Titania/Electro-Reduced Graphene Oxide Nanohybrid as an Efficient Electrochemical Sensor for the Determination of Allura Red
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Prepration of TiO2/GO Nanocomposites
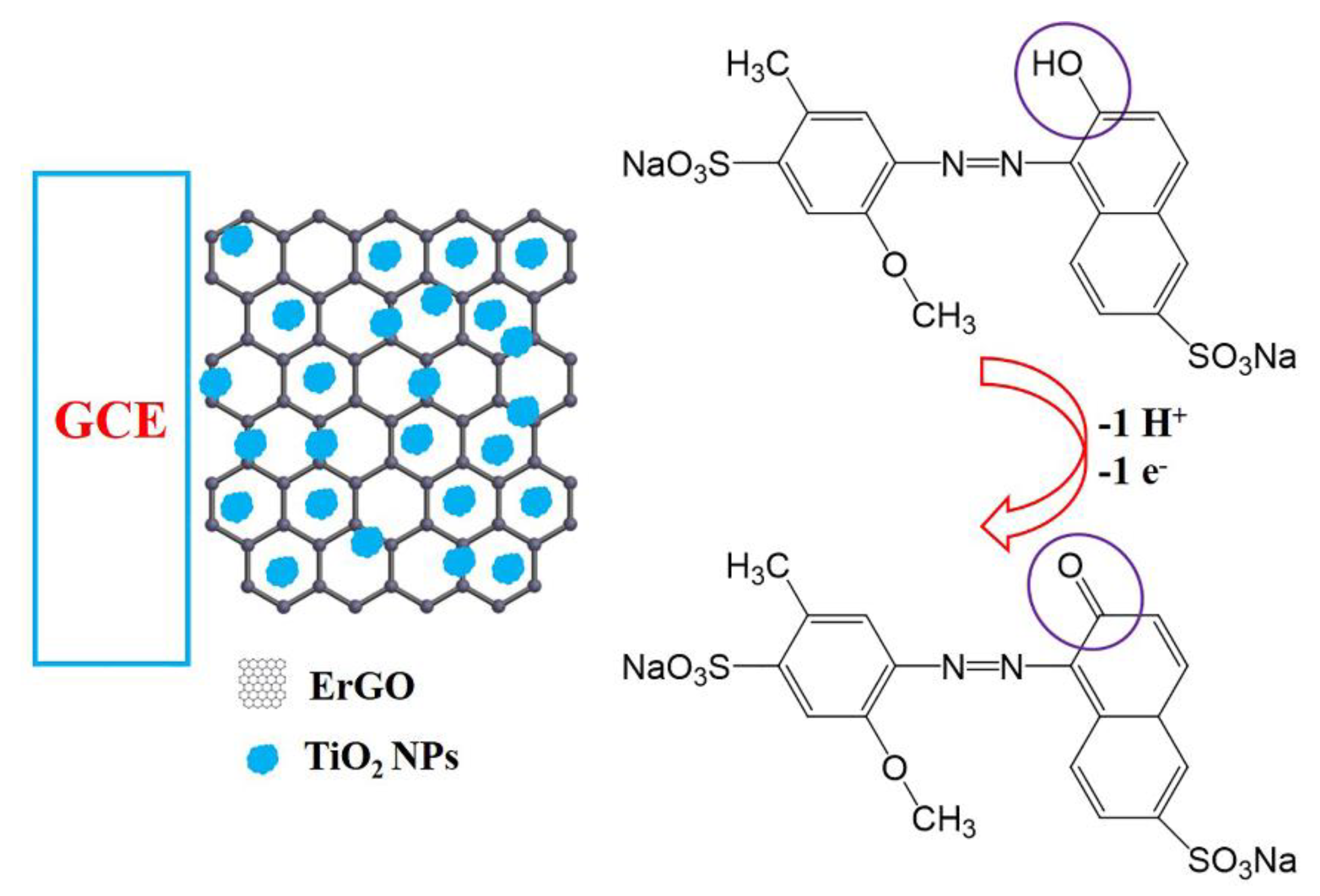
2.3. Preparation of TiO2/ErGO Modified Electrodes
2.4. Electrochemical Detection of Allura Red
3. Results and Discussions
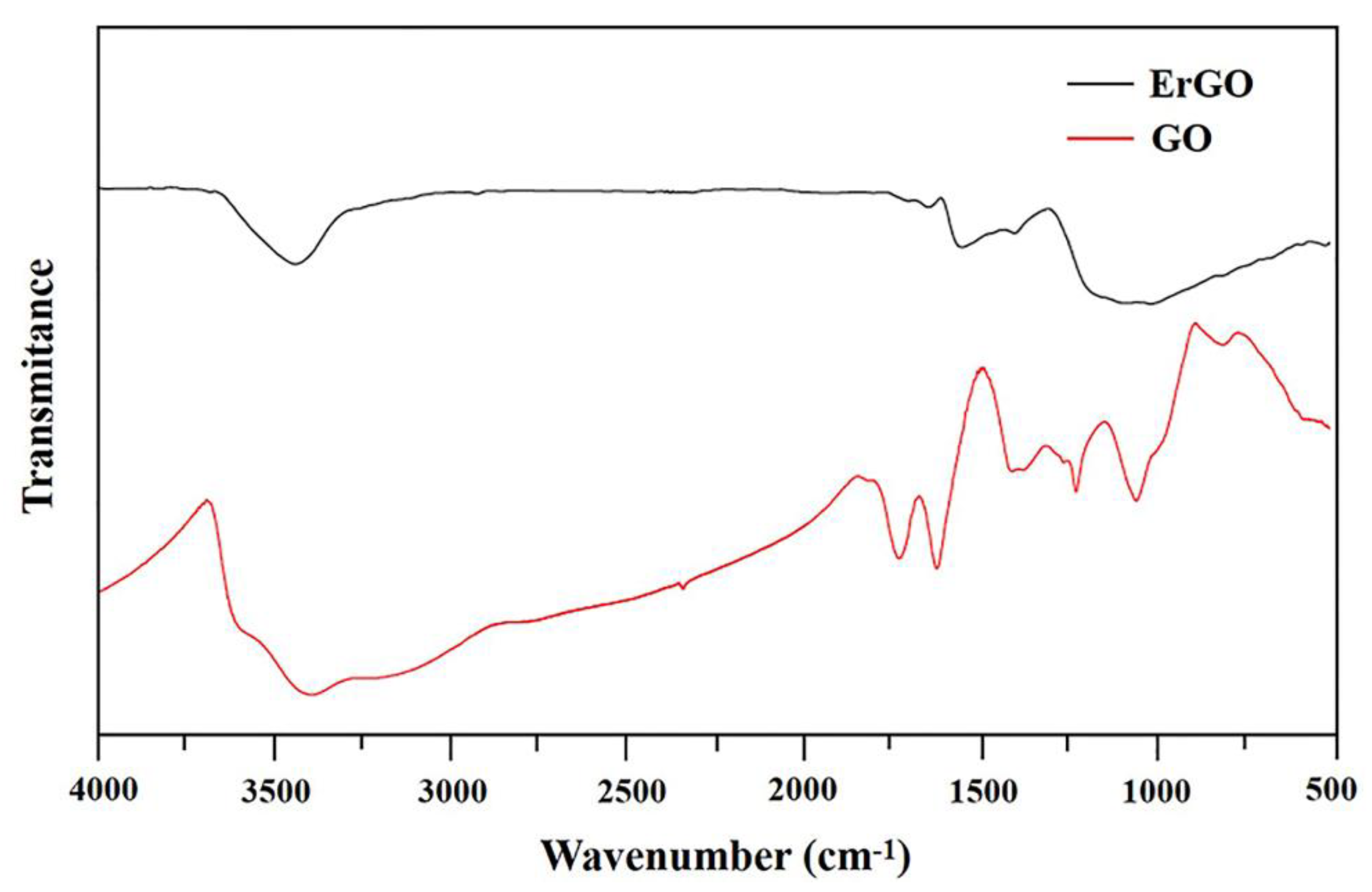
3.1. Materials Chararazation
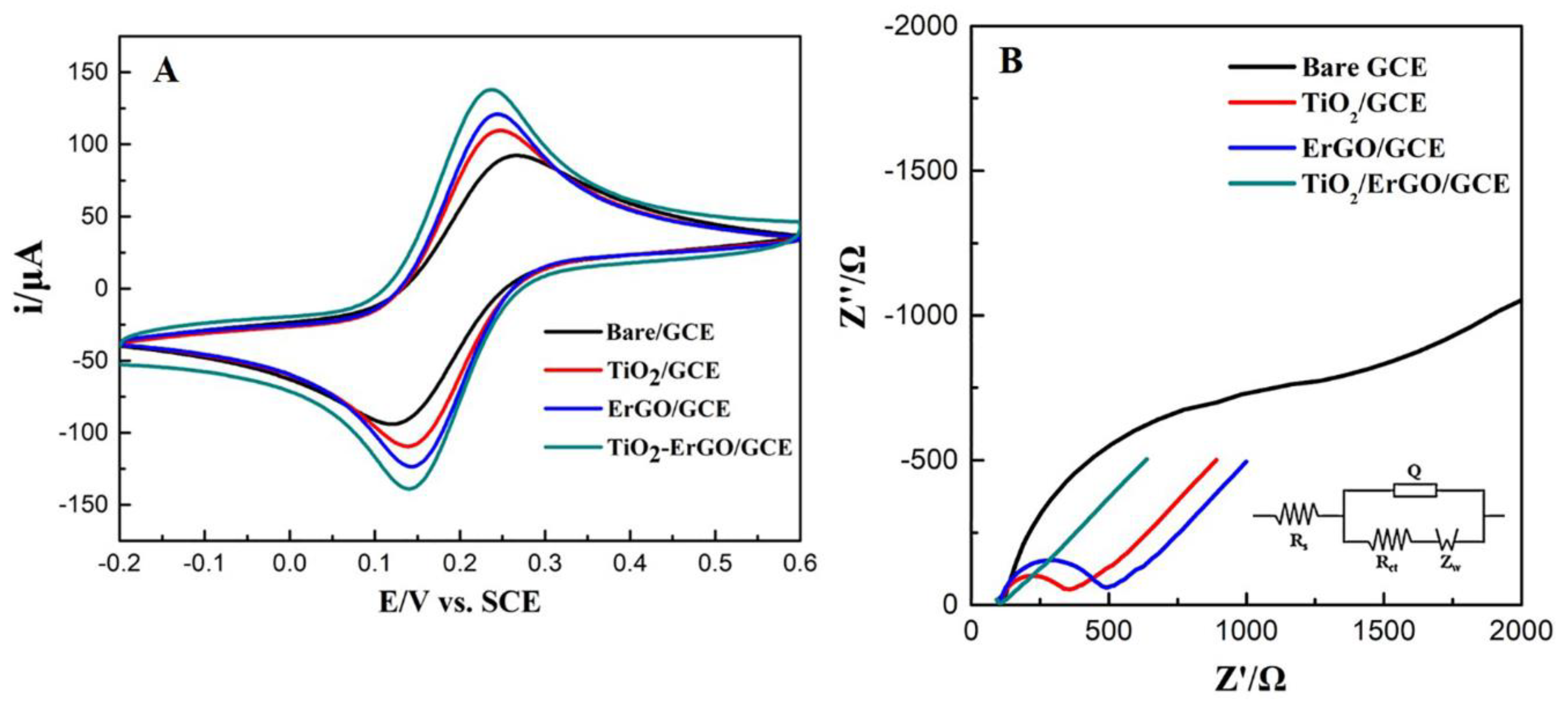
3.2. Assessement of Electrochemical Properties
3.3. Electrochemical Response of Allura Red on Different Modified Electrodes
3.4. Optimization of Analytical Conditions
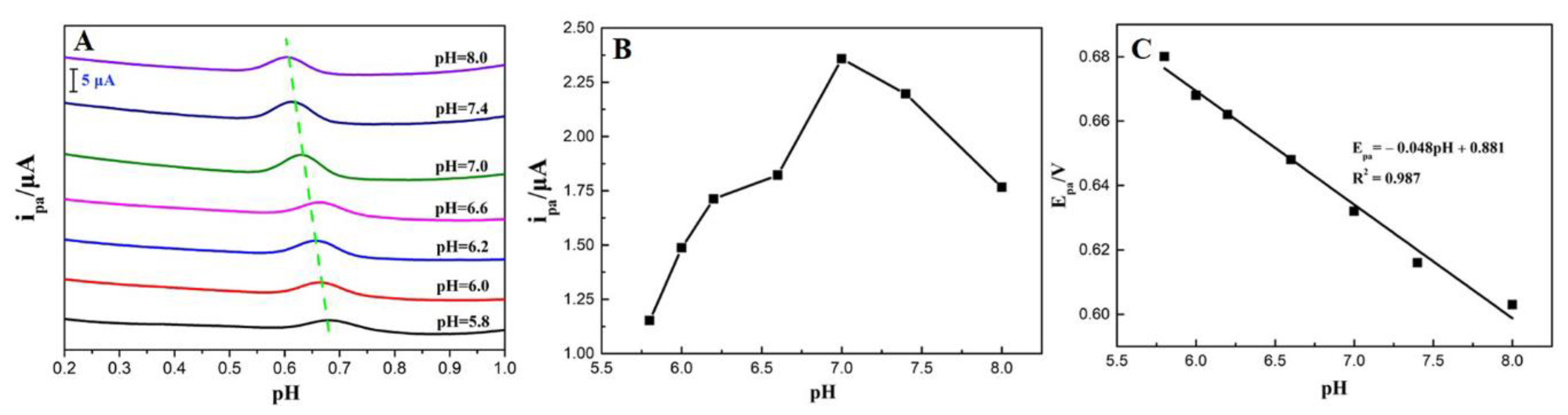
3.4.1. Effect of pH
3.4.2. Effect of Scan Rates
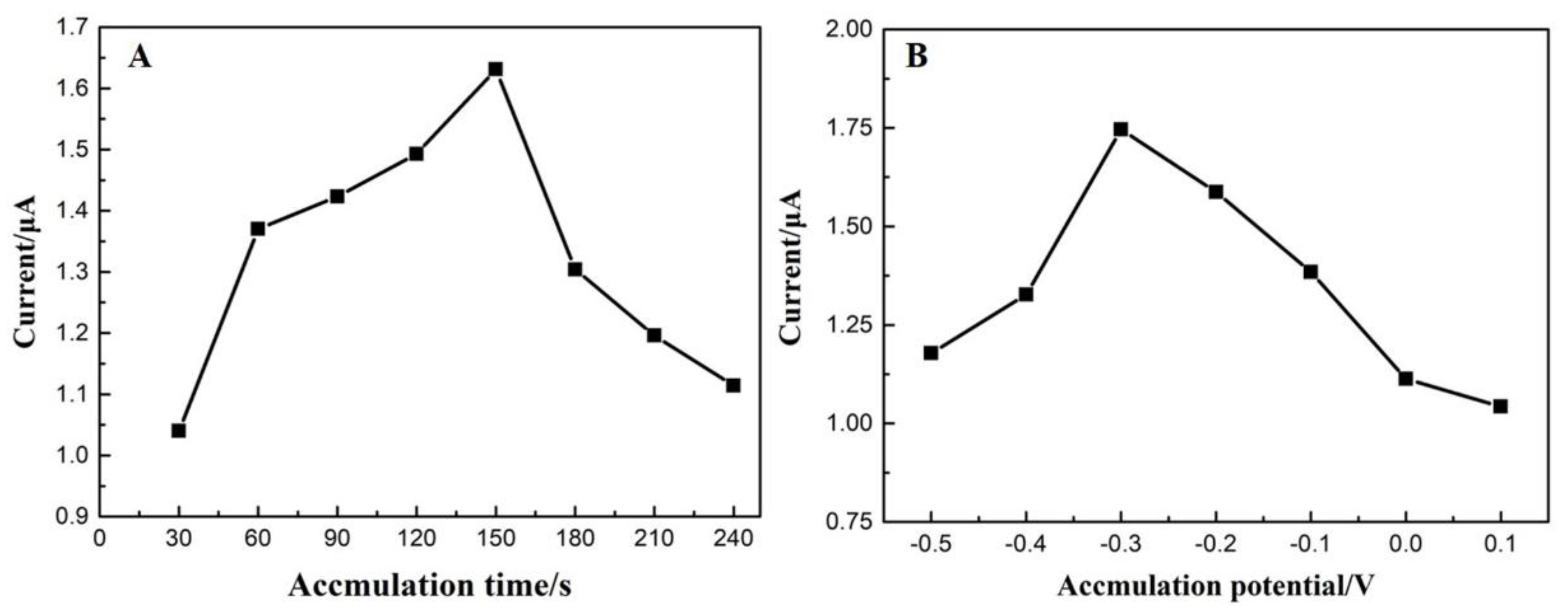
3.4.3. Effect of Accumulation Parameters
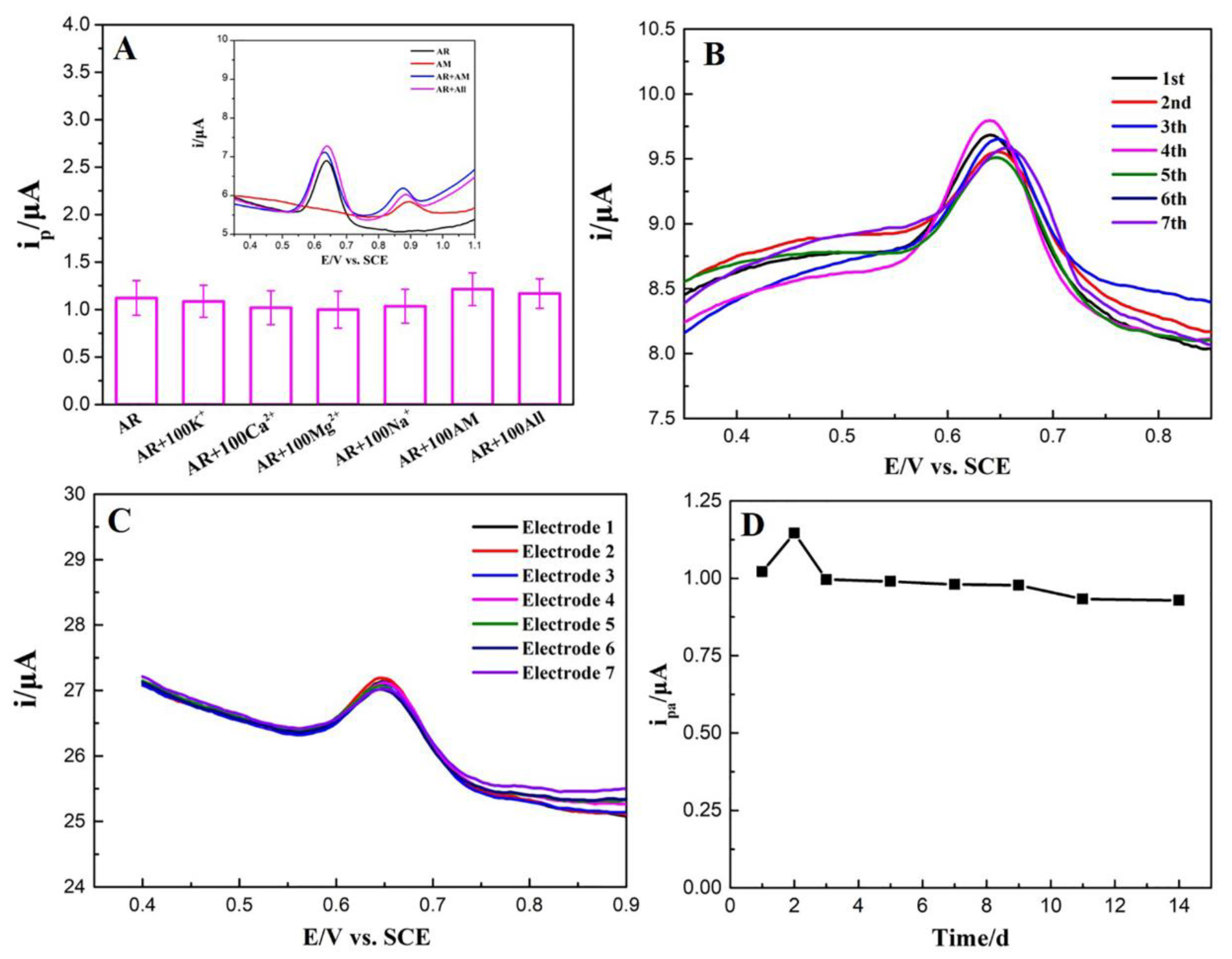
3.5. Selectivity, Reproducibility, and Stability
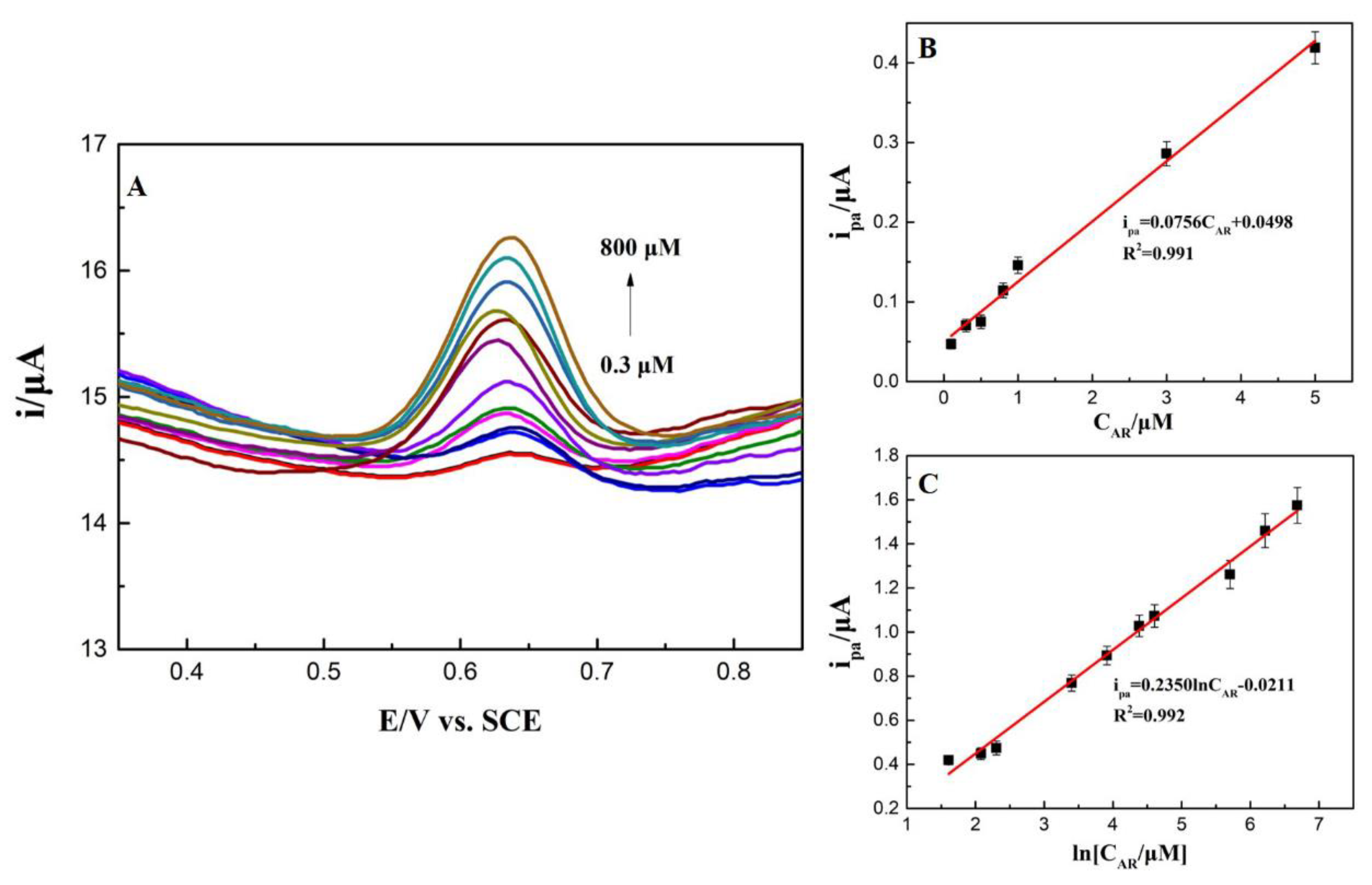
3.6. Standard Curves, Detection Range, and Detection Limit
3.7. Determination of Allura Red in Actual Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tanaka, T. Reproductive and neurobehavioural toxicity study of Ponceau 4R administered to mice in the diet. Food Chem. Toxicol. 2006, 44, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Dinç, E.; Baydan, E.; Kanbur, M.; Onur, F. Spectrophotometric multicomponent determination of sunset yellow, tartrazine and allura red in soft drink powder by double divisor-ratio spectra derivative, inverse least-squares and principal component regression methods. Talanta 2002, 58, 579–594. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products (NDA). Scientific Opinion on the appropriateness of the food azo-colours Tartrazine (E 102), Sunset Yellow FCF (E 110), Carmoisine (E 122), Amaranth (E 123), Ponceau 4R (E 124), Allura Red AC (E 129), Brilliant Black BN (E 151), Brown FK (E 154), Brown HT (E 155) and Litholrubine BK (E 180) for inclusion in the list of food ingredients set up in Annex IIIa of Directive 2000/13/EC. Efsa J. 2010, 8, 1778. [Google Scholar]
- Yu, L.; Shi, M.; Yue, X.; Qu, L. Detection of allura red based on the composite of poly (diallyldimethylammonium chloride) functionalized graphene and nickel nanoparticles modified electrode. Sens. Actuators B 2016, 225, 398–404. [Google Scholar] [CrossRef]
- Dixit, S.; Khanna, S.K.; Das, M. All India survey for analyses of colors in sweets and savories: Exposure risk in Indian population. J. Food Sci. 2013, 78, T642–T647. [Google Scholar] [CrossRef]
- Soylak, M.; Unsal, Y.E.; Tuzen, M. Spectrophotometric determination of trace levels of allura red in water samples after separation and preconcentration. Food Chem. Toxicol. 2011, 49, 1183–1187. [Google Scholar] [CrossRef]
- Minioti, K.S.; Sakellariou, C.F.; Thomaidis, N.S. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta 2007, 583, 103–110. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, Q.H.; Ma, K.; Li, H.M.; Guo, Z. Identification and determination of 34 water-soluble synthetic dyes in foodstuff by high performance liquid chromatography–diode array detection–ion trap time-of-flight tandem mass spectrometry. Food Chem. 2015, 182, 316–326. [Google Scholar] [CrossRef]
- Yoshioka, N.; Ichihashi, K.J.T. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 2008, 74, 1408–1413. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Hamza, A.; Al-Sibaai, A.A.; Bashammakh, A.S.; Al-Saidi, H.M. A new method for analysis of sunset yellow in food samples based on cloud point extraction prior to spectrophotometric determination. J. Ind. Eng. Chem. 2013, 19, 529–535. [Google Scholar] [CrossRef]
- Pourreza, N.; Rastegarzadeh, S.; Larki, A. Determination of Allura red in food samples after cloud point extraction using mixed micelles. Food Chem. 2011, 126, 1465–1469. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Shih, Y.-C.; Chen, Y.-C. Determining eight colorants in milk beverages by capillary electrophoresis. J. Chromatogr. 2002, 959, 317–325. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Chiu, C.-W.; Sue, S.-L.; Cheng, C.-F. Analysis of food colorants by capillary electrophoresis with large-volume sample stacking. J. Chromatogr. 2003, 995, 29–36. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Magesa, F.; Liu, J.; Li, G.; He, Q. Construction of effective electrochemical sensor for the determination of quinoline yellow based on different morphologies of manganese dioxide functionalized graphene. J. Food Compos. 2019, 84, 103280. [Google Scholar] [CrossRef]
- Li, G.; Zhong, P.; Ye, Y.; Wan, X.; Cai, Z.; Yang, S.; Xia, Y.; Li, Q.; Liu, J.; He, Q. A Highly Sensitive and Stable Dopamine Sensor Using Shuttle-Like α-Fe2O3 Nanoparticles/Electro-Reduced Graphene Oxide Composites. J. Electrochem. Soc. 2019, 166, B1552–B1561. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Xia, Y.; Tuo, D.; Deng, P.; Tian, Y.; Wu, Y.; Li, G.; Chen, D. Rapid and Sensitive Voltammetric Detection of Rhodamine B in Chili-Containing Foodstuffs Using MnO2 Nanorods/Electro-Reduced Graphene Oxide Composite. J. Electrochem. Soc. 2019, 166, B805–B813. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B 2018, 172, 565–572. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, C.; Gao, F. Ultrasensitive electrochemical detection of DNA based on Zn2+ assistant DNA recycling followed with hybridization chain reaction dual amplification. Biosens. Bioelectron. 2015, 63, 425–431. [Google Scholar] [CrossRef]
- Qian, Y.; Tang, D.; Du, L.; Zhang, Y.; Zhang, L.; Gao, F. A novel signal-on electrochemical DNA sensor based on target catalyzed hairpin assembly strategy. Biosens. Bioelectron. 2015, 64, 177–181. [Google Scholar] [CrossRef]
- Qian, Y.; Gao, F.; Du, L.; Zhang, Y.; Tang, D.; Yang, D. A novel label-free and enzyme-free electrochemical aptasensor based on DNA in situ metallization. Biosens. Bioelectron. 2015, 74, 483–490. [Google Scholar] [CrossRef] [PubMed]
- López-de-Alba, P.L.; López-Martínez, L.; De-León-Rodríguez, L.M. Simultaneous determination of synthetic dyes tartrazine, allura red and sunset yellow by differential pulse polarography and partial least squares. A multivariate calibration method. Electroanalysis 2002, 14, 197–205. [Google Scholar] [CrossRef]
- Chanlon, S.; Joly-Pottuz, L.; Chatelut, M.; Vittori, O.; Cretier, J. Determination of Carmoisine, Allura red and Ponceau 4R in sweets and soft drinks by Differential Pulse Polarography. J. Food Compos. Anal. 2005, 18, 503–515. [Google Scholar] [CrossRef]
- Silva, M.L.S.; Garcia, M.B.Q.; Lima, J.L.F.C.; Barrado, E. Voltammetric determination of food colorants using a polyallylamine modified tubular electrode in a multicommutated flow system. Talanta 2007, 72, 282–288. [Google Scholar] [CrossRef]
- Xie, X.Y.; Luo, H.Q.; Li, N.B. Determination of azo compounds by differential pulse voltammetry at a bismuth/poly (p-aminobenzene sulfonic acid) film electrode and application for detection in food stuffs. J. Electroanal. Chem. 2010, 639, 175–180. [Google Scholar] [CrossRef]
- Claux, B.; Vittori, O. Bismuth film electrode as an alternative for mercury electrodes: Determination of azo dyes and application for detection in food stuffs. Electroanalysis 2007, 19, 2243–2246. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Juárez, M.G.; Galán-Vidal, C.; Miranda, J.; Barrado, E. Determination of allura red and tartrazine in food samples by sequential injection analysis combined with voltammetric detection at antimony film electrode. Electroanalysis 2015, 27, 2329–2334. [Google Scholar] [CrossRef]
- Sierra-Rosales, P.; Toledo-Neira, C.; Ortúzar-Salazar, P.; Squella, J.A. MWCNT-modified Electrode for Voltammetric Determination of Allura Red and Brilliant Blue FCF in Isotonic Sport Drinks. Electroanalysis 2019, 31, 883–890. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Lu, X.; Yang, J.; Wu, K. Multi-wall carbon nanotube film-based electrochemical sensor for rapid detection of Ponceau 4R and Allura Red. Food Chem. 2010, 122, 909–913. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Wang, X.; Wang, W.; Chen, Z. Simultaneous determination of Ponceau-4R and Allura Red in soft drinks based on the ionic liquid modified expanded graphite paste electrode. Int. J. Environ. Anal. Chem. 2015, 95, 581–591. [Google Scholar] [CrossRef]
- Penagos-Llanos, J.; García-Beltrán, O.; Calderón, J.A.; Nagles, E.; Hurtado, J. Carbon Paste Composite with Co3O4 as a New Electrochemical Sensor for the Detection of Allura Red by Reduction. Electroanalysis 2019, 31, 695–703. [Google Scholar] [CrossRef]
- Penagos-Llanos, J.; García-Beltrán, O.; Calderón, J.A.; Hurtado-Murillo, J.J.; Nagles, E.; Hurtado, J.J. Simultaneous determination of tartrazine, sunset yellow and allura red in foods using a new cobalt-decorated carbon paste electrode. J. Electroanal. Chem. 2019, 852, 113517. [Google Scholar] [CrossRef]
- Zhou, W.-Y.; Liu, J.-Y.; Song, J.-Y.; Li, J.-J.; Liu, J.-H.; Huang, X.-J. Surface-electronic-state-modulated, single-crystalline (001) TiO2 nanosheets for sensitive electrochemical sensing of heavy-metal ions. Anal. Chem. 2017, 89, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.; Azab, S.; Fekry, A.; Ameer, M.J.B. Bioelectronics. Nano-TiO2 modified carbon paste sensor for electrochemical nicotine detection using anionic surfactant. Biosens. Bioelectron. 2016, 79, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.J.; Li, C.M.; Zang, J.F.; Cui, X.Q.; Qiao, Y.; Guo, J. New nanostructured TiO2 for direct electrochemistry and glucose sensor applications. Adv. Funct. Mater. 2008, 18, 591–599. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, K.-J.; Niu, D.-J.; Yang, C.-P.; Jing, Q.-S. TiO2-graphene nanocomposite for electrochemical sensing of adenine and guanine. Electrochim. Acta 2011, 56, 4685–4690. [Google Scholar] [CrossRef]
- Benvenuto, P.; Kafi, A.; Chen, A. High performance glucose biosensor based on the immobilization of glucose oxidase onto modified titania nanotube arrays. J. Electroanal. Chem. 2009, 627, 76–81. [Google Scholar] [CrossRef]
- Tong, H.; Zhao, L.; Li, D.; Zhang, X. N, Fe and WO3 modified TiO2 for degradation of formaldehyde. J. Alloy Compd. 2011, 509, 6408–6413. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Y.; Li, D.; He, W. Three-dimensional sea-urchin-like hierarchical TiO2 microspheres synthesized by a one-pot hydrothermal method and their enhanced photocatalytic activity. Mater. Res. Bull. 2013, 48, 2420–2425. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Zhou, Y.; Li, J. A situ hydrothermal synthesis of a two-dimensional MoS2/TiO2 heterostructure composite with exposed (001) facets and its visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2017, 28, 9003–9010. [Google Scholar] [CrossRef]
- Chen, C.; Cao, S.; Long, H.; Qian, G.; Tsang, Y.; Gong, L.; Yu, W.; Xiao, Y. Highly efficient photocatalytic performance of graphene oxide/TiO2–Bi2O3 hybrid coating for organic dyes and NO gas. J. Mater. Sci. Mater. Electron. 2015, 26, 3385–3391. [Google Scholar] [CrossRef]
- Dang, M.; Zhou, Y.; Li, H.; Lv, C. Preparation and photocatalytic activity of N-doped TiO2 nanotube array films. J. Mater. Sci. Mater. Electron. 2012, 23, 320–324. [Google Scholar] [CrossRef]
- Daniel, D.; Gutz, I.G.R. Microfluidic cell with a TiO2-modified gold electrode irradiated by an UV-LED for in situ photocatalytic decomposition of organic matter and its potentiality for voltammetric analysis of metal ions. Electrochem. Commun. 2007, 9, 522–528. [Google Scholar] [CrossRef]
- Wan, X.; Yang, S.; Cai, Z.; He, Q.; Ye, Y.; Xia, Y.; Li, G.; Liu, J. Facile Synthesis of MnO2 Nanoflowers/N-Doped Reduced Graphene Oxide Composite and Its Application for Simultaneous Determination of Dopamine and Uric Acid. Nanomaterials 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Ye, Y.; Wan, X.; Liu, J.; Yang, S.; Xia, Y.; Li, G.; He, Q. Morphology–Dependent Electrochemical Sensing Properties of Iron Oxide–Graphene Oxide Nanohybrids for Dopamine and Uric Acid. Nanomaterials 2019, 9, 835. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; Deng, P.; Chen, D. Facile electrochemical sensor for nanomolar rutin detection based on magnetite nanoparticles and reduced graphene oxide decorated electrode. Nanomaterials 2019, 9, 115. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Y.; Wan, X.; Yang, S.; Cai, Z.; Ye, Y.; Li, G. Morphology-dependent MnO2/nitrogen-doped graphene nanocomposites for simultaneous detection of trace dopamine and uric acid. Mater. Sci. Eng. C 2020, 109, 110615. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J. Phys. Chem. Lett. 2009, 1, 520–527. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Roh, K.-M.; Choi, J.-W.; Huang, J. A glucose biosensor based on TiO2–graphene composite. Biosens. Bioelectron. 2012, 38, 184–188. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, H.-T.; Liu, J.-H.; Yang, C.-P.; Jing, Q.-S.; Zhang, Y.-X.; Yang, X.-K.; Huang, K.-J. Hydrothermal preparation and electrochemical sensing properties of TiO2–graphene nanocomposite. Colloids Surf. B 2011, 83, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, J.-H.; Lu, H.-T.; Zhang, Q. Electrochemical behavior and voltammetric determination of paracetamol on Nafion/TiO2–graphene modified glassy carbon electrode. Colloids Surf. B 2011, 85, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, J.-H.; Lu, H.-T.; Zhang, Q. Electrochemistry and voltammetric determination of L-tryptophan and L-tyrosine using a glassy carbon electrode modified with a Nafion/TiO2-graphene composite film. Microchim. Acta 2011, 173, 241–247. [Google Scholar] [CrossRef]
- Alamdari, S.; Ghamsari, M.S.; Afarideh, H.; Mohammadi, A.; Geranmayeh, S.; Tafreshi, M.J.; Ehsani, M.H. Preparation and characterization of GO-ZnO nanocomposite for UV detection application. Opt. Mater. 2019, 92, 243–250. [Google Scholar] [CrossRef]
- Guangli, L.; Jingtao, W.; Yonghui, X.; Yiyong, W.; Yaling, T.; Jun, L.; Dongchu, C.; Quanguo, H. Towards emerging EEG applications: A novel printable flexible Ag/AgCl dry electrode array for robust recording EEG signals at forehead sites. J. Neural Eng. 2020. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Duan, Y.Y. Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sens. Actuators B 2018, 277, 250–260. [Google Scholar] [CrossRef]
- Nagles, E.; Garcia-Beltran, O. Determination of Allura Red in the Presence of Cetylpyridinium Bromide by Square-wave Adsorptive Stripping Voltammetry on a Glassy Carbon Electrode. Anal. Sci. 2018, 34, 1171–1175. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]




| Methods | Electrodes | Peak Potential (V) | Detection Range (μg/L) | LOD (μg/L) | Ref |
|---|---|---|---|---|---|
| DPV | MWCNT/GCE | 0.68 | 50–600 | 24.8 | [29] |
| SWV | CoOx/CPE | −0.25 | 49.6–496 | 24.8 | [31] |
| DPV | MWCNT/GCE | 0.72 | 496–4468 | 7.0 | [28] |
| SWV | IL-EGPE | 0.72 | 500–5000 | 0.89 | [30] |
| DPV | SbF/SPCE | −0.68 | 500–2500 | 149 | [27] |
| SWV | CoC/CPE | 0.85 | 59.6–1489 | 39.7 | [32] |
| SWV | GCE with CPB | 0.91 | 29.8–5709 | 14.9 | [57] |
| SWV | PAMI/GCE | −0.18 | 4964–148,920 | 695 | [24] |
| DPV | TiO2/ErGO/GCE | 0.62 | 149–2482; 2482–397,120 | 24.8 | This work |
| Samples | Detected (μM) | RSD (%) | Added (μM) | Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|---|
| Milk drinking | 14.28 | 4.26 | 1 | 15.34 | 2.81 | 106.0 |
| 14.28 | 4.26 | 10 | 24.69 | 0.85 | 104.1 | |
| 14.28 | 4.26 | 100 | 112.7 | 4.86 | 98.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wu, J.; Jin, H.; Xia, Y.; Liu, J.; He, Q.; Chen, D. Titania/Electro-Reduced Graphene Oxide Nanohybrid as an Efficient Electrochemical Sensor for the Determination of Allura Red. Nanomaterials 2020, 10, 307. https://doi.org/10.3390/nano10020307
Li G, Wu J, Jin H, Xia Y, Liu J, He Q, Chen D. Titania/Electro-Reduced Graphene Oxide Nanohybrid as an Efficient Electrochemical Sensor for the Determination of Allura Red. Nanomaterials. 2020; 10(2):307. https://doi.org/10.3390/nano10020307
Chicago/Turabian StyleLi, Guangli, Jingtao Wu, Hongguang Jin, Yonghui Xia, Jun Liu, Quanguo He, and Dongchu Chen. 2020. "Titania/Electro-Reduced Graphene Oxide Nanohybrid as an Efficient Electrochemical Sensor for the Determination of Allura Red" Nanomaterials 10, no. 2: 307. https://doi.org/10.3390/nano10020307
APA StyleLi, G., Wu, J., Jin, H., Xia, Y., Liu, J., He, Q., & Chen, D. (2020). Titania/Electro-Reduced Graphene Oxide Nanohybrid as an Efficient Electrochemical Sensor for the Determination of Allura Red. Nanomaterials, 10(2), 307. https://doi.org/10.3390/nano10020307






