Ni-Rich Layered Oxide with Preferred Orientation (110) Plane as a Stable Cathode Material for High-Energy Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of LiNi0.6Co0.2Mn0.2O2 (NCM622) Cathode Materials
2.2. Characterization Methods
2.3. Electrochemical Measurements
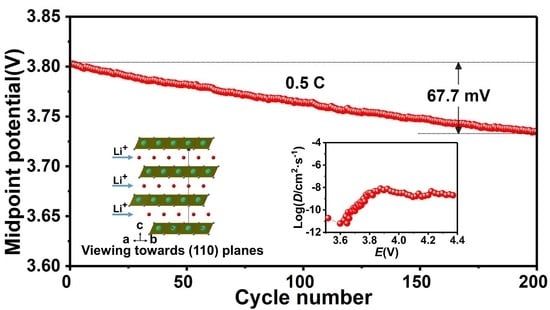
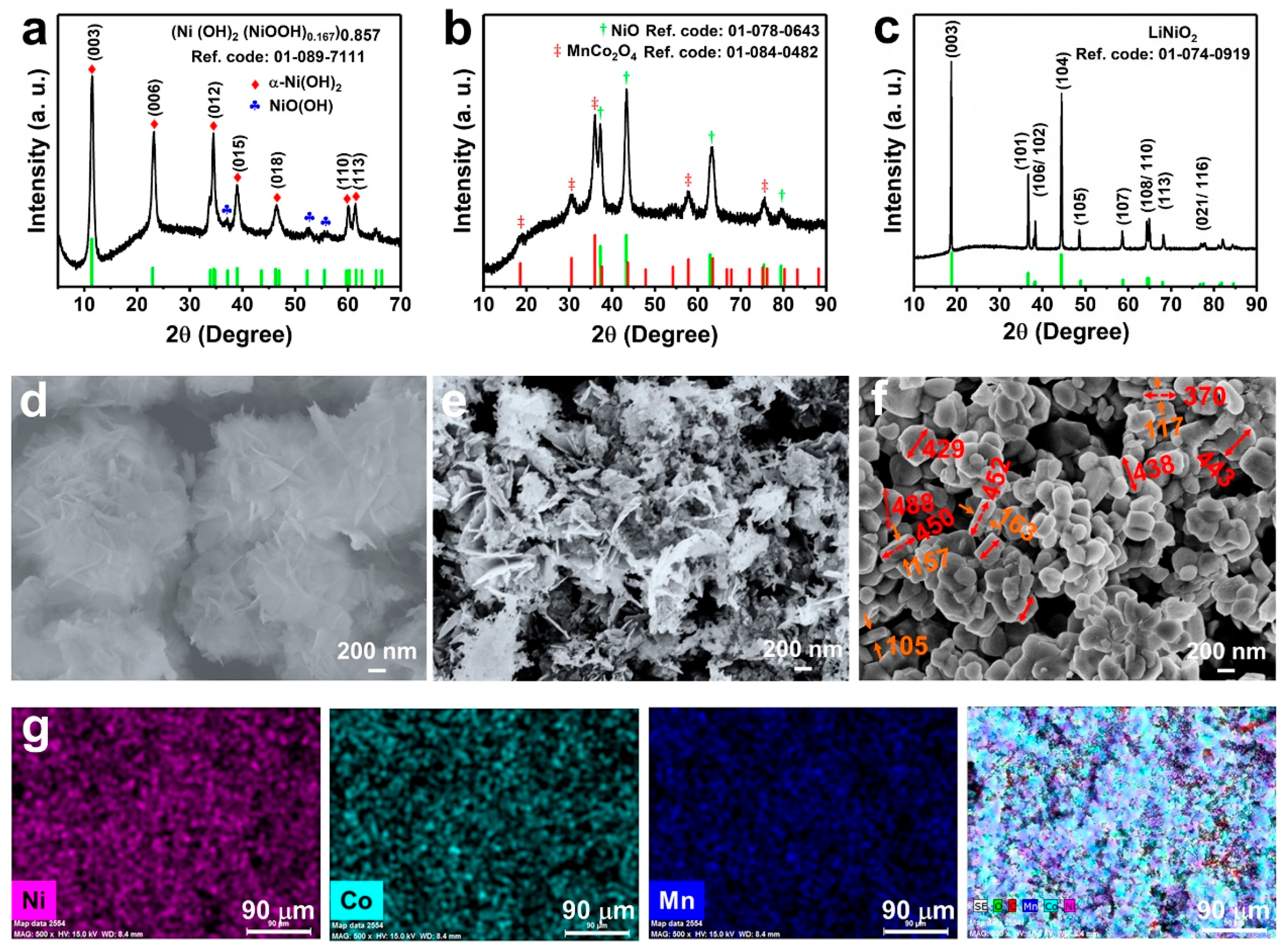
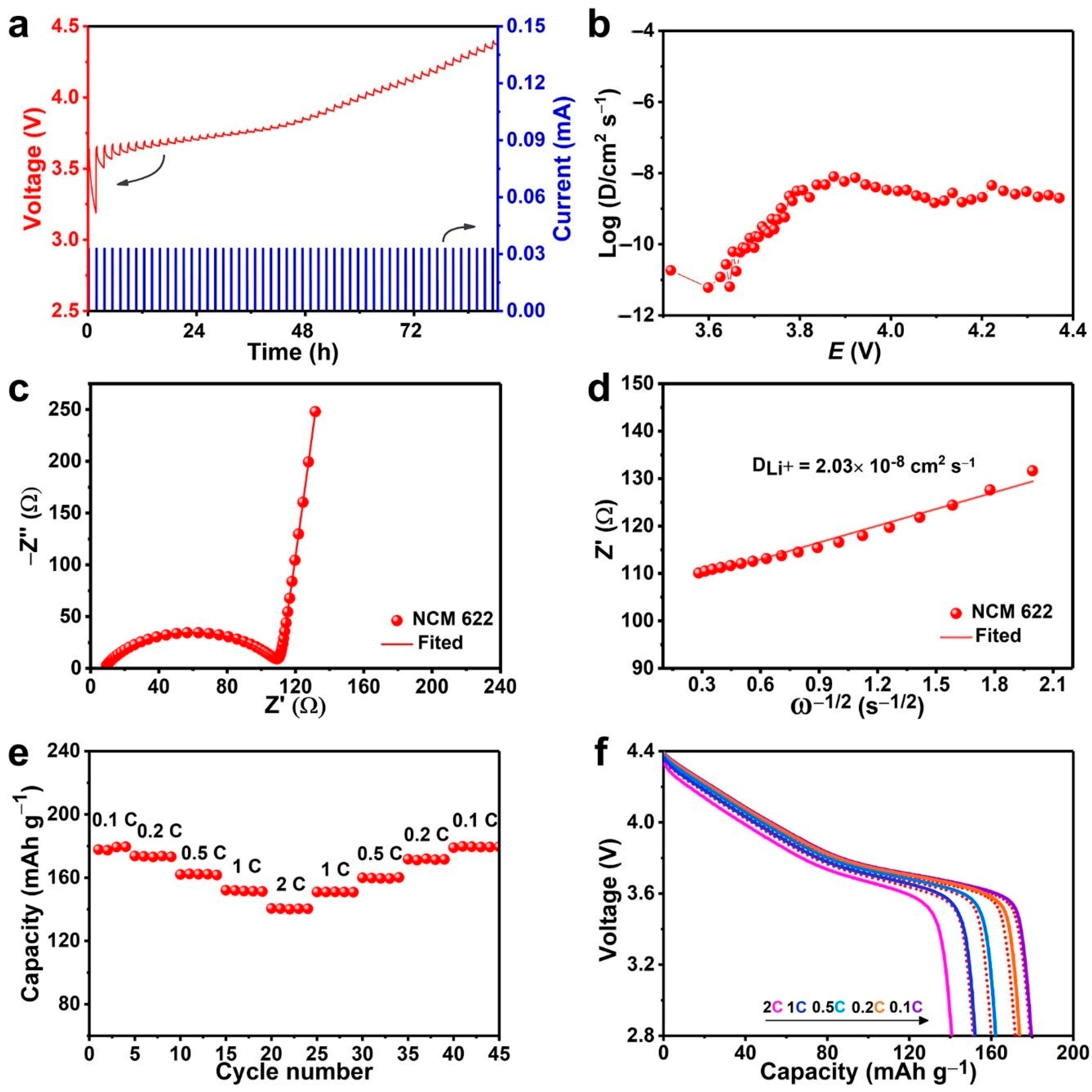
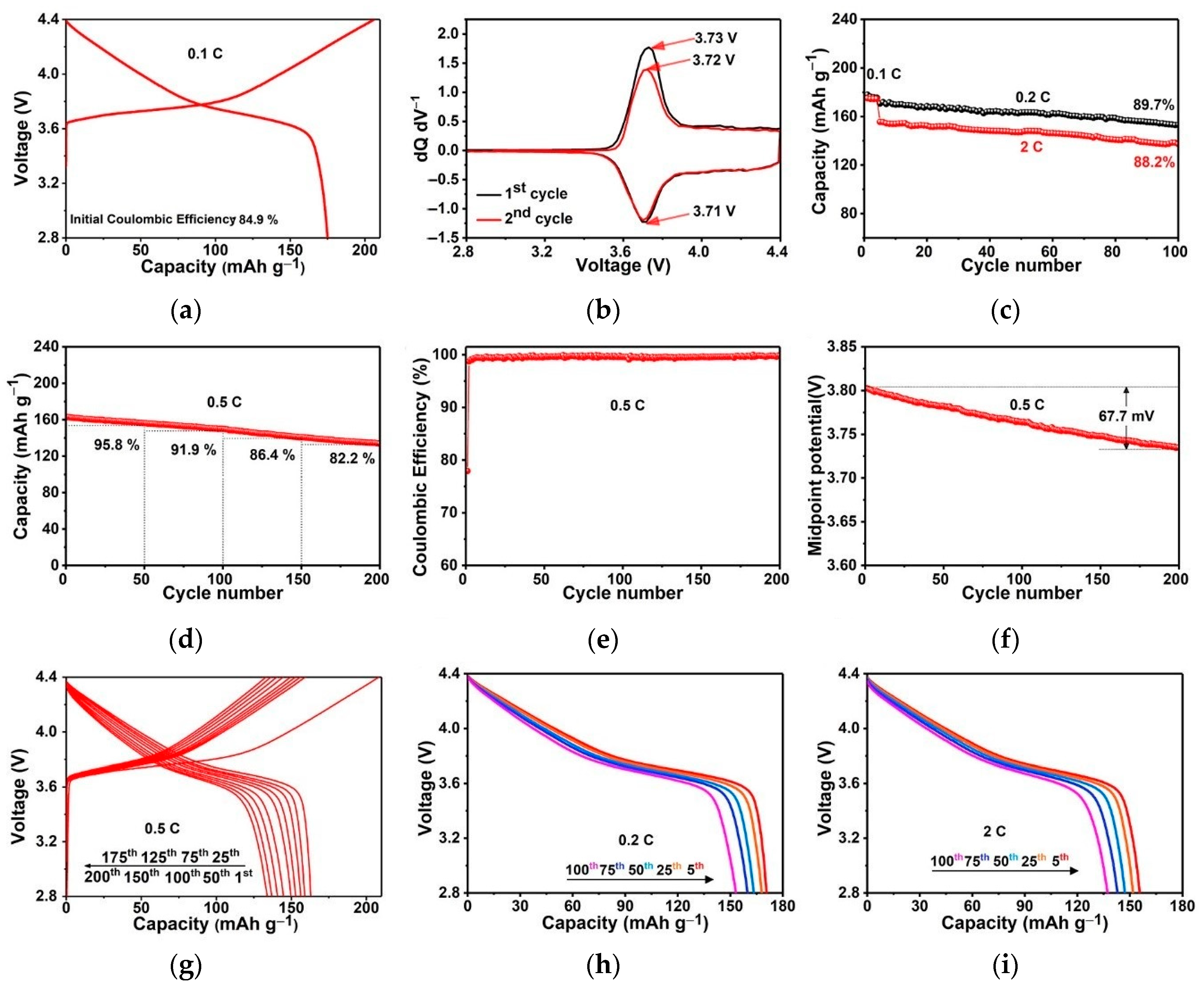
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xu, X.; Liu, J.; Liu, Z.; Li, F.; Hu, R.; Liu, J.; Hou, X.; Feng, Y.; Yu, Y.; et al. Mechanistic Understanding of Metal Phosphide Host for Sulfur Cathode in High-Energy-Density Lithium-Sulfur Batteries. ACS Nano 2019, 13, 8986–8996. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, J.; Liu, Z.; Shen, J.; Hu, R.; Liu, J.; Ouyang, L.; Zhang, L.; Zhu, M. Robust Pitaya-Structured Pyrite as High Energy Density Cathode for High-Rate Lithium Batteries. ACS Nano 2017, 11, 9033–9040. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and Reality of Ni-Rich Cathode for Commercialization. Adv. Energy Mater. 2018, 8, 1702028. [Google Scholar] [CrossRef]
- Emani, S.; Liu, C.H.; Ashuri, M.; Sahni, K.; Wu, J.P.; Yang, W.L.; Nemeth, K.; Shaw, L.L. Li3BN2 as a Transition Metal Free, High Capacity Cathode for Li-ion Batteries. Chemelectrochem 2019, 6, 320–325. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Sergey, C.; Lee, H.; Yoon, J.; Kang, Y.-M.; Yoon, W.-S. Advances in the Cathode Materials for Lithium Rechargeable Batteries. Angew. Chem. Int. Ed. 2020, 59, 2578–2605. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Zou, L.; Zhao, W.; Jia, H.; Zheng, J.; Li, L.; Abraham, D.P.; Chen, G.; Croy, J.R.; Zhang, J.-G.; Wang, C. The Role of Secondary Particle Structures in Surface Phase Transitions of Ni-Rich Cathodes. Chem. Mater. 2020, 32, 2884–2892. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Szczesna-Chrzan, A.; Trzeciak, T.; Ostrowski, A.; Szymanski, D.; Wieczorek, W.; Rarog-Pilecka, W.; Marcinek, M. On the Sensitivity of the Ni-rich Layered Cathode Materials for Li-ion Batteries to the Different Calcination Conditions. Nanomaterials 2020, 10, 2018. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Klöpsch, R.; Vortmann, B.; Hahn, H.; Nowak, S.; Amereller, M.; Gentschev, A.C.; et al. The truth about the 1st cycle Coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. 2016, 18, 3956–3965. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Huang, H.; He, W.; Zheng, H.; Deng, P.; Li, S.; Qu, B.; Wang, T. Surfactant-Assisted Synthesis of High Energy {010} Facets Beneficial to Li-Ion Transport Kinetics with Layered LiNi0.6Co0.2Mn0.2O2. ACS Sustain. Chem. Eng. 2018, 6, 6312–6320. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Novák, P.; Aschauer, U.; Berg, E. Cation Ordering and Redox Chemistry of Layered Ni-Rich LixNi1-2yCoyMnyO2: An Operando Raman Spectroscopy Study. Chem. Mater. 2019, 32, 186–194. [Google Scholar] [CrossRef]
- Sahni, K.; Ashuri, M.; He, Q.; Sahore, R.; Bloom, I.D.; Liu, Y.; Kaduk, J.A.; Shaw, L.L. H3PO4 treatment to enhance the electrochemical properties of Li(Ni1/3Mn1/3Co1/3)O2 and Li(Ni0.5Mn0.3Co0.2)O2 cathodes. Electrochim. Acta 2019, 301, 8–22. [Google Scholar] [CrossRef]
- Xin, F.; Zhou, H.; Chen, X.; Zuba, M.; Chernova, N.; Zhou, G.; Whittingham, M.S. Li-Nb-O Coating/Substitution Enhances the Electrochemical Performance of the LiNi0.8Mn0.1Co0.1O2 (NMC 811) Cathode. ACS Appl. Mater. Interfaces 2019, 11, 34889–34894. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Z.; Dai, A.; Tang, L.; Wei, H.; Li, Y.; He, Z.; Lu, J. Boosting Cell Performance of LiNi0.8Co0.15Al0.05O2 via Surface Structure Design. Small 2019, 15, 1904854. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.-L.; Deng, Y.-P.; Chen, Z. Ni-Rich/Co-Poor Layered Cathode for Automotive Li-Ion Batteries: Promises and Challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Shaw, L.; Ashuri, M. Coating-A Potent Method to Enhance Electrochemical Performance of Li(NixMnyCoz)O2 Cathodes for Li-ion Batteries. J. Adv. Mater. Lett. 2019, 10, 369–380. [Google Scholar]
- Rosy; Haber, S.; Evenstein, E.; Saha, A.; Brontvein, O.; Kratish, Y.; Bravo-Zhivotovskii, D.; Apeloig, Y.; Leskes, M.; Noked, M. Alkylated LixSiyOz Coating for Stabilization of Li-rich Layered Oxide Cathodes. Energy Storage Mater. 2020, 33, 268–275. [Google Scholar]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From Surface ZrO2 Coating to Bulk Zr Doping by High Temperature Annealing of Nickel-Rich Lithiated Oxides and Their Enhanced Electrochemical Performance in Lithium Ion Batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
- Shevtsov, A.; Han, H.; Morozov, A.; Carozza, J.C.; Savina, A.A.; Shakhova, I.; Khasanova, N.R.; Antipov, E.V.; Dikarev, E.V.; Abakumov, A.M. Protective Spinel Coating for Li1.17Ni0.17Mn0.50Co0.17O2 Cathode for Li-Ion Batteries through Single-Source Precursor Approach. Nanomaterials 2020, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.S.; Gauthier, R.; Eldesoky, A.; Murray, V.S.; Dahn, J.R. New Chemical Insights into the Beneficial Role of Al2O3 Cathode Coatings in Lithium-ion Cells. ACS Appl. Mater. Interfaces 2019, 11, 14095–14100. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-J.; Jung, H.-G.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. Improved Cycling Stability of Li[Ni0.90Co0.05Mn0.05]O2 Through Microstructure Modification by Boron Doping for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801202. [Google Scholar] [CrossRef]
- Sun, H.H.; Ryu, H.-H.; Kim, U.-H.; Weeks, J.A.; Heller, A.; Sun, Y.-K.; Mullins, C.B. Beyond Doping and Coating: Prospective Strategies for Stable High-Capacity Layered Ni-Rich Cathodes. ACS Energy Lett. 2020, 5, 1136–1146. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, J.; Li, Y.; Zheng, S.; Zhou, K.; Wang, D.; Wang, D.; Hong, C.; Gong, Z.; Yang, Y. Restraining the polarization increase of Ni-rich and low-Co cathodes upon cycling by Al-doping. J. Mater. Chem. A 2020, 8, 6893–6901. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Chen, Z.; Noh, H.-J.; Lee, D.-J.; Jung, H.-G.; Ren, Y.; Wang, S.; Yoon, C.S.; Myung, S.-T.; Amine, K. Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 2012, 11, 942–947. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Kim, M.-H.; Prakash, J.; Amine, K. Synthesis and Characterization of Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the Microscale Core-Shell Structure as the Positive Electrode Material for Lithium Batteries. J. Am. Chem. Soc. 2005, 127, 13411–13418. [Google Scholar] [CrossRef]
- Zeng, X.; Zhan, C.; Lu, J.; Amine, K. Stabilization of a High-Capacity and High-Power Nickel-Based Cathode for Li-Ion Batteries. Chem 2018, 4, 690–704. [Google Scholar] [CrossRef]
- He, L.-P.; Li, K.; Zhang, Y.; Liu, J. Structural and Electrochemical Properties of Low-Cobalt-Content LiNi0.6+xCo0.2–xMn0.2O2 (0.0 ≤ x ≤ 0.1) Cathodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 28253–28263. [Google Scholar] [CrossRef]
- Liu, J.; Kopold, P.; Wu, C.; van Aken, P.A.; Maier, J.; Yu, Y. Uniform yolk-shell Sn4P3@C nanospheres as high-capacity and cycle-stable anode materials for sodium-ion batteries. Energy Environ. Sci. 2015, 8, 3531–3538. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Shen, J.; Li, F.; Wang, Z.; Zhang, D.; Zuo, S. Fe3O4@C Nanotubes Grown on Carbon Fabric as a Free-Standing Anode for High Performance Li-Ion Batteries. Chem. Eur. J. 2020, 26, 14708–14714. [Google Scholar]
- Chen, Z.; Chao, D.; Liu, J.; Copley, M.; Lin, J.; Shen, Z.; Kim, G.-T.; Passerini, S. 1D nanobar-like LiNi0.4Co0.2Mn0.4O2 as a stable cathode material for lithium-ion batteries with superior long-term capacity retention and high rate capability. J. Mater. Chem. A 2017, 5, 15669–15675. [Google Scholar] [CrossRef]
- Kang, H.J.; Bari, G.; Lee, T.G.; Khan, T.T.; Park, J.W.; Hwang, H.J.; Cho, S.Y.; Jun, Y.S. Microporous Carbon Nanoparticles for Lithium-Sulfur Batteries. Nanomaterials 2020, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Han, C.; Li, B.; He, Y.; Lin, Z. In-Situ Crafting of ZnFe2O4 Nanoparticles Impregnated within Continuous Carbon Network as Advanced Anode Materials. ACS Nano 2016, 10, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; He, Y.; Li, B.; Zhao, S.; Wang, S.; He, Y.B.; Lin, Z. Polymer-Templated Formation of Polydopamine-Coated SnO2 Nanocrystals: Anodes for Cyclable Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2017, 56, 1869–1872. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Ding, Z.; Wang, J.; Wu, J.; Zhang, H.; Lavorgna, M. On the tailoring the 1D rod-like hierarchical nano/micro LiNi0.8Co0.15Al0.05O2 structure with exposed (101) plane by template method. J. Alloys Compd. 2019, 791, 356–363. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, W.-Y.; Zhang, J.; Wang, S.; Zhang, T.-T.; Yin, K.; Peng, X.; Jiang, Y.-C.; Liu, K.-H.; Guo, X.-D. Controlled synthesis of nickel-rich layered oxide cathodes with preferentially exposed {010} active facets for high rate and long cycling stable lithium-ion batteries. J. Alloys Compd. 2019, 775, 72–80. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Wu, B.; Xu, H.; Wang, L.; Yang, X.-Q.; Wu, F. Sphere-Shaped Hierarchical Cathode with Enhanced Growth of Nanocrystal Planes for High-Rate and Cycling-Stable Li-Ion Batteries. Nano Lett. 2015, 15, 656–661. [Google Scholar] [CrossRef]
- Chen, M.; Jin, X.; Chen, Z.; Zhong, Y.; Liao, Y.; Qiu, Y.; Cao, G.; Li, W. A cross-like hierarchical porous lithium-rich layered oxide with (110)-oriented crystal planes as a high energy density cathode for lithium ion batteries. J. Mater. Chem. A 2019, 7, 13120–13129. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Q.; Wu, X.; Chen, Z.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Synthesis of Ni-Rich Layered-Oxide Nanomaterials with Enhanced Li-Ion Diffusion Pathways as High-Rate Cathodes for Li-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 6583–6590. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. Math. Phys. Eng. Sci. 2015, 471, 20140792. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Dahn, J. The Formation of Layered Double Hydroxide Phases in the Coprecipitation Syntheses of [Ni0.80Co0.15](1-x)/0.95Alx(OH)2(anionn–)x/n (x = 0–0.2, n = 1, 2). ChemEngineering 2019, 3, 38. [Google Scholar] [CrossRef]
- Song, Y.; Wang, M.; Li, J.; Liu, Y.; Cui, H. Nanosheets self-supported structure in the orderly porous spheres of Co/Mn ions co-substituted α-Ni(OH)2 for high-performance supercapacitors. J. Sol-Gel Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Wei, W.; Ye, W.; Wang, J.; Huang, C.; Xiong, J.B.; Qiao, H.; Cui, S.; Chen, W.; Mi, L.; Yan, P. Hydrangea-like alpha-Ni1/3Co2/3(OH)2 Reinforced by Ethyl Carbamate “Rivet” for All-Solid-State Supercapacitors with Outstanding Comprehensive Performance. ACS Appl. Mater. Interfaces 2019, 11, 32269–32281. [Google Scholar] [CrossRef] [PubMed]
- Duquesne, E.; Betelu, S.; Seron, A.; Ignatiadis, I.; Perrot, H.; Debiemme-Chouvy, C. Tuning Redox State and Ionic Transfers of Mg/Fe-Layered Double Hydroxide Nanosheets by Electrochemical and Electrogravimetric Methods. Nanomaterials 2020, 10, 1832. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient water oxidation using nanostructured alpha-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Shi, J.-L.; Liang, J.-Y.; Yin, Y.-X.; Zhang, J.-N.; Yu, X.-Q.; Guo, Y.-G. Suppressing Surface Lattice Oxygen Release of Li-Rich Cathode Materials via Heterostructured Spinel Li4Mn5O12 Coating. Adv. Mater. 2018, 30, 1801751. [Google Scholar] [CrossRef]
- Meng, F.; Hu, R.; Chen, Z.; Tan, L.; Lan, X.; Yuan, B. Plasma assisted synthesis of LiNi0.6Co0.2Mn0.2O2 cathode materials with good cyclic stability at subzero temperatures. J. Energy Chem. 2021, 56, 46–55. [Google Scholar] [CrossRef]
- Mo, Y.; Guo, L.; Cao, B.; Wang, Y.; Zhang, L.; Jia, X.; Chen, Y. Correlating structural changes of the improved cyclability upon Nd-substitution in LiNi0.5Co0.2Mn0.3O2 cathode materials. Energy Storage Mater. 2019, 18, 260–268. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Wu, C.; Qian, J.; Chen, G.; Liu, L.; Wang, H.; Zhou, X.; Wu, F. Three-dimensional fusiform hierarchical micro/nano Li1.2Ni0.2Mn0.6O2 with a preferred orientation (110) plane as a high energy cathode material for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 5942–5951. [Google Scholar] [CrossRef]
- Xu, M.; Fei, L.; Zhang, W.; Li, T.; Lu, W.; Zhang, N.; Lai, Y.; Zhang, Z.; Fang, J.; Zhang, K.; et al. Tailoring Anisotropic Li-Ion Transport Tunnels on Orthogonally Arranged Li-Rich Layered Oxide Nanoplates toward High-Performance Li-Ion Batteries. Nano Lett. 2017, 17, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, R.; Cao, C. LiNi1/3Co1/3Mn1/3O2 Nanoplates with {010} Active Planes Exposing Prepared in Polyol Medium as a High-Performance Cathode for Li-Ion Battery. ACS Appl. Mater. Interfaces 2014, 6, 5075–5082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mauger, A.; Lu, Q.; Groult, H.; Perrigaud, L.; Gendron, F.; Julien, C.M. Synthesis and characterization of LiNi1/3Mn1/3Co1/3O2 by wet-chemical method. Electrochim. Acta 2010, 55, 6440–6449. [Google Scholar] [CrossRef]
- Shen, Z.; Cao, L.; Rahn, C.D.; Wang, C.Y. Least Squares Galvanostatic Intermittent Titration Technique (LS-GITT) for Accurate Solid Phase Diffusivity Measurement. J. Electrochem. Soc. 2013, 160, A1842–A1846. [Google Scholar] [CrossRef]
- Märker, K.; Reeves, P.J.; Xu, C.; Griffith, K.J.; Grey, C.P. Evolution of Structure and Lithium Dynamics in LiNi0.8Mn0.1Co0.1O2 (NMC811) Cathodes during Electrochemical Cycling. Chem. Mater. 2019, 31, 2545–2554. [Google Scholar] [CrossRef]
- Funabiki, A.; Inaba, M.; Ogumi, Z. Ac impedance analysis of electrochemical lithium intercalation into highly oriented pyrolytic graphite. J. Power Sources 1997, 68, 227–231. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, Z.; Shen, J.; Xu, X.; Zeng, L.; Li, Y.; Zhang, D.; Zuo, S.; Liu, J. Ni-Rich Layered Oxide with Preferred Orientation (110) Plane as a Stable Cathode Material for High-Energy Lithium-Ion Batteries. Nanomaterials 2020, 10, 2495. https://doi.org/10.3390/nano10122495
Li F, Liu Z, Shen J, Xu X, Zeng L, Li Y, Zhang D, Zuo S, Liu J. Ni-Rich Layered Oxide with Preferred Orientation (110) Plane as a Stable Cathode Material for High-Energy Lithium-Ion Batteries. Nanomaterials. 2020; 10(12):2495. https://doi.org/10.3390/nano10122495
Chicago/Turabian StyleLi, Fangkun, Zhengbo Liu, Jiadong Shen, Xijun Xu, Liyan Zeng, Yu Li, Dechao Zhang, Shiyong Zuo, and Jun Liu. 2020. "Ni-Rich Layered Oxide with Preferred Orientation (110) Plane as a Stable Cathode Material for High-Energy Lithium-Ion Batteries" Nanomaterials 10, no. 12: 2495. https://doi.org/10.3390/nano10122495
APA StyleLi, F., Liu, Z., Shen, J., Xu, X., Zeng, L., Li, Y., Zhang, D., Zuo, S., & Liu, J. (2020). Ni-Rich Layered Oxide with Preferred Orientation (110) Plane as a Stable Cathode Material for High-Energy Lithium-Ion Batteries. Nanomaterials, 10(12), 2495. https://doi.org/10.3390/nano10122495