Excellent, Lightweight and Flexible Electromagnetic Interference Shielding Nanocomposites Based on Polypropylene with MnFe2O4 Spinel Ferrite Nanoparticles and Reduced Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
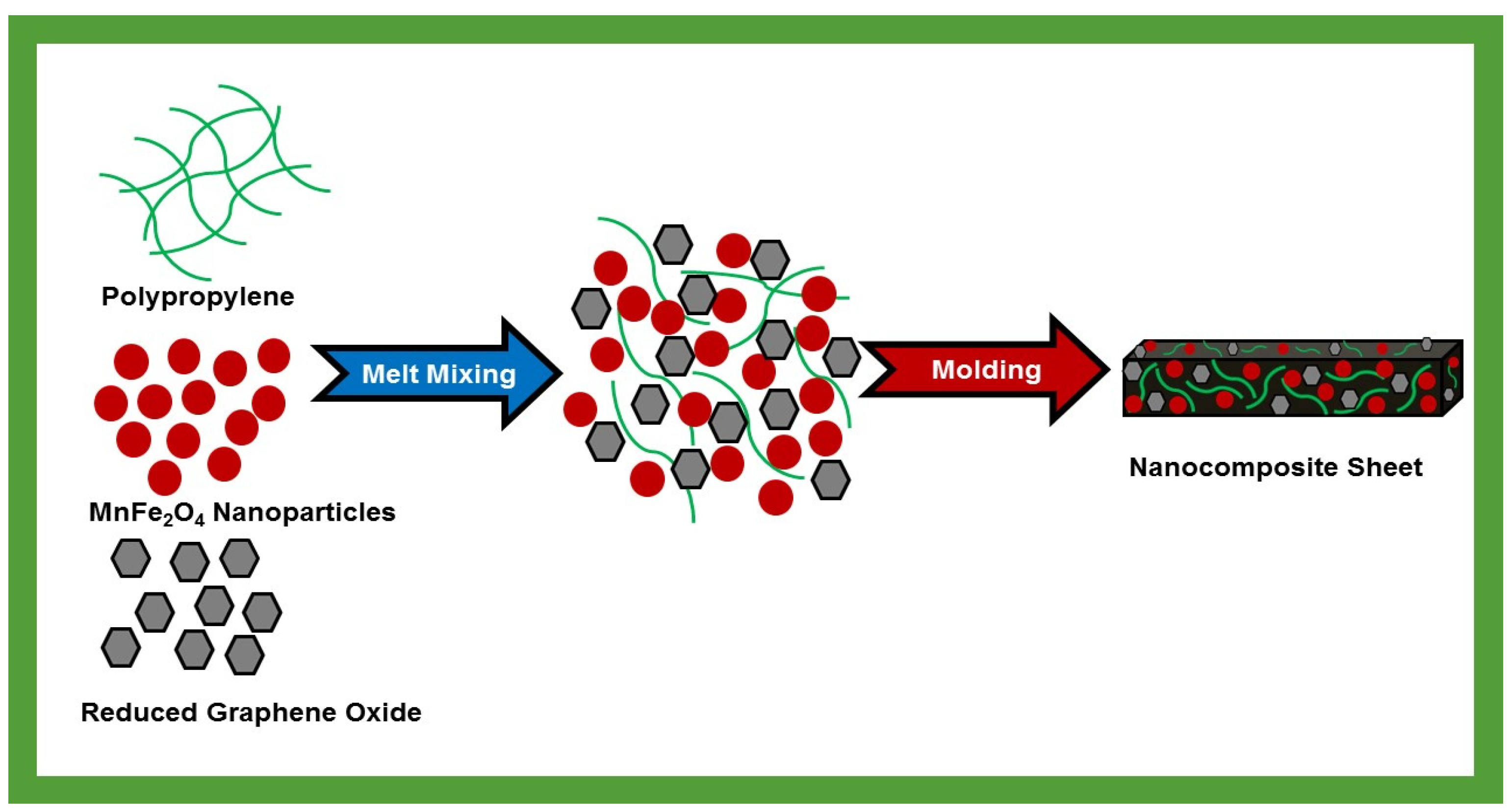
2.3. Preparation of Nanocomposites
2.4. Characterization Techniques
3. Results
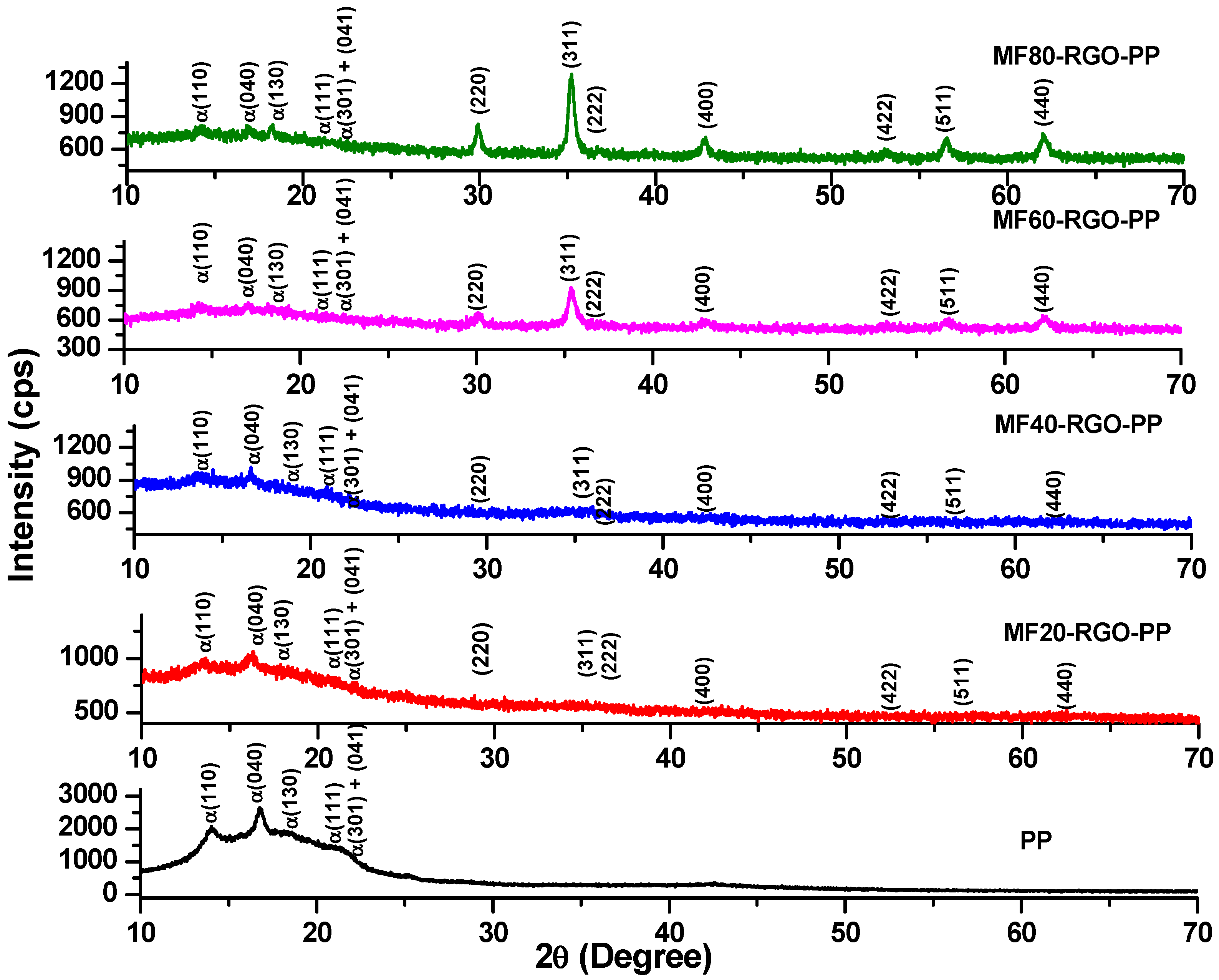
3.1. XRD Study
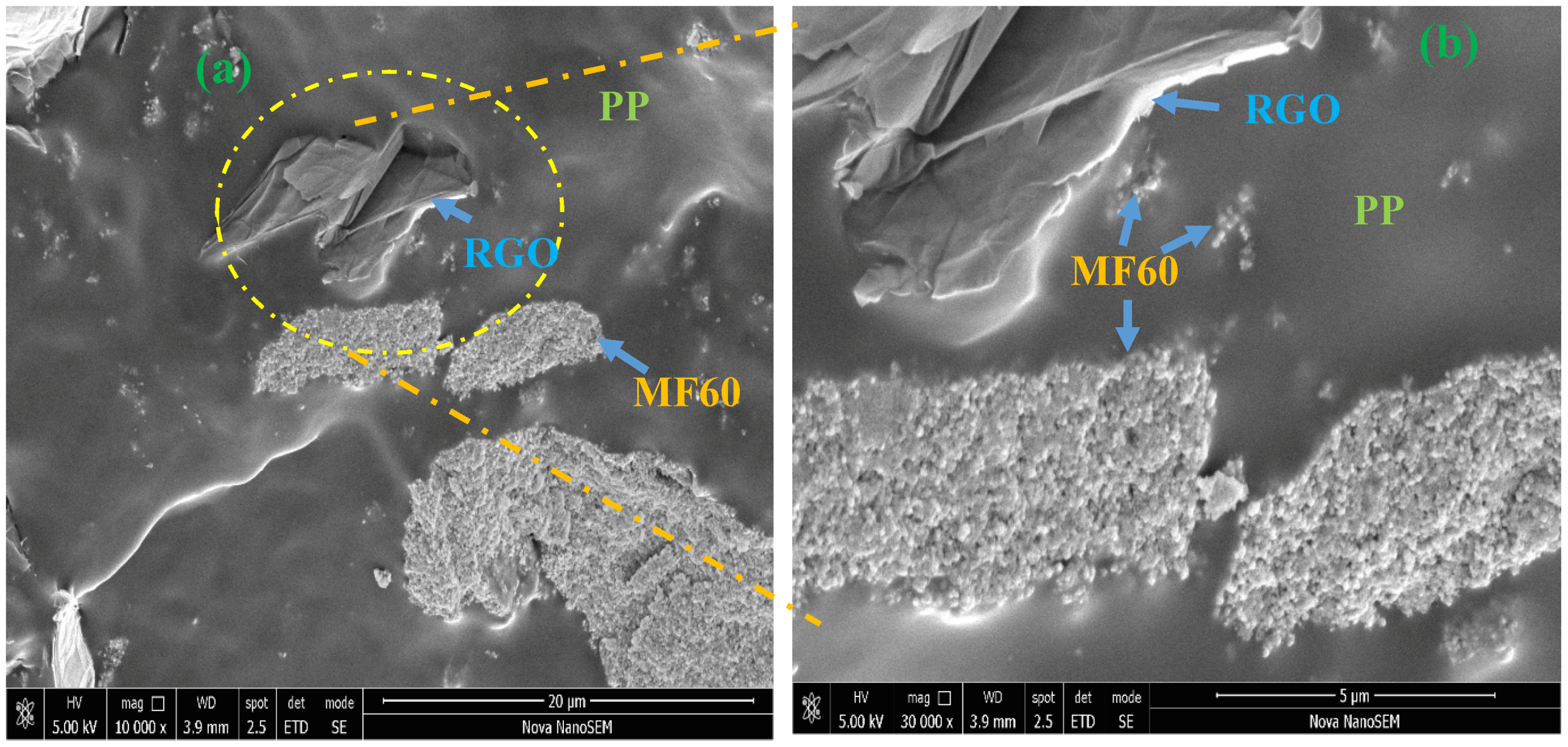
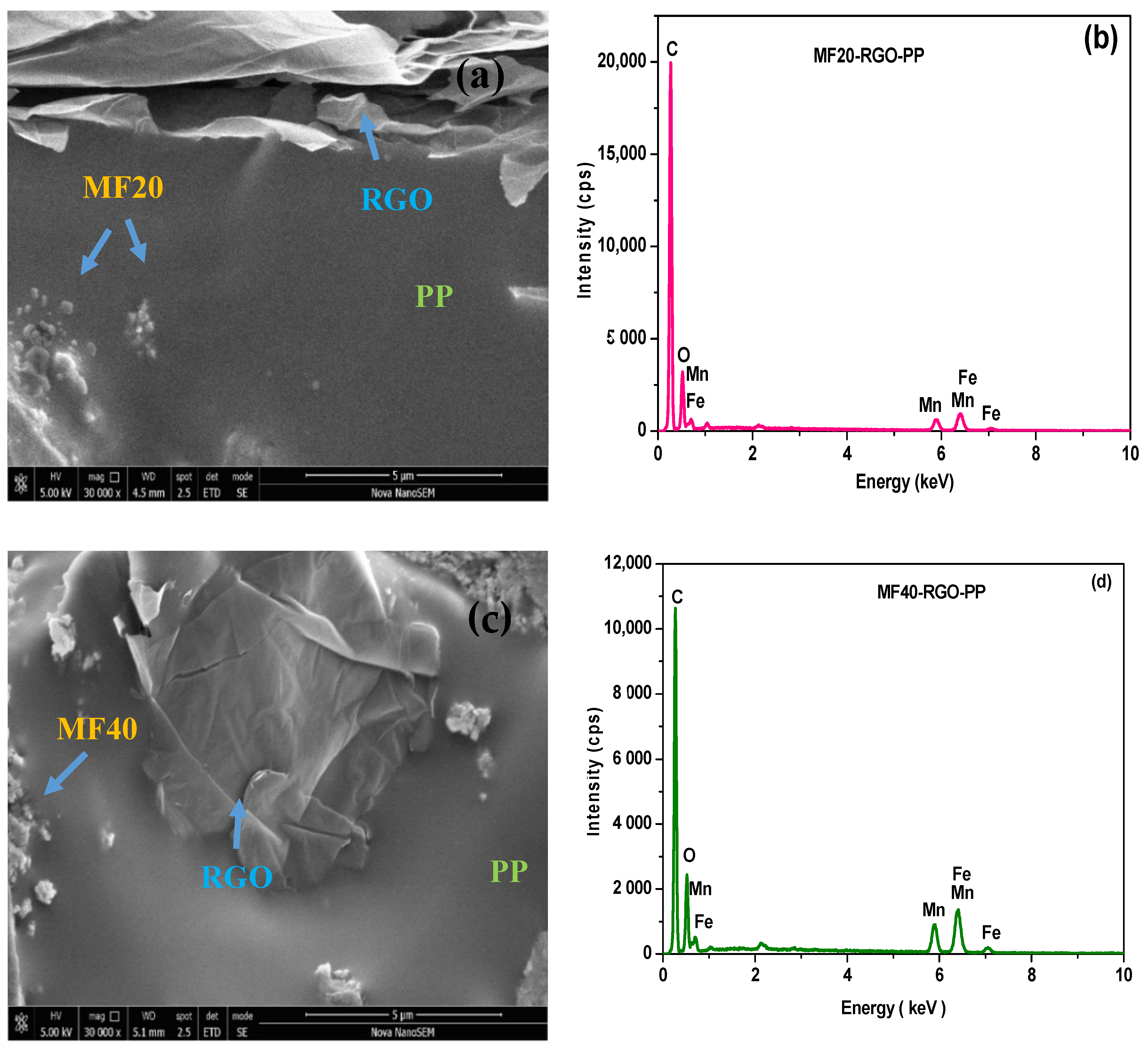
3.2. FE-SEM Study
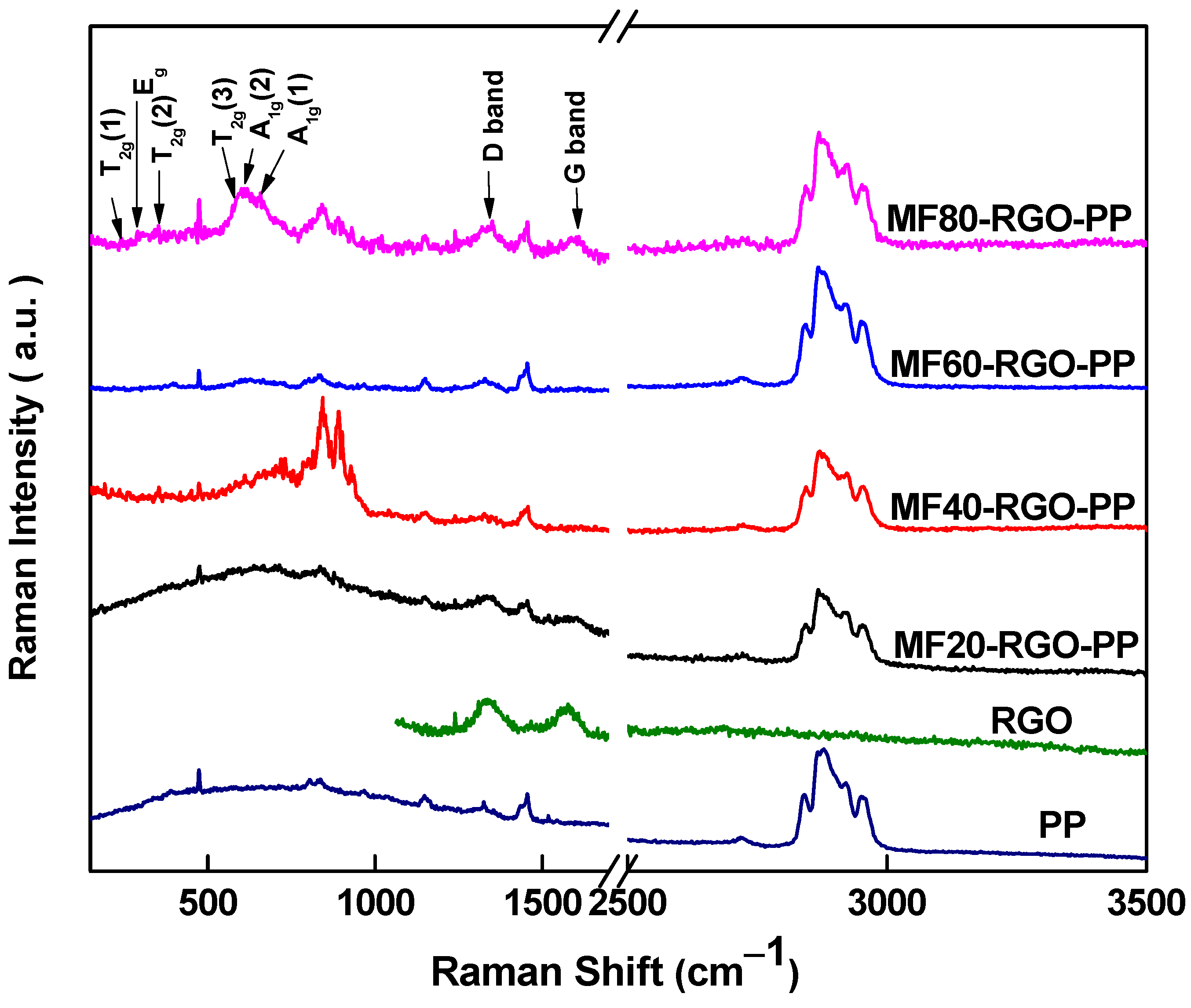
3.3. Raman Spectroscopy
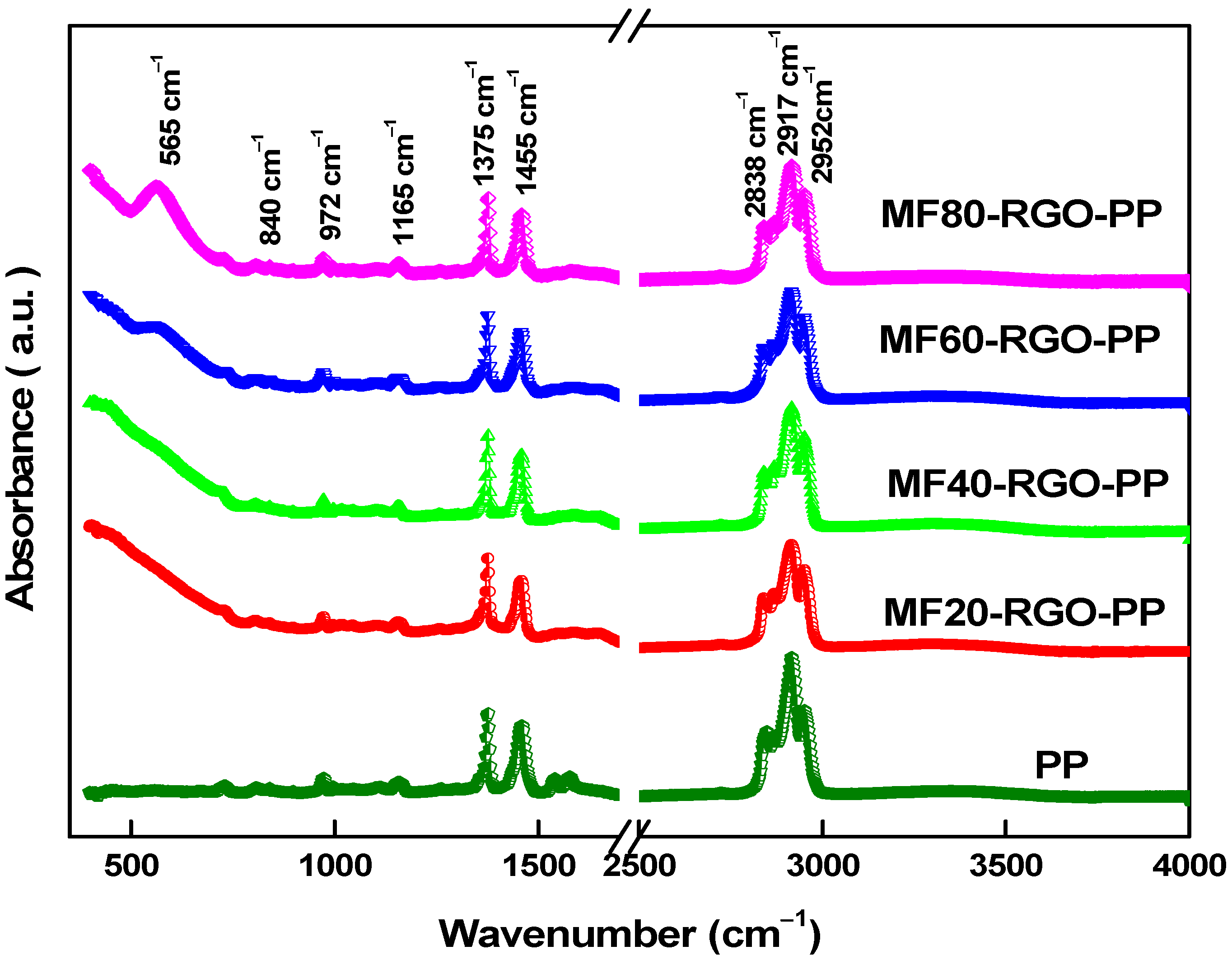
3.4. FTIR Spectroscopy
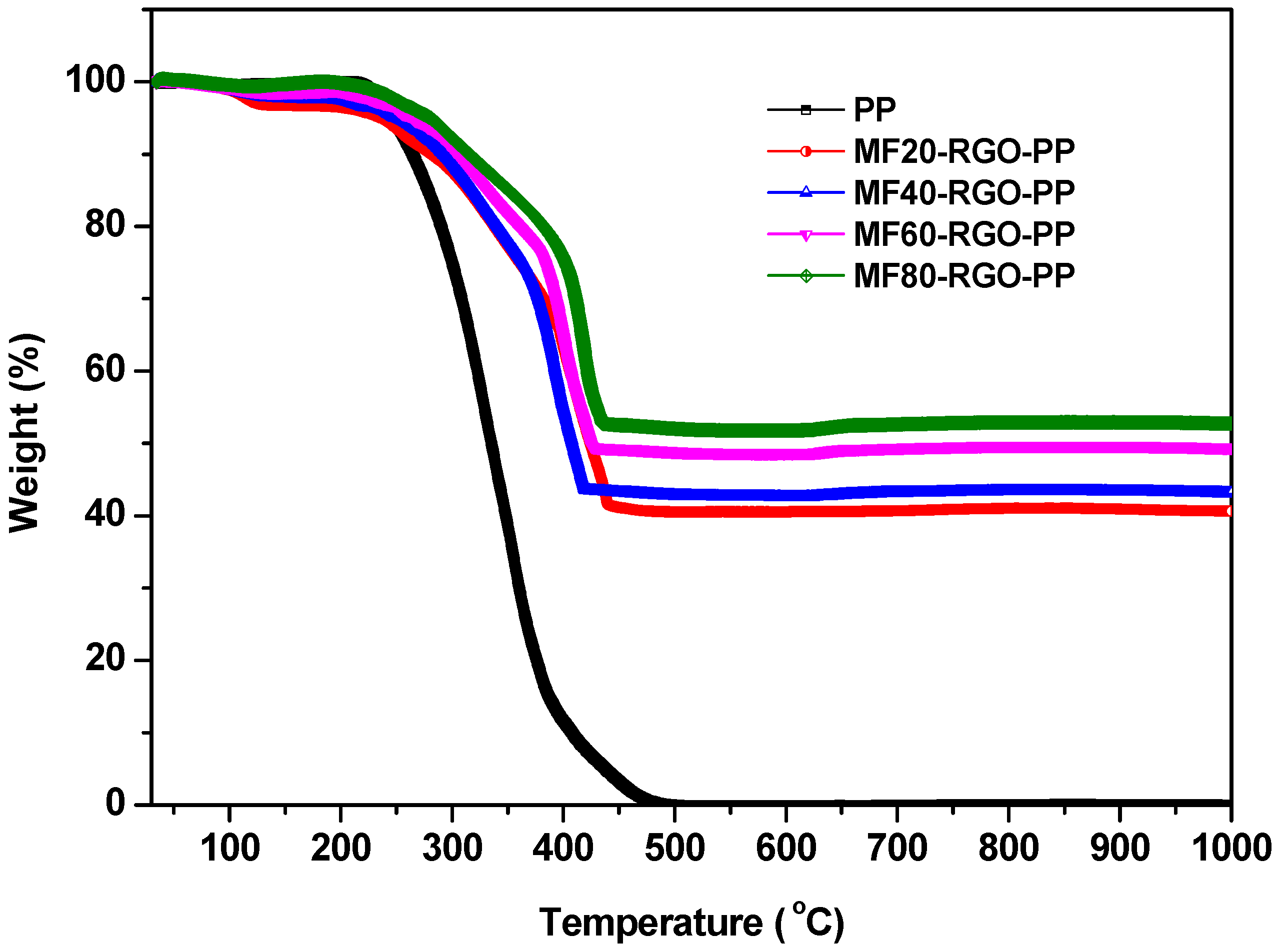
3.5. Thermogravimetric Analysis (TGA)
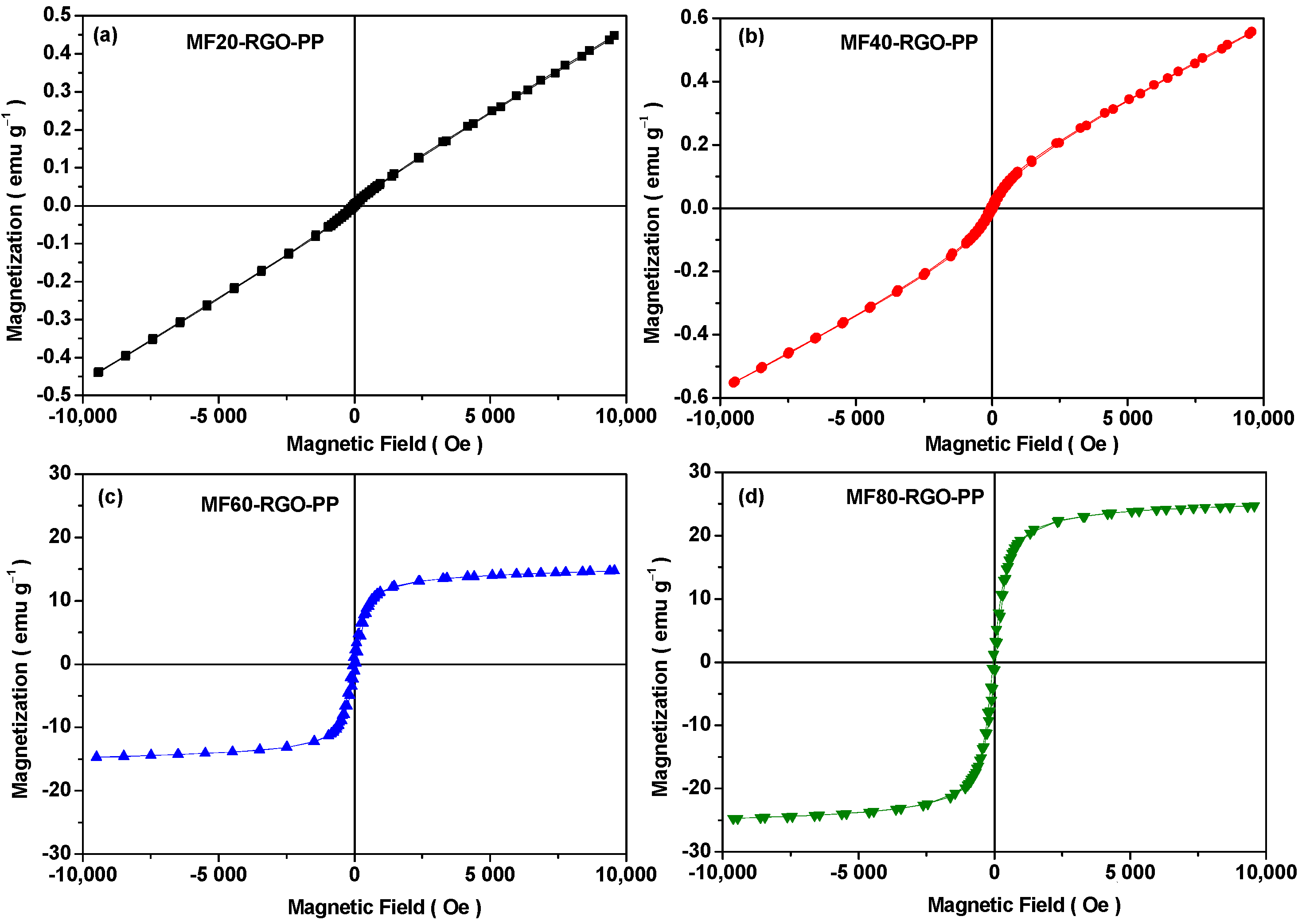
3.6. Magnetic Property
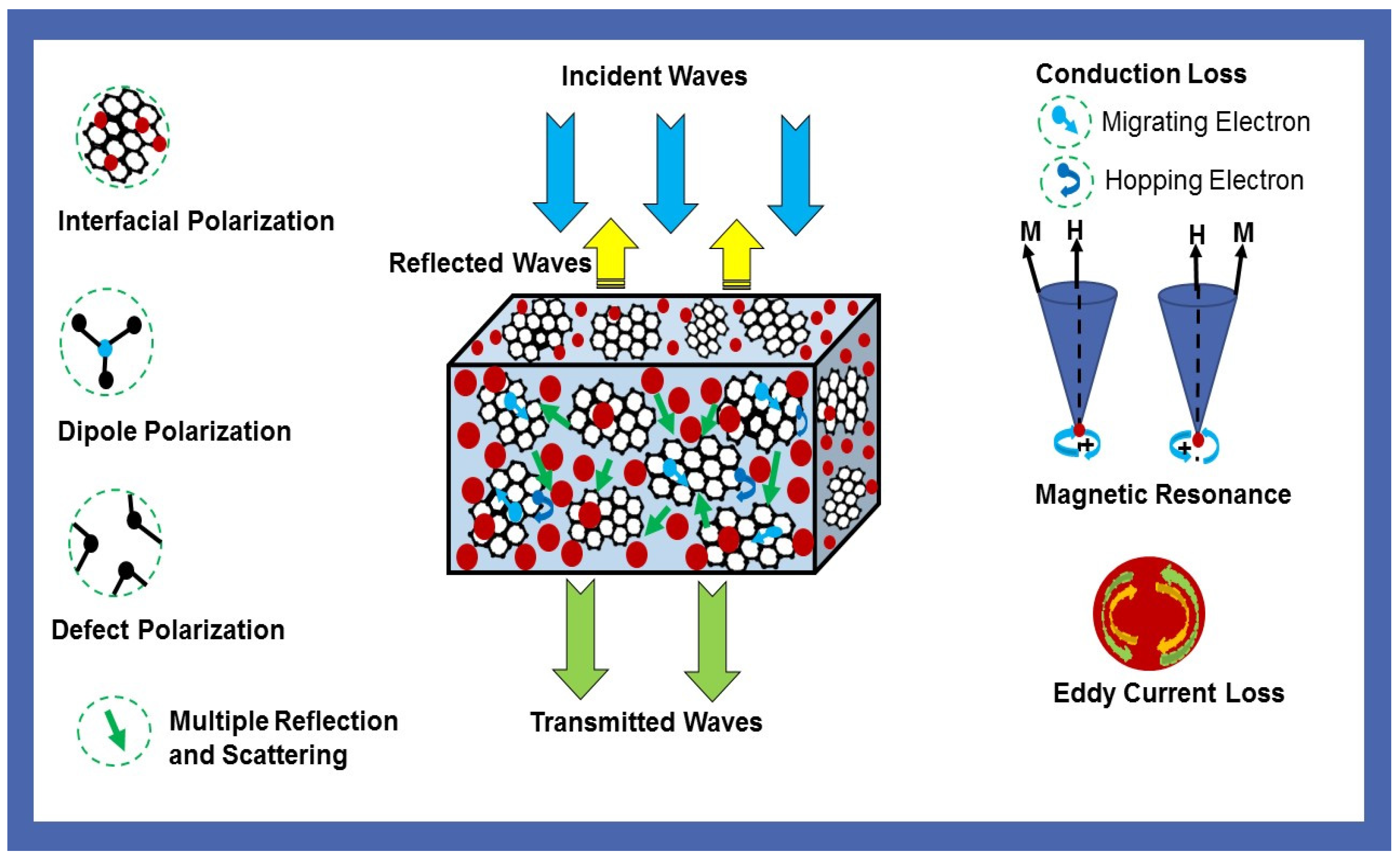
3.7. Electromagnetic Interference Shielding Effectiveness
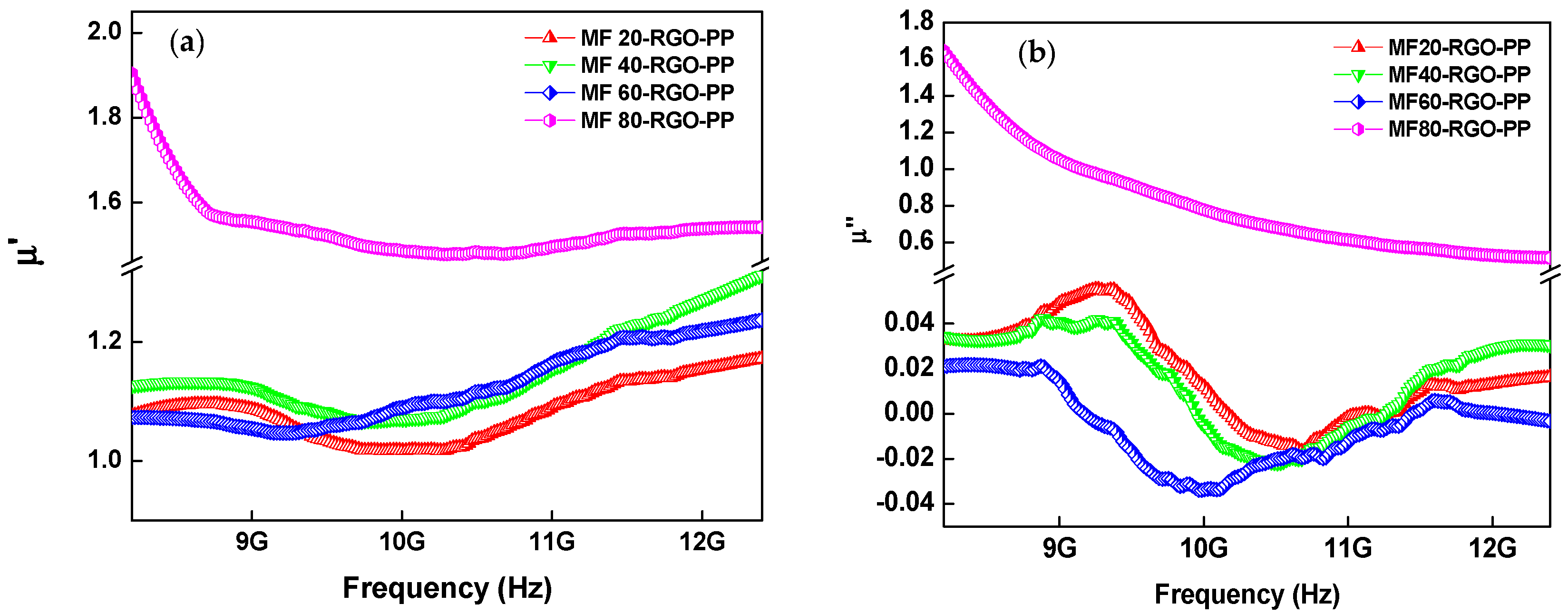
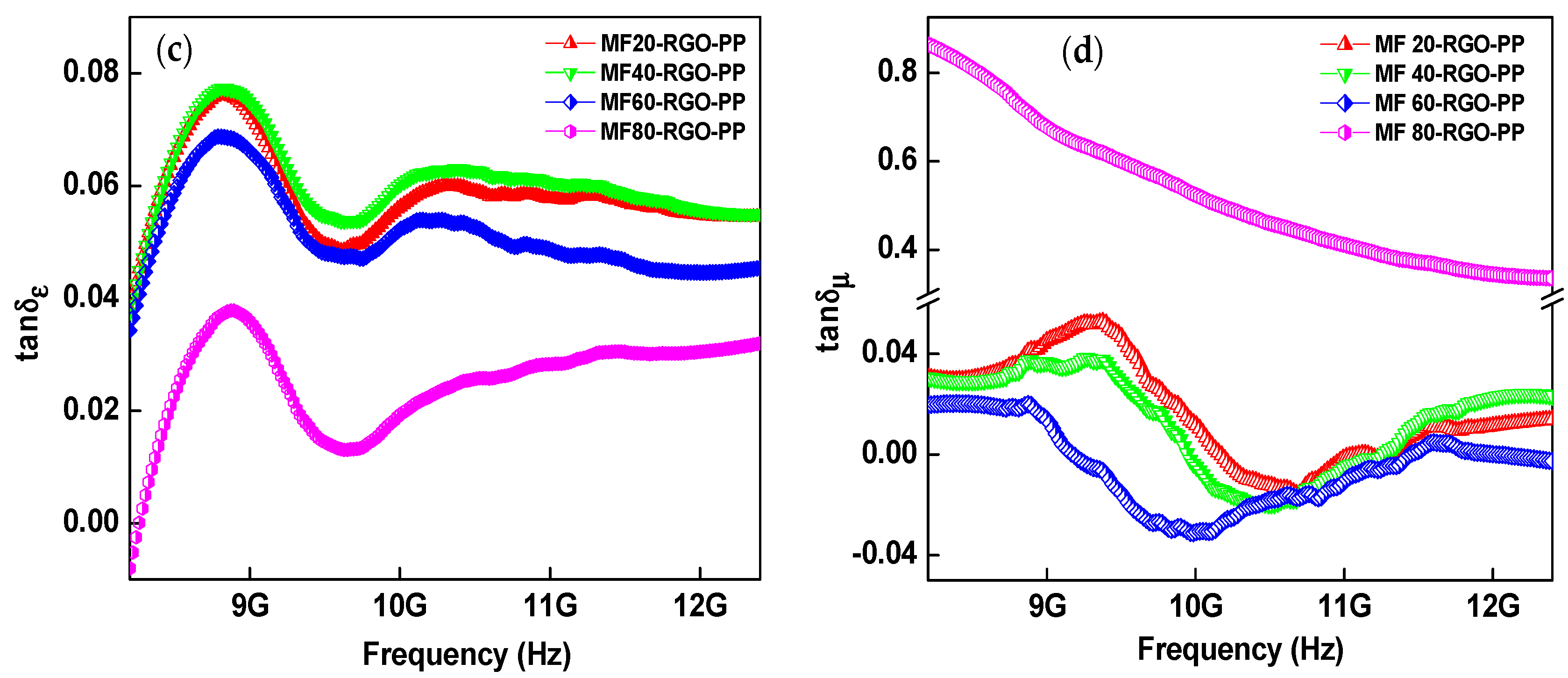
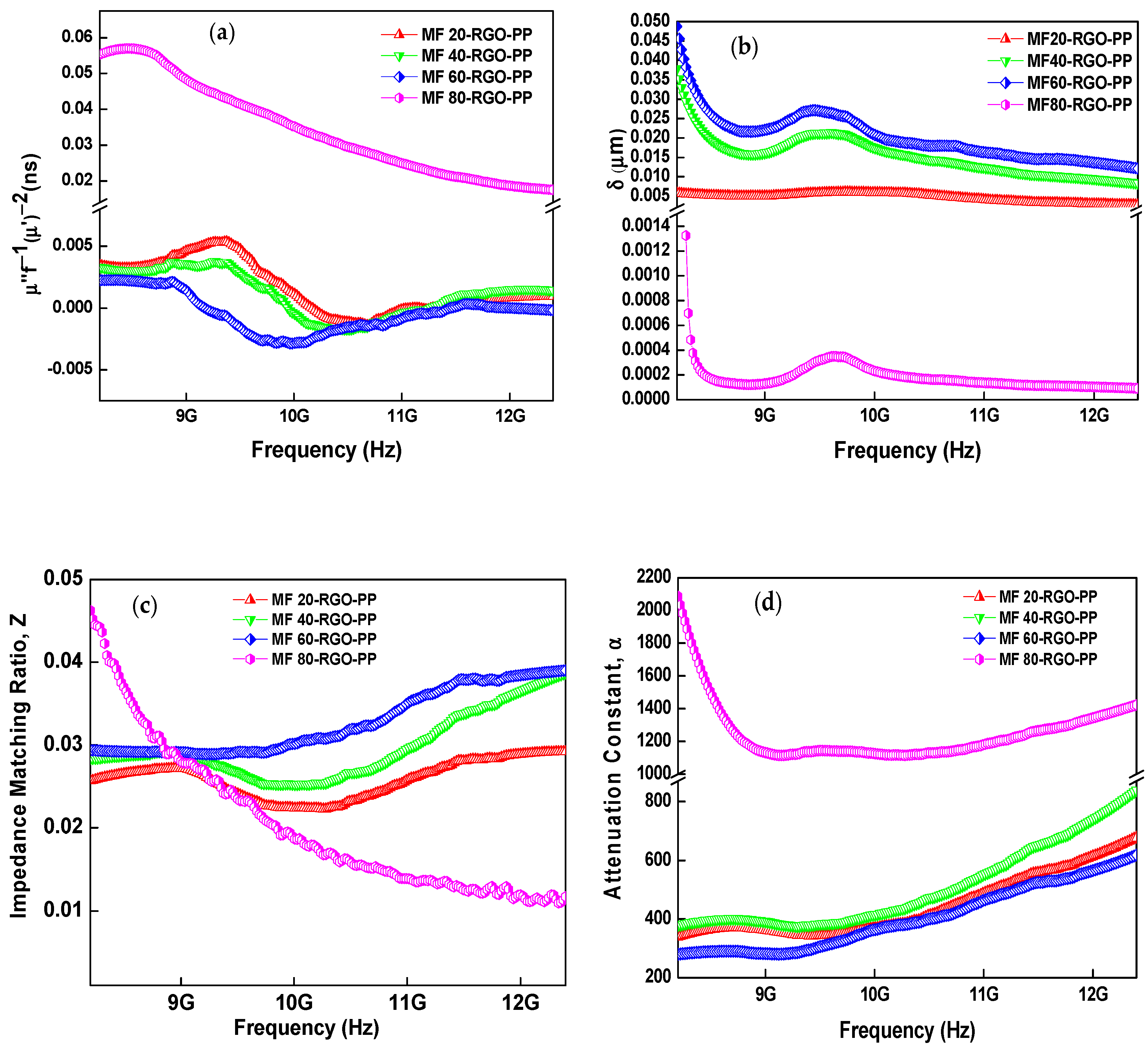
3.8. Electromagnetic Properties and Parameters
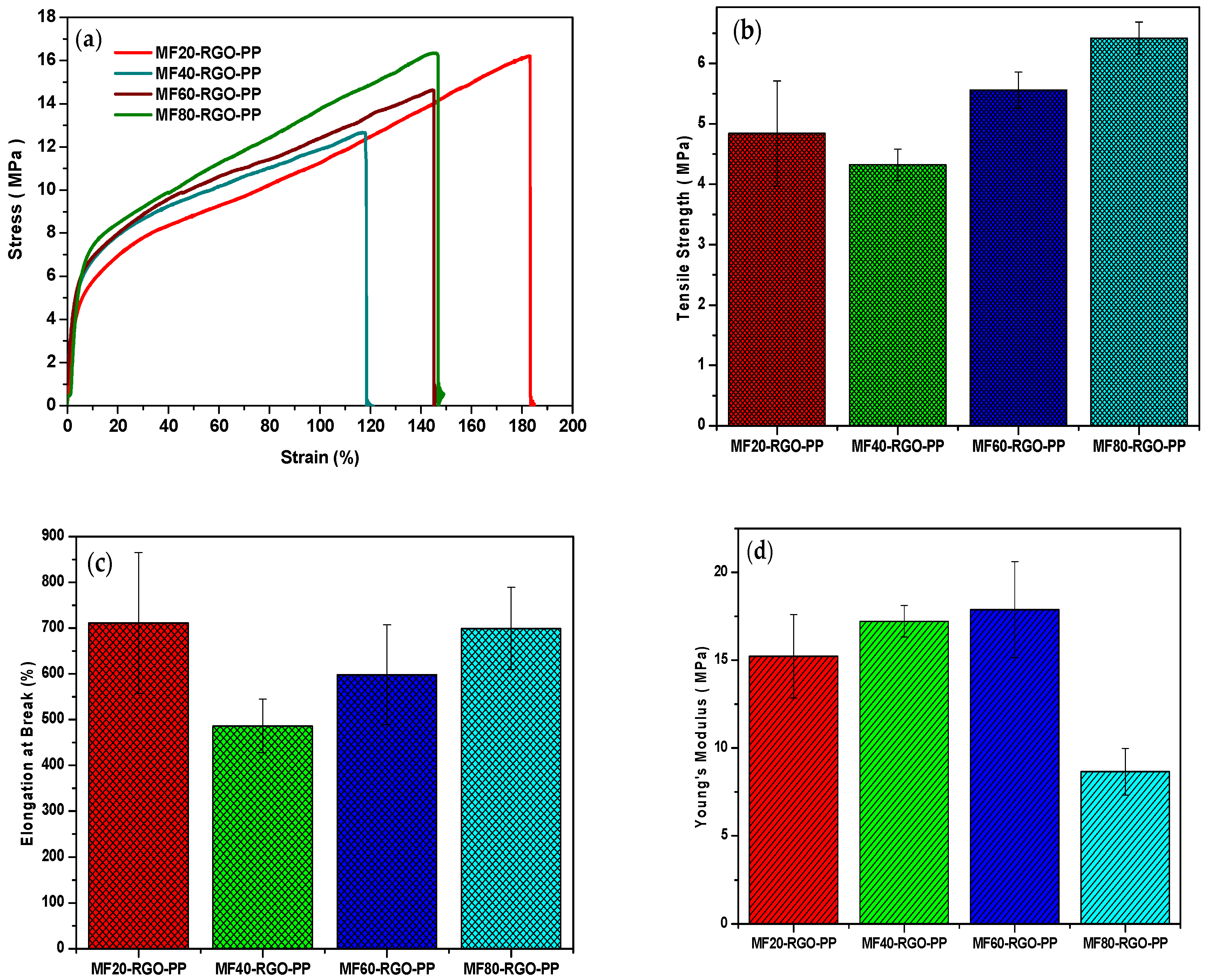
3.9. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biswas, S.; Arief, I.; Panja, S.S.; Bose, S. Absorption-Dominated Electromagnetic Wave Suppressor Derived from Ferrite-Doped Cross-Linked Graphene Framework and Conducting Carbon. ACS Appl. Mater. Interfaces 2017, 9, 3030–3039. [Google Scholar] [CrossRef]
- Cao, W.-T.; Chen, F.-F.; Zhu, Y.-J.; Zhang, Y.-G.; Jiang, Y.-Y.; Ma, M.-G.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, M.; Yu, Y.; Wen, B.; Cheng, L. A Novel Polyaniline-Coated Bagasse Fiber Composite with Core-Shell Heterostructure Provides Effective Electromagnetic Shielding Performance. ACS Appl. Mater. Interfaces 2017, 9, 809–818. [Google Scholar] [CrossRef]
- Shen, B.; Li, Y.; Zhai, W.; Zheng, W. Compressible Graphene-Coated Polymer Foams with Ultralow Density for Adjustable Electromagnetic Interference (EMI) Shielding. ACS Appl. Mater. Interfaces 2016, 8, 8050–8057. [Google Scholar] [CrossRef]
- Hsiao, S.-T.; Ma, C.-C.M.; Tien, H.-W.; Liao, W.-H.; Wang, Y.-S.; Li, S.-M.; Yang, C.-Y.; Lin, S.-C.; Yang, R.-B. Effect of Covalent Modification of Graphene Nanosheets on the Electrical Property and Electromagnetic Interference Shielding Performance of a Water-Borne Polyurethane Composite. ACS Appl. Mater. Interfaces 2015, 7, 2817–2826. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.; Zhou, W.; Luo, F. Effect of Multiwalled Carbon Nanotubes on the Electromagnetic Interference Shielding Properties of Polyimide/Carbonyl Iron Composites. Ind. Eng. Chem. Res. 2015, 54, 6589–6595. [Google Scholar] [CrossRef]
- Kim, S.; Oh, J.-S.; Kim, M.-G.; Jang, W.; Wang, M.; Kim, Y.; Seo, H.-W.; Kim, Y.-C.; Lee, J.-H.; Lee, Y.; et al. Electromagnetic Interference (EMI) Transparent Shielding of Reduced Graphene Oxide (RGO) Interleaved Structure Fabricated by Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2014, 6, 17647–17653. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Z.; Zhou, J. Fabrication and microwave absorption of reduced graphene oxide/Ni0.4Zn0.4Co0.2 Fe2O4 nanocomposites. Ceram. Int. 2016, 42, 9241–9249. [Google Scholar] [CrossRef]
- Dippong, T.; Toloman, D.; Levei, E.-A.; Cadar, O.; Mesaros, A. A possible formation mechanism and photocatalytic properties of CoFe2O4/ PVA-SiO2 nanocomposites. Thermochim. Acta 2018, 666, 103–115. [Google Scholar] [CrossRef]
- Jazirehpour, M.; Ebrahimi, S.S. Synthesis of magnetite nanostructures with complex morphologies and effect of these morphologies on magnetic and electromagnetic properties. Ceram. Int. 2016, 42, 16512–16520. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Wu, Y.; Zong, B.; Ding, J. Size-dependent microwave absorption properties of Fe3O4 nanodiscs. RSC Adv. 2016, 6, 25444–25448. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Fan, F.; Ma, M.; Sun, J.; Chen, J.; Zhang, Y.; Gu, N. Size-dependent electromagnetic properties and the related simulations of Fe3O4 nanoparticles made by microwave-assisted thermal decomposition. Colloids Surf. A 2017, 530, 191–199. [Google Scholar] [CrossRef]
- Wu, N.; Liu, X.; Zhao, C.; Cui, C.; Xia, A. Effects of particle size on the magnetic and microwave absorption properties of carbon-coated nickel nanocapsules. J. Alloy. Compd. 2016, 656, 628–634. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Machovsky, M.; Skoda, D.; Urbánek, P.; Masař, M.; Jurča, M.; Urbánek, M.; Kalina, L.; et al. NiFe2O4 Nanoparticles Synthesized by Dextrin from Corn-Mediated Sol-Gel Combustion Method and Its Polypropylene Nanocomposites Engineered with Reduced Graphene Oxide for the Reduction of Electromagnetic Pollution. ACS Omega 2019, 4, 22069–22081. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Jamatia, T.; Machovsky, M.; Skoda, D.; Urbánek, P.; Masař, M.; Urbánek, M.; Kalina, L.; et al. Impact of sonochemical synthesis condition on the structural and physical properties of MnFe2O4 spinel ferrite nanoparticles. Ultrason. Sonochem. 2020, 61, 104839. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Rakhi, R.B.; Chen, W.; Alshareef, H.N. Effect of pH induced chemical modification of hydrothermally reduced graphene oxide on supercapacitor performance. J. Power Sources 2013, 233, 313–319. [Google Scholar] [CrossRef]
- Patade, S.R.; Andhare, D.D.; Somvanshi, S.B.; Jadhav, S.A.; Khedkar, M.V.; Jadhav, K.M. Self-heating evaluation of superparamagnetic MnFe2O4 nanoparticles for magnetic fluid hyperthermia application towards cancer treatment. Ceram. Int. 2020, 46, 25576–25583. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Liao, S.-H.; Lin, Y.-F.; Wang, C.-A.; Pu, N.-W.; Tsai, H.-M.; Ma, C.-C.M. Preparation and characterization of polypropylene-graft-thermally reduced graphite oxide with an improved compatibility with polypropylene-based nanocomposite. Nanoscale 2011, 3, 1516. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuritka, I.; Vilcáková, J.; Machovský, M.; Škoda, D.; Urbánek, P.; Masar, M.; Goralik, M.; Urbánek, M.; Kalina, L.; et al. Polypropylene Nanocomposite Filled with Spinel Ferrite NiFe2O4 Nanoparticles and In-Situ Thermally-Reduced Graphene Oxide for Electromagnetic Interference Shielding Application. Nanomaterials 2019, 9, 621. [Google Scholar] [CrossRef]
- Varshney, D.; Verma, K.; Kumar, A. Structural and vibrational properties of ZnxMn1-xFe2O4(x = 0.0, 0.25, 0.50, 0.75, 1.0) mixed ferrites. Mater. Chem. Phys. 2011, 131, 413–419. [Google Scholar] [CrossRef]
- Gupta, A.; Jamatia, R.; Patil, R.A.; Ma, Y.-R.; Pal, A.K. Copper Oxide/Reduced Graphene Oxide Nanocomposite-Catalyzed Synthesis of Flavanones and Flavanones with Triazole Hybrid Molecules in One Pot: A Green and Sustainable Approach. ACS Omega 2018, 3, 7288–7299. [Google Scholar] [CrossRef]
- Wadi, V.S.; Jena, K.K.; Halique, K.; Alhassan, S.M. Enhanced Mechanical Toughness of Isotactic Polypropylene Using Bulk Molybdenum Disulfide. ACS Omega 2020, 5, 11394–11401. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, P.; Thakur, P.; Rana, K.; Chevalier, A.; Mattei, J.-L.; Queffélec, P. Enhancement of magnetic properties of Ni0.5Zn0.5Fe2O4 nanoparticles prepared by the co-precipitation method. Ceram. Int. 2016, 42, 10664–10670. [Google Scholar] [CrossRef]
- Gopanna, A.; Mandapati, R.N.; Thomas, S.P.; Rajan, K.; Chavali, M. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blends for qualitative and quantitative analysis. Polym. Bull. 2019, 76, 4259–4274. [Google Scholar] [CrossRef]
- Hassan, M.M.; Koyama, K. Enhanced thermal, mechanical and fire retarding properties of polystyrene sulphonate-grafted- nanosilica/polypropylene composites. RSC Adv. 2015, 5, 16950–16959. [Google Scholar] [CrossRef]
- He, Q.; Yuan, T.; Zhang, X.; Luo, Z.; Haldolaarachchige, N.; Sun, L.; Young, D.P.; Wei, S.; Guo, Z. Magnetically Soft and Hard Polypropylene/Cobalt Nanocomposites: Role of Maleic Anhydride Grafted Polypropylene. Macromolecules 2013, 46, 2357–2368. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Li, Y.; Sun, L.; Haldolaarachchige, N.; Young, D.P.; Southworth, C.; Khasanov, A.; Luo, Z.; Guo, Z. Surfactant-Free Synthesized Magnetic Polypropylene Nanocomposites: Rheological, Electrical, Magnetic, and Thermal Properties. Macromolecules 2011, 44, 4382–4391. [Google Scholar] [CrossRef]
- Lv, H.; Liang, X.; Ji, G.; Zhang, H.; Du, Y. Porous Three- Dimensional Flower-like Co/CoO and Its Excellent Electromagnetic Absorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 9776–9783. [Google Scholar] [CrossRef]
- Manna, K.; Srivastava, S.K. Fe3O4@Carbon@Polyaniline Trilaminar Core−Shell Composites as Superior Microwave Absorber in Shielding of Electromagnetic Pollution. ACS Sustain. Chem. Eng. 2017, 5, 10710–10721. [Google Scholar] [CrossRef]
- Shahzad, F.; Kumar, P.; Kim, Y.-H.; Hong, S.M.; Koo, C.M. Biomass-Derived Thermally Annealed Interconnected Sulfur-Doped Graphene as a Shield against Electromagnetic Interference. ACS Appl. Mater. Interfaces 2016, 8, 9361–9369. [Google Scholar] [CrossRef]
- Song, W.-L.; Gong, C.; Li, H.; Cheng, X.-D.; Chen, M.; Yuan, X.; Chen, H.; Yang, Y.; Fang, D. Graphene-Based Sandwich Structures for Frequency Selectable Electromagnetic Shielding. ACS Appl. Mater. Interfaces 2017, 9, 36119–36129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Wang, G.-S.; Cao, W.-Q.; Wei, Y.-Z.; Liang, J.-F.; Guo, L.; Cao, M.-S. Enhanced Microwave Absorption Property of Reduced Graphene Oxide (RGO)-MnFe2O4 Nanocomposites and Polyvinylidene Fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Zhang, L.; Sun, P.; Wang, J.; Feng, X.; Zhang, Y.; Dai, J.; Tang, Y. Apium-derived biochar loaded with MnFe2O4@C for excellent low frequency electromagnetic wave absorption. Ceram. Int. 2020, 46, 13641–13650. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bera, P.; Chakradhar, R.P.S.; Choudhury, B.; Pawar, S.P.; Bose, S.; Nair, R.U.; Barshilia, H.C. Enhanced microwave absorption properties of PMMA modified MnFe2O4-polyaniline nanocomposites. Phys. Chem. Chem. Phys. 2019, 21, 5068–5077. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Xavier, P.; Gupta, S.N.; Kar, G.N.; Bose, S.; Sood, A.K. Excellent Electromagnetic Interference Shielding by Graphene- MnFe2O4 -Multiwalled Carbon Nanotube Hybrids at Very Low Weight Percentage in Polymer Matrix. ChemistrySelect 2016, 1, 5995–6003. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhang, W.; Huang, S. One-pot synthesis of MnFe2O4 nanoparticles-decorated reduced graphene oxide for enhanced microwave absorption properties. Mater. Technol. 2017, 32, 32–37. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, L.; Wang, J.; Feng, X.; Zhao, L.; Rao, H.; Wang, Y.; Dai, J. Preparation of SiO2- MnFe2O4 Composites via One-Pot Hydrothermal Synthesis Method and Microwave Absorption Investigation in S-Band. Molecules 2019, 24, 2605. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhang, W.; Huang, S. Synthesis and electromagnetic absorption properties of Ag-coated reduced graphene oxide with MnFe2O4 particles. J. Magn. Magn. Mater. 2016, 404, 58–63. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K.; Bhattacharya, S.N.; Varley, R.J. Experimental and simulation study of effect of thickness on performance of (butylene adipate-co-terephthalate) and poly lactide nanocomposites incorporated with graphene as stand-alone electromagnetic interference shielding and metal-backed microwave absorbers. Compos. Sci. Technol. 2020, 195, 108186. [Google Scholar]
- Sui, M.; Fu, T.; Sun, X.; Cui, G.; Lv, X.; Gu, G. Unary and binary doping effect of M2+ (M=Mn, Co, Ni, Zn) substituted hollow Fe3O4 approach for enhancing microwave attenuation. Ceram. Int. 2018, 44, 17138–17146. [Google Scholar] [CrossRef]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.B.; Pasha, S.K.K. Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: A review. Compos. Part A 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Mishra, M.; Singh, A.P.; Singh, B.P.; Singh, V.N.; Dhawan, S.K. Conducting Ferrofluid: A High-performance Microwave Shielding Material. J. Mater. Chem. A 2014, 2, 13159–13168. [Google Scholar] [CrossRef]
- Behera, C.; Choudhary, R.N.P.; Das, P.R. Size dependent electrical and magnetic properties of mechanically-activated MnFe2O4 nanoferrite. Ceram. Int. 2015, 41, 13042–13054. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, F.; Zhang, X.; Qiao, J.; Liu, C.; Liu, J. CuNi alloy/ carbon foam nanohybrids as high-performance electromagnetic wave absorbers. Carbon 2021, 172, 488–496. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, H.; Dong, C.; Xiao, X.; Du, S.; Wang, Y. Reduced graphene oxide(RGO)/Mn3O4 nanocomposites for dielectric loss properties and electromagnetic interference shielding effectiveness at high frequency. Ceram. Int. 2016, 42, 936–942. [Google Scholar] [CrossRef]
- Yin, Y.; Zeng, M.; Liu, J.; Tang, W.; Dong, H.; Xia, R.; Yu, R. Enhanced high-frequency absorption of anisotropic Fe3O4/graphene nanocomposites. Sci. Rep. 2016, 6, 25075. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Feng, A.; Zhang, N.; Jia, Z.; Huang, Z.; Liu, X.; Wu, G. Mesoporous carbon hollow microspheres with tunable pore size and shell thickness as efficient electromagnetic wave absorbers. Compos. Part B 2019, 167, 167,690–699. [Google Scholar] [CrossRef]
- Guanglei Wu, G.; Jia, Z.; Zhou, X.; Nie, G.; Lv, H. Interlayer controllable of hierarchical MWCNTs@C@FexOy cross-linked composite with wideband electromagnetic absorption performance. Compos. Part A 2020, 128, 105687. [Google Scholar]
- Jia, Z.; Gao, Z.; Feng, A.; Zhang, Y.; Zhang, C.; Nie, G.; Wang, K.; Wu, G. Laminated microwave absorbers of A-site cation deficiency perovskite La0.8FeO3 doped at hybrid RGO carbon. Compos. Part B 2019, 176, 107246. [Google Scholar] [CrossRef]
- Meng, X.M.; Zhang, X.J.; Lu, C.; Pan, Y.F.; Wang, G.-S. Enhanced absorbing properties of three-phase composites based on a thermoplastic-ceramic matrix (BaTiO3+PVDF) and carbon black nanoparticles. J. Mater. Chem. 2014, 2, 18725–18730. [Google Scholar] [CrossRef]
- Zhao, Z.; Kou, K.; Wu, H. 2-Methylimidazole-mediated hierarchical Co3O4/N-doped carbon/short-carbon-fiber composite as high-performance electromagnetic wave absorber. J. Colloid Interface Sci. 2020, 574, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Hu, P.; Li, X.; Hong, C.; Zhang, X.; Han, J. NiCo2S4 nanosheets on 3D wood-derived carbon for microwave absorption. Chem. Eng. J. 2020, 398, 125588. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Chen, Z.; Yu, W.; Loh, K.P.; Zhong, B.; Shi, Y.; Xu, Q.-H. Efficient low-frequency microwave absorption and solar evaporation properties of γ-Fe2O3 nanocubes/graphene composites. Chem. Eng. J. 2021, 405, 126676. [Google Scholar] [CrossRef]
- Shi, X.-L.; Cao, M.-S.; Yuan, J.; Fang, X.-Y. Dual nonlinear dielectric resonance and nesting microwave absorption peaks of hollow cobalt nanochains composites with negative permeability. Appl. Phys. Lett. 2009, 95, 163108. [Google Scholar] [CrossRef]
- Sun, X.; He, J.; Li, G.; Tang, J.; Wang, T.; Guo, Y.; Xue, H. Laminated magnetic graphene with enhanced electromagnetic wave absorption properties. J. Mater. Chem. C 2013, 1, 765. [Google Scholar] [CrossRef]
- Luo, J.; Shen, P.; Yao, W.; Jiang, C.; Xu, J. Synthesis, Characterization, and Microwave Absorption Properties of Reduced Graphene Oxide/Strontium Ferrite/Polyaniline Nanocomposites. Nanoscale Res. Lett. 2016, 11, 141. [Google Scholar] [CrossRef]
- Ibrahim, I.R.; Matori, K.A.; Ismail, I.; Awang, Z.; Rusly, S.N.A.; Nazlan, R.; Idris, F.M.; Zulkimi, M.M.M.; Abdullah, N.H.; Mustaffa, M.S.; et al. A Study on Microwave Absorption Properties of Carbon Black and Ni0.6Zn0.4Fe2O4 Nanocomposites by Tuning the Matching-Absorbing Layer Structures. Sci. Rep. 2020, 10, 3135. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, L.; Zhang, Y.; Du, X.; Zhao, Y.; Yang, Z. High temperature electromagnetic interference shielding of lightweight and flexible ZrC/SiC nanofiber mats. Chem. Eng. J. 2021, 404, 126521. [Google Scholar] [CrossRef]
- Lai, H.; Li, W.; Xu, L.; Wang, X.; Jiao, H.; Fan, Z.; Lei, Z.; Yuan, Y. Scalable fabrication of highly crosslinked conductive nanofibrous films and their applications in energy storage and electromagnetic interference shielding. Chem. Eng. J. 2020, 400, 125322. [Google Scholar] [CrossRef]
- Gupta, T.K.; Singh, B.P.; Mathur, R.B.; Dhakate, S.R. Multi-walled carbon nanotube-graphene-polyaniline multiphase nanocomposite with superior electromagnetic shielding effectiveness. Nanoscale 2014, 6, 842. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, H.; Zhao, J.; Ji, G.; Du, Y. Achieving excellent bandwidth absorption by a mirror growth process of magnetic porous polyhedron structures. Nano Res. 2016, 9, 1813–1822. [Google Scholar] [CrossRef]
- Deng, Y.D.; Zheng, Y.; Zhang, D.; Han, C.; Cheng, A.; Shen, J.; Zeng, G.; Zhang, H. A novel and facile-to-synthesize three-dimensional honeycomb-like nano-Fe3O4@C composite: Electromagnetic wave absorption with wide bandwidth. Carbon 2020, 169, 118–128. [Google Scholar] [CrossRef]
- Xu, Z.; Du, Y.; Liu, D.; Wang, Y.; Ma, W.; Wang, Y.; Xu, P.; Han, X. Pea-like Fe/Fe3C Nanoparticles Embedded in Nitrogen-Doped Carbon Nanotubes with Tunable Dielectric/Magnetic Loss and Efficient Electromagnetic Absorption. ACS Appl. Mater. Interfaces 2019, 11, 4268–4277. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Lyu, Y.; Li, X.; Chen, J.; Zhang, X.; Han, J.; Hu, P. Construction of MnO nanoparticles anchored on SiC whiskers for superior electromagnetic wave absorption. J. Colloid Interface Sci. 2020, 559, 186–196. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Yan, Q.; Zheng, W.-G.; He, Z.; Yu, Z.-Z. Tough Graphene-Polymer Microcellular Foams for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2011, 3, 918–924. [Google Scholar] [CrossRef]
- Zou, H.; Li, S.; Zhang, L.; Yan, S.; Wu, H.; Zhang, S.; Tian, M. Determining factors for high performance silicone rubber microwave absorbing materials. J. Magn. Magn. Mater. 2011, 323, 1643–1651. [Google Scholar] [CrossRef]



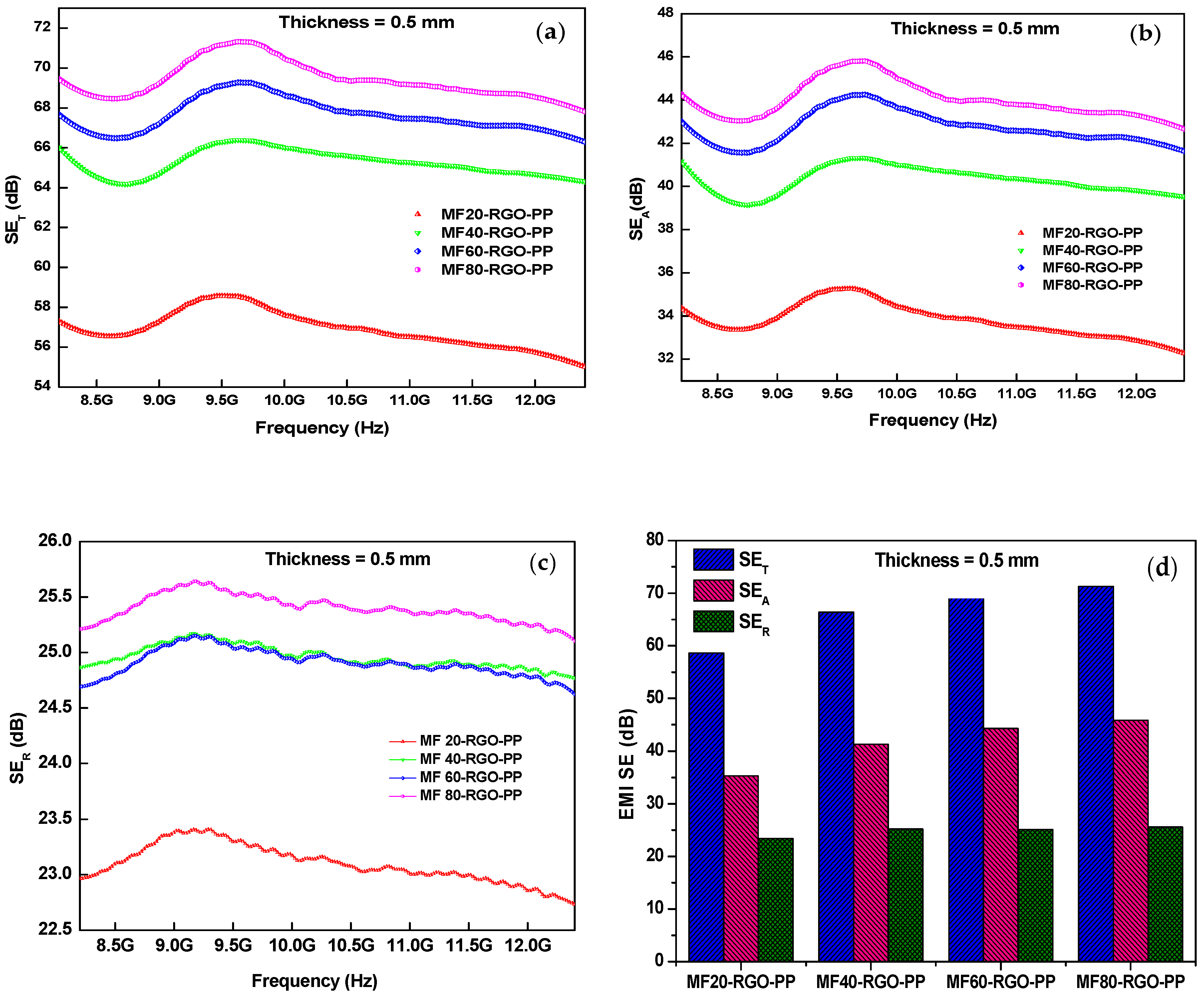
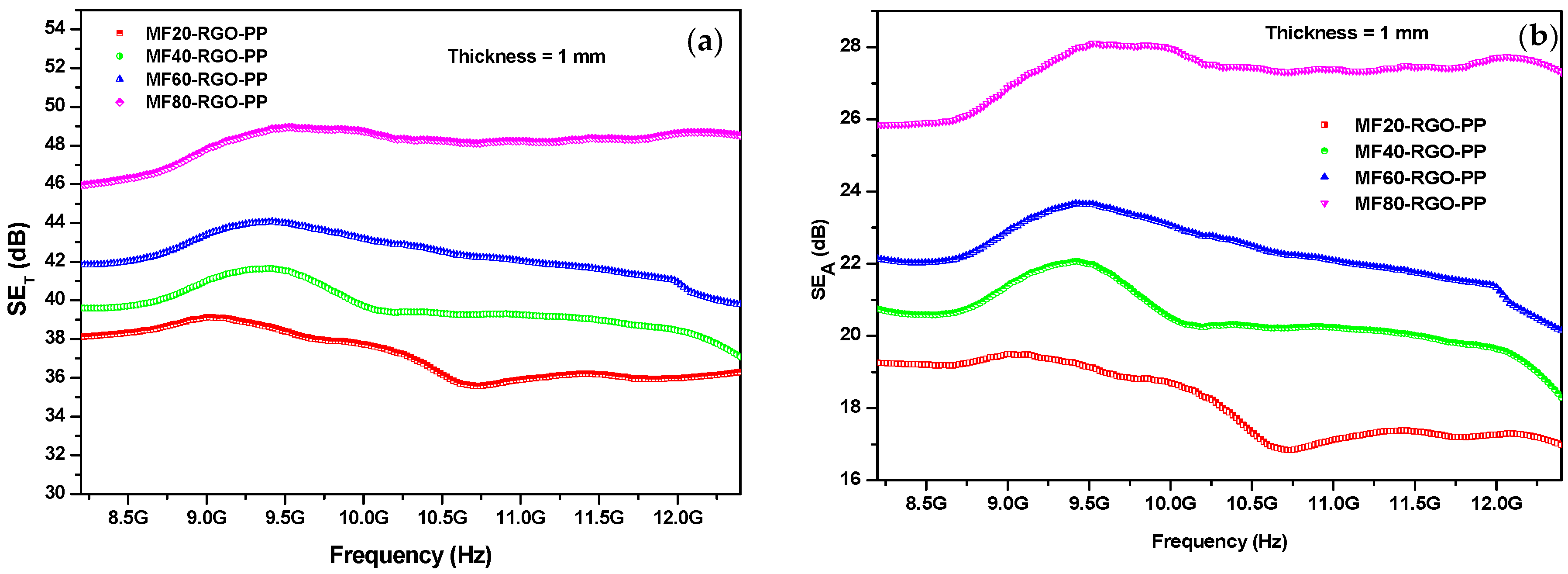
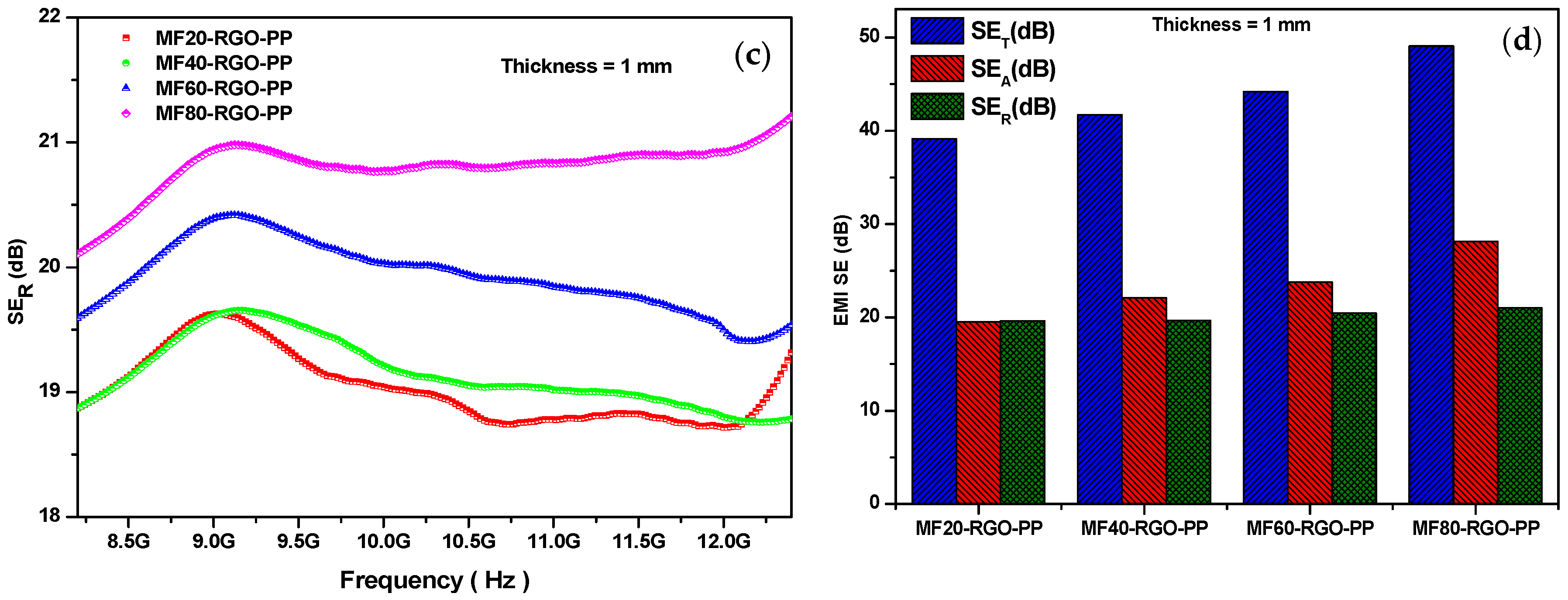
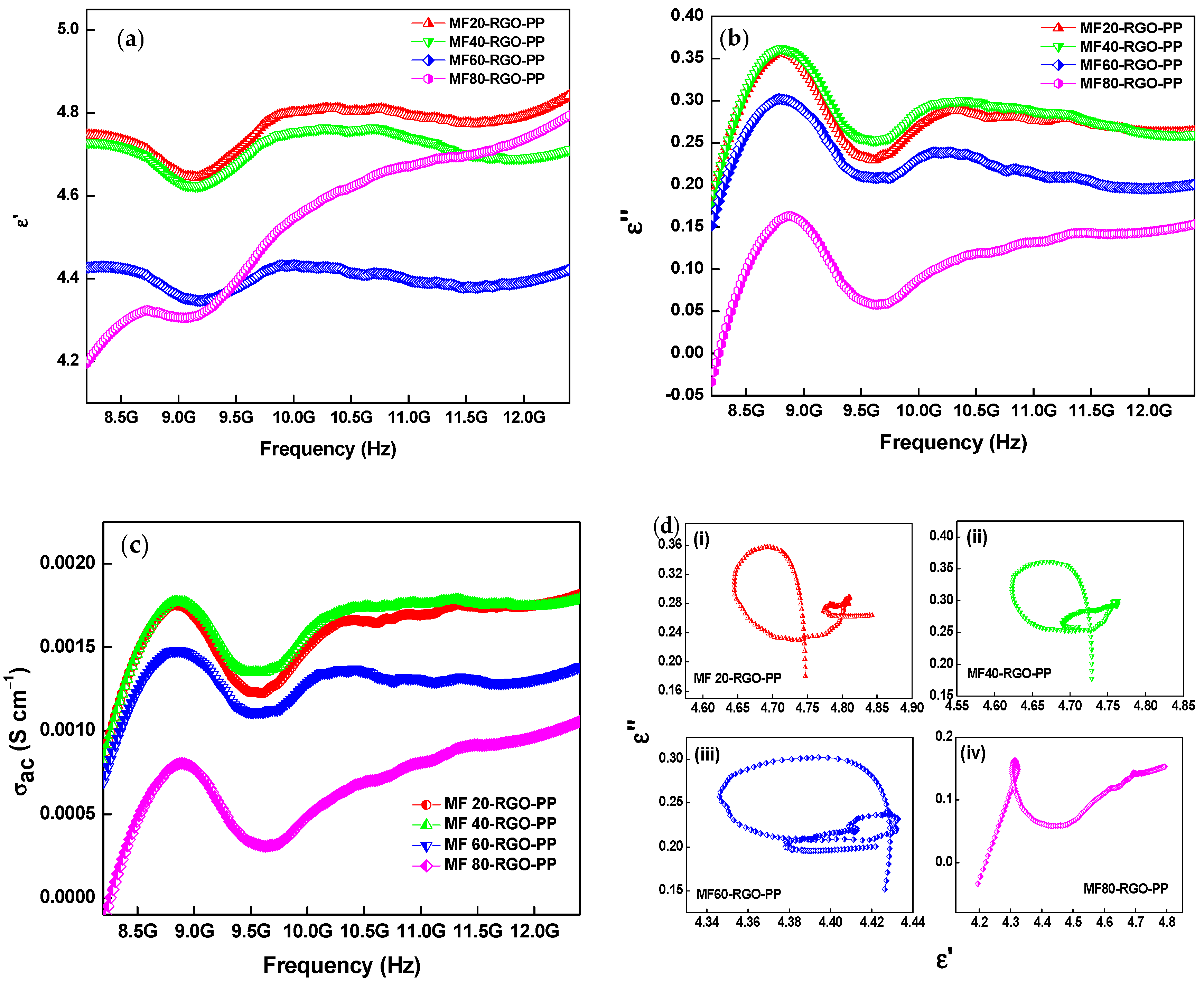
| Sample | Concentration of Mn (NO3)2 4H2O | Concentration of Fe (NO3)2 9H2O | Concentration of NaOH | Sonication Time | Reaction Temperature |
|---|---|---|---|---|---|
| MF20 | 0.17 M | 0.36 M | 1.66 M | 20 min | 65 °C |
| MF40 | 0.17 M | 0.36 M | 1.66 M | 40 min | 74 °C |
| MF60 | 0.17 M | 0.36 M | 1.66 M | 60 min | 85 °C |
| MF80 | 0.17 M | 0.36 M | 1.66 M | 80 min | 93 °C |
| No. | Shielding Material | Frequency (GHz) | Specimens Thickness (mm) | Effect of Shielding | Ref. |
|---|---|---|---|---|---|
| 1. | RGO/MnFe2O4/PVDF | 2–18 GHz | 3.0 mm | −29.0 dB | [32] |
| 2. | Biochar/MnFe2O4@C | 0.2–3 GHz | 2.5 mm | −48.92 dB | [33] |
| 3. | PMMA modified MnFe2O4-PANI | 8–12 GHz | ~44 dB | [34] | |
| 4. | PVDF/RGO-MnFe2O4/MWCNTs | 8–18 GHz | −38dB | [35] | |
| 5. | MnFe2O4/RGO | 2–18 GHz | 3.5 mm | −32.8 dB | [36] |
| 6. | SiO2-MnFe2O4 | 0.2–3 GHz | 4 mm | −14.87 dB | [37] |
| 7. | Ag/MnFe2O4/RGO | 2–18 GHz | 3.5 mm | −38 dB | [38] |
| 8. | MnFe2O4-RGO-PP | 8.2–12.4 GHz | 0.5 mm | ~71.3 dB | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.S.; Anju; Jamatia, T.; Kuřitka, I.; Vilčáková, J.; Škoda, D.; Urbánek, P.; Machovský, M.; Masař, M.; Urbánek, M.; et al. Excellent, Lightweight and Flexible Electromagnetic Interference Shielding Nanocomposites Based on Polypropylene with MnFe2O4 Spinel Ferrite Nanoparticles and Reduced Graphene Oxide. Nanomaterials 2020, 10, 2481. https://doi.org/10.3390/nano10122481
Yadav RS, Anju, Jamatia T, Kuřitka I, Vilčáková J, Škoda D, Urbánek P, Machovský M, Masař M, Urbánek M, et al. Excellent, Lightweight and Flexible Electromagnetic Interference Shielding Nanocomposites Based on Polypropylene with MnFe2O4 Spinel Ferrite Nanoparticles and Reduced Graphene Oxide. Nanomaterials. 2020; 10(12):2481. https://doi.org/10.3390/nano10122481
Chicago/Turabian StyleYadav, Raghvendra Singh, Anju, Thaiskang Jamatia, Ivo Kuřitka, Jarmila Vilčáková, David Škoda, Pavel Urbánek, Michal Machovský, Milan Masař, Michal Urbánek, and et al. 2020. "Excellent, Lightweight and Flexible Electromagnetic Interference Shielding Nanocomposites Based on Polypropylene with MnFe2O4 Spinel Ferrite Nanoparticles and Reduced Graphene Oxide" Nanomaterials 10, no. 12: 2481. https://doi.org/10.3390/nano10122481
APA StyleYadav, R. S., Anju, Jamatia, T., Kuřitka, I., Vilčáková, J., Škoda, D., Urbánek, P., Machovský, M., Masař, M., Urbánek, M., Kalina, L., & Havlica, J. (2020). Excellent, Lightweight and Flexible Electromagnetic Interference Shielding Nanocomposites Based on Polypropylene with MnFe2O4 Spinel Ferrite Nanoparticles and Reduced Graphene Oxide. Nanomaterials, 10(12), 2481. https://doi.org/10.3390/nano10122481










