Photocatalytic and Oxidative Synthetic Pathways for Highly Efficient PANI-TiO2 Nanocomposites as Organic and Inorganic Pollutant Sorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Materials Characterization
2.3. Study Of The Reaction First Step
2.4. Dye-Removal Tests
2.5. Cr(VI) Remediation Tests
3. Results
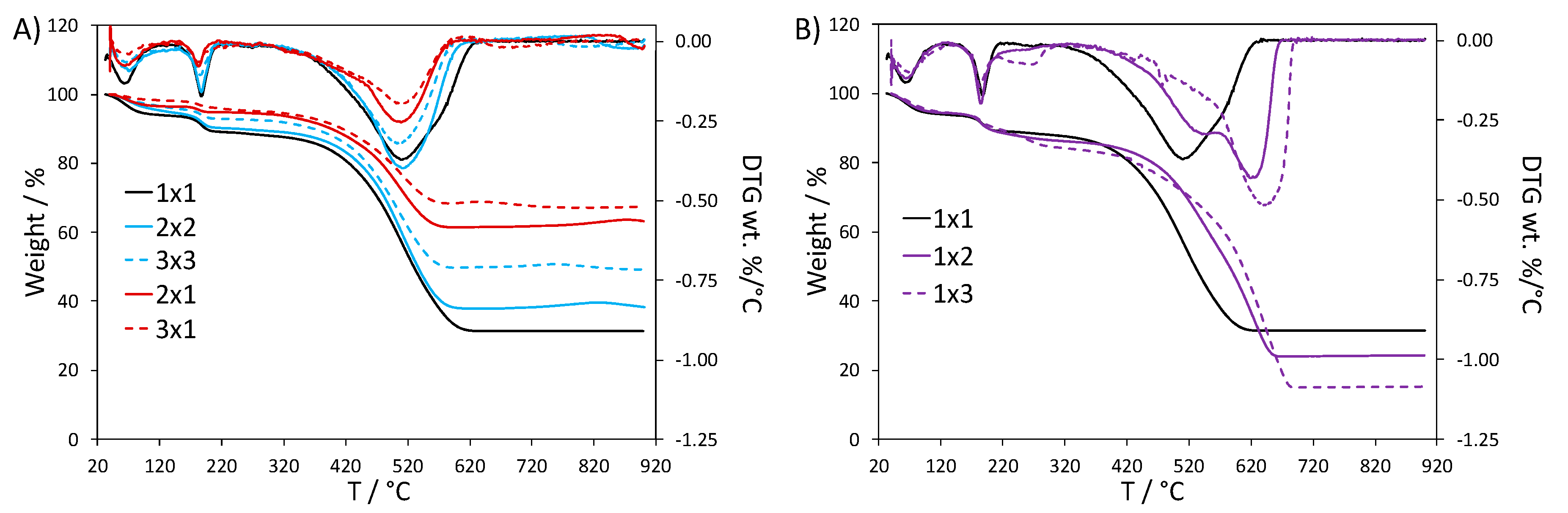
3.1. Thermogravimetric Analyses
3.2. Structural Properties
3.3. Morphological Properties
3.4. FTIR Spectroscopy
3.5. UV-vis Spectroscopy
3.6. Mass Spectra
3.7. Study Of The Oligomerization Step
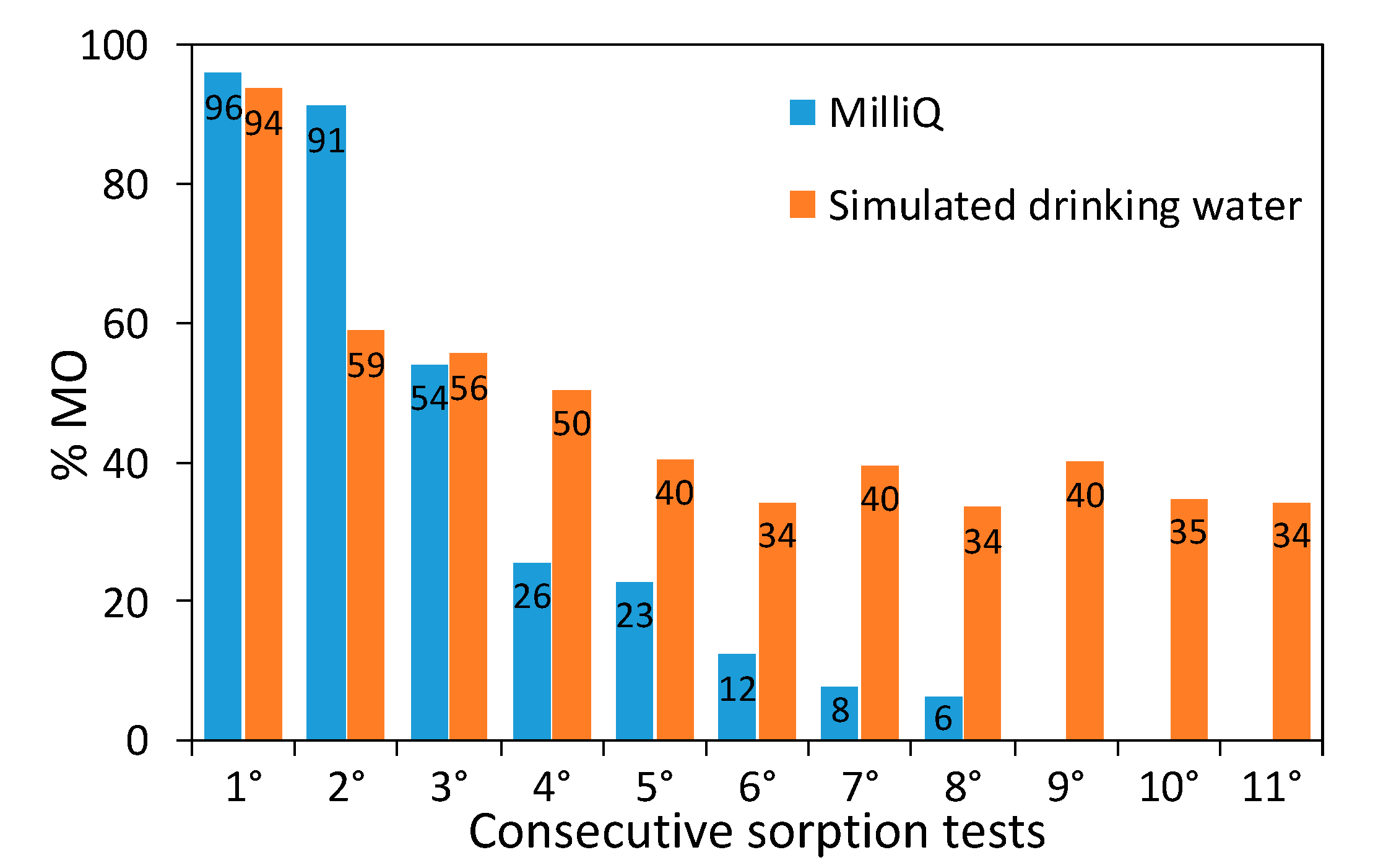
3.8. Dye Removal Tests
3.9. Chromium Remediation Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Ansari, M.O.; Parveen, N.; Oves, M.; Barakat, M.A.; Alshahri, A.; Khan, M.Y.; Cho, M.H. Facile route to a conducting ternary polyaniline@TiO2/GN nanocomposite for environmentally benign applications: Photocatalytic degradation of pollutants and biological activity. RSC Adv. 2016, 6, 111308–111317. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Wang, P.H.; Wang, T.L.; Lin, W.C.; Lin, H.Y.; Lee, M.H.; Yang, C.H. Enhanced supercapacitor performance using electropolymerization of self-doped polyaniline on carbon film. Nanomaterials 2018, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, X.; Ren, L.; Piao, Q.; Zhong, J.; Wang, Y.; Li, H.; Chen, Y.; Wang, B. Flexible Solid-State Supercapacitor Based on a Metal–Organic Framework Interwoven by Electrochemically-Deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Liang, X.; Yasmin, A.; Ge, X.; Xiao, X.; Zhu, Y.; Ge, Z.; Wang, Y.; Wu, L.; Xu, T. Angioplasty mimetic stented ion transport channels construct durable high-performance membranes. J. Mater. Chem. A 2019, 7, 10030–10040. [Google Scholar] [CrossRef]
- Cao, G.; Gao, X.; Wang, L.; Cui, H.; Lu, J.; Meng, Y.; Xue, W.; Cheng, C.; Tian, Y.; Tian, Y. Easily synthesized polyaniline@cellulose nanowhiskers better tune network structures in Ag-based adhesives: Examining the improvements in conductivity, stability, and flexibility. Nanomaterials 2019, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; He, Y.; Plachy, T.; Kazantseva, N.; Saha, P.; Cheng, Q. Hierarchical PANI/NiCo-LDH core-shell composite networks on carbon cloth for high performance asymmetric supercapacitor. Nanomaterials 2019, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Li, T.; Xiong, C.; Tang, C.; Li, H.; Zhao, T.; Dang, A. A facile method to prepare silver doped graphene combined with polyaniline for high performances of filter paper based flexible electrode. Nanomaterials 2019, 9, 1434. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Y.; Li, X.; Jiang, X.; Gao, P.; Sun, K.; Zhou, J.; Zhang, Z.; Jiang, Q. An optical sensor with polyaniline-gold hybrid nanostructures for monitoring pH in saliva. Nanomaterials 2017, 7, 67. [Google Scholar] [CrossRef]
- Michalska, A.; Golczak, S.; Langer, K.; Langer, J.J. Micro-and nanostructured polyaniline for instant identification of metal ions in solution. Nanomaterials 2019, 9, 231. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Lv, W.; Feng, J.; Yan, W. RSC Advances excellent adsorption performance on acid red G. RSC Adv. 2015, 5, 21132–21141. [Google Scholar] [CrossRef]
- He, J.; To, J.W.F.; Psarras, P.C.; Yan, H.; Atkinson, T.; Holmes, R.T.; Nordlund, D.; Bao, Z.; Wilcox, J. Tunable Polyaniline-Based Porous Carbon with Ultrahigh Surface Area for CO2 Capture at Elevated Pressure. Adv. Energy Mater. 2016, 6, 1502491. [Google Scholar] [CrossRef]
- Della Pina, C.; De Gregorio, M.A.; Clerici, L.; Dellavedova, P.; Falletta, E. Polyaniline (PANI): An innovative support for sampling and removal of VOCs in air matrices. J. Hazard. Mater. 2018, 344, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, J.; Wang, M.; Guo, R. Fe3O4/PANI/MnO2 core-shell hybrids as advanced adsorbents for heavy metal ions. J. Mater. Chem. A 2017, 5, 4058–4066. [Google Scholar] [CrossRef]
- Gong, K.; Guo, S.; Zhao, Y.; Hu, Q.; Liu, H.; Sun, D.; Li, M.; Qiu, B.; Guo, Z. Bacteria cell templated porous polyaniline facilitated detoxification and recovery of hexavalent chromium. J. Mater. Chem. A 2018, 6, 16824–16832. [Google Scholar] [CrossRef]
- Shumakovich, G.; Kurova, V.; Vasil’eva, I.; Pankratov, D.; Otrokhov, G.; Morozova, O.; Yaropolov, A. Laccase-mediated synthesis of conducting polyaniline. J. Mol. Catal. B Enzym. 2012, 77, 105–110. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Ou, B.; Zhao, S.; Wang, Z.; Wang, S. Electrochemical preparation of polyaniline nanowires with the used electrolyte solution treated with the extraction process and their electrochemical performance. Nanomaterials 2018, 8, 103. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Electrochemical synthesis of polyaniline and its comparison with chemically synthesized polyaniline. J. Appl. Polym. Sci. 2007, 104, 1900–1904. [Google Scholar] [CrossRef]
- Cionti, C.; Della Pina, C.; Meroni, D.; Falletta, E.; Ardizzone, S. Triply green polyaniline: UV irradiation-induced synthesis of a highly porous PANI/TiO2 composite and its application in dye removal. Chem. Commun. 2018, 54, 10702–10705. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Falletta, E.; Pifferi, V.; Falciola, L.; Cappelletti, G.; Ardizzone, S. Emerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium. Photochem. Photobiol. Sci. 2017, 16, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Keivani, M.B. Polyaniline Conducting Electroactive Polymers Thermal and Environmental Stability Studies. E-Journal Chem. 2006, 3, 202–217. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Li, X.; Lu, D. Surface properties of polyaniline/nano-TiO2 composites. Appl. Surf. Sci. 2004, 229, 395–401. [Google Scholar] [CrossRef]
- Lee, I.S.; Lee, J.Y.; Sung, J.H.; Choi, H.J. Synthesis and electrorheological characteristics of polyaniline-titanium dioxide hybrid suspension. Synth. Met. 2005, 152, 173–176. [Google Scholar] [CrossRef]
- Gurunathan, K.; Trivedi, D.C. Studies on polyaniline and colloidal TiO2 composites. Mater. Lett. 2000, 45, 262–268. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Z.; Li, Y.; Sun, L.; Zhang, T. Synthesis, characterization and thermal analysis of polyaniline/ZrO2 composites. Thermochim. Acta 2006, 441, 191–194. [Google Scholar] [CrossRef]
- Ansari, M.O.; Mohammad, F. Sensors and Actuators B: Chemical Thermal stability, electrical conductivity and ammonia sensing studies on p -toluenesulfonic acid doped polyaniline: Titanium dioxide (p TSA/Pani:TiO2) nanocomposites. Sensors Actuators B. Chem. 2011, 157, 122–129. [Google Scholar] [CrossRef]
- Schnitzler, D.C.; Meruvia, M.S.; Hümmelgen, I.A.; Zarbin, A.J.G. Preparation and Characterization of Novel Hybrid Materials Formed from (Ti,Sn)O2 Nanoparticles and Polyaniline. Chem. Mater. 2003, 15, 4658–4665. [Google Scholar] [CrossRef]
- Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Roković, M.K.; Žic, M. Stability and synergistic effect of polyaniline/TiO2 photocatalysts in degradation of Azo dye in wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef]
- Alves, W.F.; Venancio, E.C.; Leite, F.L.; Kanda, D.H.F.; Malmonge, L.F.; Malmonge, J.A.; Mattoso, L.H.C. Thermo-analyses of polyaniline and its derivatives. Thermochim. Acta 2010, 502, 43–46. [Google Scholar] [CrossRef]
- Chandrakanthi, N.; Careem, M.A. Thermal stability of polyaniline. Polym. Bull. 2000, 44, 101–108. [Google Scholar] [CrossRef]
- Lu, X.; Tan, C.Y.; Xu, J.; He, C. Thermal degradation of electrical conductivity of polyacrylic acid doped polyaniline: Effect of molecular weight of the dopants. Synth. Met. 2003, 138, 429–440. [Google Scholar] [CrossRef]
- Nabid, M.R.; Golbabaee, M.; Moghaddam, A.B. Polyaniline/TiO2 Nanocomposite: Enzymatic Synthesis and Electrochemical Properties. Int. J. Electrochem. Sci. 2008, 3, 1117–1126. [Google Scholar]
- Li, X.; Chen, W.; Bian, C.; He, J.; Xu, N.; Xue, G. Surface modification of TiO2 nanoparticles by polyaniline. Appl. Surf. Sci. 2003, 217, 16–22. [Google Scholar] [CrossRef]
- Pouget, J.P.; Józefowicz, M.E.; Epstein, A.J.; Tang, X.; MacDiarmid, A.G. X-ray Structure of Polyaniline. Macromolecules 1991, 24, 779–789. [Google Scholar] [CrossRef]
- Katoch, A.; Burkhart, M.; Hwang, T.; Kim, S.S. Synthesis of polyaniline/TiO2 hybrid nanoplates via a sol–gel chemical method. Chem. Eng. J. 2012, 192, 262–268. [Google Scholar] [CrossRef]
- Singh, R.; Choudhary, R.B.; Kandulna, R. Robust optical and electrical properties of TiO2-sensitized polymeric (PANI–TiO2) nanocomposites for hybrid solar cell application. Bull. Mater. Sci. 2019, 42, 202. [Google Scholar] [CrossRef]
- Reddy, K.R.; Karthik, K.V.; Prasad, S.B.B.; Soni, S.K.; Jeong, H.M.; Raghu, A. V Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 2016, 120, 169–174. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, M. Polyaniline/TiO2 Composite Nanotubes. J. Phys. Chem. B 2003, 107, 6748–6753. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Gao, H.; Cui, W. Three-dimensional structure of PANI/CdS NRs-SiO2 hydrogel for photocatalytic hydrogen evolution with high activity and stability. Nanomaterials 2019, 9, 427. [Google Scholar] [CrossRef]
- Rimoldi, L.; Pargoletti, E.; Meroni, D.; Falletta, E.; Cerrato, G.; Turco, F.; Cappelletti, G. Concurrent role of metal (Sn, Zn) and N species in enhancing the photocatalytic activity of TiO2 under solar light. Catal. Today 2018, 313, 40–46. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Bandosz, T.J. Defectous UiO-66 MOF Nanocomposites as Reactive Media of Superior Protection against Toxic Vapors. ACS Appl. Mater. Interfaces, 2019; acsami.9b17314. [Google Scholar]
- Falletta, E.; Costa, P.; Della Pina, C.; Lanceros-Mendez, S. Development of high sensitive polyaniline based piezoresistive films by conventional and green chemistry approaches. Sensors Actuators, A Phys. 2014, 220, 13–21. [Google Scholar] [CrossRef]
- Šeděnková, I.; Trchová, M.; Stejskal, J. Thermal degradation of polyaniline films prepared in solutions of strong and weak acids and in water - FTIR and Raman spectroscopic studies. Polym. Degrad. Stab. 2008, 93, 2147–2157. [Google Scholar] [CrossRef]
- Meroni, D.; Lo Presti, L.; Di Liberto, G.; Ceotto, M.; Acres, R.G.; Prince, K.C.; Bellani, R.; Soliveri, G.; Ardizzone, S. A Close Look at the Structure of the TiO2-APTES Interface in Hybrid Nanomaterials and Its Degradation Pathway: An Experimental and Theoretical Study. J. Phys. Chem. C 2017, 121, 430–440. [Google Scholar] [CrossRef]
- Della Pina, C.; Ferretti, A.M.; Ponti, A.; Falletta, E. A green approach to magnetically-hard electrically-conducting polyaniline/CoFe2O4 nanocomposites. Compos. Sci. Technol. 2015, 110, 138–144. [Google Scholar] [CrossRef]
- Albuquerque, J.E.; Mattoso, L.H.C.; Balogh, D.T.; Faria, R.M.; Masters, J.G.; MacDiarmid, A.G. Simple method to estimate the oxidation state of polyanilines. Synth. Met. 2000, 113, 19–22. [Google Scholar] [CrossRef]
- Laska, J.; Widlarz, J. Spectroscopic and structural characterization of low molecular weight fractions of polyaniline. Polymer (Guildf). 2005, 46, 1485–1495. [Google Scholar] [CrossRef]
- Dolan, A.R.; Wood, T.D. Synthesis and characterization of low molecular weight oligomers of soluble polyaniline by electrospray ionization mass spectrometry. Synth. Met. 2004, 143, 243–250. [Google Scholar] [CrossRef]
- Rimoldi, L.; Giordana, A.; Cerrato, G.; Falletta, E.; Meroni, D. Insights on the photocatalytic degradation processes supported by TiO2/WO3 systems. The case of ethanol and tetracycline. Catal. Today 2019, 328, 210–215. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Cho, M.H. Route to High Surface Area, Mesoporosity of Polyaniline-Titanium Dioxide Nanocomposites via One Pot Synthesis for Energy Storage Applications. Ind. Eng. Chem. Res. 2016, 55, 116–124. [Google Scholar] [CrossRef]
- Li, S.C.; Diebold, U. Reactivity of TiO2 rutile and anatase surfaces toward nitroaromatics. J. Am. Chem. Soc. 2010, 132, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Losovyj, Y.; Paliwal, V.K.; Diebold, U. Photoemission study of azobenzene and aniline adsorbed on TiO2 anatase (101) and rutile (110) surfaces. J. Phys. Chem. C 2011, 115, 10173–10179. [Google Scholar] [CrossRef]
- Saroyan, H.S.; Bele, S.; Giannakoudakis, D.A.; Samanidou, V.F.; Bandosz, T.J.; Deliyanni, E.A. Degradation of endocrine disruptor, bisphenol-A, on an mixed oxidation state manganese oxide/modified graphite oxide composite: A role of carbonaceous phase. J. Colloid Interface Sci. 2019, 539, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Golczak, S.; Kanciurzewska, A.; Fahlman, M.; Langer, K.; Langer, J.J. Comparative XPS surface study of polyaniline thin films. Solid State Ionics 2008, 179, 2234–2239. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Li, S.; Cheng, J.; Zhang, Y.; Zhou, J.; Yang, D.; Tong, D.; Wang, B. Interfacial Engineered Polyaniline/Sulfur-Doped TiO2 Nanotube Arrays for Ultralong Cycle Lifetime Fiber-Shaped, Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 18390–18399. [Google Scholar] [CrossRef]
- Reif, A.; Freemann, H.S. Environmental Chemistry of Dyes and Pigments; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Kumar, R.; Ansari, M.O.; Parveen, N.; Barakat, M.A.; Cho, M.H. Simple route for the generation of differently functionalized PVC@graphene-polyaniline fiber bundles for the removal of Congo red from wastewater. RSC Adv. 2015, 5, 61486–61494. [Google Scholar] [CrossRef]
- Yan, B.; Chen, Z.; Cai, L.; Chen, Z.; Fu, J.; Xu, Q. Fabrication of polyaniline hydrogel: Synthesis, characterization and adsorption of methylene blue. Appl. Surf. Sci. 2015, 356, 39–47. [Google Scholar] [CrossRef]
- Mahanta, D.; Madras, G.; Radhakrishnan, S.; Patil, S. Adsorption of Sulfonated Dyes by Polyaniline Emeraldine Salt and Its Kinetics. J. Phys. Chem. B 2008, 112, 10153–10157. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, R. Conducting Polyaniline/Iron Oxide Composite: A Novel Adsorbent for the Removal of Amido Black 10B. J. Chem. Eng. Data 2010, 55, 3489–3493. [Google Scholar] [CrossRef]
- Gómez, V.; Larrechi, M.S.; Callao, M.P. Kinetic and adsorption study of acid dye removal using activated carbon. Chemosphere 2007, 69, 1151–1158. [Google Scholar] [CrossRef]
- Guo, X.; Fei, G.T.; Su, H.; De Zhang, L. High-performance and reproducible polyaniline nanowire/tubes for removal of Cr(VI) in aqueous solution. J. Phys. Chem. C 2011, 115, 1608–1613. [Google Scholar] [CrossRef]
- Sarin, V.; Pant, K.K. Removal of chromium from industrial waste by using eucalyptus bark. Bioresour. Technol. 2006, 97, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, H.; Wang, B. Removal of Chromium ( VI ) from Aqueous Solutions Using Polyaniline Doped with Sulfuric Acid. Ind. Eng. Chem. Res. 2010, 49, 9998–10004. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Barakat, M.A. DBSA doped polyaniline/multi-walled carbon nanotubes composite for high efficiency removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2013, 228, 748–755. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of hexavalent chromium ions using polyaniline/silica gel composite. J. Water Process Eng. 2014, 1, 37–45. [Google Scholar] [CrossRef]
- Zuo, J.; Torres, E. Comparison of Adsorption of Mercaptopropyltrimethoxysilane on Amphiphilic TiO2 and Hydroxylated SiO2. Langmuir 2010, 26, 15161–15168. [Google Scholar] [CrossRef]
- Kumar, A.; Mathur, N. Photocatalytic degradation of aniline at the interface of TiO2 suspensions containing carbonate ions. J. Colloid Interface Sci. 2006, 300, 244–252. [Google Scholar] [CrossRef]
- Canle L., M.; Santaballa, J.A.; Vulliet, E. On the mechanism of TiO2-photocatalyzed degradation of aniline derivatives. J. Photochem. Photobiol. A Chem. 2005, 175, 192–200. [Google Scholar] [CrossRef]
- Wenhua, L.; Hong, L.; Sao, C.; Jianqing, Z.; Chunan, C. Kinetics of photocatalytic degradation of aniline in water over TiO2 supported on porous nickel. J. Photochem. Photobiol. A Chem. 2000, 131, 125–132. [Google Scholar] [CrossRef]
- Castagna, R.; Tunesi, M.; Saglio, B.; Della Pina, C.; Sironi, A.; Albani, D.; Bertarelli, C.; Falletta, E. Ultrathin electrospun PANI nanofibers for neuronal tissue engineering. J. Appl. Polym. Sci. 2016, 133, 43885. [Google Scholar] [CrossRef]
- Sapurina, I.; Stejskal, J. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yuan, X.; Tao, J.; Peng, S.; Yang, W.; Dong, X.; Zhang, C.; Luo, Y. Polyaniline modified mesoporous titanium dioxide that enhances oxo-biodegradation of polyethylene films for agricultural plastic mulch application. Polym. Int. 2019, 68, 1332–1340. [Google Scholar] [CrossRef]
- Vaez, M.; Alijani, S.; Omidkhah, M.; Zarringhalam Moghaddam, A. Synthesis, characterization and optimization of N-TiO2/PANI nanocomposite for photodegradation of acid dye under visible light. Polym. Compos. 2018, 39, 4605–4616. [Google Scholar] [CrossRef]

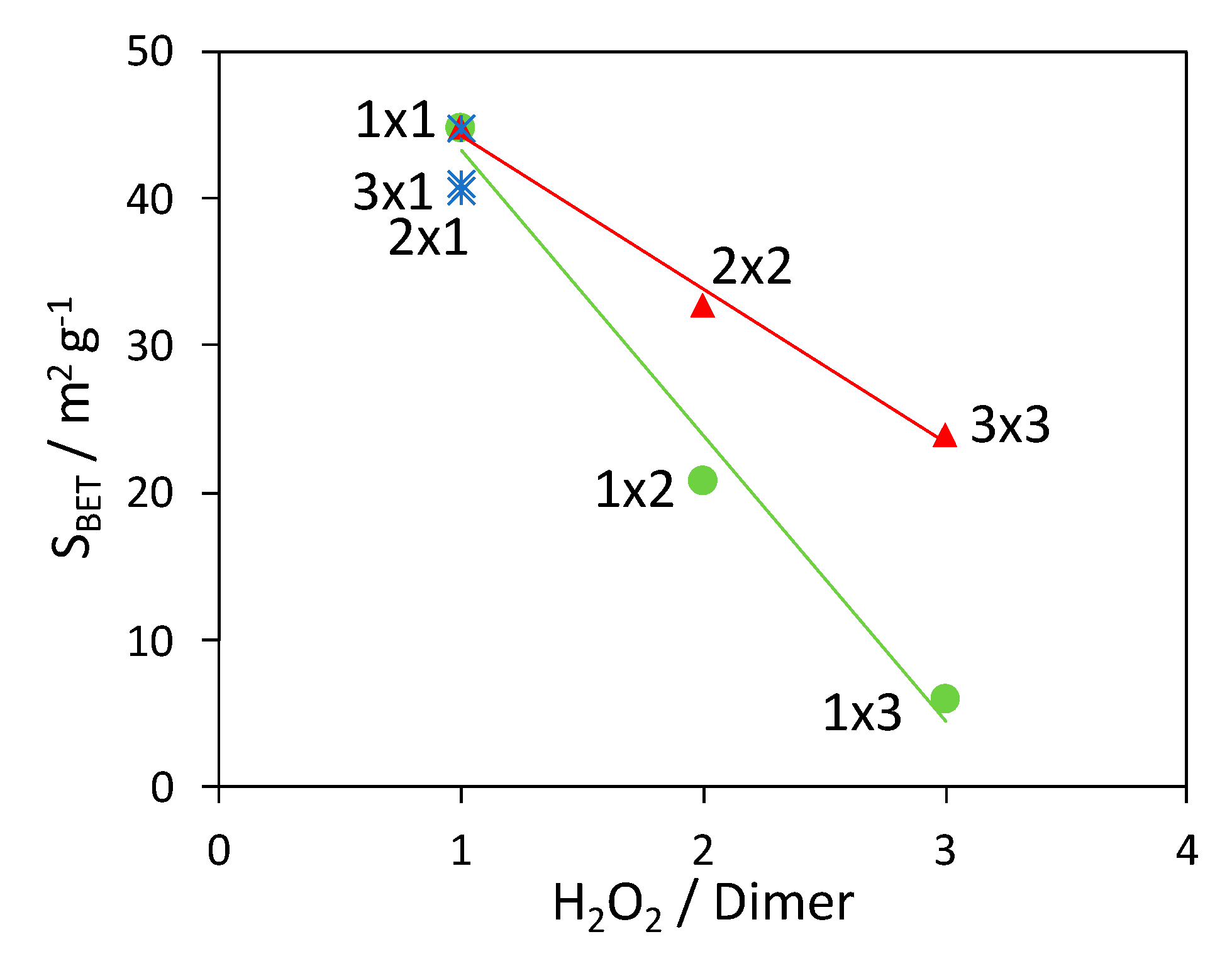

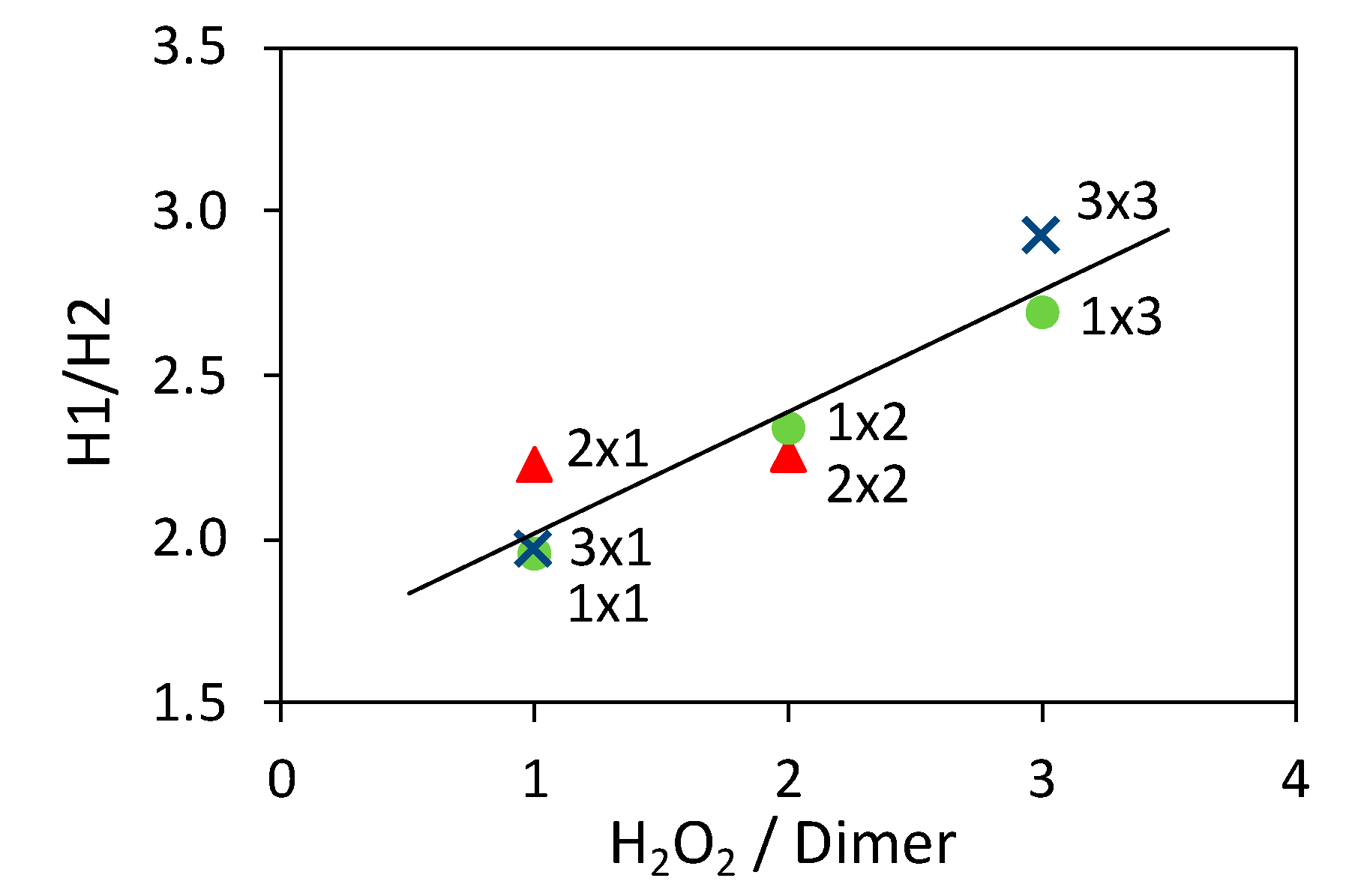



| Sample | Molar Ratios | Sample Composition1 | SBET (m2 g−1) | SBET Mix 2 (m2 g−1) | ||
|---|---|---|---|---|---|---|
| TiO2/Dimer | H2O2/Dimer | %PANI | %TiO2 | |||
| 1 × 1 | 0.4 | 1.0 | 58 | 31 | 44.8 | 17.2 |
| 2 × 2 | 0.8 | 2.0 | 52 | 38 | 32.8 | 20.6 |
| 3 × 3 | 1.2 | 3.0 | 43 | 50 | 24.0 | 26.3 |
| 2 × 1 | 0.8 | 1.0 | 34 | 61 | 40.5 | 31.5 |
| 3 × 1 | 1.2 | 1.0 | 27 | 69 | 41.0 | 35.3 |
| 1 × 2 | 0.4 | 2.0 | 64 | 24 | 20.8 | 13.9 |
| 1 × 3 | 0.4 | 3.0 | 75 | 15 | 6.0 | 9.8 |
| Region | TiO2 | NoUV | UV | |||
|---|---|---|---|---|---|---|
| Position (eV) | % Area | Position (eV) | % Area | Position (eV) | % Area | |
| Ti 2p | 458.5 | 100 | 458.5 | 51 | 458.5 | 28 |
| - | 459.7 | 49 | 459.7 | 72 | ||
| C 1s | 284.7 | 81 | 284.6 | 65 | 284.8 | 68 |
| 285.9 | 14 | 286.1 | 35 | 286.4 | 24 | |
| 288.4 | 5 | - | 288.4 | 8 | ||
| O 1s | 529.7 | 85 | 529.8 | 57 | 530.1 | 53 |
| 531.4 | 15 | 531.2 | 43 | 531.2 | 39 | |
| - | - | 532.9 | 8 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cionti, C.; Della Pina, C.; Meroni, D.; Falletta, E.; Ardizzone, S. Photocatalytic and Oxidative Synthetic Pathways for Highly Efficient PANI-TiO2 Nanocomposites as Organic and Inorganic Pollutant Sorbents. Nanomaterials 2020, 10, 441. https://doi.org/10.3390/nano10030441
Cionti C, Della Pina C, Meroni D, Falletta E, Ardizzone S. Photocatalytic and Oxidative Synthetic Pathways for Highly Efficient PANI-TiO2 Nanocomposites as Organic and Inorganic Pollutant Sorbents. Nanomaterials. 2020; 10(3):441. https://doi.org/10.3390/nano10030441
Chicago/Turabian StyleCionti, Carolina, Cristina Della Pina, Daniela Meroni, Ermelinda Falletta, and Silvia Ardizzone. 2020. "Photocatalytic and Oxidative Synthetic Pathways for Highly Efficient PANI-TiO2 Nanocomposites as Organic and Inorganic Pollutant Sorbents" Nanomaterials 10, no. 3: 441. https://doi.org/10.3390/nano10030441
APA StyleCionti, C., Della Pina, C., Meroni, D., Falletta, E., & Ardizzone, S. (2020). Photocatalytic and Oxidative Synthetic Pathways for Highly Efficient PANI-TiO2 Nanocomposites as Organic and Inorganic Pollutant Sorbents. Nanomaterials, 10(3), 441. https://doi.org/10.3390/nano10030441