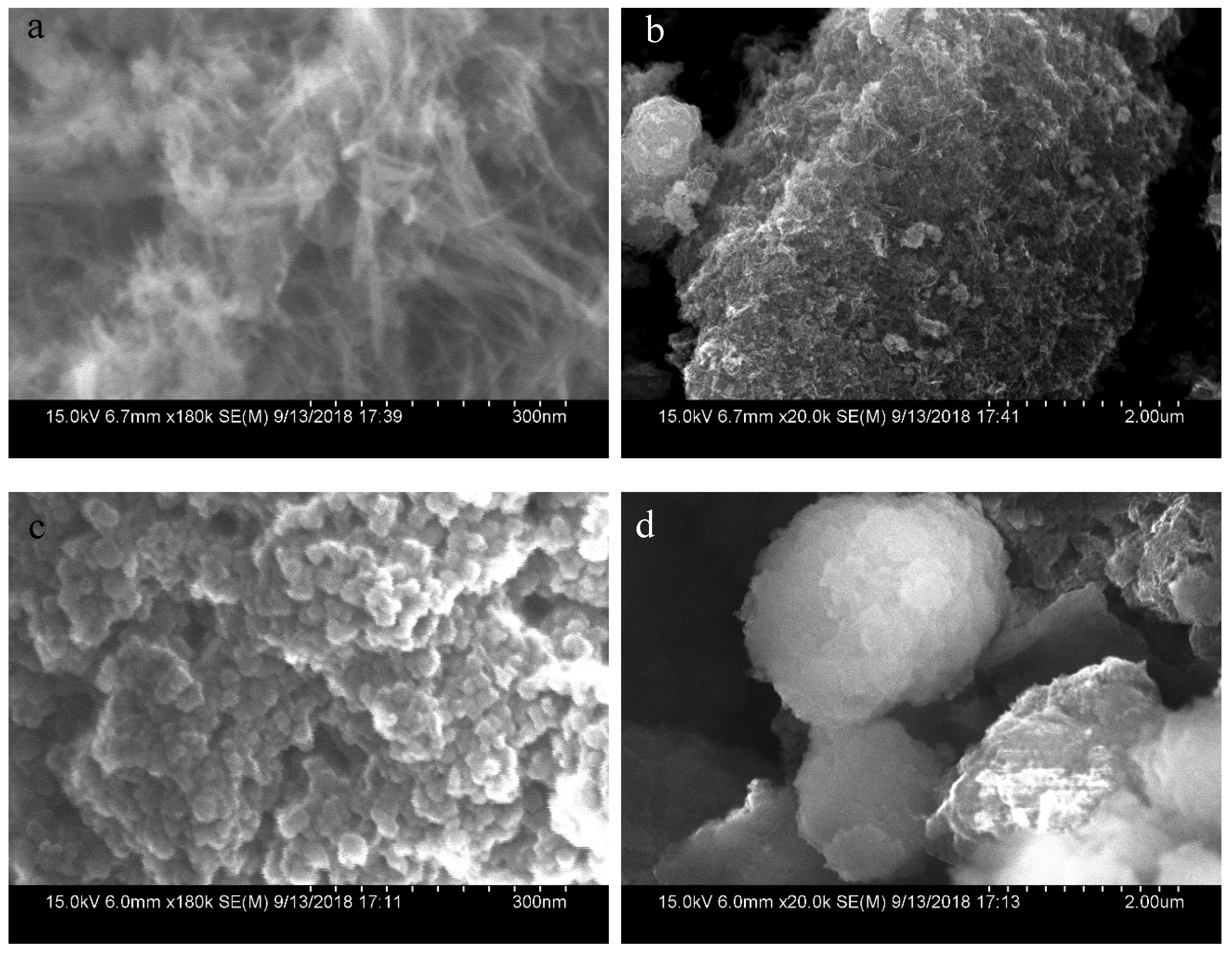
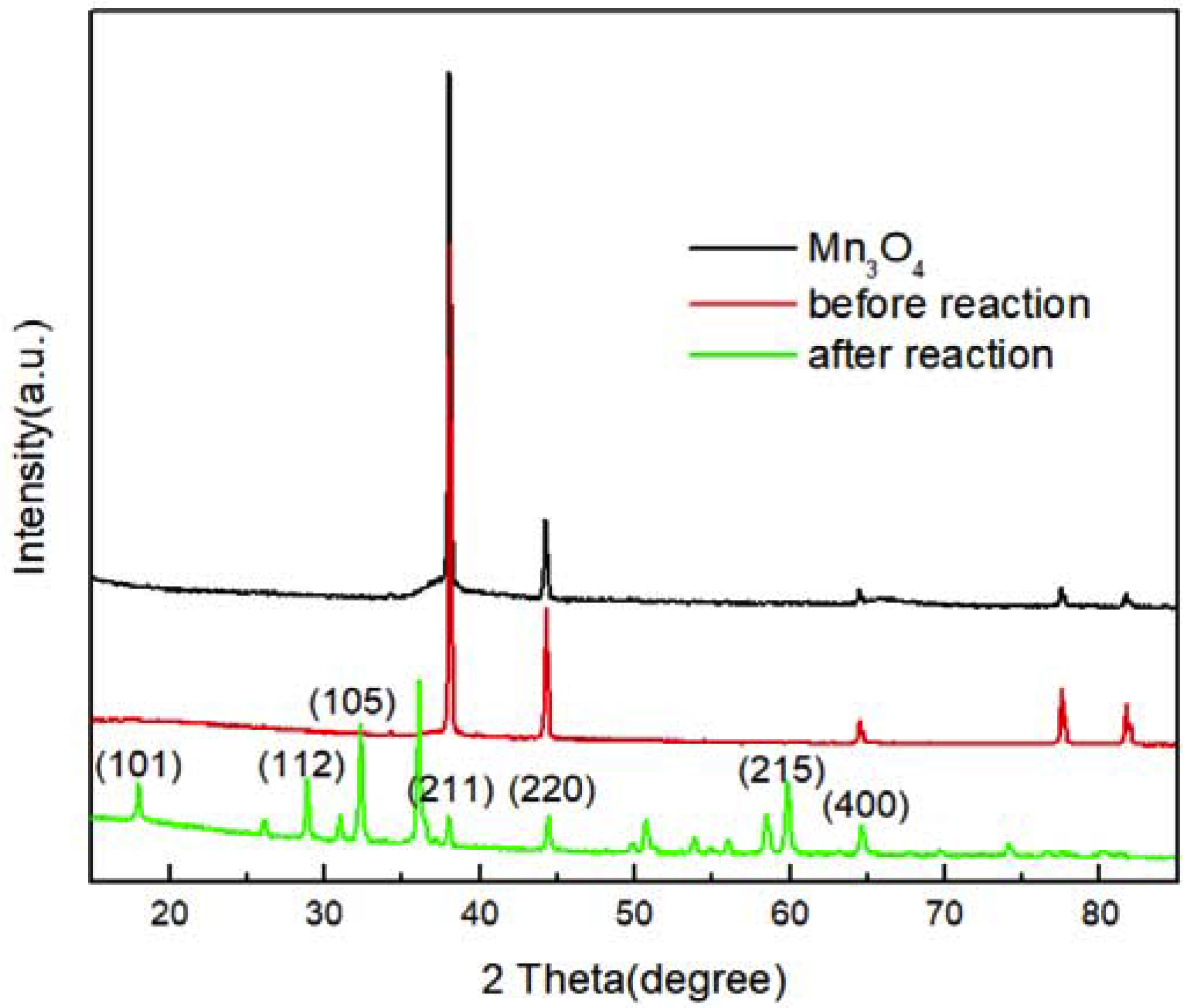
3.1. Composition and Morphologies of the Prepared Mn3O4
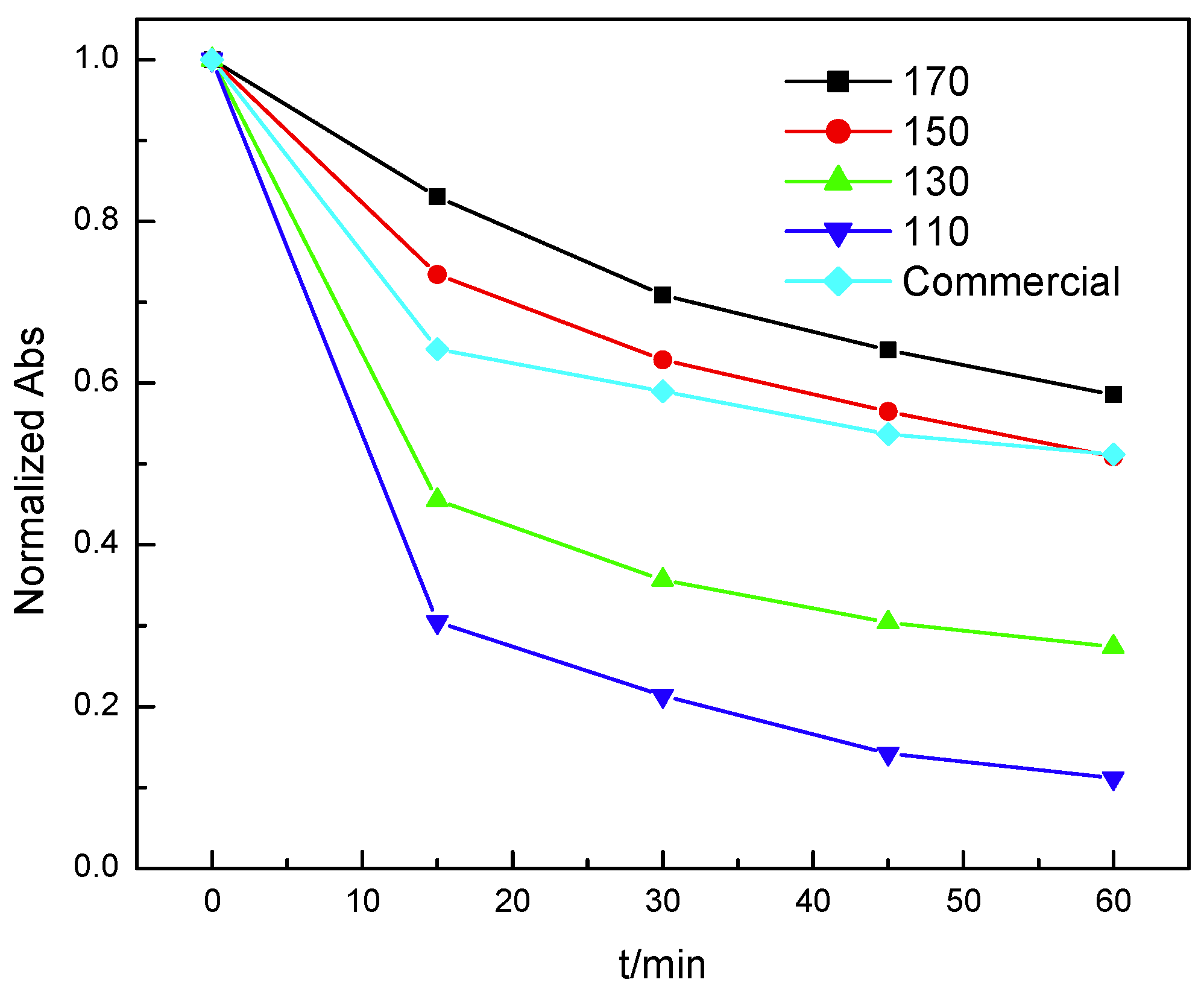
3.2. Treatment of Phenol with Mn3O4 at Room Temperature
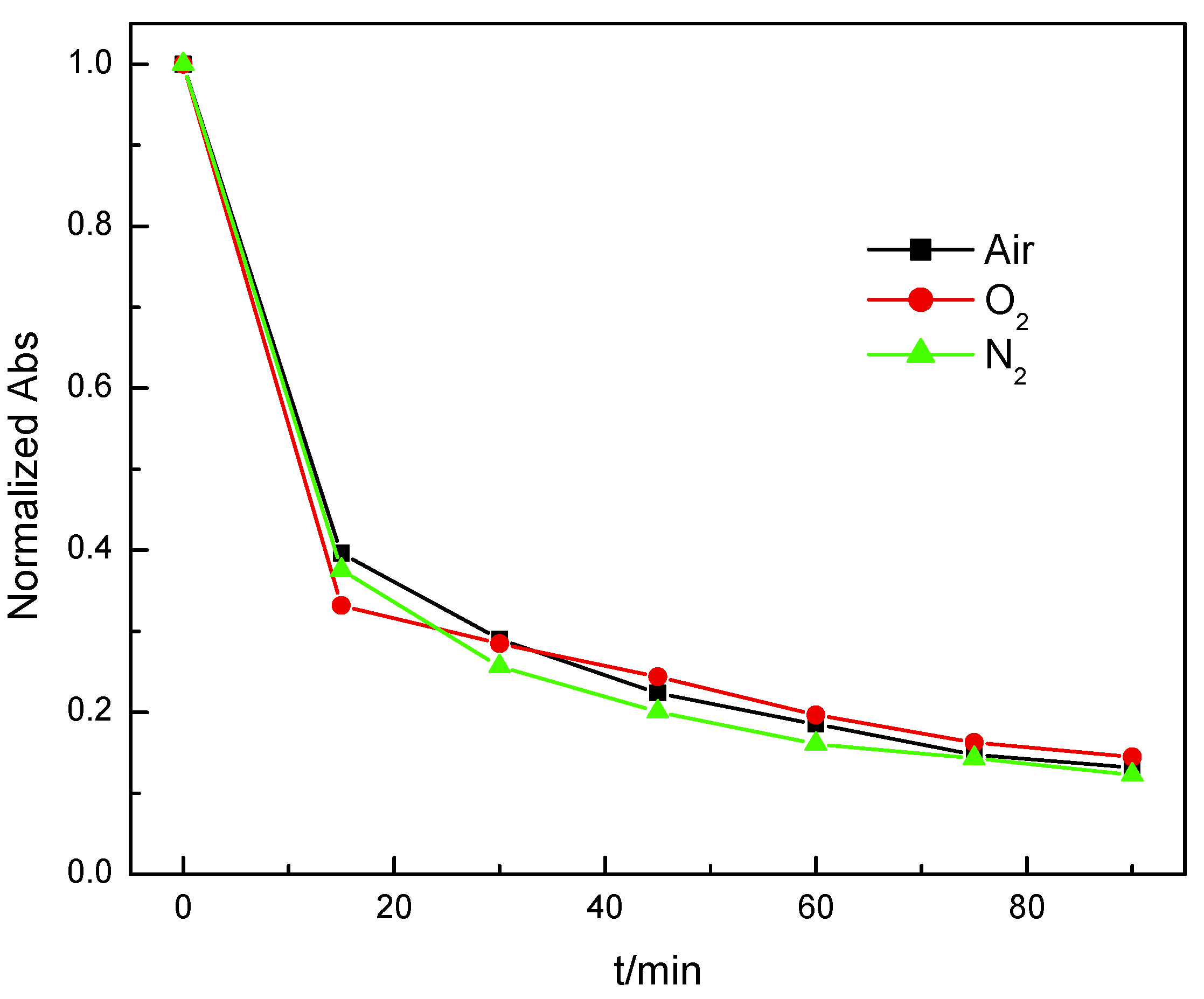
3.3. Analyse of the Function of Mn3O4 in the Treatment of Phenol
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoseinpour, V.; Ghaemi, N. Green synthesis of manganese nanoparticles: Applications and future perspective-A review. J. Photochem. Photobiol. B Biol. 2018, 189, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Asaikkutti, A.; Bhavan, P.S.; Vimala, K.; Karthik, M.; Cheruparambath, P. Dietary supplementation of green synthesized manganese-oxide nanoparticles and its effect on growth performance, muscle composition and digestive enzyme activities of the giant freshwater prawn Macrobrachium rosenbergii. J. Trace Elem. Med. Biol. 2016, 35, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Shaik, D.P.; Pitcheri, R.; Qiu, Y.; Hussain, O.M. Hydrothermally synthesized porous Mn3O4 nanoparticles with enhanced electrochemical performance for supercapacitors. Ceram. Int. 2019, 45, 2226–2233. [Google Scholar] [CrossRef]
- Yang, L.X.; Zhu, Y.J.; Tong, H.; Wang, W.W.; Cheng, G.F. Low temperature synthesis of Mn3O4 polyhedral nanocrystals and magnetic study. J. Solid State Chem. 2006, 179, 1225–1229. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Zhang, X.; Xia, Z.; Wang, H.L.; Sun, J.; Dong, R.T.; Wang, F. Crystallographically-oriented Mn oxide nanorod/nanowire arrays electrodes. J. Alloy. Compd. 2015, 620, 390–398. [Google Scholar] [CrossRef]
- Shin, J.; Richter, G.; Gianola, D.S. Suppressing instabilities in defect-scarce nanowires by controlling the energy release rate during incipient plasticity. Mater. Des. 2020, 189, 108460. [Google Scholar] [CrossRef]
- Veeramani, H.; Aruguete, D.; Monsegue, N.; Murayama, M.; Dippon, U.; Kappler, A.; Hochella, M.F. Low-Temperature Green Synthesis of Multivalent Manganese Oxide Nanowire. ACS Sustain. Chem. Eng. 2013, 19, 1070–1074. [Google Scholar] [CrossRef]
- Sambasivam, S.; Li, G.J.; Jeong, J.H.; Choi, B.C.; Lim, K.T.; Kim, S.S.; Song, T.K. Structural, optical, and magnetic properties of single-crystalline Mn3O4 nanowires. J. Nanopart. Res. 2012, 14, 1138–1146. [Google Scholar] [CrossRef]
- Fang, C.X.; Gao, X.M.; Zhang, X.C.; Zhu, J.H.; Sun, S.P.; Wang, X.N.; Wu, W.D.; Wu, Z.X. Facile synthesis of alkaline-earth metal manganites for the efficient degradation of phenolic compounds via catalytic ozonation and evaluation of the reaction mechanism. J. Colloid Interf. Sci. 2019, 551, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Y.; Wu, C.Y.; Zhou, Y.X.; Zuo, J.E.; Song, G.Q.; Tan, Y. Ozonation reactivity characteristics of dissolved organic matter in secondary petrochemical wastewater by single ozone, ozone/H2O2, and ozone/catalyst. Chemosphere 2019, 233, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Niki, E.; Shiokawa, H.; Kamiya, Y. Ozonation of organic compounds. 2. Ozonation of phenol in water. J. Org. Chem. 1979, 44, 2137–2142. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; Torres-Socías, E.D.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processe. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Y.; Xia, X.; Wang, L.; Dong, W. Fe1–xZnxS ternary solid solution as an efficient Fenton–like catalyst for ultrafast degradation of phenol. J. Hazard. Mater. 2018, 353, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, V.; Teixeira, A.R.; Lucas, M.S.; Silva, A.M.T.; Peres, J.A. Pillared interlayered natural clays as heterogeneous photocatalysts for H2O2-assisted treatment of a winery wastewater. Sep. Purif. Technol. 2019, 228, 115768. [Google Scholar] [CrossRef]
- Li, H.P.; Cheng, R.Q.; Liu, Z.L.; Du, C.F. Waste control by waste: Fenton-like oxidation of phenol over Cu modified ZSM-5 from coal gangue. Sci. Total Environ. 2019, 683, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Han, R.L.; Zhang, Y.H.; Xie, Y.L. Application of Mn3O4 nanowires in the dye waste water treatment at room temperature. Sep. Purif. Technol. 2020, 234, 116119. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Kinetics of wet peroxide oxidation of phenol with a gold/activated carbon catalyst. Chem. Eng. J. 2014, 253, 486–492. [Google Scholar] [CrossRef]
- Bin-Dahman, O.A.; Saleh, T.A. Synthesis of carbon nanotubes grafted with PEG and its efficiency for the removal of phenol from industrial wastewater. Environ. Nanotechnol. Monit. Manag. 2020, 13. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 263–288. [Google Scholar] [CrossRef] [PubMed]




| Mn3O4 (Commercial) | Mn3O4 (130 °C) | Mn3O4 (110 °C) | |
|---|---|---|---|
| BET surface area/(m2·g−1) Adsorption average pore diameter/nm | 14.48 21.5 | 189.69 10.3 | 248.8 10.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, R.; Chen, M.; Liu, X.; Zhang, Y.; Xie, Y.; Sui, Y. Controllable Synthesis of Mn3O4 Nanowires and Application in the Treatment of Phenol at Room Temperature. Nanomaterials 2020, 10, 461. https://doi.org/10.3390/nano10030461
Han R, Chen M, Liu X, Zhang Y, Xie Y, Sui Y. Controllable Synthesis of Mn3O4 Nanowires and Application in the Treatment of Phenol at Room Temperature. Nanomaterials. 2020; 10(3):461. https://doi.org/10.3390/nano10030461
Chicago/Turabian StyleHan, Runlin, Min Chen, Xiaobing Liu, Yuhang Zhang, Yongli Xie, and Yan Sui. 2020. "Controllable Synthesis of Mn3O4 Nanowires and Application in the Treatment of Phenol at Room Temperature" Nanomaterials 10, no. 3: 461. https://doi.org/10.3390/nano10030461
APA StyleHan, R., Chen, M., Liu, X., Zhang, Y., Xie, Y., & Sui, Y. (2020). Controllable Synthesis of Mn3O4 Nanowires and Application in the Treatment of Phenol at Room Temperature. Nanomaterials, 10(3), 461. https://doi.org/10.3390/nano10030461






