Functional Bioglass—Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin Films Deposition
2.3. Thin Films Characterization
2.3.1. Morfological, Structural and Tribological
2.3.2. Drug Release Behavior
2.3.3. Electrochemical Investigation
2.3.4. Microbiological Assay
3. Results and Discussion
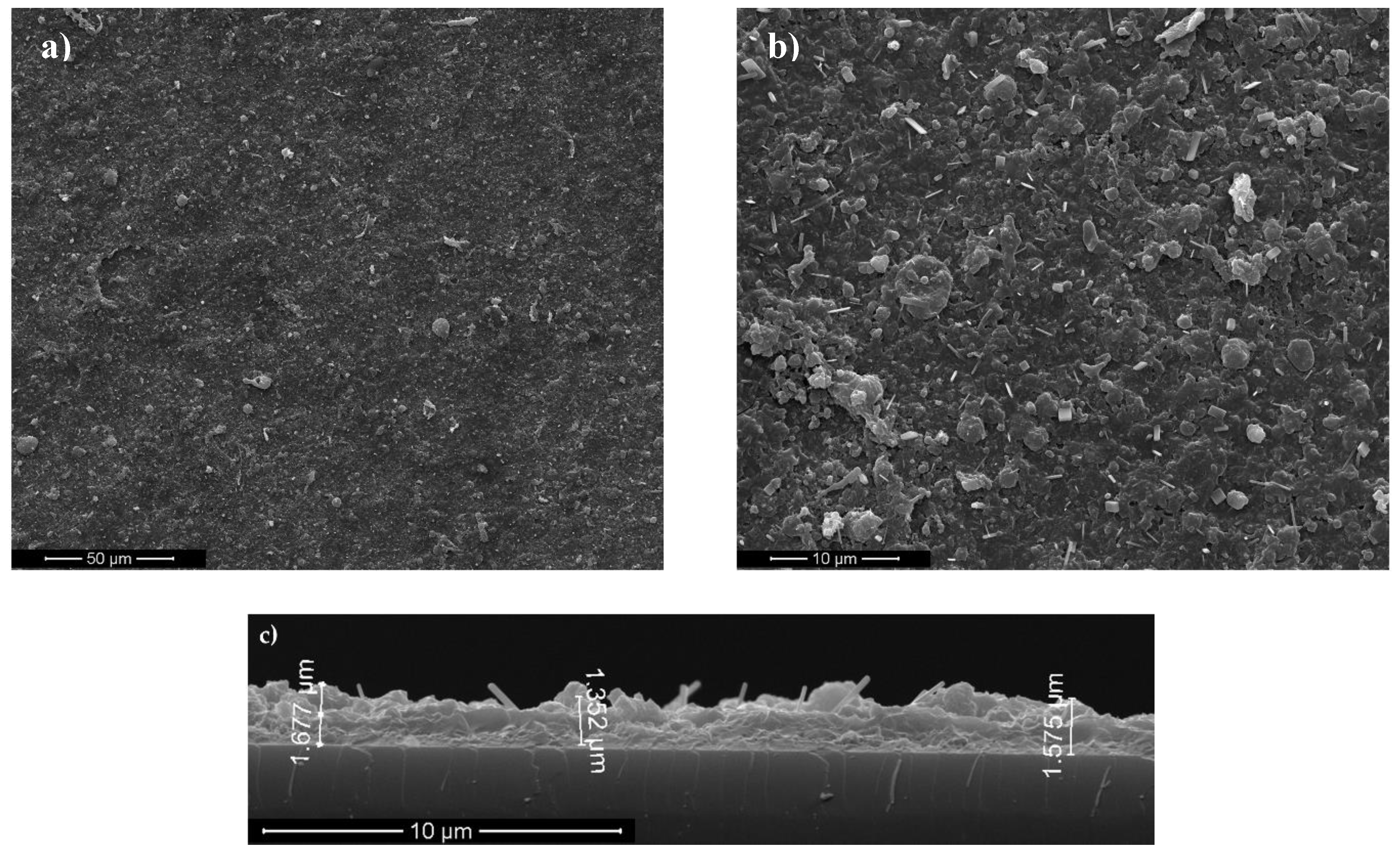
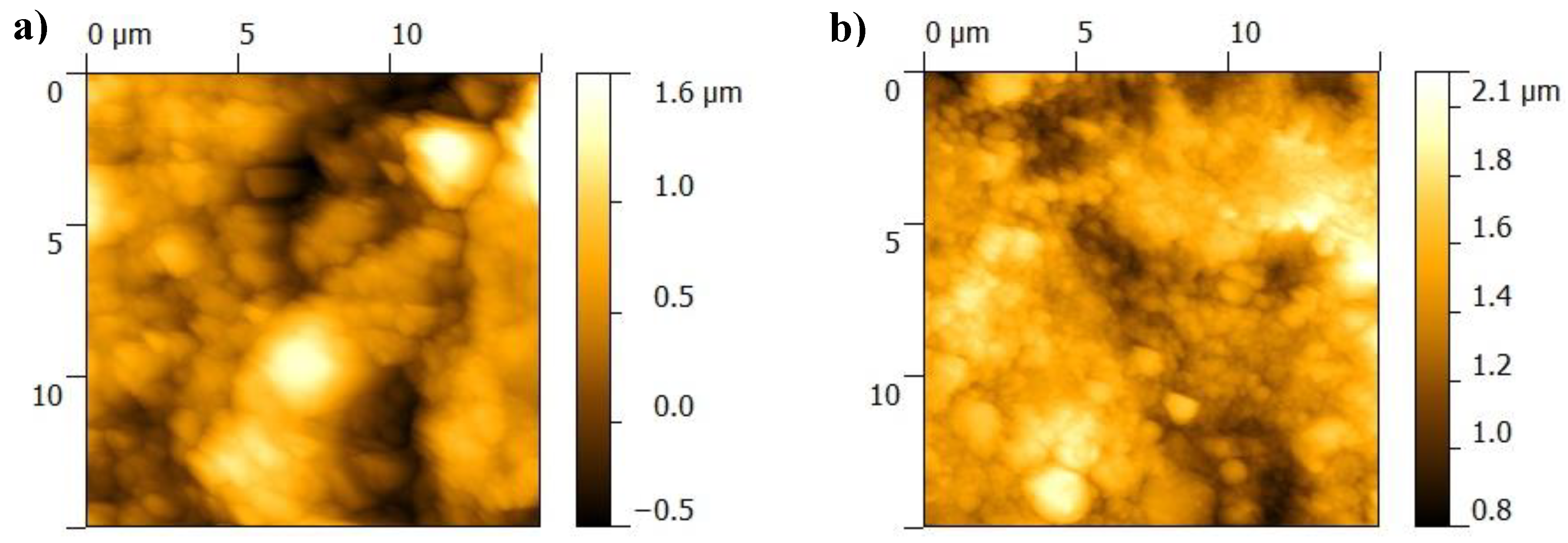
3.1. Surface Characterization of As-Deposited Structures
3.2. Characterization of Structures after Immersion in SBF
3.3. Biological Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Kumar, R.; Kumar, S.; Prakash, C. Biomechanical Properties of Orthopedic and Dental Implants: A Comprehensive Review. Handb. Res. Green Eng. Tech. Mod. Manuf. 2019, 1–13. [Google Scholar] [CrossRef]
- Scholz, M.S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Gilbert, M.E.; Muflahi, S.A.; Pernice, M.F.; Trevarthen, J.A.; et al. The use of composite materials in modern orthopaedic medicine and prosthetic devices: A review. Compos. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C 2019, 104, 109895. [Google Scholar] [CrossRef] [PubMed]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Floroian, L.; Samoila, C.; Badea, M.; Munteanu, D.; Ristoscu, C.; Sima, F.; Negut, I.; Chifiriuc, M.C.; Mihailescu, I.N. Stainless steel surface biofunctionalization with PMMA-bioglass coatings: Compositional, electrochemical corrosion studies and microbiological assay. J. Mater. Sci. Mater. Med. 2015, 26, 195. [Google Scholar] [CrossRef]
- Motealleh, A.; Eqtesadi, S.; Perera, F.H.; Ortiz, A.L.; Miranda, P.; Pajares, A.; Wendelbo, R. Reinforcing 13–93 bioglass scaffolds fabricated by robocasting and pressureless spark plasma sintering with graphene oxide. J. Mech. Behav. Biomed. Mater. 2019, 97, 108–116. [Google Scholar] [CrossRef]
- Krasny, M.; Krasny, K.; Zadurska, M.; Fiedor, P. Evaluation of treatment outcomes and clinical indications for antibiotic prophylaxis in patients undergoing implantation procedures. Adv. Med. Sci. 2016, 61, 113–116. [Google Scholar] [CrossRef]
- Lindeboom, J.A.; Frenken, J.W.; Tuk, J.G.; Kroon, F.H. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone-grafting procedures: Preoperative single-dose penicillin versus preoperative single-dose clindamycin. Int. J. Oral Maxillofac. Surg. 2006, 35, 433–436. [Google Scholar] [CrossRef]
- Jaeger, M.; Maier, D.; Kern, W.V.; Südkamp, N.P. Antibiotics in trauma and orthopedic surgery—A primer of evidence-based recommendations. Injury 2006, 37, S74–S80. [Google Scholar] [CrossRef]
- Infection Prevention in Total Knee and Total Hip Arthroplasties. Abstract—Europe PMC. Available online: https://europepmc.org/article/med/18309390 (accessed on 8 January 2020).
- Floroian, L.; Badea, M.; Samoila, C.; Floroian, D.; Ristoscu, C.G.; Mihailescu, N.; Negut, I.; Mihailescu, I.N. Implant Structure, Nanocomposition Coverage and Surface Functioning Process Dental and Bone Implants, RO131045 (A0), OSIM BOPI nr; OSIM: Bucharest, Romania, 2016.
- Floroian, L.; Ristoscu, C.; Mihailescu, N.; Negut, I.; Badea, M.; Ursutiu, D.; Chifiriuc, M.C.; Urzica, I.; Dyia, H.M.; Bleotu, C.; et al. Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings. Molecules 2016, 21, 740. [Google Scholar] [CrossRef]
- Rius, D.R.; Gallardo, M.G.; Planella, J.M.M. Method for Applying an Antibacterial Protection to a Dental Implant, and Dental Implant Obtained. U.S. Patent Application No. 14/729,788, 7 January 2016. [Google Scholar]
- Sanpo, N.; Tan, M.L.; Cheang, P.; Khor, K.A. Antibacterial Property of Cold-Sprayed HA-Ag/PEEK Coating. J. Ther. Spray Technol. 2009, 18, 10–15. [Google Scholar] [CrossRef]
- Chung, C.J.; Lin, H.I.; Tsou, H.K.; Shi, Z.Y.; He, J.L. An antimicrobial TiO2 coating for reducing hospital-acquired infection. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Ryu, H.S.; Hong, S.H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. Part A 2009, 88, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Macha, I.J.; Karacan, I.; Ben-Nissan, B.; Cazalbou, S.; Müller, W.H. Development of antimicrobial composite coatings for drug release in dental, orthopaedic and neural prostheses applications. SN Appl. Sci. 2018, 1, 68. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Prolongation of bactericidal efficiency of chitosan—Bioactive glass coating by drug controlled release. Prog. Org. Coat. 2020, 139, 105440. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. In vitro bactericidal and drug release properties of vancomycin-amino surface functionalized bioactive glass nanoparticles. Mater. Chem. Phys. 2020, 241, 122423. [Google Scholar] [CrossRef]
- Floroian, L.; Ristoscu, C.; Candiani, G.; Pastori, N.; Moscatelli, M.; Mihailescu, N.; Negut, I.; Badea, M.; Gilca, M.; Mihailescu, I.N.; et al. Antimicrobial thin films based on ayurvedic plants extracts embedded in a bioactive glass matrix. Appl. Surf. Sci. 2017, 417, 224–233. [Google Scholar] [CrossRef]
- Rose, W.E.; Otto, D.P.; Aucamp, M.E.; Miller, Z.; de Villiers, M.M. Prevention of Biofilm Formation by Methacrylate-Based Copolymer Films Loaded With Rifampin, Clarithromycin, Doxycycline Alone or in Combination. Pharm. Res. 2015, 32, 61–73. [Google Scholar] [CrossRef]
- Risk Assessment of Antibiotic Resistance Development by Antibiotic-Loaded Bone Cements: Is it a Clinical Concern? | EFORT Open Reviews. Available online: https://online.boneandjoint.org.uk/doi/full/10.1302/2058-5241.4.180104 (accessed on 9 January 2020).
- Sousa, B.C.D.; Gomes, F.D.A.; Ferreira, C.M.; Rocha, M.M.D.N.P.; Barros, E.B.; Albuquerque, D.S.D. Persistent extra-radicular bacterial biofilm in endodontically treated human teeth: Scanning electron microscopy analysis after apical surgery. Microsc. Res. Tech. 2017, 80, 662–667. [Google Scholar] [CrossRef]
- Cardoso, F.G.D.R.; Chung, A.; Martinho, F.C.; Camargo, C.H.R.; Carvalho, C.A.T.; Gomes, B.P.F.D.A.; Valera, M.C. Investigation of Bacterial Contents From Persistent Endodontic Infection and Evaluation of Their Inflammatory Potential. Braz. Dent. J. 2016, 27, 412–418. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Gould, K.; Şen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjäderhane, L.; Dummer, P.M.H. European Society of Endodontology position statement: The use of antibiotics in endodontics. Int. Endod. J. 2018, 51, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Natural Therapeutic Options in Endodontics—A Review. Abstract—Europe PMC. Available online: https://europepmc.org/article/PMC/4911752 (accessed on 8 January 2020).
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Tanaskovic, D.; Jokic, B.; Socol, G.; Popescu, A.; Mihailescu, I.N.; Petrovic, R.; Janackovic, D.J. Synthesis of functionally graded bioactive glass-apatite multistructures on Ti substrates by pulsed laser deposition. Appl. Surf. Sci. 2007, 254, 1279–1282. [Google Scholar] [CrossRef]
- Gyorgy, E.; Grigorescu, S.; Socol, G.; Mihailescu, I.N.; Janackovic, D.; Dindune, A.; Kanepe, D.; Palcevskis, E.; Zdrentu, E.L.; Petrescu, S.M. Bioactive glass and hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2007, 253, 7981–7986. [Google Scholar] [CrossRef]
- Baxter, D.; Yeh, J. The use of polymethyl methacrylate (PMMA) in neurosurgery. In Biomaterials for Spinal Surgery; Ambrosio, L., Tanner, E., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 365–384. ISBN 978-1-84569-986-4. [Google Scholar]
- Zhang, L.; Wu, D.; Chen, Y.; Wang, X.; Zhao, G.; Wan, H.; Huang, C. Surface modification of polymethyl methacrylate intraocular lenses by plasma for improvement of antithrombogenicity and transmittance. Appl. Surf. Sci. 2009, 255, 6840–6845. [Google Scholar] [CrossRef]
- Butoi, B.; Staicu, D. Cured pmma thin films for greatly improving the electro-optical response time in nematic liquid crystal pixels. Dig. J. Nanomater. Biostruct. 2019, 14, 175–182. [Google Scholar]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Kankariya, A.R.; Patel, A.R.; Kunte, S.S. The effect of different concentrations of water soluble azadirachtin (neem metabolite) on Streptococcus mutans compared with chlorhexidine. J. Indian Soc. Pedod. Prev. Dent. 2016, 34, 105. [Google Scholar] [CrossRef]
- Lakshmi, T.; Krishnan, V.; Rajendran, R.; Madhusudhanan, N. Azadirachta indica: A herbal panacea in dentistry—An update. Pharm. Rev. 2015, 9, 41–44. [Google Scholar] [CrossRef]
- Mustafa, M. Antibacterial Efficacy of Neem (Azadirachta indica) Extract against Enterococcus faecalis: An in vitro Study. J. Contemp. Dent. Pract. 2016, 17, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Chandrappa, P.M.; Dupper, A.; Tripathi, P.; Arroju, R.; Sharma, P.; Sulochana, K. Antimicrobial activity of herbal medicines (tulsi extract, neem extract) and chlorhexidine against Enterococcus faecalis in Endodontics: An in vitro study. J. Int. Soc. Prev. Community Dent. 2015, 5, S89–S92. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.S.; Athira, S.; Chandramohan, S.; Ranjith, K.; Raj, V.V.; Manjula, V.D. Comparison of efficacy of herbal disinfectants with chlorhexidine mouthwash on decontamination of toothbrushes: An experimental trial. J. Int. Soc. Prev. Community Dent. 2016, 6, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.S.; Penmatsa, T.; Kumar, A.K.; Reddy, M.N.; Gautam, N.S.; Gautam, N.R. Antibacterial activity of aqueous extracts of Indian chewing sticks on dental plaque: An in vitro study. J. Pharm. Bioallied Sci. 2014, 6, S140–S145. [Google Scholar]
- Quelemes, P.V.; Perfeito, M.L.G.; Guimarães, M.A.; dos Santos, R.C.; Lima, D.F.; Nascimento, C.; Silva, M.P.N.; Soares, M.J.; Ropke, C.D.; Eaton, P.; et al. Effect of neem (Azadirachta indica A. Juss) leaf extract on resistant Staphylococcus aureus biofilm formation and Schistosoma mansoni worms. J. Ethnopharmacol. 2015, 175, 287–294. [Google Scholar] [CrossRef]
- Mistry, K.S.; Sanghvi, Z.; Parmar, G.; Shah, S.; Pushpalatha, K. Antibacterial efficacy of Azadirachta indica, Mimusops elengi and 2% CHX on multispecies dentinal biofilm. J. Conserv. Dent. 2015, 18, 461–466. [Google Scholar] [CrossRef]
- Kang, J.J.; Samad, M.A.; Kim, K.S.; Bae, S. Comparative Anti-inflammatory Effects of Anti-arthritic Herbal Medicines and Ibuprofen. Nat. Prod. Commun. 2014, 9, 1934578X1400900932. [Google Scholar] [CrossRef]
- McGill, R.A.; Chrisey, D.B. Method of Producing a Film Coating by Matrix Assisted Pulsed Laser Deposition. U.S. Patent No. 6,025,036, 15 February 2000. [Google Scholar]
- Cristescu, R.; Popescu, C.; Socol, G.; Visan, A.; Mihailescu, I.N.; Gittard, S.D.; Miller, P.R.; Martin, T.N.; Narayan, R.J.; Stamatin, I.; et al. Deposition of antibacterial of poly(1,3-bis-(p-carboxyphenoxy propane)-co-(sebacic anhydride)) 20:80/gentamicin sulfate composite coatings by MAPLE. Appl. Surf. Sci. 2011, 257, 5287–5292. [Google Scholar] [CrossRef]
- Schou, J. Fundamentals of Laser-Assisted Fabrication of Inorganic and Organic Films. In Functionalized Nanoscale Materials, Devices and Systems; Springer: Dordrecht, The Netherlands, 2008; pp. 241–256. [Google Scholar] [CrossRef]
- Visan, A.; Grossin, D.; Stefan, N.; Duta, L.; Miroiu, F.M.; Stan, G.E.; Sopronyi, M.; Luculescu, C.; Freche, M.; Charvillat, C.; et al. Biomimetic nanocrystalline apatite coatings synthesized by Matrix Assisted Pulsed Laser Evaporation for medical applications. Mater. Sci. Eng. B 2014, 181, 56–63. [Google Scholar] [CrossRef]
- Cristescu, R.; Popescu, C.; Popescu, A.C.; Grigorescu, S.; Duta, L.; Mihailescu, I.N.; Caraene, G.; Albulescu, R.; Albulescu, L.; Stamatin, I.; et al. Functionalized polyvinyl alcohol derivatives thin films for controlled drug release and targeting systems: MAPLE deposition and morphological, chemical and in vitro characterization. Appl. Surf. Sci. 2009, 255, 5600–5604. [Google Scholar] [CrossRef]
- Applied Nanoindentation in Advanced Materials, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017.
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 10993-12:2012 Biological Evaluation of Medical Devices Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 2012.
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Eason, R. Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-05211-2. [Google Scholar]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-56486-4. [Google Scholar]
- Kavitha, R.J.; Abinaya, R.; Subha, B.; Ravichandran, K. Surface Reactivity of Quick Alkali Mediated Bivalent Metal Ions (Zn-Mg-Sr) Doped Bioactive Glass. Int. J. Innov. Res. Sci. Eng. 2014, 1, 2347–3207. [Google Scholar]
- Sugumaran, D.; Karim, K.J.A. Removal of copper (II) ion using chitosan-graft-poly(methyl methacrylate) as adsorbent. Eproc. Chem. 2017, 2, 1–11. [Google Scholar]
- Tommasini, F.J.; Ferreira, L.D.C.; Tienne, L.G.P.; Aguiar, V.D.O.; Silva, M.H.P.D.; Rocha, L.F.D.M.; Marques, M.D.F.V. Poly (Methyl Methacrylate)-SiC Nanocomposites Prepared Through in Situ Polymerization. Mater. Res. 2018. [Google Scholar] [CrossRef]
- Tanwar, D. Production And Characterization Of Neem Oil Methyl Ester. Int. J. Eng. Res. 2013, 2, 8. [Google Scholar]
- Banerjee, K.; Thiagarajan, N.; Thiagarajan, P. Azadirachta indica A. Juss Based Emollient Cream for Potential Dermatological Applications. Indian J. Pharm. Sci. 2016, 78, 320–325. [Google Scholar] [CrossRef]
- Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques, and Applications; John Wiley & Sons: Weinheim, Germany, 2019; ISBN 978-3-527-34139-9. [Google Scholar]












| Name | Peak BE | FWHM (eV) | Area (P) CPS.eV | Atomic % |
|---|---|---|---|---|
| C 1s | 284.10 | 3.57 | 604263.05 | 56.74 |
| O 1s | 531.21 | 304 | 814956.33 | 29.49 |
| Si 2p | 101.08 | 2.77 | 50090.55 | 4.91 |
| N 1s | 398.97 | 2.82 | 37012.54 | 2.17 |
| Ca 2p | 346.22 | 2.45 | 131256.80 | 2.11 |
| P 2p | 132.01 | 2.63 | 4006.47 | 0.26 |
| Na 1s | 1071.8 | 5.36 | 13990.24 | 0.21 |
| Sample | RMS (µm) | Ra (µm) |
|---|---|---|
| BGNPMMA/Ti | 0.331 | 0.268 |
| BGN/PMMA/SS | 0.272 | 0.219 |
| Sample | RMS (µm) | Ra (µm) |
|---|---|---|
| BGN/PMMA/Ti | 0.630 | 0.537 |
| BGN/PMMA/SS | 0.357 | 0.281 |
| Sample | Time (days) | Max Phase Angle (grade) | |
|---|---|---|---|
| BGN/PMMA/SS | 0 | 68.14 | |
| 3 | 58.53 | 53.41 | |
| 7 | 61.46 | 41.15 | |
| 14 | 59.80 | 53.76 | |
| 21 | 60.55 | 54.14 | |
| 28 | 65.60 | ||
| SS | 0 | 65.68 | |
| 14 | 63.53 | ||
| 21 | 66.00 | 47.39 | |
| 28 | 65.02 | 37.52 | |
| 40 | 65.68 | 41.65 | |
| 50 | 66.16 | 48.23 | |
| Bacteria (CTRL) | Studied Samples | Bacteria vs CTRL (% mean ± st. dev.) | Antibacterial Efficiency (% vs CTRL) |
|---|---|---|---|
| Staphylococcus aureus | BGN/PMMA/SS | 5.1 ± 1.2 | 95.1 |
| SS | 7.9 ± 2.5 | 79.0 | |
| Escherichia coli | BGN/PMMA/SS | 59.1 ± 3.8 | 38.2 |
| SS | 64.5 ± 4.6 | 30.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negut, I.; Floroian, L.; Ristoscu, C.; Mihailescu, C.N.; Mirza Rosca, J.C.; Tozar, T.; Badea, M.; Grumezescu, V.; Hapenciuc, C.; Mihailescu, I.N. Functional Bioglass—Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery. Nanomaterials 2020, 10, 385. https://doi.org/10.3390/nano10020385
Negut I, Floroian L, Ristoscu C, Mihailescu CN, Mirza Rosca JC, Tozar T, Badea M, Grumezescu V, Hapenciuc C, Mihailescu IN. Functional Bioglass—Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery. Nanomaterials. 2020; 10(2):385. https://doi.org/10.3390/nano10020385
Chicago/Turabian StyleNegut, Irina, Laura Floroian, Carmen Ristoscu, Cristian N. Mihailescu, Julia Claudia Mirza Rosca, Tatiana Tozar, Mihaela Badea, Valentina Grumezescu, Claudiu Hapenciuc, and Ion N. Mihailescu. 2020. "Functional Bioglass—Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery" Nanomaterials 10, no. 2: 385. https://doi.org/10.3390/nano10020385
APA StyleNegut, I., Floroian, L., Ristoscu, C., Mihailescu, C. N., Mirza Rosca, J. C., Tozar, T., Badea, M., Grumezescu, V., Hapenciuc, C., & Mihailescu, I. N. (2020). Functional Bioglass—Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery. Nanomaterials, 10(2), 385. https://doi.org/10.3390/nano10020385









