Strategy for Encapsulation of CdS Quantum Dots into Zeolitic Imidazole Frameworks for Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
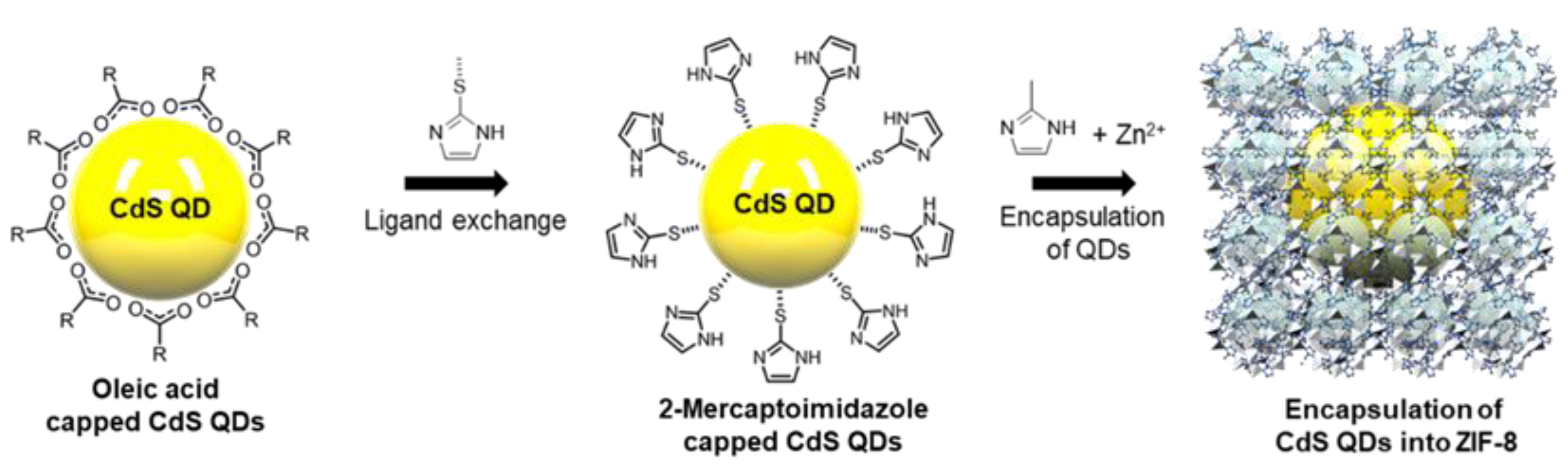
2.2. Synthesis of Oleic Acid Capped CdS QDs
2.3. Preparation of 2-Mercaptoimidazole Capped CdS
2.4. Encapsulation of CdS QDs into ZIF-8
2.5. Test for Photocatalytic Performance
2.6. Instrumentation
3. Results
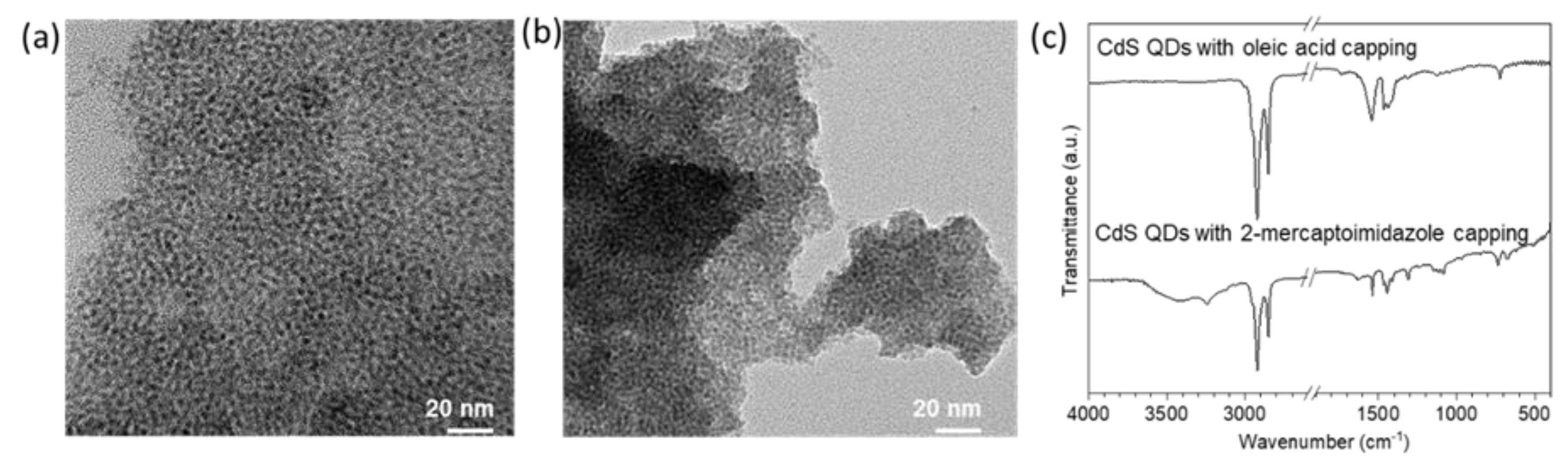
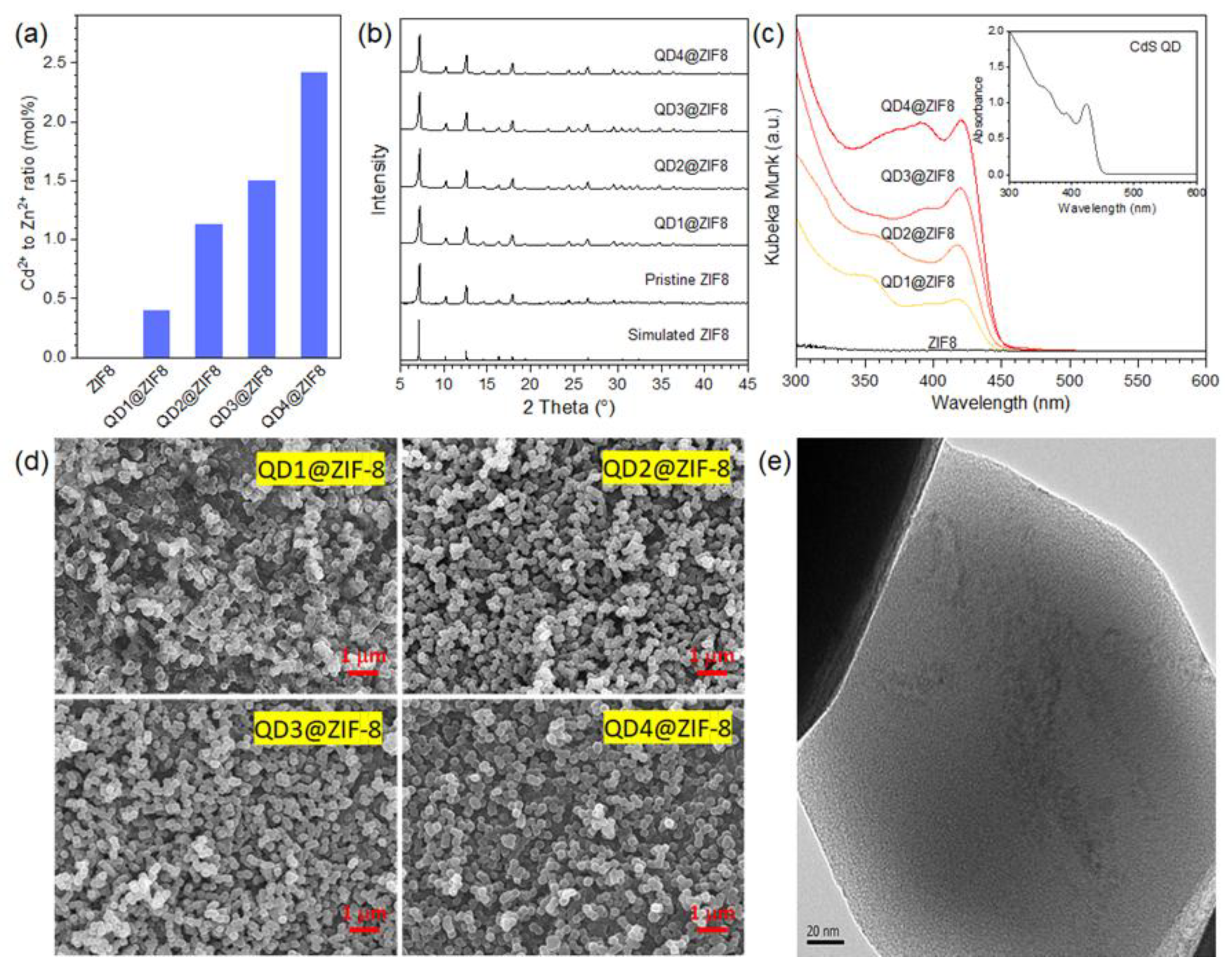
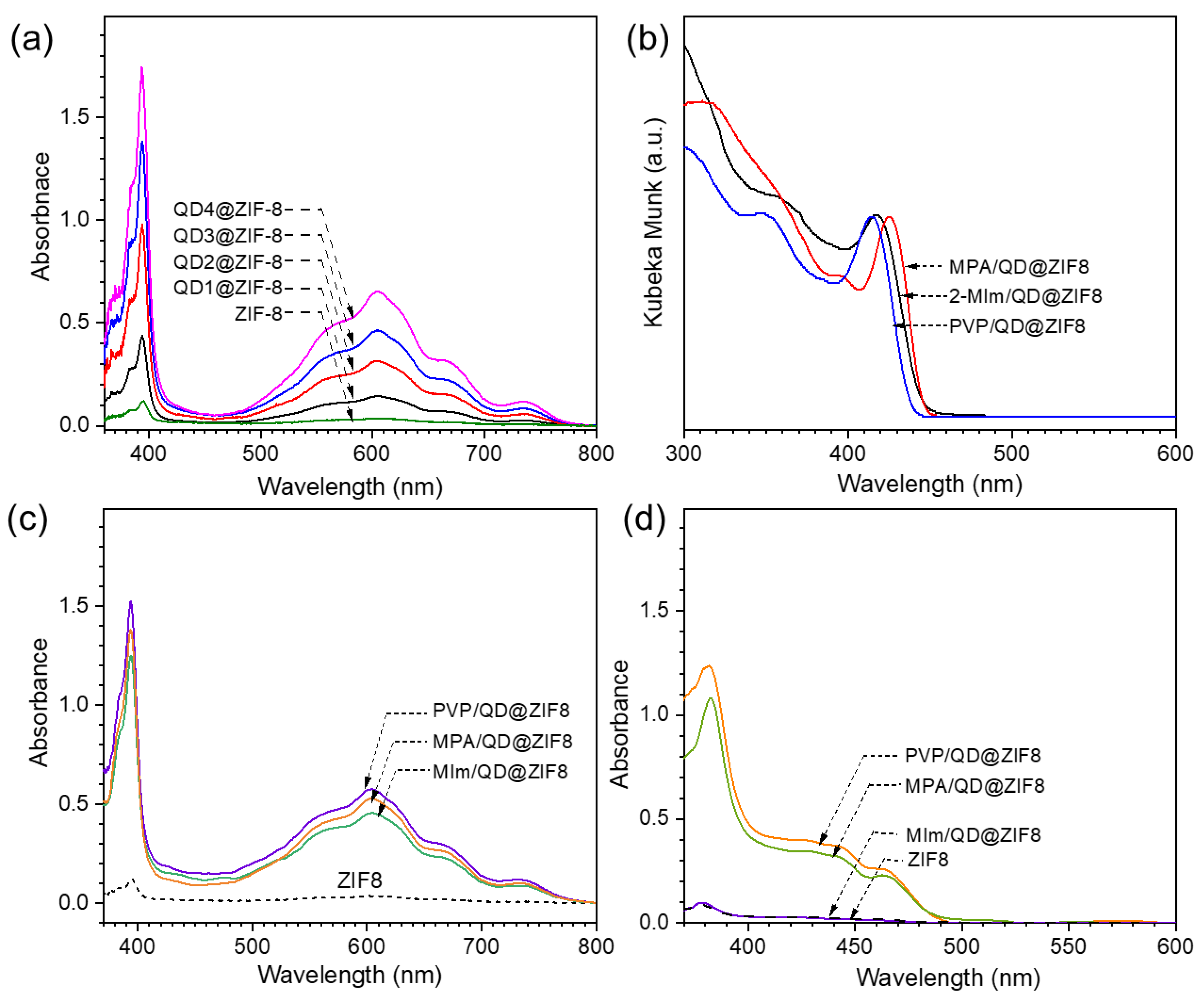
3.1. Characterization of ZIF-8 Encapsulated CdS QDs
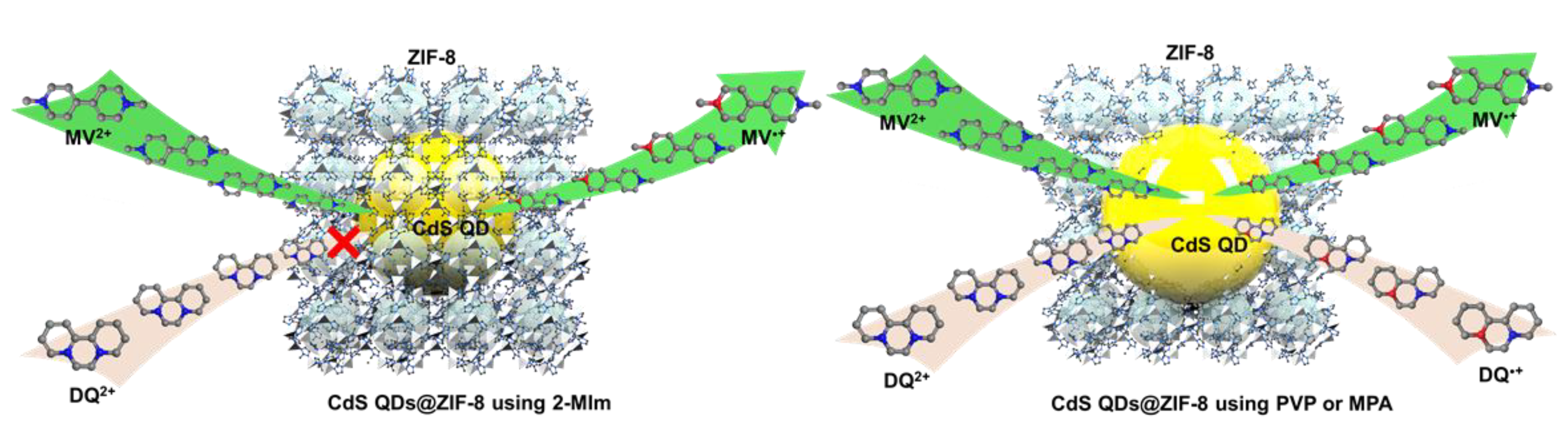
3.2. Photocatalytic Performance of ZIF-8 Encapsulated CdS QDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, Y.J.; Chen, D.; Yu, Z.T.; Zou, Z.G. Cadmium sulfide-based nanomaterials for photocatalytic hydrogen production. J. Mater. Chem. A 2018, 6, 11606–11630. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Fang, H.; Jia, W.; Li, H.; Yang, L.; Wang, K. Cadmium sulfide quantum dots stabilized by aromatic amino acids for visible light-induced photocatalytic degradation of organic dyes. New J. Chem. 2015, 39, 6951–6957. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C. Highly Efficient Photocatalyst Based on a CdS Quantum Dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 25377–25386. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.M.; Brown, P.R.; Bulović, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.; Lee, M.W.; Lee, S.; Lee, J.; Cho, S.; Lee, H.; Cha, H.G.; Kim, H.S. Enhancing Photocatalytic β-O-4 Bond Cleavage in Lignin Model Compounds by Silver-Exchanged Cadmium Sulfide. ACS Catal. 2020, 10, 8465–8475. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Demchenko, D.O.; Robinson, R.D.; Sadtler, B.; Erdonmez, C.K.; Alivisatos, A.P.; Wang, L.W. Formation mechanism and properties of CdS-Ag2S nanorod superlattices. ACS Nano 2008, 2, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Grim, J.Q.; Manna, L.; Moreels, I. A sustainable future for photonic colloidal nanocrystals. Chem. Soc. Rev. 2015, 44, 5897–5914. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tang, Y.; Li, Z.; Ding, X.; Yu, B.; Lin, L. Largely Enhancing Luminous Efficacy, Color-Conversion Efficiency, and Stability for Quantum-Dot White LEDs Using the Two-Dimensional Hexagonal Pore Structure of SBA-15 Mesoporous Particles. ACS Appl. Mater. Interfaces 2019, 11, 18808–18816. [Google Scholar] [CrossRef]
- Amjadi, M.; Jalili, R. Molecularly imprinted mesoporous silica embedded with carbon dots and semiconductor quantum dots as a ratiometric fluorescent sensor for diniconazole. Biosens. Bioelectron. 2017, 96, 121–126. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoon, K.B. Preparation and characterization of CdS and PbS quantum dots in zeolite Y and their applications for nonlinear optical materials and solar cell. Coord. Chem. Rev. 2014, 263–264, 239–256. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.S. Work-Function Engineering in Lead Sulfide and Cadmium Sulfide Quantum Dots incorporated into Zeolite y using Ion Exchange. Part. Part. Syst. Charact. 2016, 33, 126–131. [Google Scholar] [CrossRef]
- Qiu, Y.; Xing, Z.; Guo, M.; Zhao, T.; Wang, Y.; Chen, P.; Li, Z.; Pan, K.; Zhou, W. Cadmium sulfide quantum dots/dodecahedral polyoxometalates/oxygen-doped mesoporous graphite carbon nitride with Z-scheme and Type-II as tandem heterojunctions for boosting visible-light-driven photocatalytic performance. J. Colloid Interface Sci. 2021, 582, 752–763. [Google Scholar] [CrossRef]
- Song, Y.; Fang, W.; Liu, C.; Sun, Z.; Li, F.; Xu, L. Effect of mixed Mo/W polyoxometalate modification on photoelectrocatalytic activity of CdS nanocrystals for arsenic(III) oxidation. J. Phys. Chem. Solids 2020, 141, 109395. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Liang, R.; He, Z.; Zhou, C.; Yan, G.; Wu, L. Mof-derived porous Fe2O3 nanoparticles coupled with cds quantum dots for degradation of bisphenol a under visible light irradiation. Nanomaterials 2020, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. CdS-decorated UiO-66(NH2) nanocomposites fabricated by a facile photodeposition process: An efficient and stable visible-light-driven photocatalyst for selective oxidation of alcohols. J. Mater. Chem. A 2013, 1, 11473–11482. [Google Scholar] [CrossRef]
- Shen, B.; Wang, B.; Zhu, L.; Jiang, L. Properties of cobalt-and nickel-doped ZIF-8 framework materials and their application in heavy-metal removal from wastewater. Nanomaterials 2020, 10, 1636. [Google Scholar] [CrossRef]
- Liang, K.; Coghlan, C.J.; Bell, S.G.; Doonan, C.; Falcaro, P. Enzyme encapsulation in zeolitic imidazolate frameworks: A comparison between controlled co-precipitation and biomimetic mineralization. Chem. Commun. 2016, 52, 473–476. [Google Scholar] [CrossRef]
- Paul, A.; Vyas, G.; Paul, P.; Srivastava, D.N. Gold-nanoparticle-encapsulated zif-8 for a mediator-free enzymatic glucose sensor by amperometry. ACS Appl. Nano Mater. 2018, 1, 3600–3607. [Google Scholar] [CrossRef]
- Basnayake, S.A.; Tan, K.; Leonard, M.; Chabal, Y.; Balkus, K.J. Encapsulation of red sulfur chromophores in a zeolitic imidazolate framework (ZIF-8) via solvent assisted linker exchange. Microporous Mesoporous Mater. 2016, 219, 172–177. [Google Scholar] [CrossRef]
- Esken, D.; Turner, S.; Lebedev, O.I.; Van Tendeloo, G.; Fischer, R.A. Au@ZIFs: Stabilization and encapsulation of cavity-size matching gold clusters inside functionalized zeolite imidazolate frameworks, ZIFs. Chem. Mater. 2010, 22, 6393–6401. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Das, G.; Thote, J.; Banerjee, R. Photocatalytic metal-organic framework from CdS quantum dot incubated luminescent metallohydrogel. J. Am. Chem. Soc. 2014, 136, 14845–14851. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Nath, M.; Mohiyuddin, S.; Packirisamy, G. Multifunctional CdSNPs@ZIF-8: Potential Antibacterial Agent against GFP-Expressing Escherichia coli and Staphylococcus aureus and Efficient Photocatalyst for Degradation of Methylene Blue. ACS Omega 2018, 3, 8288–8308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal Growth of ZIF-8, ZIF-67, and Their Mixed-Metal Derivatives. J. Am. Chem. Soc. 2018, 140, 1812–1823. [Google Scholar] [CrossRef]
- Son, Y.R.; Ryu, S.G.; Kim, H.S. Rapid adsorption and removal of sulfur mustard with zeolitic imidazolate frameworks ZIF-8 and ZIF-67. Microporous Mesoporous Mater. 2020, 293, 109819. [Google Scholar] [CrossRef]
- Ellis, J.E.; Zeng, Z.; Hwang, S.I.; Li, S.; Luo, T.Y.; Burkert, S.C.; White, D.L.; Rosi, N.L.; Gassensmith, J.J.; Star, A. Growth of ZIF-8 on molecularly ordered 2-methylimidazole/single-walled carbon nanotubes to form highly porous, electrically conductive composites. Chem. Sci. 2019, 10, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Hou, B.; Cho, Y.; Kim, B.S.; Hong, J.; Park, J.B.; Ahn, S.J.; Sohn, J.I.; Cha, S.; Kim, J.M. Highly Monodispersed PbS Quantum Dots for Outstanding Cascaded-Junction Solar Cells. ACS Energy Lett. 2016, 1, 834–839. [Google Scholar] [CrossRef]
- Robinson, R.D.; Sadtler, B.; Demchenko, D.O.; Erdonmez, C.K.; Wang, L.W.; Alivisatos, A.P. Spontaneous superlattice formation in nanorods through partial cation exchange. Science 2007, 317, 355–358. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, K.; Uematsu, T.; Torimoto, T.; Kuwabata, S. Direct surface modification of semiconductor quantum dots with metal-organic frameworks. CrystEngComm 2019, 21, 5568–5577. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.R.; Kwak, M.; Lee, S.; Kim, H.S. Strategy for Encapsulation of CdS Quantum Dots into Zeolitic Imidazole Frameworks for Photocatalytic Activity. Nanomaterials 2020, 10, 2498. https://doi.org/10.3390/nano10122498
Son YR, Kwak M, Lee S, Kim HS. Strategy for Encapsulation of CdS Quantum Dots into Zeolitic Imidazole Frameworks for Photocatalytic Activity. Nanomaterials. 2020; 10(12):2498. https://doi.org/10.3390/nano10122498
Chicago/Turabian StyleSon, Ye Rim, Minseok Kwak, Songyi Lee, and Hyun Sung Kim. 2020. "Strategy for Encapsulation of CdS Quantum Dots into Zeolitic Imidazole Frameworks for Photocatalytic Activity" Nanomaterials 10, no. 12: 2498. https://doi.org/10.3390/nano10122498
APA StyleSon, Y. R., Kwak, M., Lee, S., & Kim, H. S. (2020). Strategy for Encapsulation of CdS Quantum Dots into Zeolitic Imidazole Frameworks for Photocatalytic Activity. Nanomaterials, 10(12), 2498. https://doi.org/10.3390/nano10122498






