High Electronically Conductive Tungsten Phosphate Glass-Ceramics
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
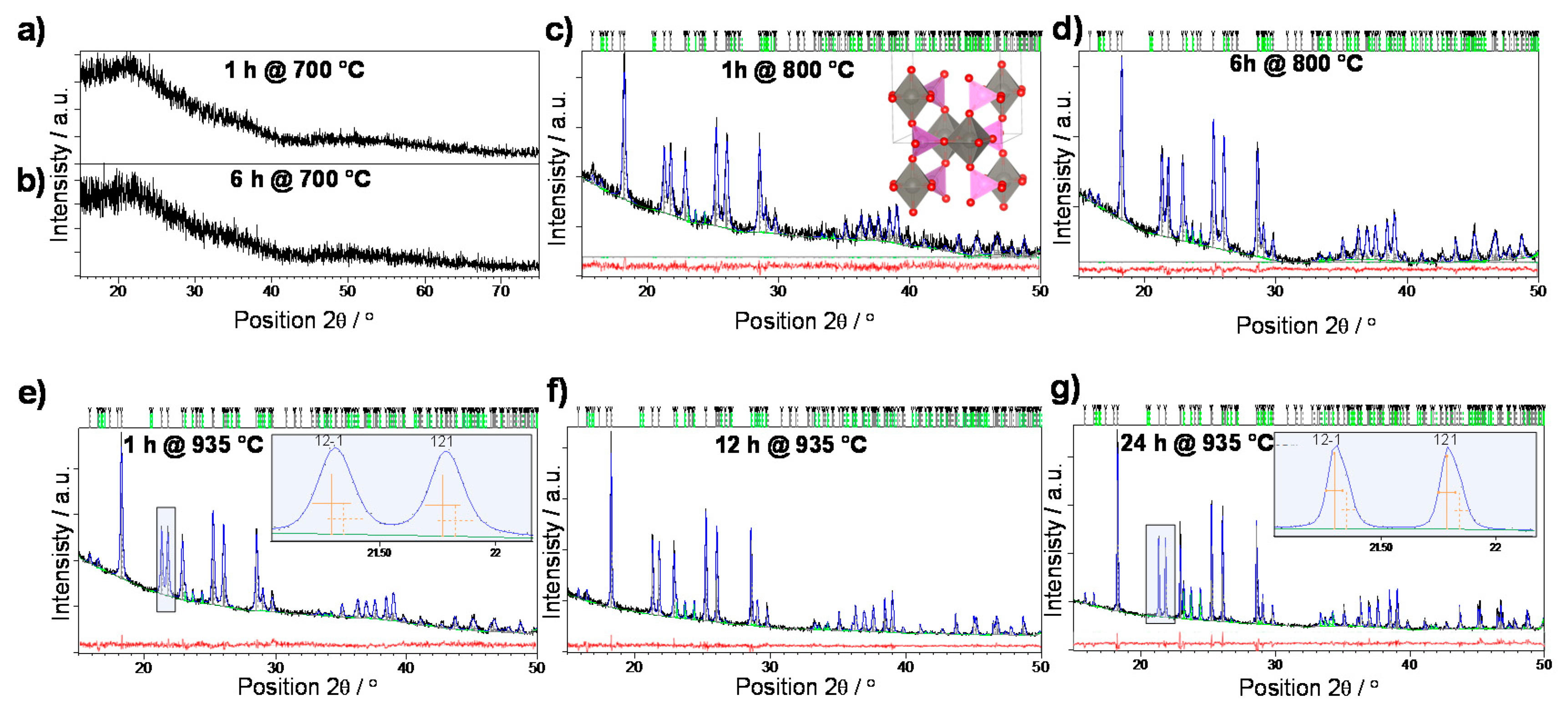
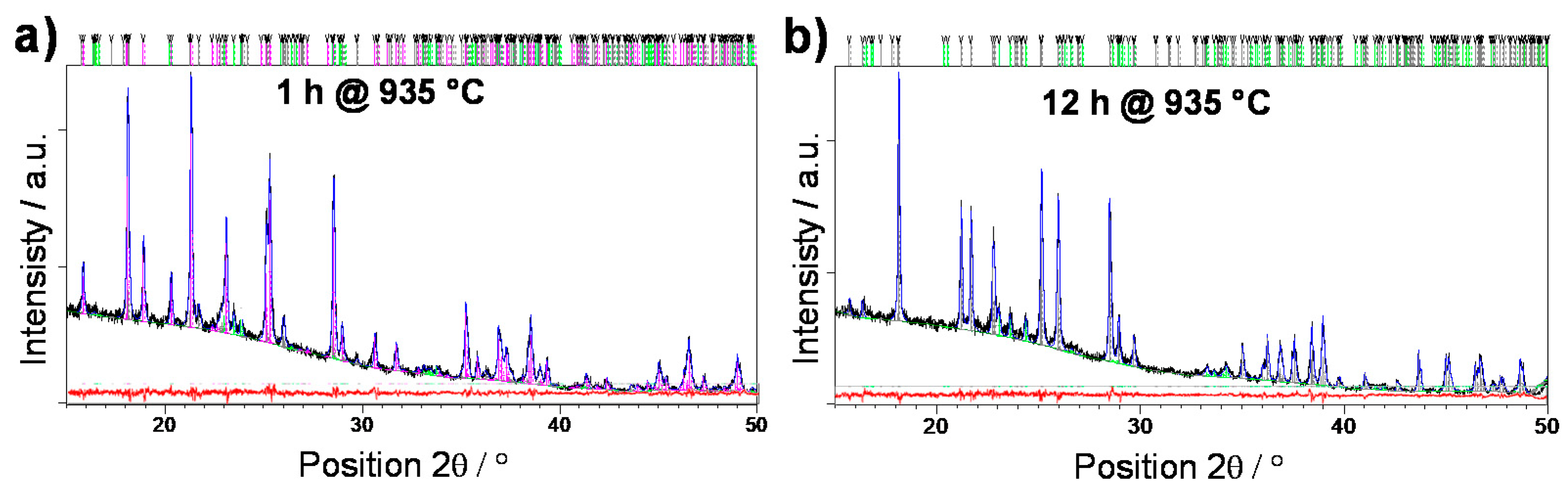
3.1. Crystallization
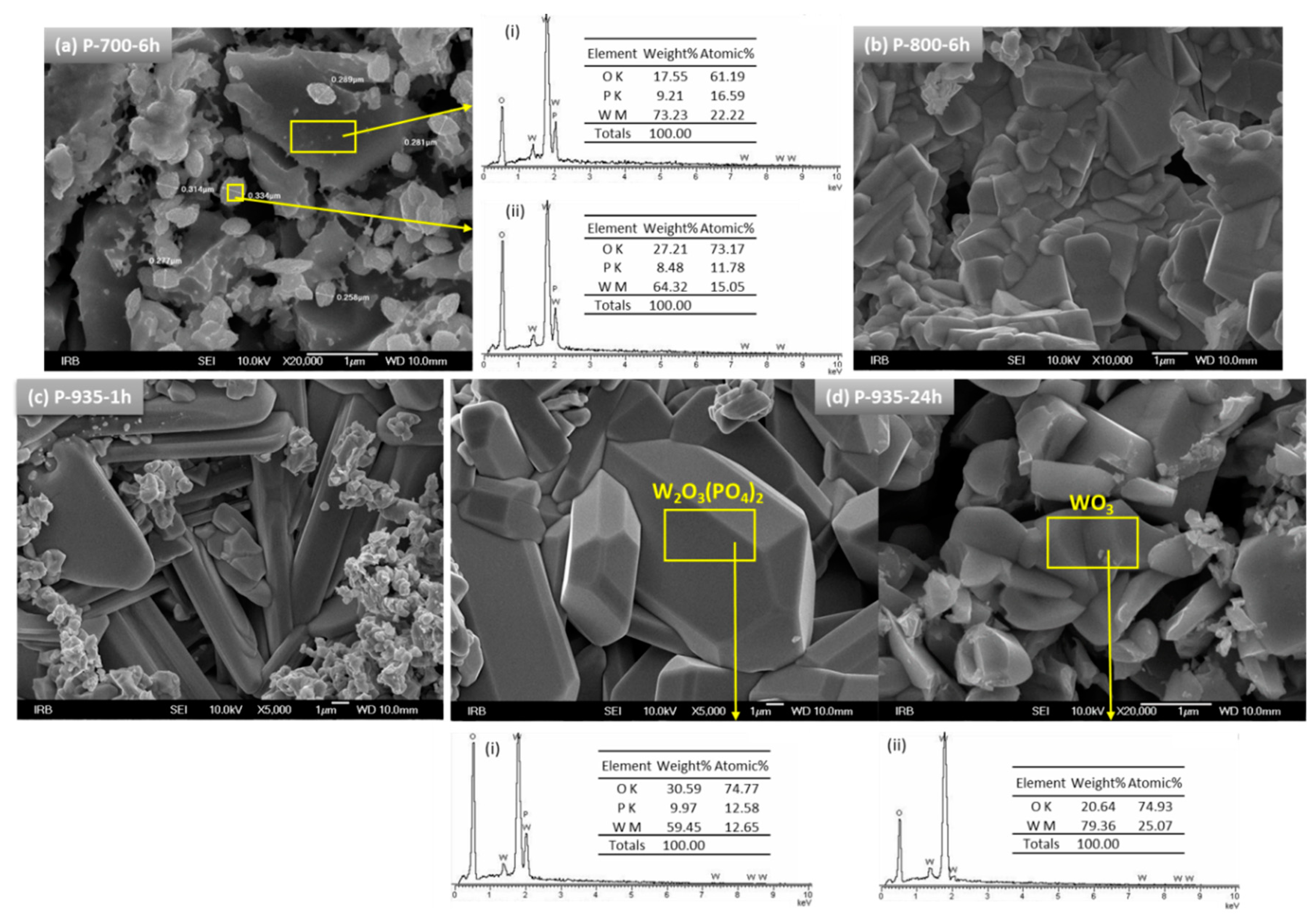
3.2. Structural Characterization
SEM Analysis
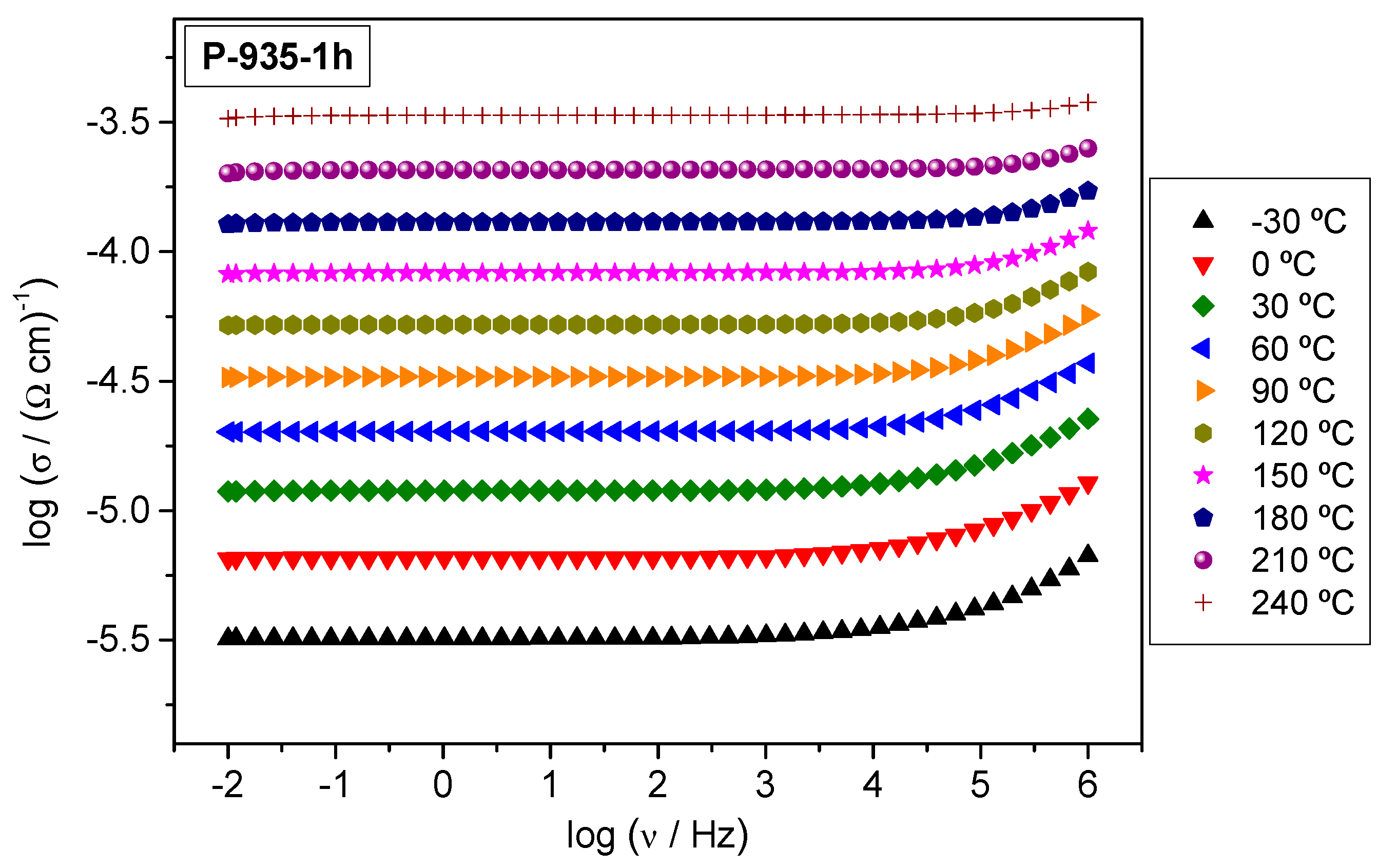
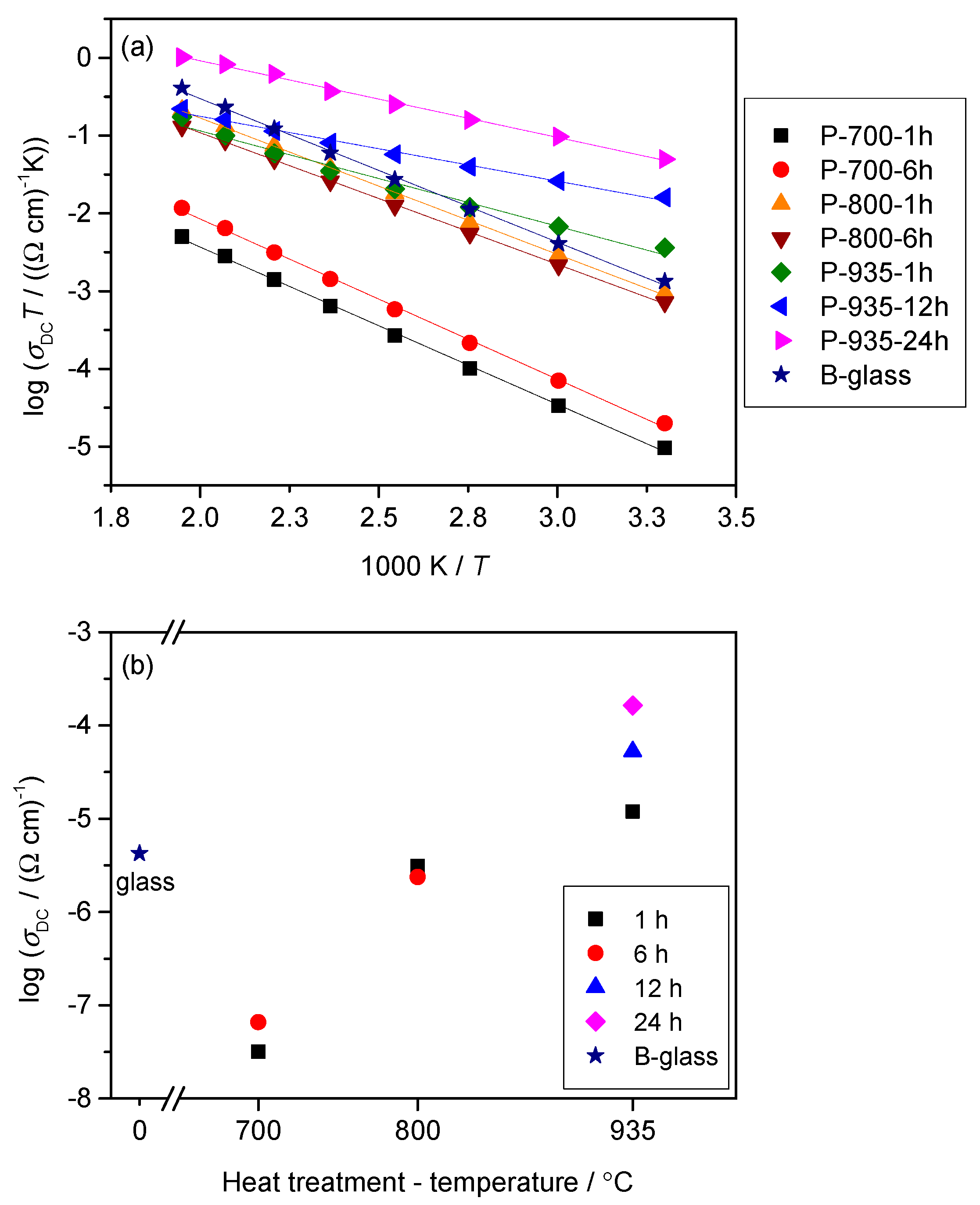
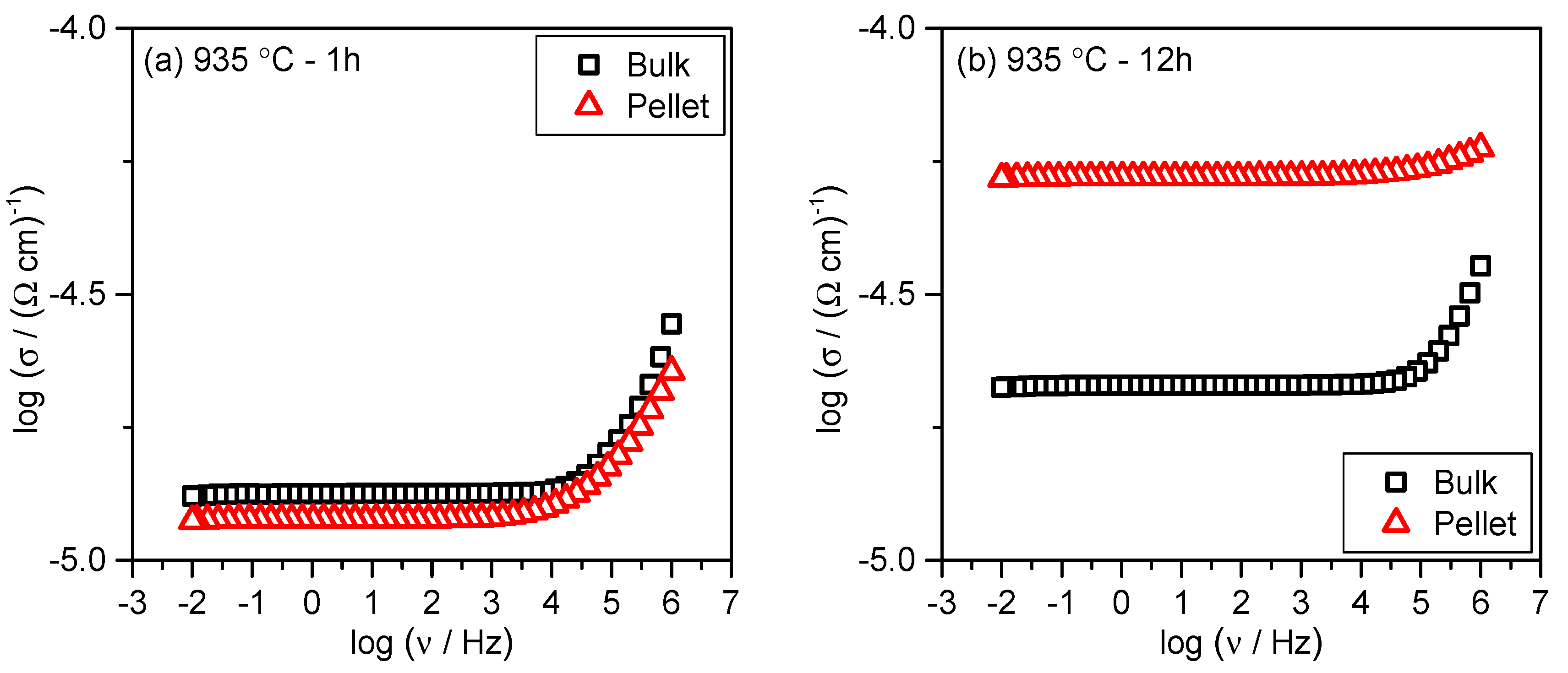
3.3. Electrical Transport
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deubener, J.; Allix, M.; Davis, M.J.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Zanotto, E.D. Bright future for glass-ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar]
- Schirmeisen, A.; Taskiran, A.; Fuchs, H.; Bracht, H.; Murugavel, S.; Roling, B. Fast Interfacial Ionic Conduction in Nanostructured Glass Ceramics. Phys. Rev. Lett. 2007, 98, 225901–225904. [Google Scholar] [CrossRef] [PubMed]
- Sayer, M.; Mansingh, A. Transport properties of semiconducting phosphate glasses. Phys. Rev. B 1972, 6, 4629. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in glasses containing transition metal ions. J. Non-Cryst. Solids 1968, 1, 1–17. [Google Scholar] [CrossRef]
- Austin, I.G.; Mott, N.F. Polarons in crystalline and non-crystalline materials. Adv. Phys. 1969, 18, 41–102. [Google Scholar] [CrossRef]
- Mackenzie, J.D.; Nasu, H. The electrical conductivity of transition metal oxide-based glasses. In Physics of Disordered Materials; Adler, D., Ed.; Plenum Press: New York, NY, USA, 1985; pp. 469–482. [Google Scholar]
- Murawski, L.; Chung, C.H.; Mackenzie, J.D. Electrical properties of semiconducting oxide glasses. J. Non-Cryst. Solids 1979, 32, 91–104. [Google Scholar] [CrossRef]
- Moguš-Milanković, A.; Day, D.E.; Šantić, B. DC conductivity and polarisation in iron phosphate glasses. Phys. Chem. Glasses 1999, 40, 69–74. [Google Scholar]
- Šantić, A.; Moguš-Milanković, A. Charge carrier dynamics in materials with disordered structures: A case study of iron phosphate glasses. Croat. Chem. Acta 2012, 85, 245–254. [Google Scholar] [CrossRef]
- Garbarczyk, J.E.; Jozwiak, P.; Wasiucionek, M.; Nowinski, J.L. Enhancement of electrical conductivity in lithium vanadate glasses by nanocrystallization. Solid State Ion. 2004, 175, 691–694. [Google Scholar] [CrossRef]
- Garbarczyk, J.E.; Jozwiak, P.; Wasiucionek, M.; Nowinski, J.L. Effect of nanocrystallization on the electronic conductivity of vanadate–phosphate glasses. Solid State Ion. 2006, 177, 2585–2588. [Google Scholar] [CrossRef]
- Garbarczyk, J.E.; Jozwiak, P.; Wasiucionek, M.; Nowinski, J.L. Nanocrystallization as a method of improvement of electrical properties and thermal stability of V2O5-rich glasses. J. Power Sources 2007, 173, 743–747. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Garbarczyk, J.E.; Gorzkowska, I.; Wasiucionek, M.; Nowinski, J.L.; Gierlotka, S.; Jozwiak, P. Correlation between electrical properties and microstructure of nanocrystallized V2O5–P2O5 glasses. J. Power Sources 2009, 194, 73–80. [Google Scholar] [CrossRef]
- Garbarczyk, J.E.; Pietrzak, T.K.; Wasiucionek, M.; Kaleta, A.; Dorau, A.; Nowinski, J.L. High electronic conductivity in nanostructured materials based on lithium-iron-vanadate-phosphate glasses. Solid State Ion. 2014, 272, 53–59. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Michalski, P.P.; Kaleta, A.; Garbarczyk, J.E. Highly conductive cathode materials for Li-ion batteries prepared by thermal nanocrystallization of selected oxide glasses. Mater. Sci. Eng. B 2016, 213, 140–147. [Google Scholar] [CrossRef]
- Pavić, L.; Skoko, Ž.; Gajović, A.; Su, D.; Moguš-Milanković, A. Electrical transport in iron phosphate glass-ceramics. J. Non-Cryst. Solids 2018, 505, 44–53. [Google Scholar] [CrossRef]
- Moguš-Milanković, A.; Sklepić, K.; Skoko, Ž.; Mikac, L.; Musić, S.; Day, D.E. Influence of nanocrystallization on the electronic conductivity of zinc iron phosphate glass. J. Am. Ceram. Soc. 2012, 95, 303–311. [Google Scholar] [CrossRef]
- Wu, C.-M.; Naseem, S.; Chou, M.-H.; Wang, J.-H.; Jian, Y.-Q. Recent Advances in Tungsten-Oxide-Based Materials and Their Applications. Front. Mater. 2019, 27, 49. [Google Scholar] [CrossRef]
- Poirier, G.; Cassanjes, F.C.; Messadeq, Y.; Ribeiro, S.J. Crystallization of monoclinic WO3 in tungstate fluorophosphate glasses. J. Non-Cryst. Solids 2009, 35, 441–446. [Google Scholar] [CrossRef]
- Poirier, G.; de Araujo, C.C.; Messadeq, Y.; Ribeiro, S.J. Tungsten fluorophosphate glass as optical limiters. J. Appl. Phys. 2002, 9, 100221–100223. [Google Scholar]
- Poirier, G.; Nalin, M.; Messadeq, Y.; Ribeiro, S.J. Photochromic properties of tungstate-based glasses. Solid State Ion. 2007, 178, 871–875. [Google Scholar] [CrossRef]
- Schlenker, C.; Le Touze, C.; Hess, C.; Rotger, A.; Dumas, J.; Marcus, J.; Greenblatt, M.; Teweldemedhin, Z.S.; Ottolenghi, A.; Foury, P.; et al. Transport properties of the charge density wave quasi two-dimensional compounds (PO2)4(WO3)2m. Synth. Met. 1995, 70, 1263–1266. [Google Scholar] [CrossRef]
- Roussel, P.; Labbe, P.H.; Groult, D. Symmetry and twins in the monophosphate tungsten bronze series (PO2)4(WO3)2m (2 ≤ m ≤14). Acta Crystallogr. B 2000, 56, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Roussel, P.; Perez, O.; Labbe, P.H. Phosphate tungsten bronze series: Crystallographic and structural properties of low-dimensional conductors. Acta Crystallogr. B 2001, 57, 603–632. [Google Scholar] [CrossRef] [PubMed]
- Aitken, B.G. Conductive WO3−x glass-ceramics. In The Physics of Non-Crystalline Solids; Pye, L.D., LaCourse, W.C., Stevens, H.J., Eds.; Taylor & Francis: London, UK, 1992; pp. 548–552. [Google Scholar]
- Salje, E.; Guttler, B. Anderson transition and intermediate polaron formation in WO3−x: Transport properties and optical absorption. Philos. Mag. B 1984, 50, 607–620. [Google Scholar] [CrossRef]
- Moore, L.; Dutta, I.; Wheaton, B.; Stapleton, E.; Parysek, P.; Aitken, B. A new identification of the conducting phase in tungsten-titanium-phosphate glass-ceramics. J. Am. Ceram. Soc. 2020, 103, 3552–3651. [Google Scholar] [CrossRef]
- Nikolić, J.; Pavić, L.; Šantić, A.; Mošner, P.; Koudelka, L.; Pajić, D.; Moguš-Milanković, A. Novel insights into electrical transport mechanism in ionic-polaronic glasses. J. Am. Ceram. Soc. 2018, 101, 1221–1235. [Google Scholar] [CrossRef]
- Pavić, L.; Šantić, A.; Nikolić, J.; Mošner, P.; Koudelka, L.; Pajić, D.; Moguš-Milanković, A. Nature of mixed electrical transport in Ag2O-ZnO-P2O5 glasses containing WO3 and MoO3. Electrochim. Acta 2018, 276, 434–445. [Google Scholar] [CrossRef]
- Pavić, L.; Nikolić, J.; Graça, M.P.F.; Costa, B.F.O.; Valente, M.A.; Skoko, Ž.; Šantić, A.; Moguš-Milanković, A. Effect of controlled crystallization on polaronic transport in phosphate-based glass-ceramics. Int. J. Appl. Glass Sci. 2020, 11, 97–111. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Šubčík, J. Study of Structure and Properties of Phosphate Glasses Modified by Heavy Metal Oxides. Ph.D. Thesis, University of Pardubice, Pardubice, Czech Republic, 2010. [Google Scholar]
- Kierkegaard, P.; Asbrink, S. The Crystal Structure of W2O3(PO4)2. Determination of a Superstructure by Means of Least-Squares Calculations. Acta Chem. Scand. 1964, 18, 2329–2338. [Google Scholar] [CrossRef]
- Tanisaki, S. On the Phase Transition of Tungsten Trioxide below Room Temperature. J. Phys. Soc. Jpn. 1960, 15, 566–573. [Google Scholar] [CrossRef]
- Hanawa, M.; Imoto, H. Structures of Two New Polymorphic Forms of Hexavalent Tungsten Oxide Phosphates. J. Solid State Chem. 1999, 144, 325–329. [Google Scholar] [CrossRef]
- Mardare, C.C.; Hassel, A.W. Review on the Versatility of Tungsten Oxide Coatings. Phys. Status Solidi A 2019, 216, 1900047. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Wang, M.-C.; Chen, S.-F.; Li, Y.-H.; Shen, T.-S.; Bor, H.-Y.; Wei, C.-N. Electrical and Physical Characteristics of WO3/Ag/WO3 Sandwich Structure Fabricated with Magnetic-Control Sputtering Metrology. Sensors 2018, 18, 2803. [Google Scholar] [CrossRef] [PubMed]
- Gillet, M.; Lemire, C.; Gillet, E.; Aguir, K. The role of surface oxygen vacancies upon WO3 conductivity. Surf. Sci. 2003, 532, 519–525. [Google Scholar] [CrossRef]
- Khyzhum, O.Y. XPS, XES and XAS studies of the electronic structure of tungsten oxides. J. Alloys Compd. 2000, 305, 1–6. [Google Scholar] [CrossRef]
| Sample | Heat Treatment Conditions | Composition (in wt. %) | ||
|---|---|---|---|---|
| T/°C | t/h | W2O3(PO4)2 | WO3 | |
| P *-700-1h | 700 | 1 | - | - |
| P-700-6h | 700 | 6 | - | - |
| P-800-1h | 800 | 1 | 97.5(1) | 2.5(1) |
| P-800-6h | 800 | 6 | 96.0(2) | 4.0(1) |
| P-935-1h | 935 | 1 | 95.7(2) | 4.3(1) |
| P-935-12h | 935 | 12 | 90.9(1) | 9.1(1) |
| P-935-24h | 935 | 24 | 82.0(1) | 18.0(2) |
| B *-935-1h | 935 | 1 | 76.1(2) (orthorombic) 19.1(2) (monoclinic) | 4.8(2) |
| B-935-12h | 935 | 12 | 91.8(1) | 8.2(1) |
| Sample | σDC/(Ω cm)–1 at 30 °C ± 1.0% | EDC/eV ± 1.0% |
|---|---|---|
| B-Glass | 4.26 × 10–6 | 0.37 |
| P-700-1h | 3.18 × 10–8 | 0.35 |
| P-700-6h | 6.57 × 10–8 | 0.35 |
| P-800-1h | 3.11 × 10–6 | 0.30 |
| P-800-6h | 2.38 × 10–6 | 0.29 |
| P-935-1h | 1.20 × 10–5 | 0.18 |
| P-935-12h | 5.26 × 10–5 | 0.13 |
| P-935-24h | 1.64 × 10–4 | 0.17 |
| B-935-1h | 1.19 × 10–5 | 0.29 |
| B-935-12h | 2.14 × 10–5 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renka, S.; Klaser, T.; Burazer, S.; Mošner, P.; Kalenda, P.; Šantić, A.; Moguš-Milanković, A. High Electronically Conductive Tungsten Phosphate Glass-Ceramics. Nanomaterials 2020, 10, 2515. https://doi.org/10.3390/nano10122515
Renka S, Klaser T, Burazer S, Mošner P, Kalenda P, Šantić A, Moguš-Milanković A. High Electronically Conductive Tungsten Phosphate Glass-Ceramics. Nanomaterials. 2020; 10(12):2515. https://doi.org/10.3390/nano10122515
Chicago/Turabian StyleRenka, Sanja, Teodoro Klaser, Sanja Burazer, Petr Mošner, Petr Kalenda, Ana Šantić, and Andrea Moguš-Milanković. 2020. "High Electronically Conductive Tungsten Phosphate Glass-Ceramics" Nanomaterials 10, no. 12: 2515. https://doi.org/10.3390/nano10122515
APA StyleRenka, S., Klaser, T., Burazer, S., Mošner, P., Kalenda, P., Šantić, A., & Moguš-Milanković, A. (2020). High Electronically Conductive Tungsten Phosphate Glass-Ceramics. Nanomaterials, 10(12), 2515. https://doi.org/10.3390/nano10122515






