A SnO2/CeO2 Nano-Composite Catalyst for Alizarin Dye Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SnO2/CeO2 Nano-Composite Catalyst
2.3. Characterization of Nano-Composite Catalyst
2.4. Dye Uptake Study
2.5. Adsorption Studies
3. Results and Discussions
3.1. Characterization of the Nano-Composite Catalyst
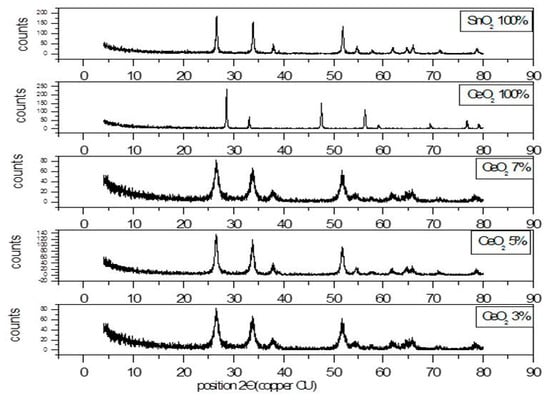
3.1.1. X-ray Diffraction Pattern of SnO2/CeO2 Nano-Composite
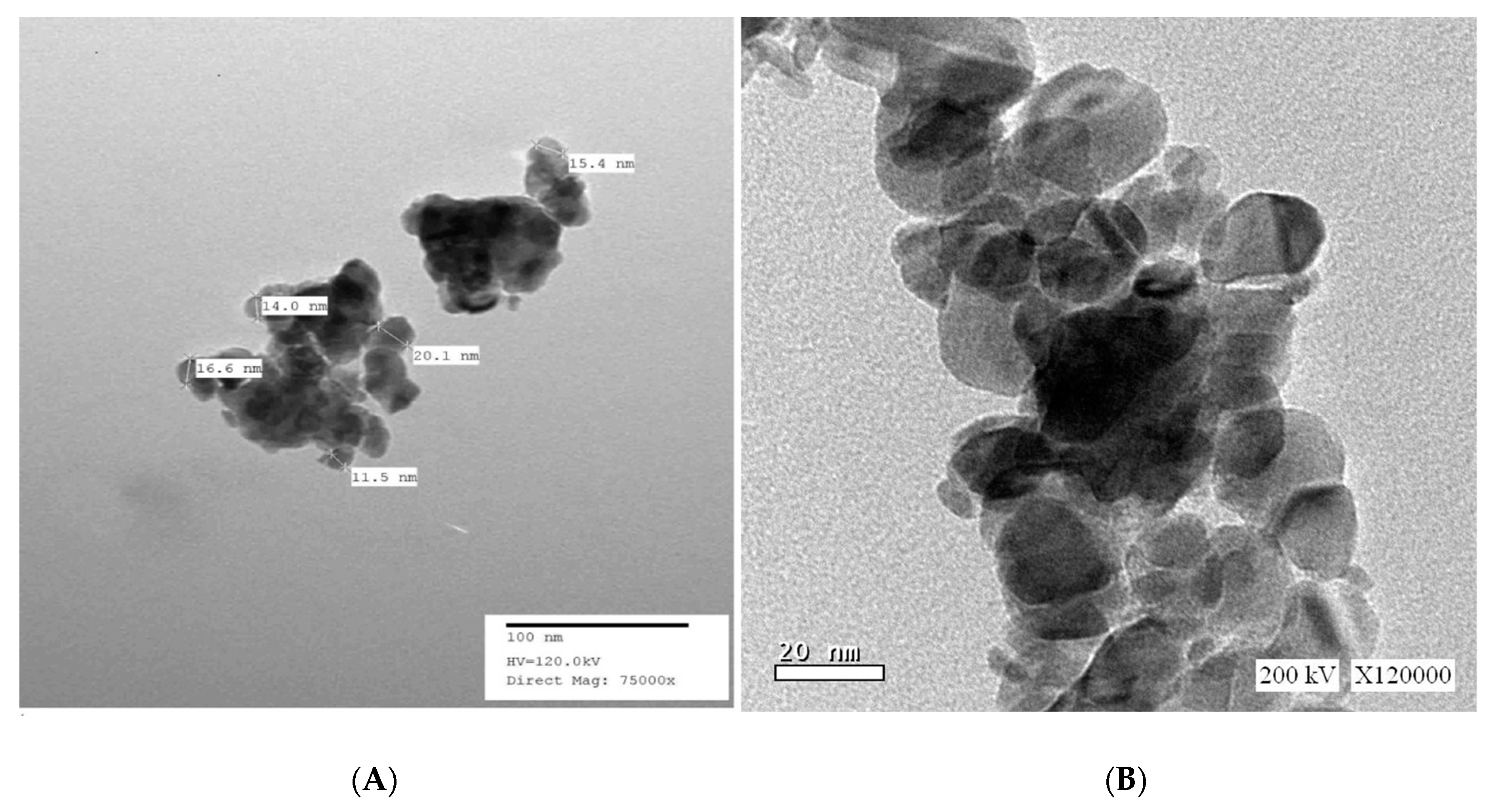
3.1.2. High-Resolution Transmission Electron Microscopy (HR-TEM) of SnO2/CeO2 Nano-Composite
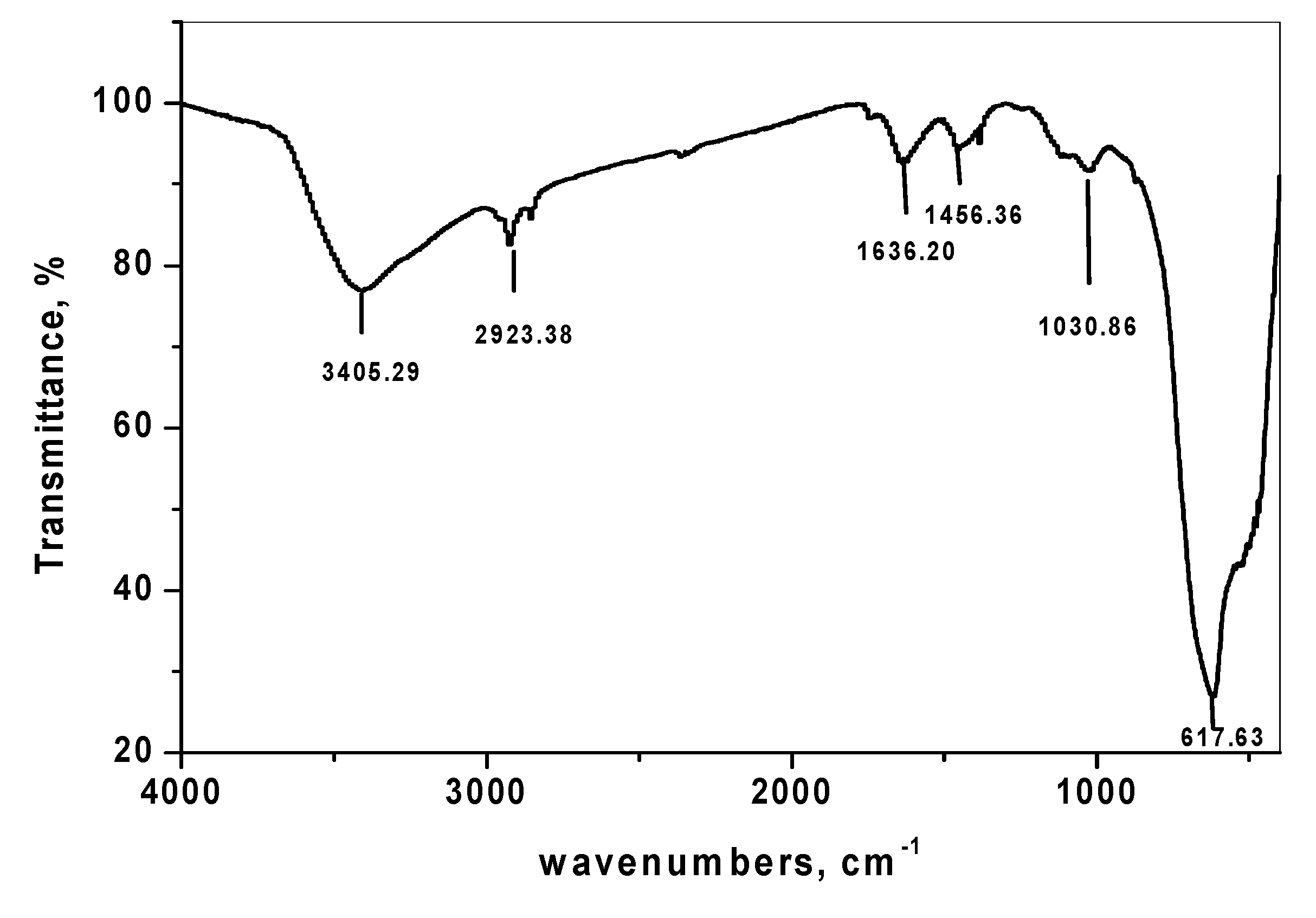
3.1.3. Fourier Transform Infrared Spectroscopy of SnO2/CeO2 Nano-Composite
3.1.4. Porous Structure
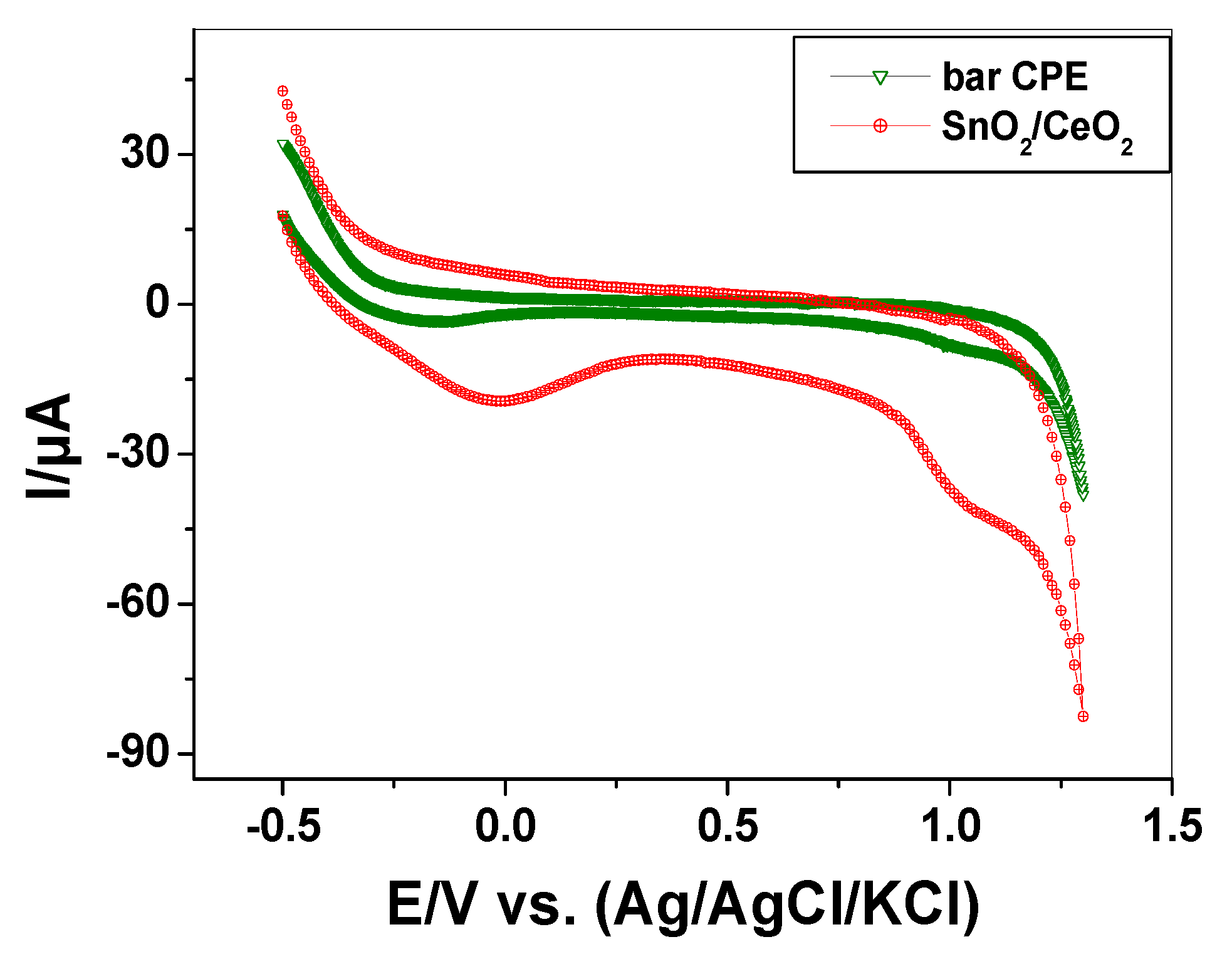
3.1.5. Electrocatalytic Behavior of SnO2/CeO2 Nano-Composite
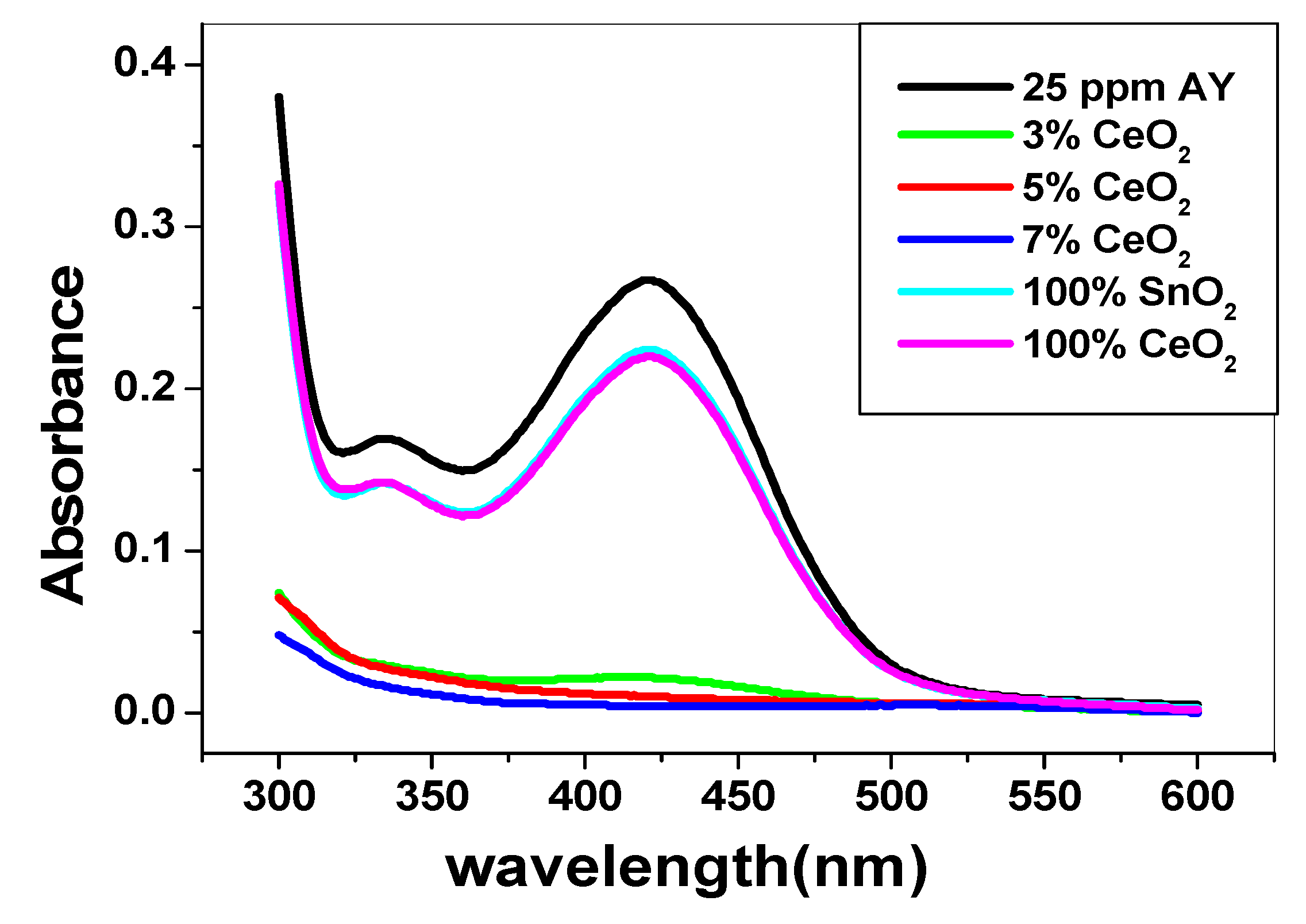
3.2. Use of SnO2/CeO2 Nano-Composite for Alizarin Dye Removal
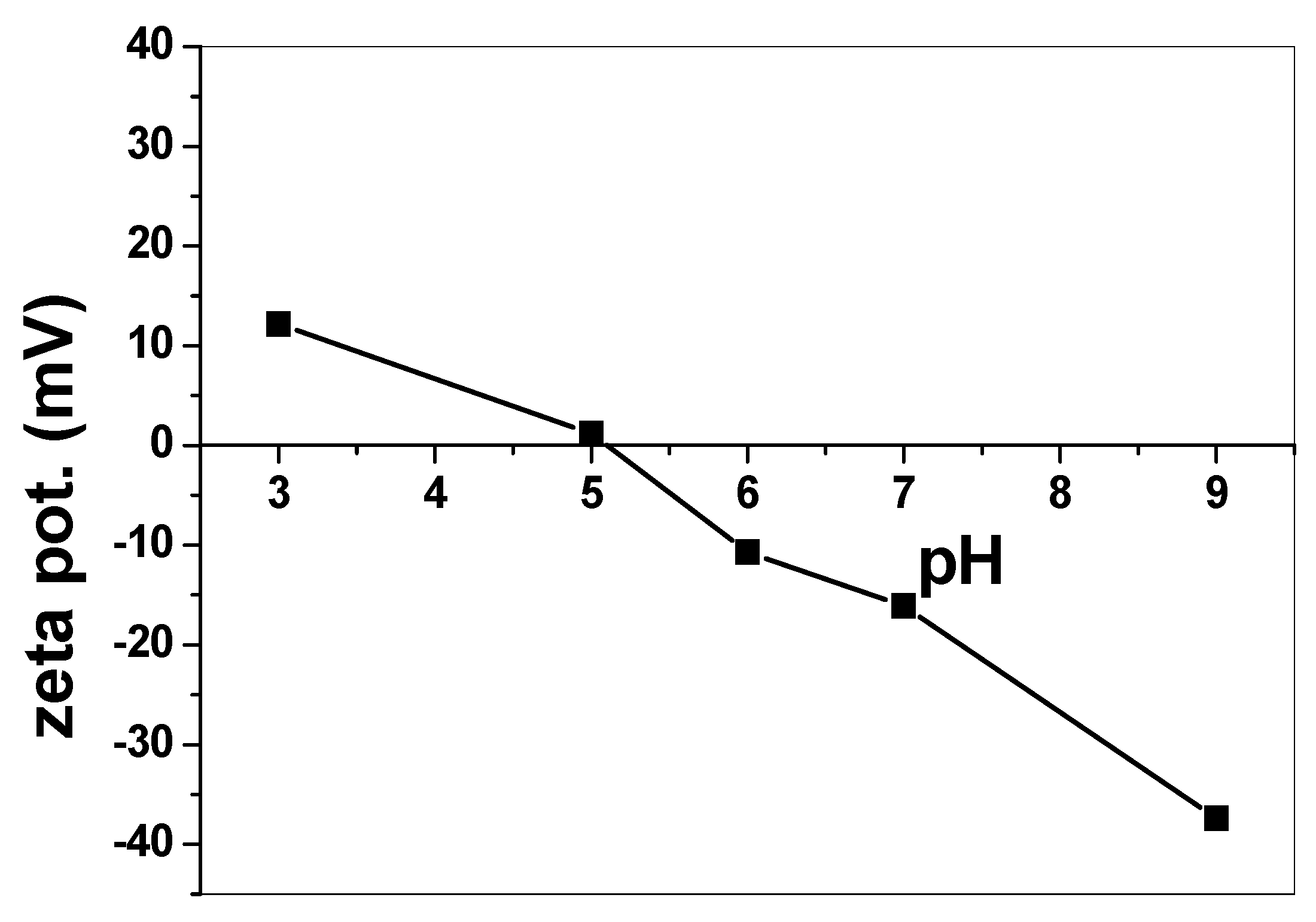
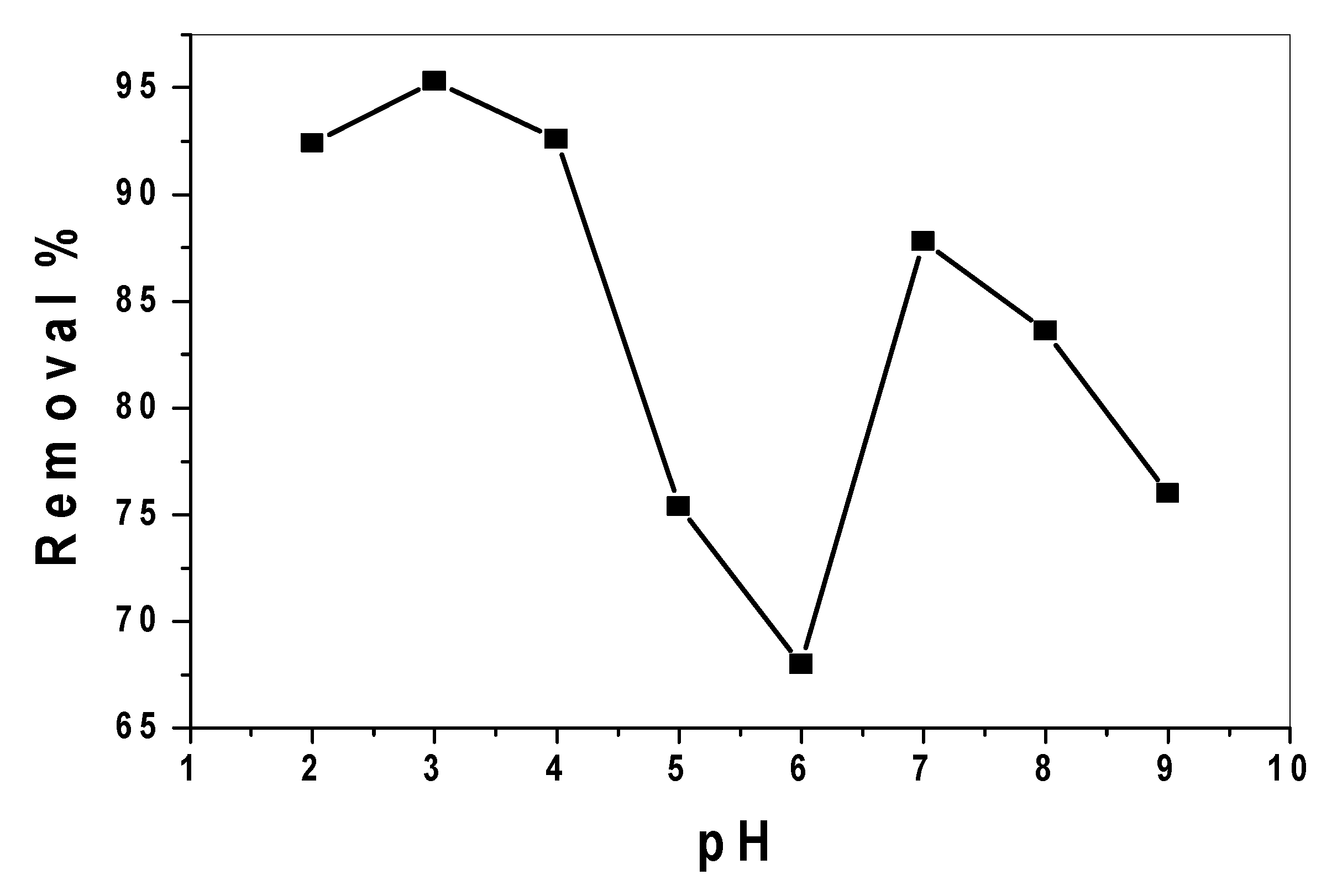
3.2.1. Effect of pH and Catalytic Activity of SnO2/CeO2 Nano-Composite
3.2.2. Effect of Contact Time
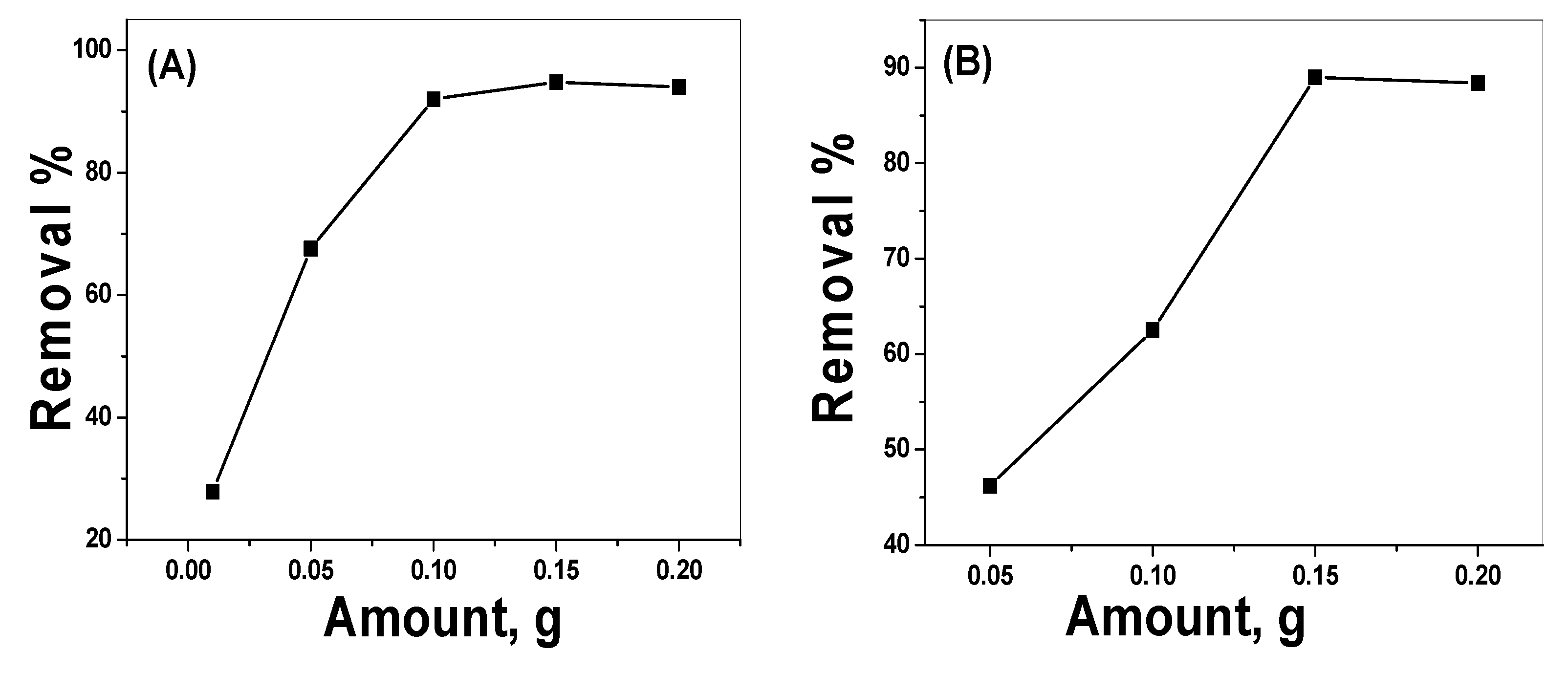
3.2.3. Effect of the Catalyst Dose
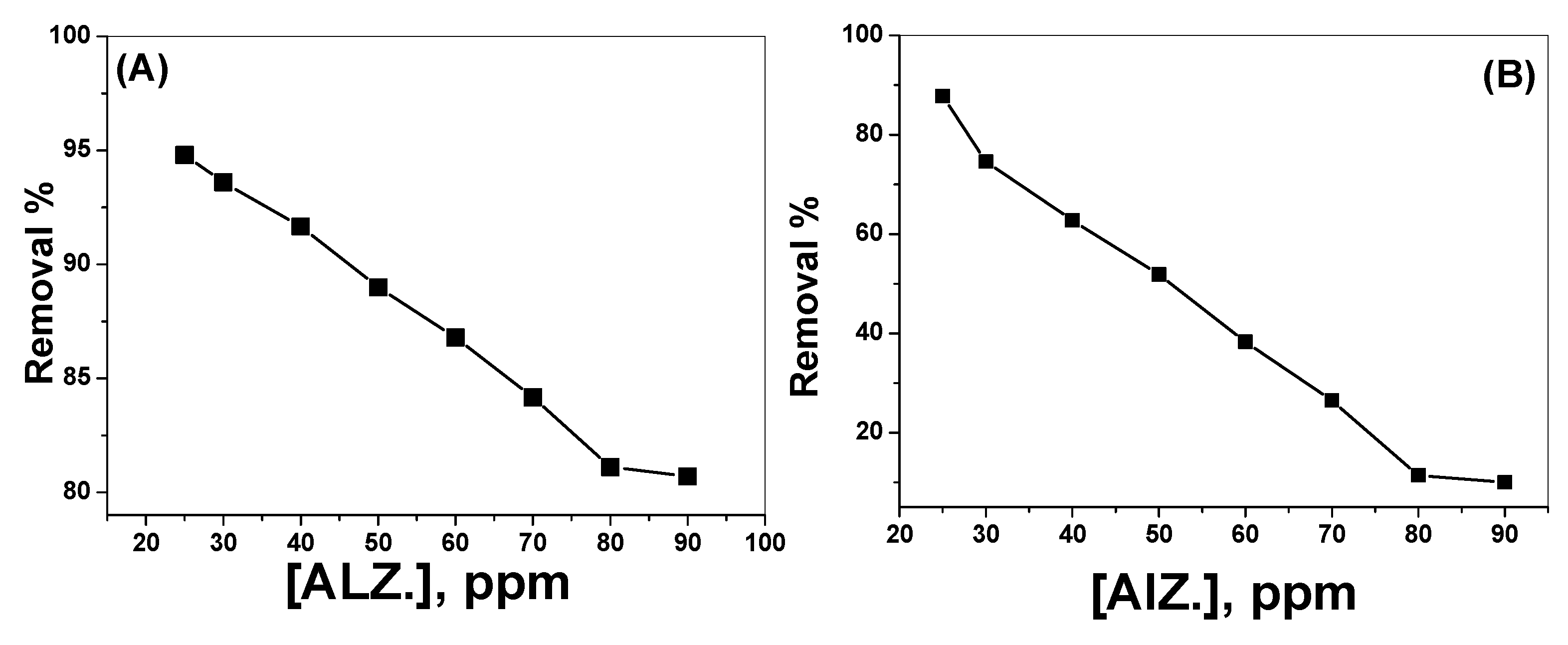
3.2.4. Effect of Alizarin Dye Concentration
3.3. Removal of Alizarin-3-Methylimino-Diacetic Acid (AMA)
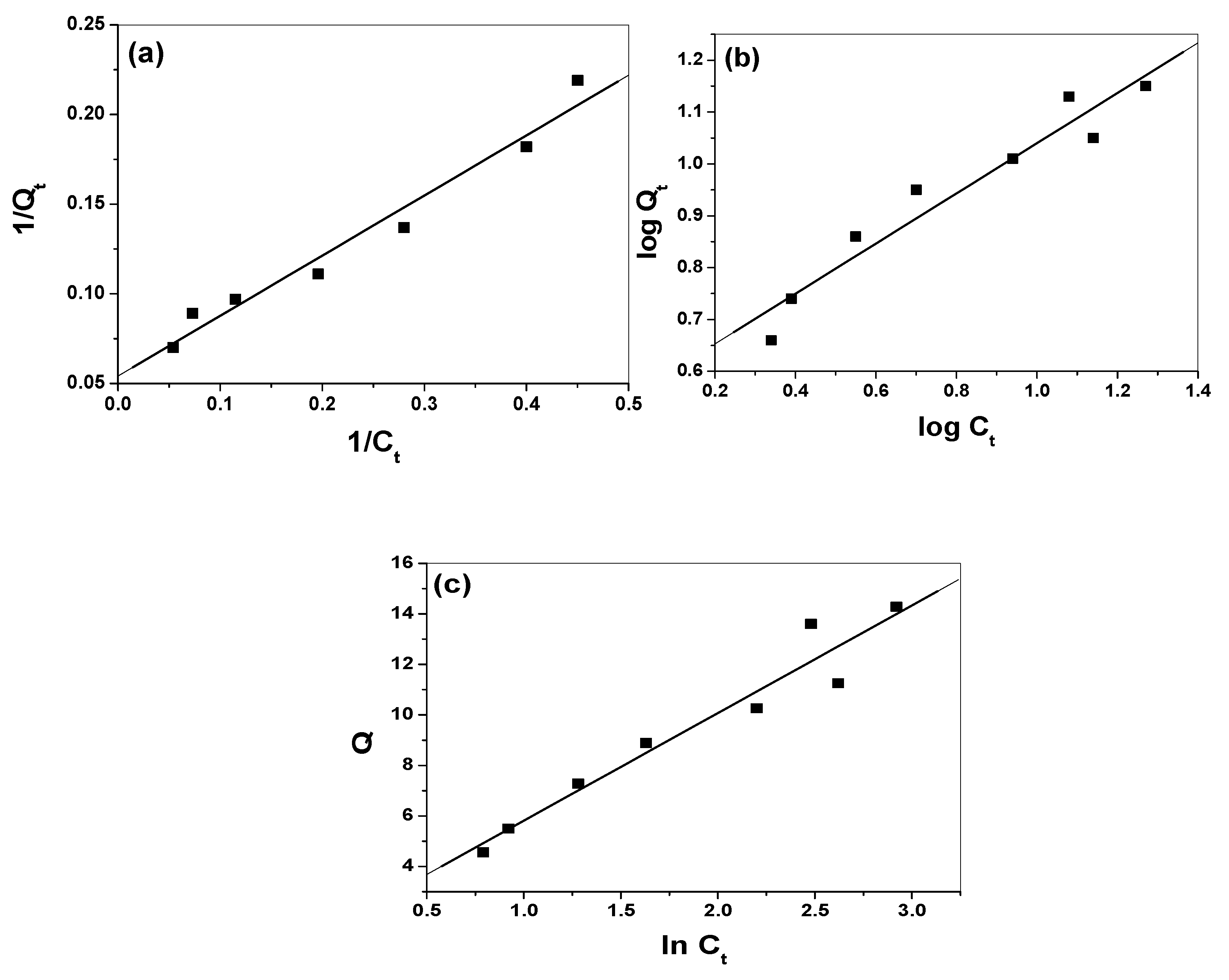
3.4. Modeling of Adsorption
3.5. Regeneration of SnO2/CeO2 Nano-Composite
3.6. Comparison with Other Sorbents for Removal of Alizarin
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Namasivayam, C.; Kavitha, D. Removal of congo red from water by adsorption onto activated carbon prepared from coir pith an agricultural solid waste. Dyes Pigm. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Parra, S.; Stanca, S.E.; Guasaquillo, I.; Thampi, K.R. Photo-catalytic degradation of atrazine using suspended and supported TiO2. Appl. Catal. B-Environ. 2004, 51, 107–116. [Google Scholar] [CrossRef]
- Zucca, P.; Vinci, C.; Sollai, F.; Rescigno, A.; Sanjust, E. Degradation of Alizarin Red S under mild experimental conditions by immobilized 5,10,15,20-tetrakis (4-sulfonatophenyl) porphine-Mn(III) as a biomimetic peroxidase like catalyst. J. Mol. Catal. A-Chem. 2008, 288, 97–102. [Google Scholar] [CrossRef]
- Riu, J.; Schönsee, I.; Barceló, D.; Ràfols, C. Determination of sulphonated azo dyes in water and wastewater. TrAC Tr. Anal. Chem. 1997, 16, 405–419. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Kurup, L.; Singh, A.K. Process development for the removal and recovery of hazardous dye erythrosine from wastewater by waste materials bottom ash and de-oiled soya as adsorbents. J. Hazard. Mater. 2006, 138, 95–105. [Google Scholar] [CrossRef]
- Joshi, K.M.; Shrivastava, V.S. Degradation of Alizarin Red-S (A Textiles Dye) by Photocatalysis using ZnO and TiO2 as Photocatalyst. Int. J. Environ. Sci. 2011, 2, 8–21. [Google Scholar]
- Seddigi, Z.S. Removal of Alizarin Yellow Dye from Water Using Zinc Doped WO3 Catalyst. Bull. Environ. Contam. Toxicol. 2010, 84, 564–567. [Google Scholar] [CrossRef]
- Xiong, Z.; Xu, A.; Li, H.; Ruan, X.; Xia, D.; Zeng, Q. Highly Efficient Photodegradation of Alizarin Green in TiO2 Suspensions Using a Microwave Powered Electrodeless Discharged Lamp. Ind. Eng. Chem. Res. 2013, 52, 362–369. [Google Scholar]
- Roopaei, H.; Zohdi, A.R.; Abbasi, Z.; Bazrafkan, M. Preparation of New Photocatalyst for Removal of Alizarin Red-S from Aqueous Solution. Ind. J. Sci. Tech. 2014, 7, 1882–1887. [Google Scholar]
- Panizza, M.; Oturan, M.A. Degradation of Alizarin Red by Electro-Fenton Process Using A Graphite-Felt Cathode. Electrochim. Acta 2011, 56, 7084–7087. [Google Scholar] [CrossRef]
- Hasan, M.; Kumar, R.; Barakat, M.A.; Lee, M. Synthesis of PVC/CNT nanocomposite fibers using a simple deposition technique for the application of Alizarin Red S (ARS) removal. RSC Adv. 2015, 5, 14393–14399. [Google Scholar] [CrossRef]
- Fayazi, M.; Motlagh, M.G.; Taher, M.A. The adsorption of basic dye (Alizarin red S) from aqueous solution onto activated carbon/γ-Fe2O3nano-composite: Kinetic and equilibrium studies. Mat. Sci. Semicond. Proc. 2015, 40, 35–43. [Google Scholar] [CrossRef]
- Hanif, S.; Shahzad, A. Removal of chromium(VI) and dye Alizarin Red S (ARS) using polymer-coated iron oxide (Fe3O4) magnetic nanoparticles by co-precipitation method. J. Nanopart. Res. 2014, 16, 2429–2444. [Google Scholar] [CrossRef]
- Jadhava, H.V.; Khetre, S.M.; Bamane, S.R. Removal of alizarin red-S from aqueous solution by adsorption on nanocrystalline Cu0.5Zn0.5Ce3O5. Der Chem. Sin. 2011, 2, 68–75. [Google Scholar]
- Ahmad, R.; Kumar, R. Comparative adsorption study for the removal of Alizarin Red S and patent Blue VF by using mentha waste. Curr. World Environ. 2008, 3, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Ghaedia, M.; Najibia, A.; Hossainiana, H.; Shokrollahia, A.; Soylakbc, M. Kinetic and equilibrium study of Alizarin Red S removal by activated carbon. Toxicol. Environ. Chem. 2012, 94, 40–48. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Yamini, Y.; Dayeni, M.; Seidi, S.; Tahmasebi, E. Adsorptive removal of alizarin red-S and alizarin yellow GG from 2 aqueous solutions using polypyrrole-coated magnetic nanoparticles. J. Environ. Chem. Eng. 2015, 3, 529–540. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Li, X.; Luo, C.; Lu, F.; Qiu, H. Removal of alizarin red from water environment using magnetic chitosan with Alizarin Red as imprinted molecules. Colloid. Surf. B 2012, 91, 250–257. [Google Scholar] [CrossRef]
- Salam, N.A.; Buhari, M. Adsorption of Alizarin and Fluorescein Dyes on Adsorbent prepared from Mango Seed. Pac. J. Sci. Technol. 2014, 15, 232–244. [Google Scholar]
- Shakeel, F.; Haq, N.; Alanazi, F.K.; Alsarra, I.A. Removal of alizarin red from aqueous solution by ethyl acetate green nanoemulsions. Water Sci. Technol. 2014, 70, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Crt, T.; Maz, A. Biosorption of heavy metals using rice milling byproducts: characterization and application for removal of metals from aqueous effluents. Chemosphere 2004, 54, 987–995. [Google Scholar]
- Mayo, J.T.; Yavuz, C.; Yean, S.; Cong, L.; Shipley, H.; Yu, W.; Falkner, J.; Kan, A.; Tomson, M.; Colvin, V.L. The effect of nanocrystalline magnetite size on arsenic removal. Sci. Technol. Adv. Mater. 2007, 8, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Messih, M.F.; Ahmed, M.A.; El-Sayed, A.S. Photocatalytic decolorization of Rhodamine B dye using novel mesoporous SnO2–TiO2 nano mixed oxides prepared by sol–gel method. J. Photochem. Photobiol. A-Chem. 2013, 260, 1–8. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. CeO2-ZnO hexagonal nanodisks: Efficient material for the degradation of direct blue 15 dye and its simulated dye bath effluent under solar ligh. J. Alloy. Compd. 2015, 620, 67–73. [Google Scholar] [CrossRef]
- Tomić, N.M.; Dohčević-Mitrović, Z.D.; Paunović, N.M.; Mijin, D.Z.; Radić, N.D.; Grbić, B.V.; Aškrabić, S.M.; Babić, B.M.; Bajuk-Bogdanović, D.V. Nanocrystalline CeO2−δ as Effective Adsorbent of Azo Dyes. Langmuir 2014, 30, 11582–11590. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhang, G.; Zhang, Q. MnO2/CeO2 for catalytic ultrasonic degradation of methyl orange. Ultrason. Sonochem. 2014, 21, 991–996. [Google Scholar] [CrossRef]
- Pradhan, G.K.; Parida, K.M. Fabrication of iron-cerium mixed oxide: an efficient photocatalyst for dye degradation. Int. J. Eng. Sci. Technol. 2010, 2, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Guo, K. Diffraction of Crystal and Pseudo Crystal; Beijing University Publishing House: Beijing, China, 1999. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- El-Desoky, H.S.; Ghoneim, M.M.; El-badawy, F.M. Carbon Nanotubes Modified Electrode for Enhanced Voltammetric Sensing of Mebeverine Hydrochloride in Formulations and Human Serum Samples. J. Electrochem. Soc. 2017, 164, B212–B222. [Google Scholar] [CrossRef]
- Lurie, J. Handbook of Analytical Chemistry; Mir Publishers: Moscow, Russia, 1975. [Google Scholar]
- Takahashi, S.; Suzuki, I.; Sugawara, T.; Seno, M.; Minaki, D.; Anzai, J. Alizarin Red S-Confined Layer-By-Layer Films as Redox-Active Coatings on Electrodes for the Voltammetric Determination of L-Dop. Materials 2017, 10, 581. [Google Scholar] [CrossRef] [Green Version]
- Balarak, D.; Mostafapour, F.K.; Azarpira, H.; Joghataei, A. Langmuir, Freundlich, Temkin and Dubinin–radushkevich Isotherms Studies of Equilibrium Sorption of Ampicilin unto Montmorillonite Nanoparticles. J. Pharm. Res. Int. 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Esmat, M.; Farghali, A.A.; Khedr, M.H.; El-Sherbiny, I.M. Alginate-based nanocomposites for efficient removal of heavy metal ions. Int. J. Biol. Macromol. 2017, 102, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubayee, W.T.; Abdul-Rasheed, O.F.; Ali, N.M. Preparation of a Modified Nanoalumina Sorbent for the Removal of Alizarin Yellow R and Methylene Blue Dyes from Aqueous Solutions. J. Chem. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Wu, K.; Yu, J.; Jiang, X. Multi-walled carbon nanotubes modified by polyaniline for the removal of alizarin yellow R fromaqueous solutions. Adsorp. Sci. Technol. 2018, 36, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Hashemian, S.; Rahimi, M.; Kerdegari, A.A. CuFe2O4@graphene nanocomposite as a sorbent for removal of alizarine yellow azo dye from aqueous solutions. Desalin. Water Treat. 2015, 57, 1–12. [Google Scholar] [CrossRef]
- Ali, O.; Mohamed, S. Adsorption of copper ions and alizarin red S from aqueous solutions onto a polymeric nanocomposite in single and binary systems. Turkish J. Chem. 2017, 41, 967–986. [Google Scholar] [CrossRef]
- Gautam, P.K.; Gautam, R.K.; Banerjee, S.; Chattopadhyaya, M.C.; Pandey, J.D. Adsorptive Removal of Alizarin Red S by a Novel Biosorbent of an Invasive Weed Mikania micrantha. Natl. Acad. Sci. Lett. 2017, 40, 113–116. [Google Scholar] [CrossRef]
- Liang, Y.; He, Y.; Yu-hang Zhang, Y.; Zhu, Q. Adsorption Property of Alizarin Red S by NiFe2O4/Polyaniline Magnetic Composite. J. Environ. Chem. Eng. 2018, 6, 416–425. [Google Scholar] [CrossRef]
- Absalan, G.; Bananejad, A.; Ghaemi, M. Removal of Alizarin Red and Purpurin from Aqueous Solutions Using Fe3O4 Magnetic Nanoparticles. Anal. Bioanal. Chem. Res. 2017, 4, 65–77. [Google Scholar]
- Adlnasab, L.; Ezoddin, M.; Karimi, M.A.; Hatamikia, N. MCM-41@Cu–Fe–LDH magnetic nanoparticles modified with cationic surfactant for removal of Alizarin Yellow from water samples and its determination with HPLC. Res. Chem. Intermed. 2018, 44, 3249–3265. [Google Scholar] [CrossRef]
- Wanassi, B.; Hariz, I.B.; Ghimbeu, C.M.; Vaulot, C.; Jeguirim, M. Green Carbon Composite-Derived Polymer Resin and Waste Cotton Fibers for the Removal of Alizarin Red S Dye. Energies 2017, 10, 1321. [Google Scholar] [CrossRef] [Green Version]
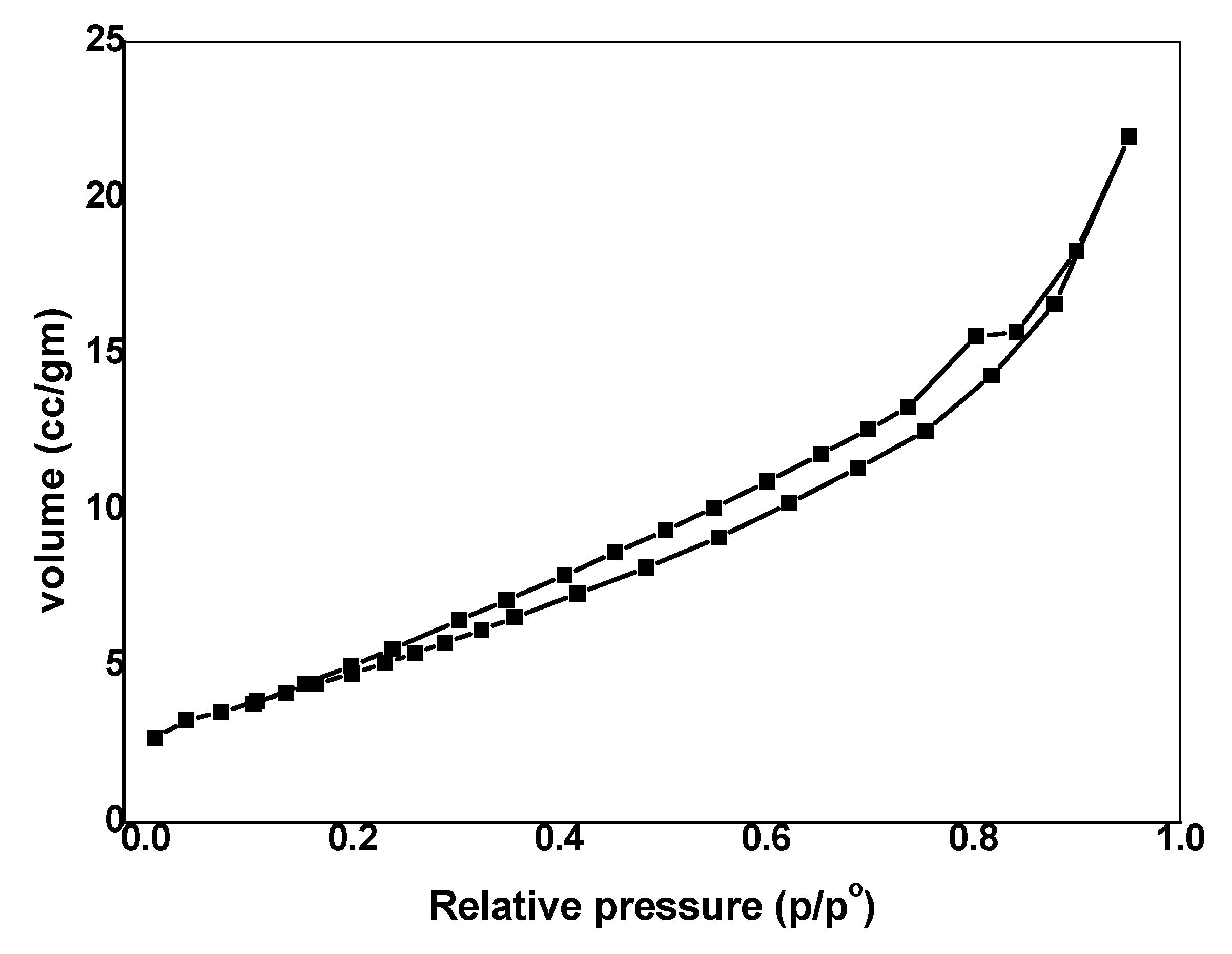

| Sample | Surface Area (m2 g−1) | Average Pore Volume, (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| SnO2/CeO2 NPs | 18.970 | 3.399 × 10−2 | 7.164 |
| System | Standard Redox Potential, E° (V) | Ref. |
|---|---|---|
Ce4+(aq) + e−  Ce3+(aq) Ce3+(aq) | +1.61 | [32] |
Sn4+(aq) + 2 e−  Sn2+(aq) Sn2+(aq) | +0.15 | |
Sn2+(aq) + 2 e−  Sn(s) Sn(s) | −0.14 | |
Alizarin  Alizarin oxidation product Alizarin oxidation product | −0.59 | [33] |
| Adsorbent Type | Maximum Adsorption Capacity, mg/g | Contact Time | Optimum pH | Best Fit Isotherm | Adsorbent Dosage, gm | Removal, % | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO/TiO2 | 12.5 | 120 min | 8 | Langmuir | 5.0 | 84.4–92.9 | [7] |
| Zinc doped WO3 catalyst | NR | 10 min | NR | NR | 0.4 | 80 | [8] |
| α-Fe2O3/NiS | NR | NR | 5 | NR | 1.0 | 88.3 | [10] |
| Activated carbon/γ-Fe2O3 nano-composite | 108.6 | 60 min | 2 | Langmuir | 0.01 | 99.4 | [13] |
| Poly METAC/Fe3O4 magnetic nanoparticles | NR | 2 days | NR | NR | NR | 80–96 | [14] |
| Nanocrystalline Cu0.5Zn0.5Ce3O5 | NR | 5 min | NR | Freundlich | 0.2 | 83% | [15] |
| PPy-coated Fe3O4 nanoparticles | 116.3 | 60 min | 4–5.4 | Langmuir | 0.1–0.12 | 78.7 | [18] |
| Chitosan-coated Fe3O4 nanoparticles | 40.12 | 50 min | 3 | Langmuir | 0.1 | NR | [19] |
| 2,4-dinitrophenyl hydrazine/Nano 𝛾-Alumina | 47.8 | 60 min | 4 | Langmuir | 0.05 | 95.6 | [36] |
| MWCNTs/PANI | 884.8 | 50 min | 8.5 | Langmuir | 0.02 | N.R | [37] |
| Fe3O4 nanoparticles | 45.8 | 5 min | 5 | Langmuir | 0.02 | 99 | [42] |
| CuFe2O4@graphene nanocomposite | 145 | 40 min | 3 | Langmuir | 0.5 | 95 | [38] |
| Chitosan/ZnO nanorod composite | 36.4 | 27 h | 2 | Freundlich | 0.1 | 85 | [39] |
| Fe3O4@MCM@Cu–Fe–LDH | 121.9 | 10 min | 9 | Langmuir | 0.03 | N.R | [43] |
| Biosorbent from Mikaniamicrantha | 46.5 | N.R | 2 | Freundlich | 0.1 | N.R | [40] |
| Green carbon composite-derived polymer resin and waste cotton fibers | 104 | 1day | 3 | Freundlich | NR | N.R | [44] |
| NiFe2O4/Polyaniline Magnetic Composite | 186 | 90 min | 4-8.6 | Langmuir | 0.03 | 96 | [41] |
| SnO2/CeO2 nano-composite | 18.5 | 30 min | 3 | Freundlich and Langmuir | 0.15 | 96.4 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.S.M.; Kamel, A.H.; Hassan, A.A.; Amr, A.E.-G.E.; El-Naby, H.A.; Elsayed, E.A. A SnO2/CeO2 Nano-Composite Catalyst for Alizarin Dye Removal from Aqueous Solutions. Nanomaterials 2020, 10, 254. https://doi.org/10.3390/nano10020254
Hassan SSM, Kamel AH, Hassan AA, Amr AE-GE, El-Naby HA, Elsayed EA. A SnO2/CeO2 Nano-Composite Catalyst for Alizarin Dye Removal from Aqueous Solutions. Nanomaterials. 2020; 10(2):254. https://doi.org/10.3390/nano10020254
Chicago/Turabian StyleHassan, Saad S. M., Ayman H. Kamel, Amr A. Hassan, Abd El-Galil E. Amr, Heba Abd El-Naby, and Elsayed A. Elsayed. 2020. "A SnO2/CeO2 Nano-Composite Catalyst for Alizarin Dye Removal from Aqueous Solutions" Nanomaterials 10, no. 2: 254. https://doi.org/10.3390/nano10020254
APA StyleHassan, S. S. M., Kamel, A. H., Hassan, A. A., Amr, A. E.-G. E., El-Naby, H. A., & Elsayed, E. A. (2020). A SnO2/CeO2 Nano-Composite Catalyst for Alizarin Dye Removal from Aqueous Solutions. Nanomaterials, 10(2), 254. https://doi.org/10.3390/nano10020254