Synthesis, Optical Properties, and Sensing Applications of LaF3:Yb3+/Er3+/Ho3+/Tm3+ Upconversion Nanoparticles
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of LaF3:Yb3+/Er3+/Ho3+/Tm3+ UCNPs and LaF3:Yb3+/Er3+@LaF3:Yb3+ Core/Shell UCNPs
2.3. Synthesis of PEG–Imidazole
2.4. Preparation of Hydrophilic UCNPs via Ligand Exchange with PEG–Imidazole
2.5. Assay for the Detection of Hg2+
2.6. Characterization
3. Results and Discussion
3.1. Synthesis of LaF3:Yb3+0.20, Er3+0.02 core UCNPs
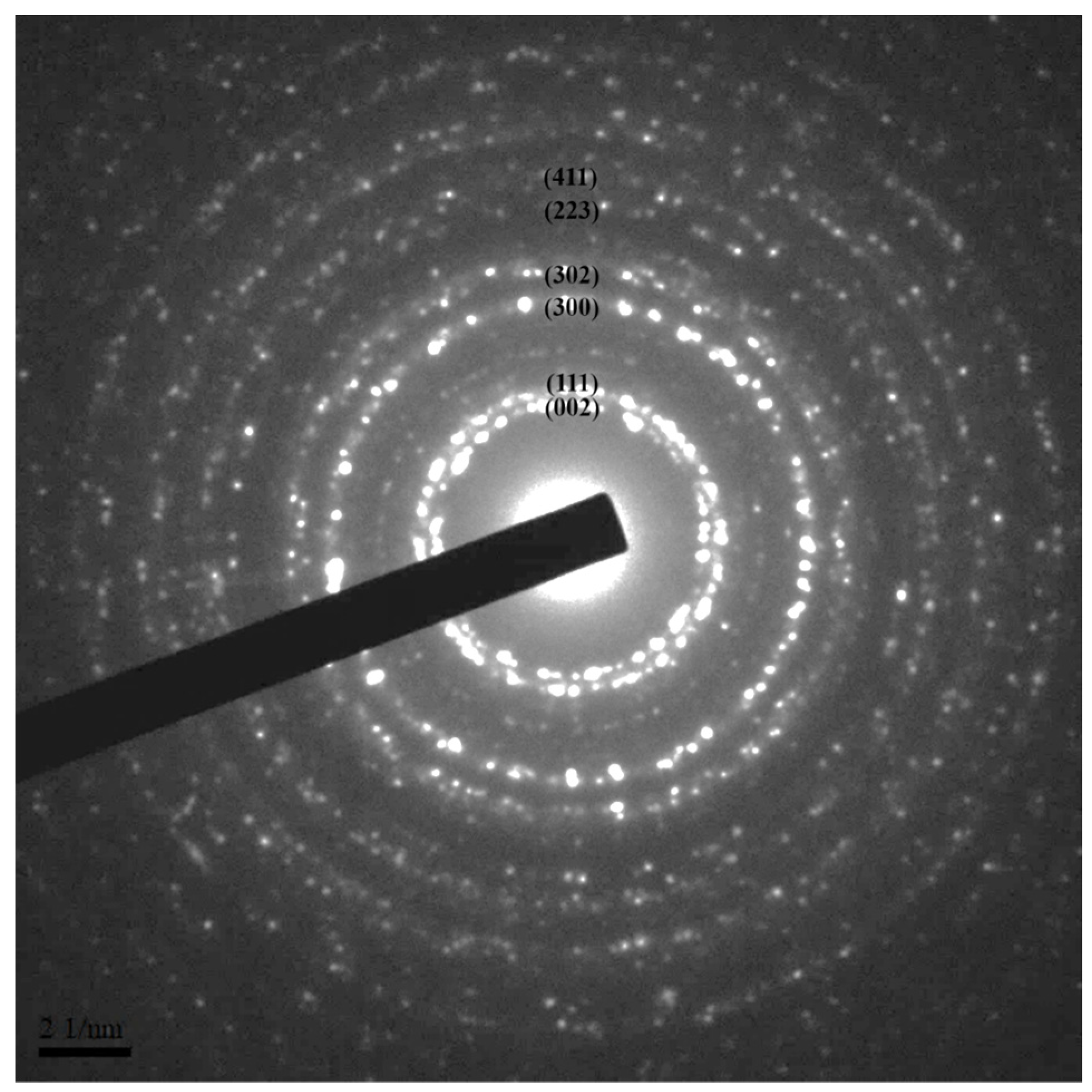
3.2. XRD of LaF3:Yb3+0.20, Er3+0.02 UCNPs
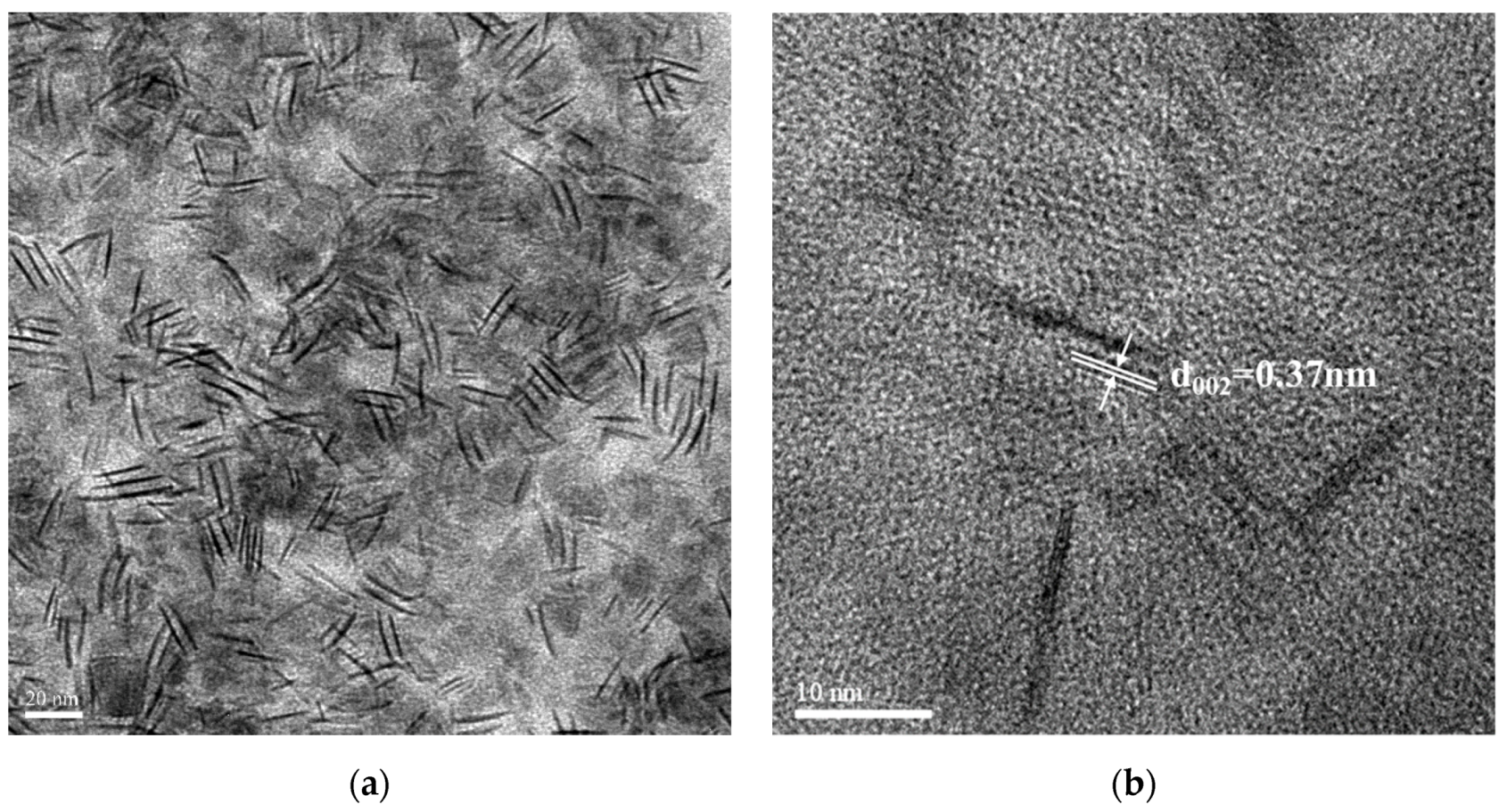
3.3. Crystal Morphology of the LaF3:Yb3+0.20, Er3+0.02 UCNPs
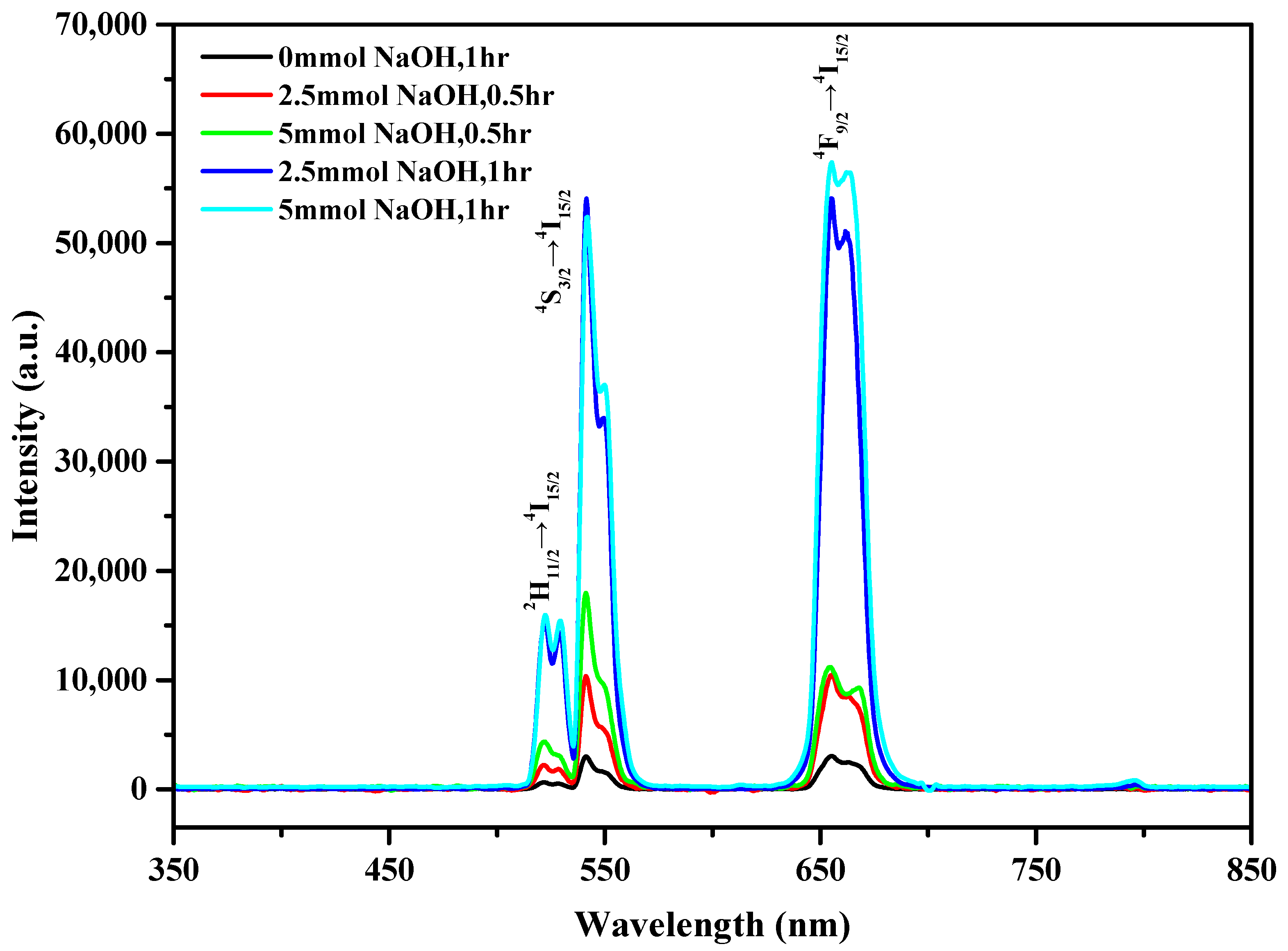
3.4. Optical Properties of the LaF3:Yb3+0.20, Er3+0.02 UCNPs
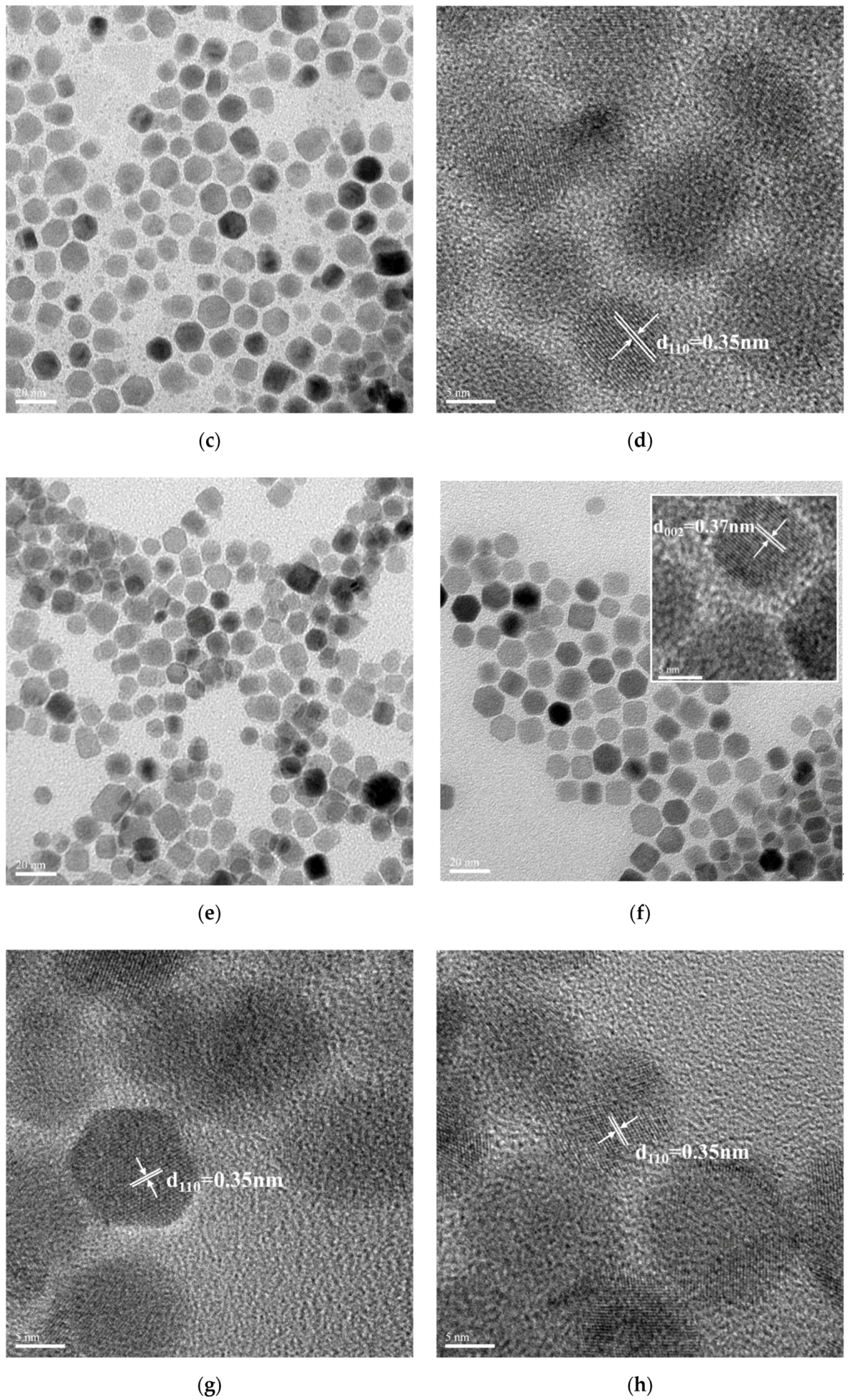
3.5. Structural, Morphological, and Optical Properties of the LaF3:Yb3+0.20, Er3+0.02@ LaF3:Yb3+0.20 Core/Shell UCNPs
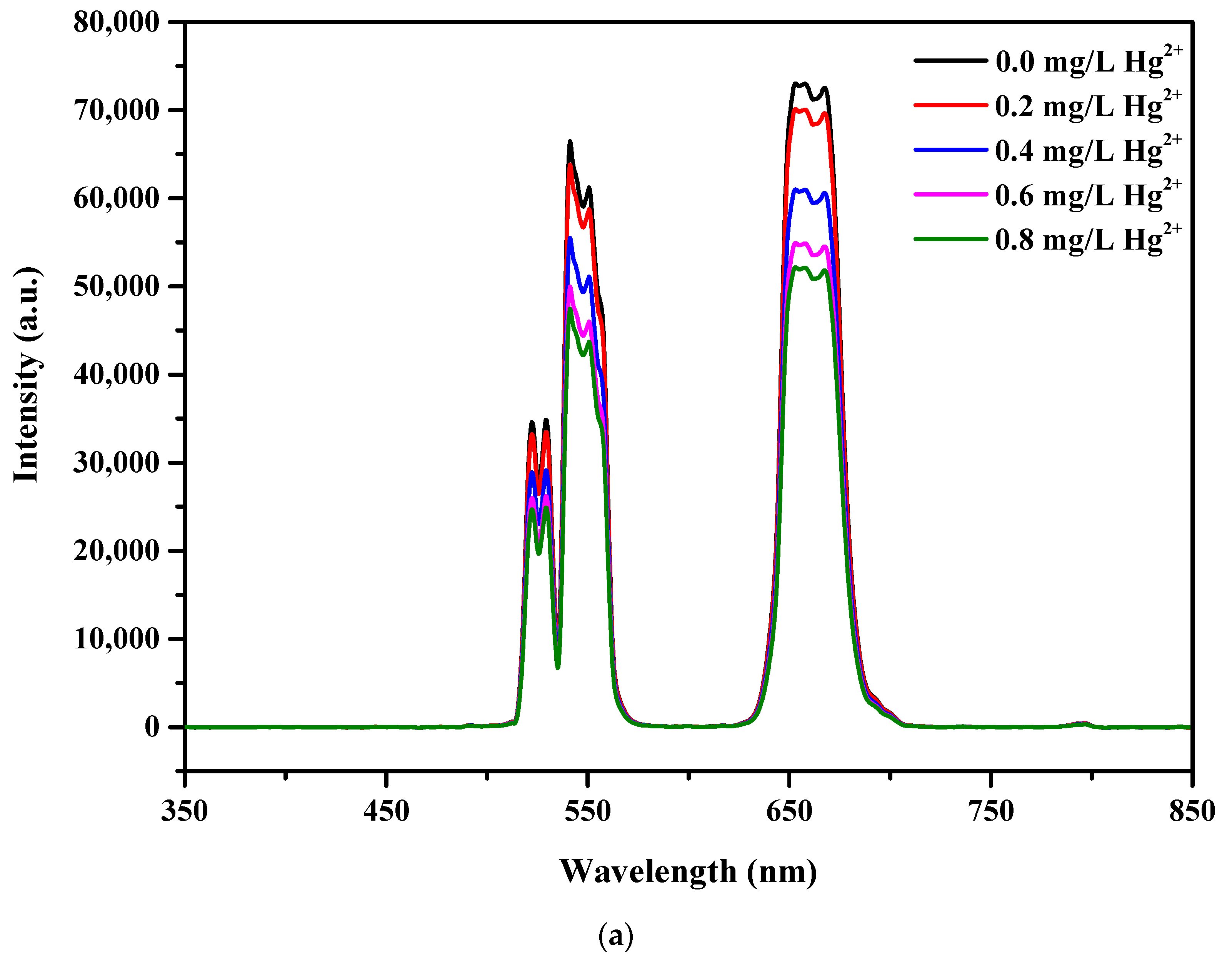
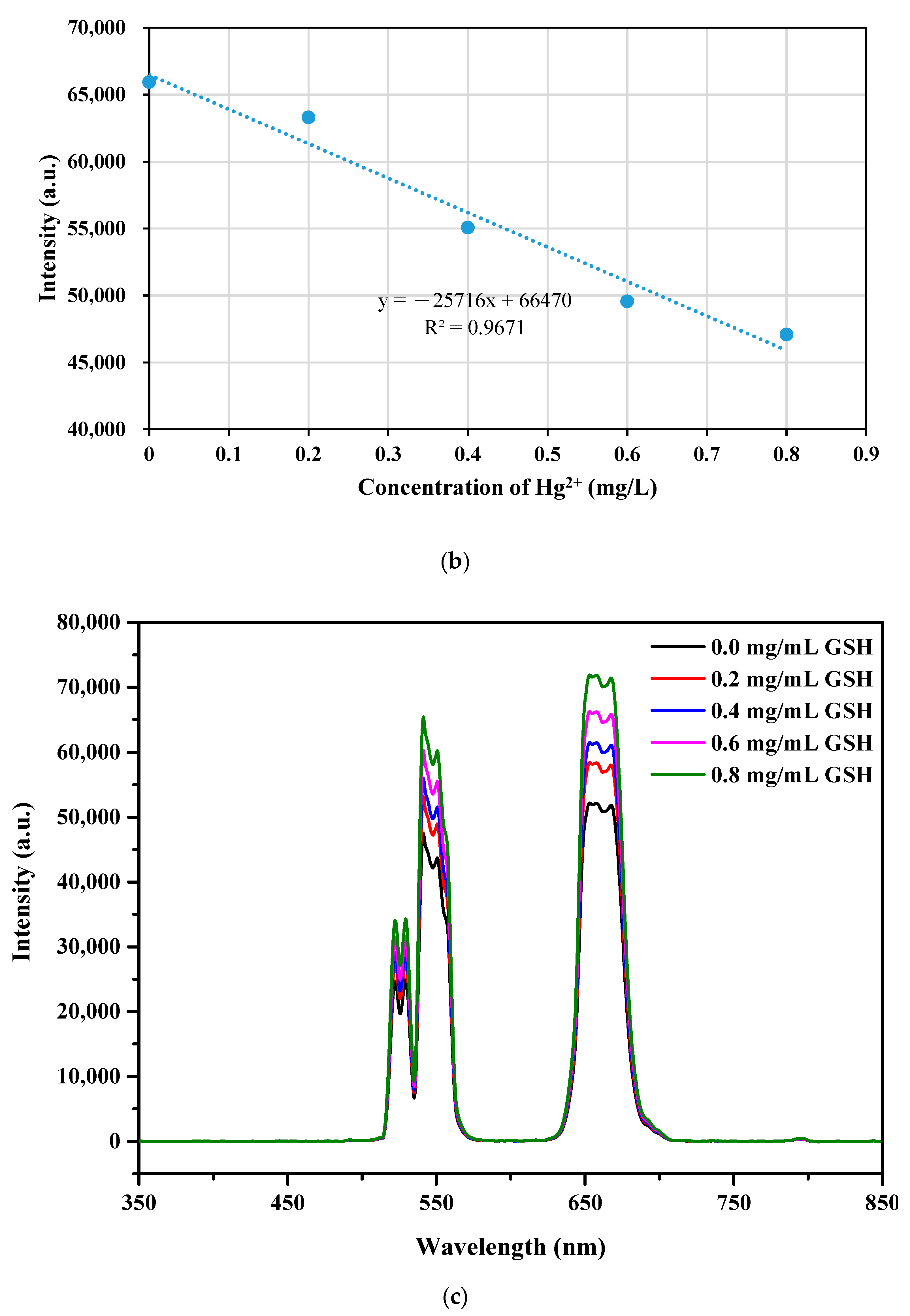
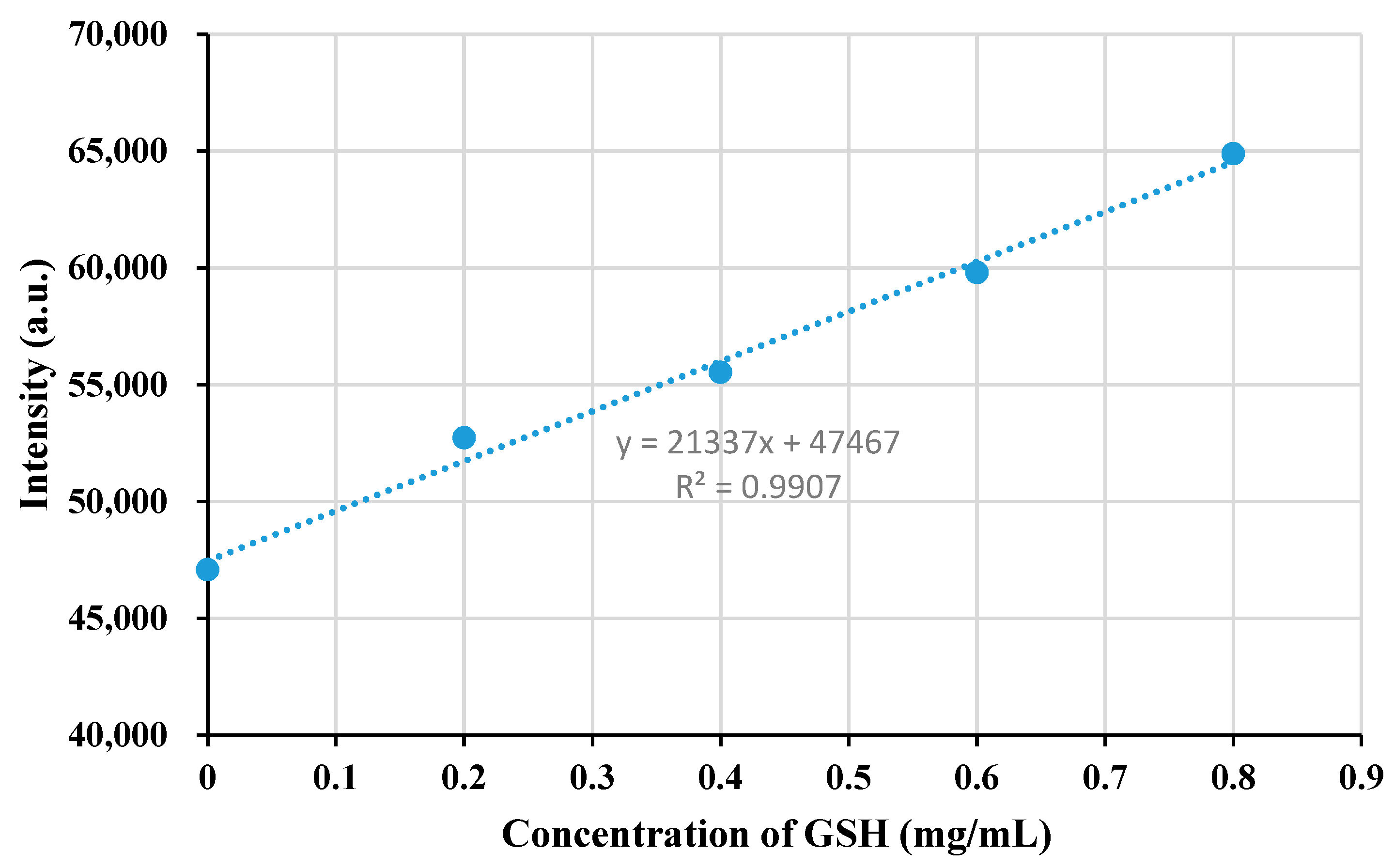
3.6. Detection of Hg2+ in Aqueous Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, L.; Wei, R.; Feng, J.; Zhang, H. Tailored lanthanide-doped upconversion nanoparticles and their promising bioapplication prospects. Coord. Chem. Rev. 2018, 364, 10–32. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, L.; Zhang, F. Exploiting lanthanide-doped upconversion nanoparticles with core/shell structures. Nano Today 2019, 25, 68–84. [Google Scholar] [CrossRef]
- Bagheri, A.; Arandiyan, H.; Boyer, C.; Lim, M. Lanthanide-doped upconversion nanoparticles: Emerging intelligent light-activated drug delivery systems. Adv. Sci. 2016, 3, 1500437. [Google Scholar] [CrossRef]
- Chen, C.; Li, C.; Shi, Z. Current advances in lanthanide-doped upconversion nanostructures for detection and bioapplication. Adv. Sci. 2016, 3, 1600029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, F.; Xu, Z.; Chaker, M.; Ma, D. Are lanthanide-doped upconversion materials good candidates for photocatalysis? Nanoscale Horiz. 2019, 4, 579–591. [Google Scholar] [CrossRef]
- Kang, D.; Jeon1, E.; Kim, S.; Lee, J. Lanthanide-doped upconversion nanomaterials: Recent advances and applications. BioChip J. 2020, 14, 124–135. [Google Scholar] [CrossRef]
- Lee, G.; Park, Y.I. Lanthanide-doped upconversion nanocarriers for drug and gene delivery. Nanomaterials 2018, 8, 511. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- DaCosta, M.V.; Doughan, S.; Han, Y.; Krull, U.J. Lanthanide upconversion nanoparticles and applications in bioassays and bioimaging: A review. Anal. Chim. Acta. 2014, 832, 1–33. [Google Scholar] [CrossRef]
- Feng, W.; Sun, L.D.; Zhang, Y.W.; Yan, C.H. Synthesis and assembly of rare earth nanostructures directed by the principle of coordination chemistry in solution-based process. Coord. Chem. Rev. 2010, 254, 1038–1053. [Google Scholar]
- Chatterjee, D.K.; Gnanasammandhan, M.K.; Zhang, Y. Small upconverting fluorescent nanoparticles for biomedical applications. Small 2010, 6, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, H.; Hu, H.; Yu, M.; Li, F.; Zhang, Q.; Huang, C. Versatile synthesis strategy for carboxylic acid−functionalized upconverting nanophosphors as biological labels. J. Am. Chem. Soc. 2008, 130, 3023–3029. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, J. Rare earth fluoride nano-/microcrystals: Synthesis, surface modification and application. J. Mater. Chem. 2010, 20, 6831–6847. [Google Scholar] [CrossRef]
- Mai, H.X.; Zhang, Y.W.; Si, R.; Yan, Z.G.; Sun, L.D.; You, L.P.; Yan, C.H. High-quality sodium rare-earth fluoride nanocrystals: Controlled synthesis and optical properties. J. Am. Chem. Soc. 2006, 128, 6426–6436. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chen, Z.; Cao, T.; Zhang, Q.; Yu, M.; Li, F.; Yi, T.; Huang, C. Hydrothermal synthesis of hexagonal lanthanide-doped LaF3 nanoplates with bright upconversion luminescence. Nanotechnology 2008, 19, 375702. [Google Scholar] [CrossRef]
- Lv, R.; Yang, G.; He, F.; Dai, Y.; Gai, S.; Yang, P. LaF3:Ln mesoporous spheres: Controllable synthesis, tunable luminescence and application for dual-modal chemo-/photo-thermal therapy. Nanoscale 2014, 6, 14799–14809. [Google Scholar] [CrossRef]
- Nie, L.; Shen, Y.; Zhang, X.; Wang, X.; Liu, B.; Wang, Y.; Pan, Y.; Xie, X.; Huang, L.; Huang, W. Selective synthesis of LaF3 and NaLaF4 nanocrystals via lanthanide ion doping. J. Mater. Chem. C 2017, 5, 9188–9193. [Google Scholar] [CrossRef]
- Kasturi, S.; Marikumar, R.; Vaidyanathan, S. Trivalent rare-earth activated hexagonal lanthanum fluoride (LaF3:RE3+, where RE = Tb, Sm, Dy and Tm) nanocrystals: Synthesis and optical properties. Luminescence 2018, 33, 897–906. [Google Scholar] [CrossRef]
- Secu, C.E.; Matei, E.; Negrila, C.; Secu, M. The influence of the nanocrystals size and surface on the Yb/Er doped LaF3 luminescence properties. J. Alloys Compd. 2019, 791, 1098–1104. [Google Scholar] [CrossRef]
- Cheng, X.; Ma, X.; Zhang, H.; Ren, Y.; Zhu, K. Optical temperature sensing properties of Yb3+/Er3+ codoped LaF3 upconversion phosphor. Physica B Condens. Matter 2017, 521, 270–274. [Google Scholar]
- Pudovkin, M.S.; Koryakovtseva, D.A.; Lukinova, E.V.; Korableva, S.L.; Khusnutdinova, R.S.; Kiiamov, A.G.; Nizamutdinov, A.S.; Semashko, V.V. Luminescence nanothermometry based on Pr3+:LaF3 single core and Pr3+:LaF3/LaF3 core/shell nanoparticles. Adv. Mater. Sci. Eng. 2019, 2618307. [Google Scholar] [CrossRef]
- Razumkova, I.A.; Denisenko, Y.G.; Boyko, A.N.; Ikonnikov, D.A.; Aleksandrovsky, A.S.; Azarapin, N.O.; Andreev, O.V. Synthesis and upconversion luminescence in LaF3:Yb3+, Ho3+, GdF3: Yb3+, Tm3+ and YF3:Yb3+, Er3+ obtained from sulfide precursors. Z. Anorg. Allg. Chem. 2019, 645, 1393–1401. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Yang, P.; Lian, H.; Lin, J. Hydrothermal synthesis of lanthanide fluorides LnF3 (Ln= La to Lu) nano-/microcrystals with multiform structures and morphologies. Chem. Mater. 2008, 20, 4317–4326. [Google Scholar] [CrossRef]
- Tian, Y.; Jiao, X.; Zhang, J.; Sui, N.; Chen, D.; Hong, G. Molten salt synthesis of LaF3: Eu3+ nanoplates with tunable size and their luminescence properties. J. Nanopart. Res. 2010, 12, 161–168. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Li, Y. Down- and up-conversion luminescent nanorods. Adv. Mater. 2007, 19, 3304–3307. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Sun, X.; Si, R.; You, L.P.; Yan, C.H. Single-crystalline and monodisperse LaF3 triangular nanoplates from a single-source precursor. J. Am. Chem. Soc. 2005, 127, 3260–3261. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Yang, T.; Feng, W.; Li, C.; Li, F. Sub-10 nm hexagonal lanthanide-doped NaLuF4 upconversion nanocrystals for sensitive bioimaging in vivo. J. Am. Chem. Soc. 2011, 133, 17122–17125. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Li, F. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef]
- Stouwdam, J.W.; van Veggel, F.C. Near-infrared emission of redispersible Er3+, Nd3+, and Ho3+ doped LaF3 nanoparticles. Nano Lett. 2002, 2, 733–737. [Google Scholar] [CrossRef]
- Bao, L.; Li, Z.; Tao, Q.; Xie, J.; Mei, Y.; Xiong, Y. Controlled synthesis of uniform LaF3 polyhedrons, nanorods and nanoplates using NaOH and ligands. Nanotechnology 2013, 24, 145604. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Tan, X.; Luo, S.; Shi, C. Fluorinated near-infrared fluorescent probes for specific detection of Hg2+ in an aqueous medium and mitochondria of living cells. New J. Chem. 2017, 41, 8899–8904. [Google Scholar] [CrossRef]
- Rodrigues, E.M.; Gálico, D.A.; Mazali, I.O.; Sigoli, F.A. Temperature probing and emission color tuning by morphology and size control of upconverting β-NaYb0. 67Gd0. 30F4: Tm0. 015: Ho0. 015 nanoparticles. Methods Appl. Fluoresc. 2017, 5, 024012. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Mahalingam, V.; Morgan, C.G.; Capobianco, J.A. The active-core/active-shell approach: A strategy to enhance the upconversion luminescence in lanthanide-doped nanoparticles. Adv. Funct. Mater. 2009, 19, 2924–2929. [Google Scholar] [CrossRef]
- Ghosh, P.; Oliva, J.; Rosa, E.D.L.; Haldar, K.K.; Solis, D.; Patra, A. Enhancement of upconversion emission of LaPO4:Er@Yb core−shell nanoparticles/nanorods. J. Phys. Chem. C 2008, 112, 9650–9658. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, Q.; Zhang, Y.; Shi, Y.; Ding, K.; Zhao, D.; Stucky, G.D. Fluorescence upconversion microbarcodes for multiplexed biological detection: Nucleic acid encoding. Adv. Mater. 2011, 23, 3775–3779. [Google Scholar] [CrossRef]
- Chien, H.W.; Wu, C.H.; Yang, C.H.; Wang, T.L. Multiple doping effect of LiYF4: Yb3+/Er3+/Ho3+/Tm3+@LiYF4:Yb3+ core/shell nanoparticles and its application in Hg2+ sensing detection. J. Alloys Compd. 2019, 806, 272–282. [Google Scholar] [CrossRef]
- Eom, Y.; Abbas, M.; Noh, H.; Kim, C. Morphology-controlled synthesis of highly crystalline Fe3O4 and CoFe2O4 nanoparticles using a facile thermal decomposition method. RSC Adv. 2016, 6, 15861–15867. [Google Scholar] [CrossRef]
- Baziulyte-Paulaviciene, D.; Traskina, N.; Vargalis, R.; Katelnikovas, A.; Sakirzanovas, S. Thermal decomposition synthesis of Er3+-activated NaYbF4 upconverting microparticles for optical temperature sensing. J. Lumin. 2019, 215, 116672. [Google Scholar] [CrossRef]
- Kostiv, U.; Engstová, H.; Krajnik, B.; Šlouf, M.; Proks, V.; Podhorodecki, A.; Ježek, P.; Horák, D. Monodisperse core-shell NaYF4:Yb3+/Er3+@NaYF4:Nd3+-PEG-GGGRGDSGGGY-NH2 nanoparticles excitable at 808 and 980 nm: Design, surface engineering, and application in life sciences. Front. Chem. 2020, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Runowski, M.; Stopikowska, N.; Szeremeta, D.; Goderski, S.; Skwierczyńska, M.; Lis, S. Upconverting lanthanide fluoride core@shell nanorods for luminescent thermometry in the first and second biological windows: β-NaYF4:Yb3+–Er3+@SiO2 temperature sensor. ACS Appl. Mater. Interfaces 2019, 11, 13389–13396. [Google Scholar] [CrossRef] [PubMed]
- Joubert, M.F. Photon avalanche upconversion in rare earth laser materials. Opt. Mater. 1999, 11, 181–203. [Google Scholar] [CrossRef]
- Chung, Y.C.; Yang, C.H.; Lee, R.H.; Wang, T.L. Dual stimuli-responsive block copolymers for controlled release triggered by upconversion luminescence or temperature variation. ACS Omega 2019, 4, 3322–3328. [Google Scholar] [CrossRef] [PubMed]
- Generalova, A.N.; Rocheva, V.V.; Nechaev, A.V.; Khochenkov, D.A.; Sholina, N.V.; Semchishen, V.A.; Zubov, V.P.; Koroleva, A.V.; Chichkov, B.N.; Khaydukov, E.V. PEG-modified upconversion nanoparticles for in vivo optical imaging of tumors. RSC Adv. 2016, 6, 30089–30097. [Google Scholar] [CrossRef]
- Boyer, J.C.; Manseau, M.P.; Murray, J.I.; van Veggel, F.C.J.M. Surface modification of upconverting NaYF4 nanoparticles with PEG−phosphate ligands for NIR (800 nm) biolabeling within the biological window. Langmuir 2010, 26, 1157–1164. [Google Scholar] [CrossRef]
- Gee, A.; Xu, X. Surface functionalisation of upconversion nanoparticles with different moieties for biomedical applications. Surfaces 2018, 1, 9. [Google Scholar] [CrossRef]
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem. Commun. 2012, 48, 1147–1149. [Google Scholar] [CrossRef]
- Xia, Y.S.; Zhu, C.Q. Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 2008, 75, 215–222. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Wang, Y.; Lou, N.; Ma, Q.; Su, X. Sensitive Hg (II) ion detection by fluorescent multilayer films fabricated with quantum dots. Sens. Actuators B 2009, 139, 476–482. [Google Scholar] [CrossRef]
- Huang, L.J.; Yu, R.Q.; Chu, X. DNA-functionalized upconversion nanoparticles as biosensors for rapid, sensitive, and selective detection of Hg2+ in complex matrices. Analyst 2015, 140, 4987–4990. [Google Scholar] [CrossRef] [PubMed]
- Mah, V.; Jalilehvand, F. Mercury(II) complex formation with glutathione in alkaline aqueous solution. J. Biol. Inorg. Chem. 2008, 13, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Mah, V.; Jalilehvand, F. Glutathione complex formation with mercury (II) in aqueous solution at physiological pH. Chem. Res. Toxicol. 2010, 23, 1815–1823. [Google Scholar] [CrossRef]


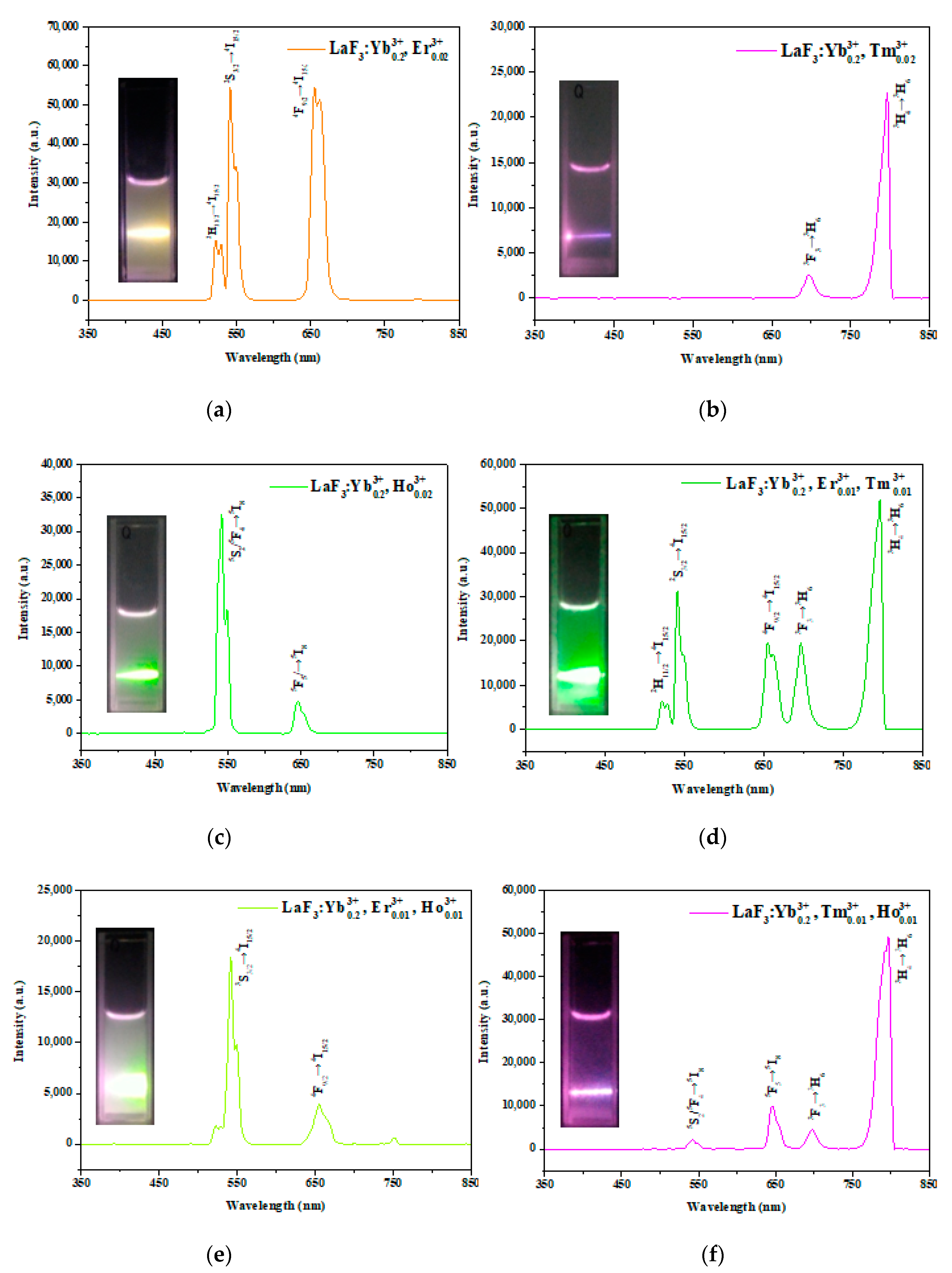
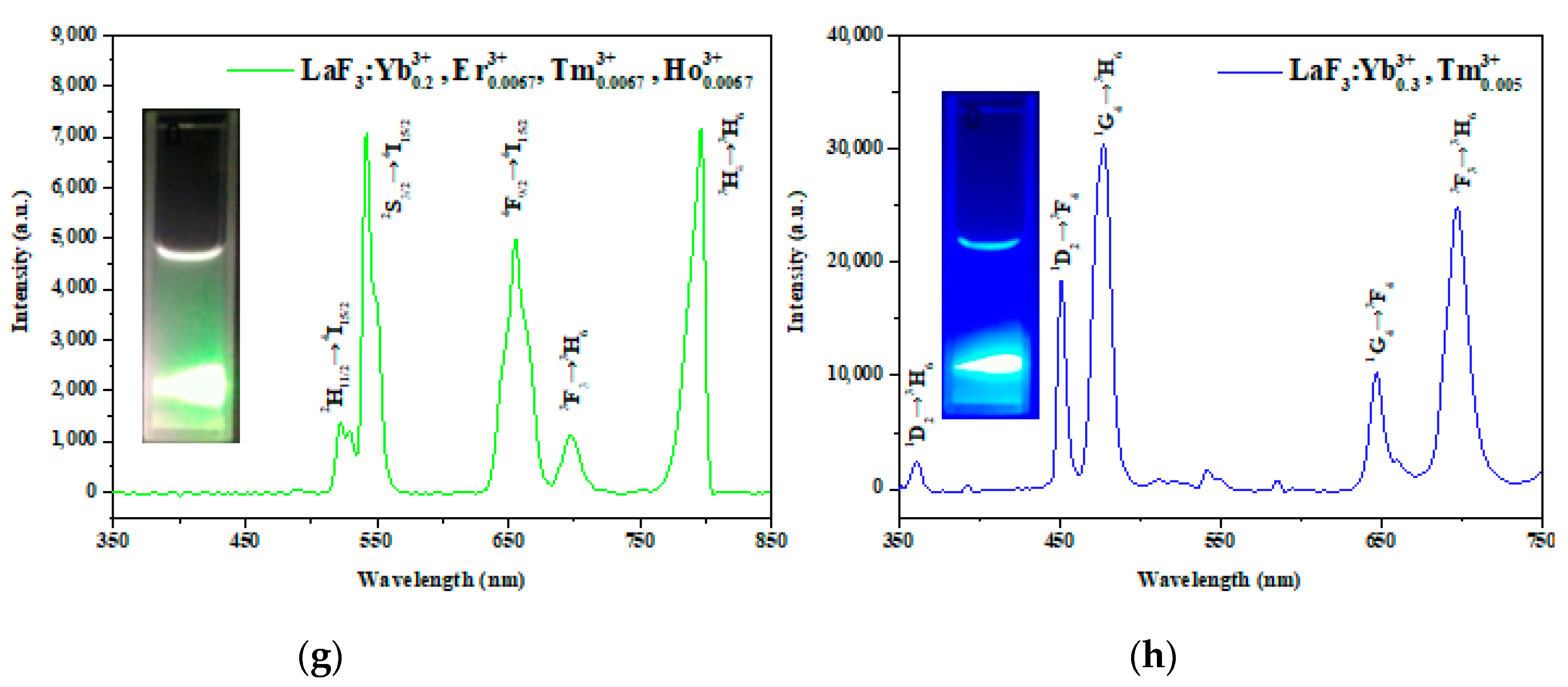

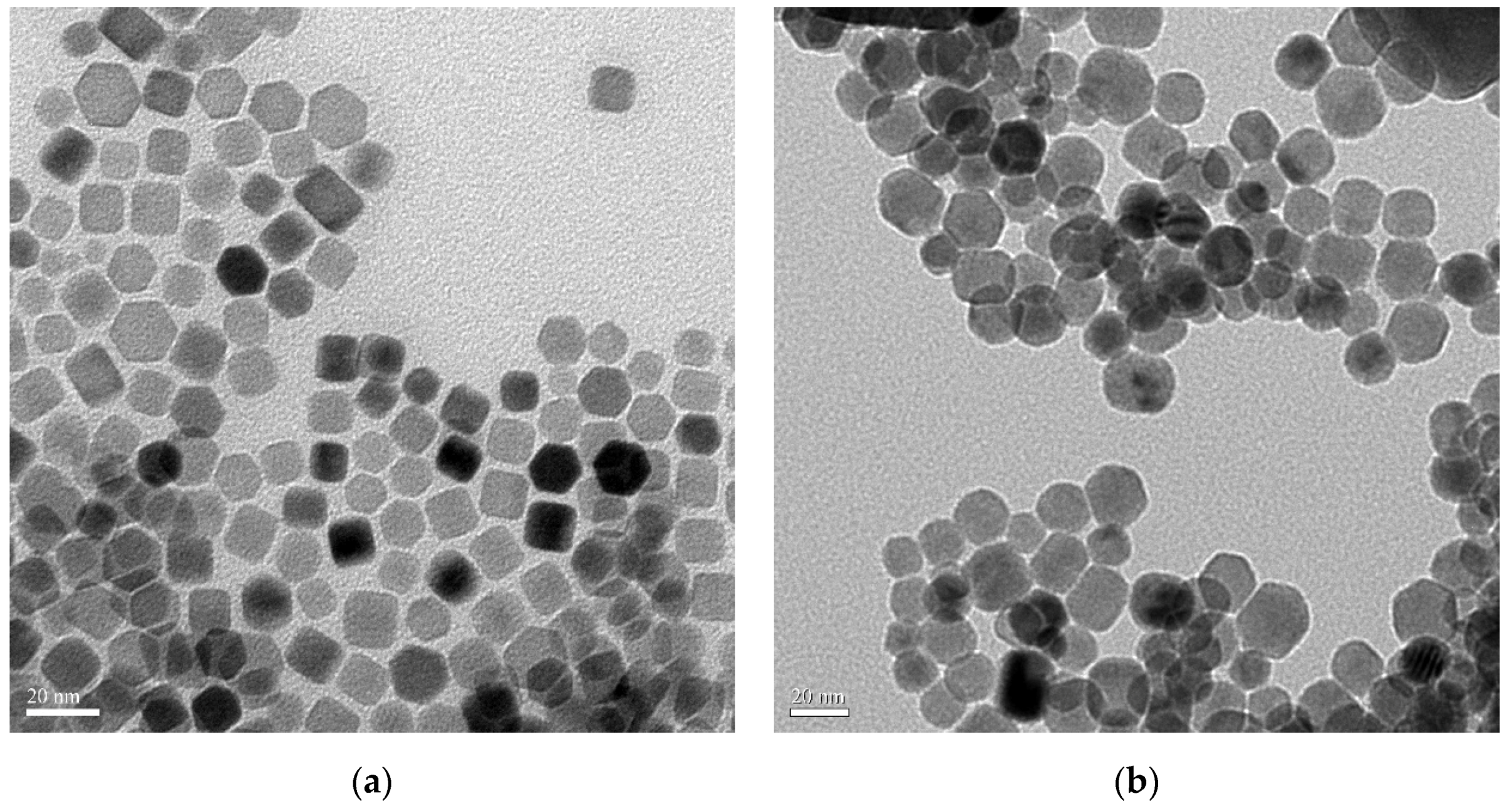
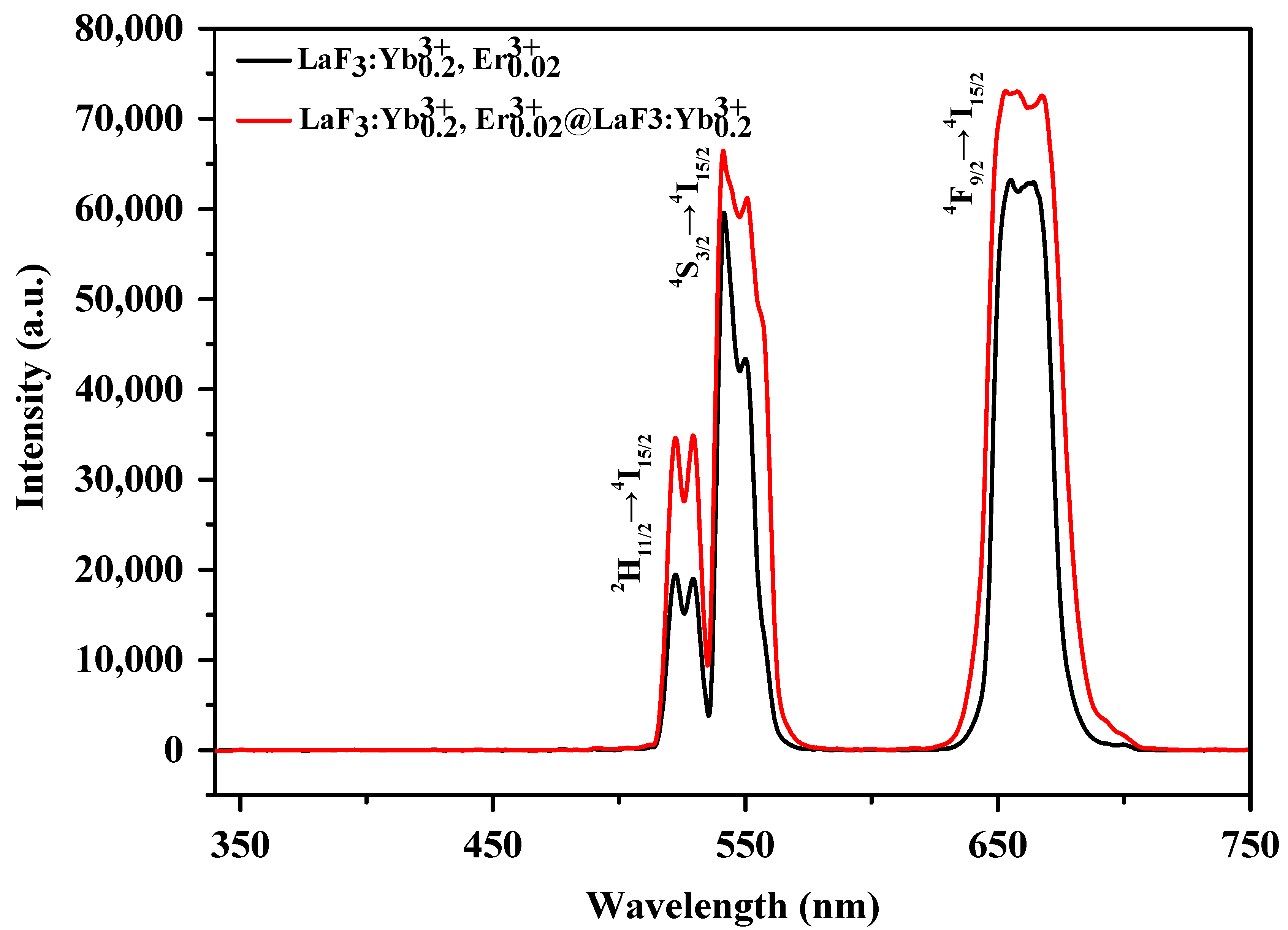
| Nanoparticle Samples | Ion Concentration (mmol) | ||||
|---|---|---|---|---|---|
| Yb3+ | Er3+ | Ho3+ | Tm3+ | ||
| No. 1 | LaF3:/ | 0.20 | 0.02 | 0 | 0 |
| No. 2 | LaF3:/ | 0.20 | 0 | 0 | 0.02 |
| No. 3 | LaF3:/ | 0.20 | 0 | 0.02 | 0 |
| No. 4 | LaF3:// | 0.20 | 0.01 | 0 | 0.01 |
| No. 5 | LaF3:// | 0.20 | 0.01 | 0.01 | 0 |
| No. 6 | LaF3:// | 0.20 | 0 | 0.01 | 0.01 |
| No. 7 | LaF3:/// | 0.20 | 0.0067 | 0.0067 | 0.0067 |
| No. 8 | LaF3:/ | 0.30 | 0 | 0 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, H.-W.; Huang, C.-H.; Yang, C.-H.; Wang, T.-L. Synthesis, Optical Properties, and Sensing Applications of LaF3:Yb3+/Er3+/Ho3+/Tm3+ Upconversion Nanoparticles. Nanomaterials 2020, 10, 2477. https://doi.org/10.3390/nano10122477
Chien H-W, Huang C-H, Yang C-H, Wang T-L. Synthesis, Optical Properties, and Sensing Applications of LaF3:Yb3+/Er3+/Ho3+/Tm3+ Upconversion Nanoparticles. Nanomaterials. 2020; 10(12):2477. https://doi.org/10.3390/nano10122477
Chicago/Turabian StyleChien, Hsiu-Wen, Chien-Hao Huang, Chien-Hsin Yang, and Tzong-Liu Wang. 2020. "Synthesis, Optical Properties, and Sensing Applications of LaF3:Yb3+/Er3+/Ho3+/Tm3+ Upconversion Nanoparticles" Nanomaterials 10, no. 12: 2477. https://doi.org/10.3390/nano10122477
APA StyleChien, H.-W., Huang, C.-H., Yang, C.-H., & Wang, T.-L. (2020). Synthesis, Optical Properties, and Sensing Applications of LaF3:Yb3+/Er3+/Ho3+/Tm3+ Upconversion Nanoparticles. Nanomaterials, 10(12), 2477. https://doi.org/10.3390/nano10122477





