Combined Toxicity of TiO2 Nanospherical Particles and TiO2 Nanotubes to Two Microalgae with Different Morphology
Abstract
1. Introduction
2. Methods
2.1. Test Material and Test Medium
2.2. Physicochemical Analysis
2.3. Algal Growth Assays
2.4. Oxidative Stress Biomarker Assays
2.5. Assessment and Prediction for Mixture Toxicity
2.6. Statistical Analysis
3. Results and Discussion
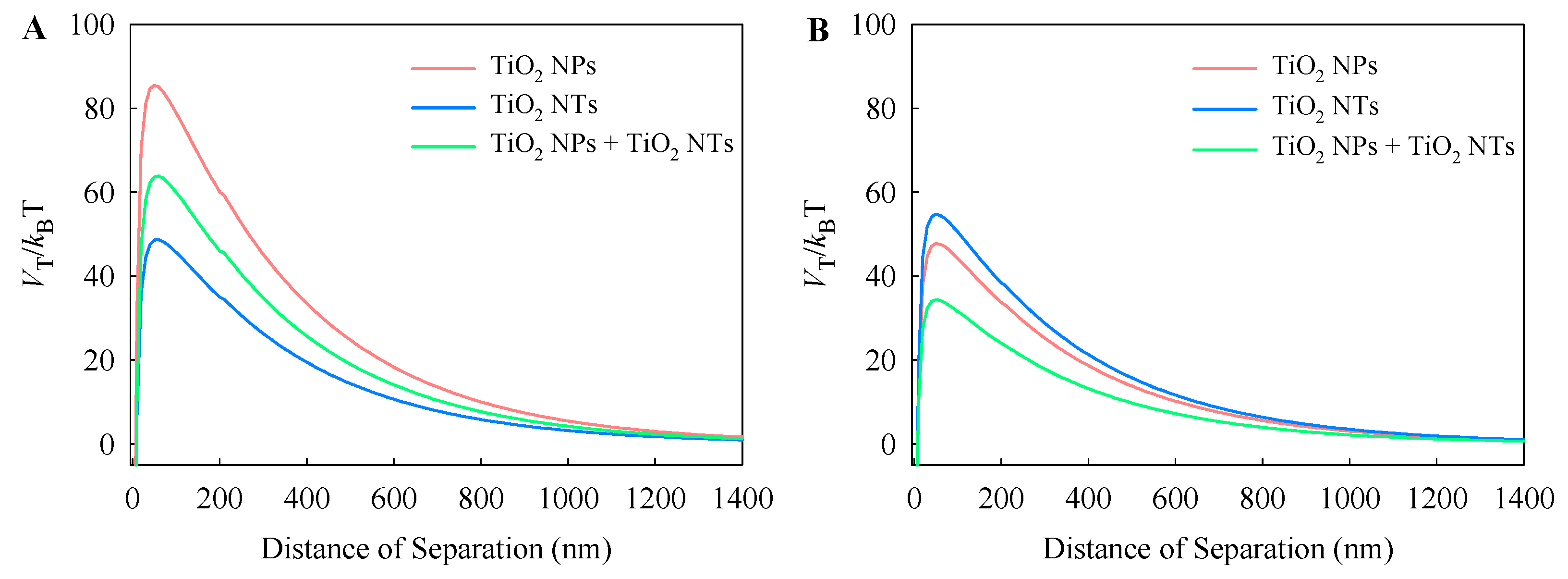
3.1. Physicochemical Characterizations
3.2. Toxicity of Single and Mixtures of TiO2 NPs to Algal
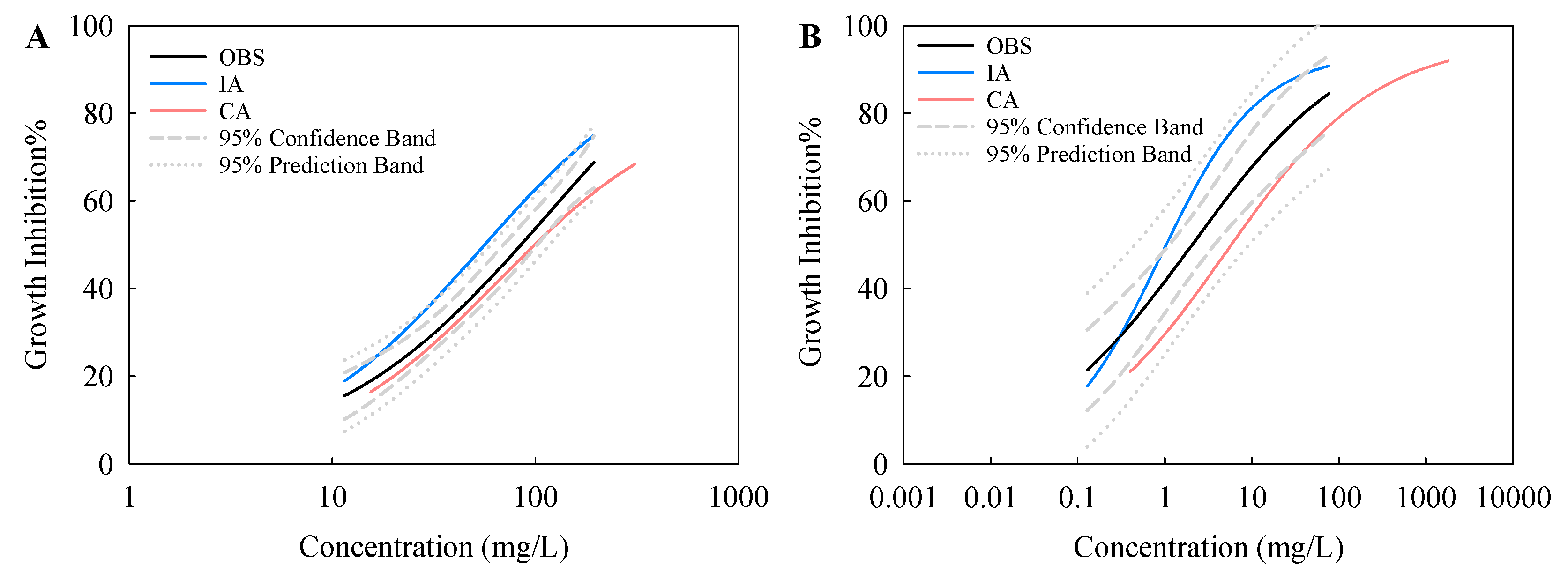
3.3. Assessment and Prediction of Joint Toxicity of TiO2 NPs and NTs
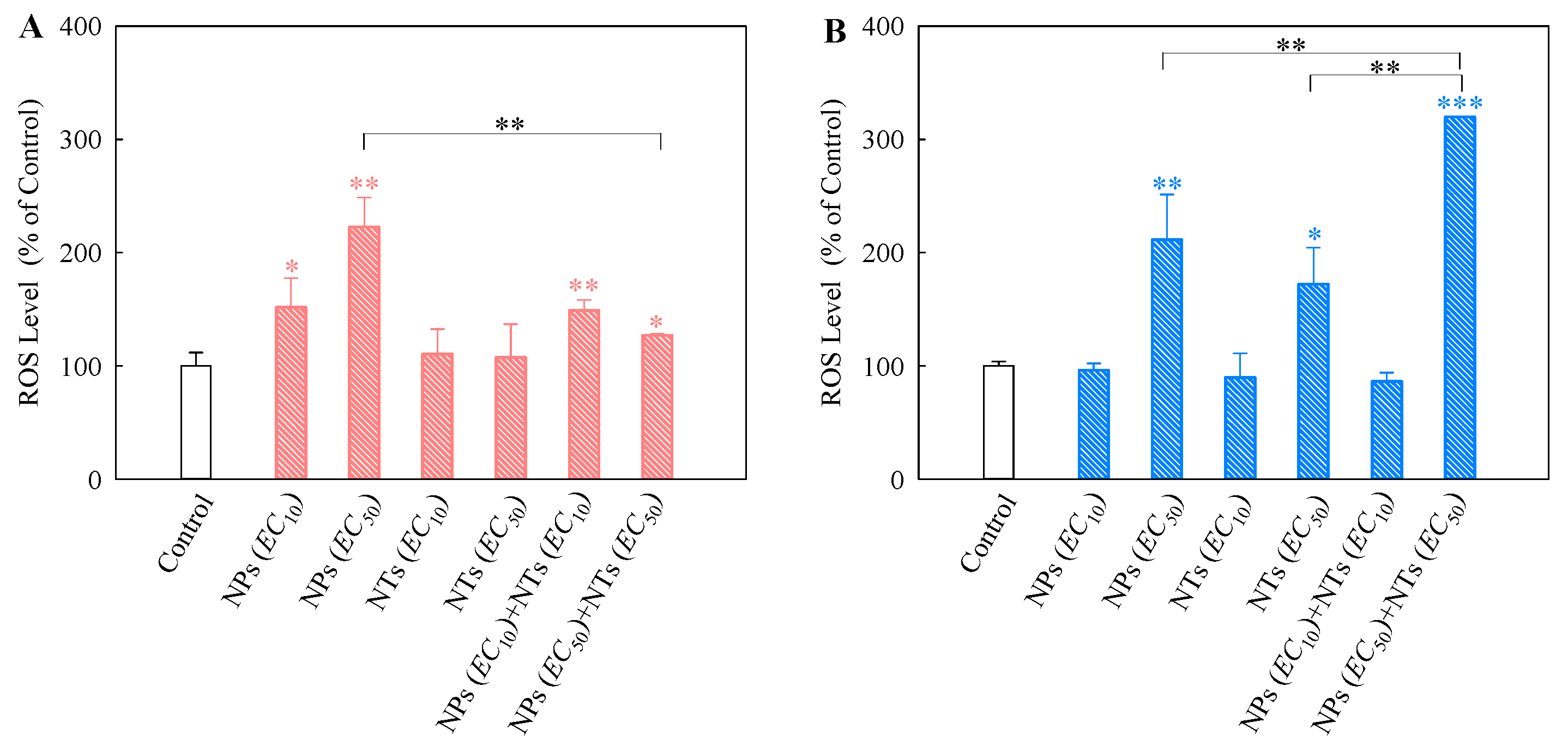
3.4. Cellular Oxidative Stress Effects of Single and Mixtures of TiO2 NPs and NTs on Algal Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klaper, R.D. The known and unknown about the environmental safety of nanomaterials in commerce. Small 2020, 16, e2000690. [Google Scholar] [CrossRef] [PubMed]
- Roma, J.; Matos, A.R.; Vinagre, C.; Duarte, B. Engineered metal nanoparticles in the marine environment: A review of the effects on marine fauna. Mar. Environ. Res. 2020, 161, 105110. [Google Scholar] [CrossRef] [PubMed]
- Damasco, J.A.; Ravi, S.; Perez, J.D.; Hagaman, D.E.; Melancon, M.P. Understanding nanoparticle toxicity to direct a safe-by-design approach in cancer nanomedicine. Nanomaterials 2020, 10, 2186. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wu, Y.; Li, X.; Wei, B.; Li, S.; Wang, X. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere 2018, 193, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Vijver, M.G.; Richardson, M.K.; Ahmad, F.; Peijnenburg, W.J. Particle-specific toxic effects of differently shaped zinc oxide nanoparticles to zebrafish embryos (Danio rerio). Environ. Toxicol. Chem. 2014, 33, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard. Mater. 2020, 123913. [Google Scholar] [CrossRef]
- Martin, O.; Scholze, M.; Ermler, S.; McPHIE, J.; Bopp, S.K.; Kienzler, A.; Parissis, N.; Kortenkamp, A. Ten years of research on synergisms and antagonisms in chemical mixtures: A systematic review and quantitative reappraisal of mixture studies. Environ. Int. 2020, 146, 106206. [Google Scholar] [CrossRef]
- Roy, B.; Suresh, P.; Chandrasekaran, N.; Mukherjee, A. Antibiotic tetracycline enhanced the toxic potential of photo catalytically active P25 titanium dioxide nanoparticles towards freshwater algae Scenedesmus obliquus. Chemosphere 2020, 128923. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Seenivasan, R.; Jenkins, D.; Chandrasekaran, N.; Mukherjee, A. Combined effects of nano-TiO2 and hexavalent chromium towards marine crustacean Artemia salina. Aquat. Toxicol. 2020, 225, 105541. [Google Scholar] [CrossRef]
- Fu, J.; Guo, Y.; Yang, L.; Han, J.; Zhou, B. Nano-TiO2 enhanced bioaccumulation and developmental neurotoxicity of bisphenol a in zebrafish larvae. Environ. Res. 2020, 187, 109682. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Assessment of toxic interaction of nano zinc oxide and nano copper oxide on germination of Raphanus sativus seeds. Environ. Monit. Assess. 2019, 191, 703. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, A. Binary mixture of nanoparticles in sewage sludge: Impact on spinach growth. Chemosphere 2020, 254, 126794. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wu, J.; Liu, M.; Zhu, G.; Chen, L.; Chang, Y.; Lu, H. Toxicity of binary mixtures of metal oxide nanoparticles to Nitrosomonas europaea. Chemosphere 2016, 153, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, J.; Su, Y.; Li, W.; Wilkinson, K.J.; Xie, B. Acute toxicity evaluation of nanoparticles mixtures using luminescent bacteria. Environ. Monit. Assess. 2020, 192, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Wang, Z.; Wang, S.; Peijnenburg, W.J. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions. Nanotoxicology 2018, 12, 423–438. [Google Scholar] [CrossRef]
- Wu, B.; Wu, J.; Liu, S.; Shen, Z.; Chen, L.; Zhang, X.-X.; Ren, H.-Q. Combined effects of graphene oxide and zinc oxide nanoparticle on human A549 cells: Bioavailability, toxicity and mechanisms. Environ. Sci. Nano 2019, 6, 635–645. [Google Scholar] [CrossRef]
- Illarionov, G.A.; Morozova, S.M.; Chrishtop, V.V.; Einarsrud, M.-A.; Morozov, M.I. Memristive TiO2: Synthesis, technologies, and applications. Front. Chem. 2020, 8, 724. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Reddy, P.V.G.; Reddy, B.R.P.; Reddy, M.V.K.; Reddy, K.R.; Shetti, N.P.; Saleh, T.A.; Aminabhavi, T.M. A review on multicomponent reactions catalysed by zero-dimensional/one-dimensional titanium dioxide (TiO2) nanomaterials: Promising green methodologies in organic chemistry. J. Environ. Manag. 2020, 111603. [Google Scholar] [CrossRef]
- Chakraborty, D.; Ethiraj, K.; Chandrasekaran, N.; Mukherjee, A. Mitigating the toxic effects of CdSe quantum dots towards freshwater alga Scenedesmus obliquus: Role of eco-corona. Environ. Pollut. 2020, 116049. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Z.; Yan, Y.; Li, J.; Yan, C.; Xing, B. Titanium dioxide nanoparticles enhance inorganic arsenic bioavailability and methylation in two freshwater algae species. Environ. Pollut. 2018, 238, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ning, F.; Cao, X.; Yao, H.; Wang, Z.; Xing, B. Photo-transformation of graphene oxide in the presence of co-existing metal ions regulated its toxicity to freshwater algae. Water Res. 2020, 176, 115735. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xie, C.; Ma, Y.; Wang, L.; He, X.; Shi, W.; Liu, X.; Liu, Y.; Zhang, Z. Size-dependent toxicity of ThO2 nanoparticles to green algae Chlorella pyrenoidosa. Aquat. Toxicol. 2019, 209, 113–120. [Google Scholar] [CrossRef] [PubMed]
- OECD Guidelines for the Testing of Chemicals. In Proceedings of the OECD Freshwater Alga and Cyanobacteria, Growth Inhibition Test No. 201, Paris, France, 28 July 2011; Available online: http://www.oecd.org (accessed on 12 December 2020).
- Cosgrove, T. Colloid Science Principles, Methods and Applications; Blackwell Publishing Ltd.: Bristol, UK, 2005. [Google Scholar]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ouyang, S.; Mu, L.; An, J.; Zhou, Q. Effects of graphene oxide and oxidized carbon nanotubes on the cellular division, microstructure, uptake, oxidative stress, and metabolic profiles. Environ. Sci. Technol. 2015, 49, 10825–10833. [Google Scholar] [CrossRef]
- Kim, K.T.; Klaine, S.J.; Lin, S.; Ke, P.C.; Kim, S.D. Acute toxicity of a mixture of copper and single-walled carbon nanotubes to Daphnia magna. Environ. Toxicol. Chem. 2010, 29, 122–126. [Google Scholar] [CrossRef]
- George, S.; Lin, S.; Ji, Z.; Thomas, C.R.; Li, L.; Mecklenburg, M.; Meng, H.; Wang, X.; Zhang, H.; Xia, T.; et al. Surface defects on plate-shaped silver nanoparticles contribute to its hazard potential in a fish gill cell line and zebrafish embryos. ACS Nano 2012, 6, 3745–3759. [Google Scholar] [CrossRef]
- Demir, E. An in vivo study of nanorod, nanosphere, and nanowire forms of titanium dioxide using Drosophila melanogaster: Toxicity, cellular uptake, oxidative stress, and DNA damage. J. Toxicol. Environ. Heal. 2020, 83, 456–469. [Google Scholar] [CrossRef]
- Demir, E. A review on nanotoxicity and nanogenotoxicity of different shapes of nanomaterials. J. Appl. Toxicol. 2020, 41, 118–147. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, J.; Shi, L.; Liu, Y.; Pan, B.; Xing, B. The mechanisms and environmental implications of engineered nanoparticles dispersion. Sci. Total. Environ. 2020, 722, 137781. [Google Scholar] [CrossRef]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano 2 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, N.; Wang, S.; Meng, Y.; Fang, H.; Wang, Z.; Wang, D.G. Dissolved organic matter modulates algal oxidative stress and membrane system responses to binary mixtures of nano-metal-oxides (nCeO2, nMgO and nFe3O4) and sulfadiazine. Nanomaterials 2019, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Baas, J.; Peijnenburg, W.J.G.M.; Vijver, M.G. Evaluating the combined toxicity of Cu and ZnO nanoparticles: Utility of the concept of additivity and a nested experimental design. Environ. Sci. Technol. 2016, 50, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Martín-De-Lucía, I.; Gonçalves, S.F.; Leganés, F.; Fernández-Piñas, F.; Rosal, R.; Loureiro, S. Combined toxicity of graphite-diamond nanoparticles and thiabendazole to Daphnia magna. Sci. Total. Environ. 2019, 688, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Tsugita, M.; Morimoto, N.; Nakayama, M. SiO2 and TiO2 nanoparticles synergistically trigger macrophage inflammatory responses. Part. Fibre Toxicol. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Wang, Z.; Ye, N.; Fang, H.; Wang, D. TiO2, SiO2 and ZrO2 nanoparticles synergistically provoke cellular oxidative damage in freshwater microalgae. Nanomaterials 2018, 8, 95. [Google Scholar] [CrossRef]
- Han, B.; Pei, Z.; Shi, L.; Wang, Q.; Li, C.; Zhang, B.; Su, X.; Zhang, N.; Zhou, L.; Zhao, B.; et al. TiO2 nanoparticles caused DNA damage in lung and extra-pulmonary organs through ROS-activated FOXO3a signaling pathway after intratracheal administration in rats. Int. J. Nanomed. 2020, 15, 6279–6294. [Google Scholar] [CrossRef]
- Wani, M.R.; Shadab, G. Titanium dioxide nanoparticle genotoxicity: A review of recent in vivo and in vitro studies. Toxicol. Ind. Heal. 2020, 36, 514–530. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Cesaroni, F.; Colacurci, N.; Rocco, L. In vitro effects of titanium dioxide nanoparticles (TiO2NPs) on cadmium chloride (CdCl2) genotoxicity in human sperm cells. Nanomaterials 2020, 10, 1118. [Google Scholar] [CrossRef]
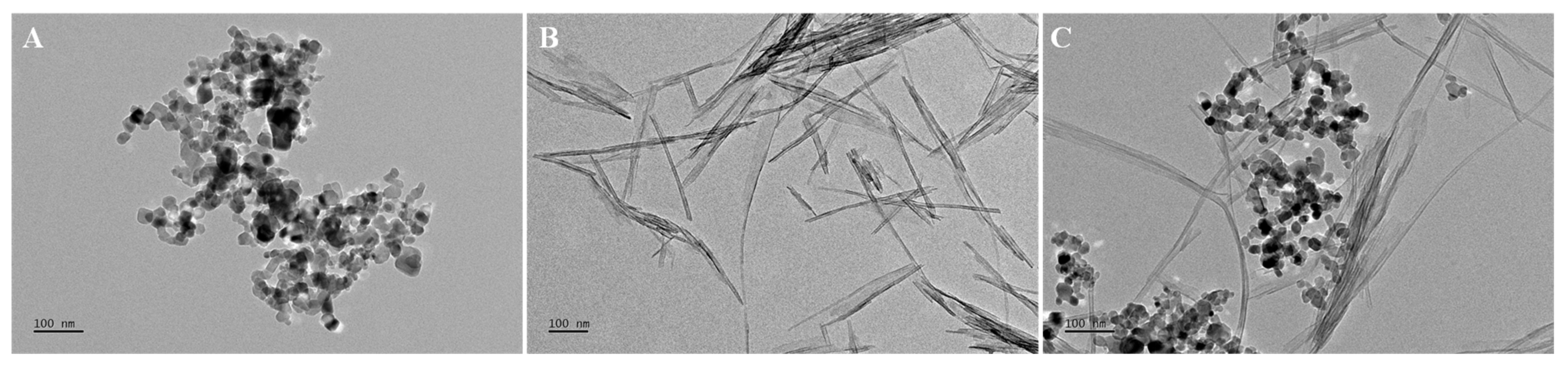


| Test Materials | 0 h | 96 h | ||
|---|---|---|---|---|
| ZP (mV) | HD (nm) | ZP (mV) | HD (nm) | |
| TiO2 NPs | −14.4 ± 0.2 | 969 ± 357 | −13.2 ± 0.3 | 643 ± 84.5 |
| TiO2 NTs | −12.5 ± 1.0 | 748 ± 201 | −13.7 ± 2.3 | 681 ± 94.5 |
| TiO2 NPs + NTs | −12.8 ± 0.5 | 943 ± 99 | −12.9 ± 0.4 | 477 ± 21.1 |
| Materials | Scenedesmus obliquus | Chlorella pyrenoidosa | |||
|---|---|---|---|---|---|
| Single Toxicity | EC10 (mg/L) | EC50 (mg/L) | EC10 (mg/L) | EC50 (mg/L) | |
| TiO2 NPs | 2.33 | 19.75 | 0.13 | 5.38 | |
| [1.75–3.44] | [17.02–23.00] | [0.11–0.14] | [3.36–8.23] | ||
| TiO2 NTs | 13.16 | 211.26 | 0.002 | 4.87 | |
| [5.08–25.75] | [152.01–427.73] | [0.002–0.004] | [2.81–9.66] | ||
| Combined Toxicity | EC50 (mg/L) | EC50 (mg/L) | |||
| TiO2 NPs + NTs | OBS | 85.04 [69.50–102.54] | 2.05 [1.16–3.88] | ||
| IA | 56.30 [56.17–56.42] | 1.08 [0.91–1.34] | |||
| CA | 99.46 [99.09–99.81] | 5.96 [4.97–7.36] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jin, S.; Zhang, F.; Wang, D. Combined Toxicity of TiO2 Nanospherical Particles and TiO2 Nanotubes to Two Microalgae with Different Morphology. Nanomaterials 2020, 10, 2559. https://doi.org/10.3390/nano10122559
Wang Z, Jin S, Zhang F, Wang D. Combined Toxicity of TiO2 Nanospherical Particles and TiO2 Nanotubes to Two Microalgae with Different Morphology. Nanomaterials. 2020; 10(12):2559. https://doi.org/10.3390/nano10122559
Chicago/Turabian StyleWang, Zhuang, Shiguang Jin, Fan Zhang, and Degao Wang. 2020. "Combined Toxicity of TiO2 Nanospherical Particles and TiO2 Nanotubes to Two Microalgae with Different Morphology" Nanomaterials 10, no. 12: 2559. https://doi.org/10.3390/nano10122559
APA StyleWang, Z., Jin, S., Zhang, F., & Wang, D. (2020). Combined Toxicity of TiO2 Nanospherical Particles and TiO2 Nanotubes to Two Microalgae with Different Morphology. Nanomaterials, 10(12), 2559. https://doi.org/10.3390/nano10122559