FRET-Based Analysis of AgInS2/ZnAgInS/ZnS Quantum Dot Recombination Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AIS/ZAIS/ZnS QDs
2.3. Solubilization of AIS/ZAIS/ZnS QDs and Formation of the QD-Dye Complexes
2.4. Equipment
3. Results and Discussion
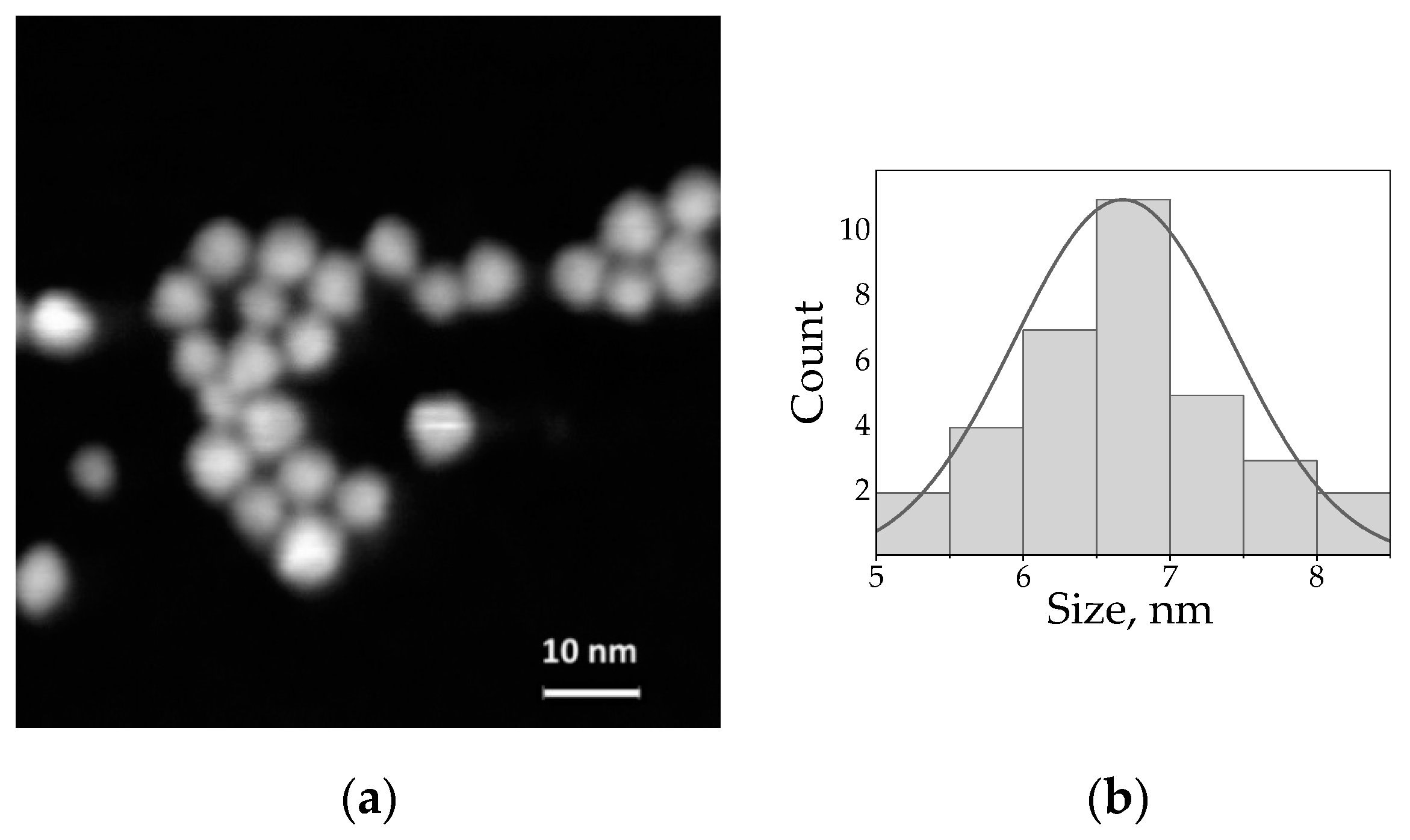
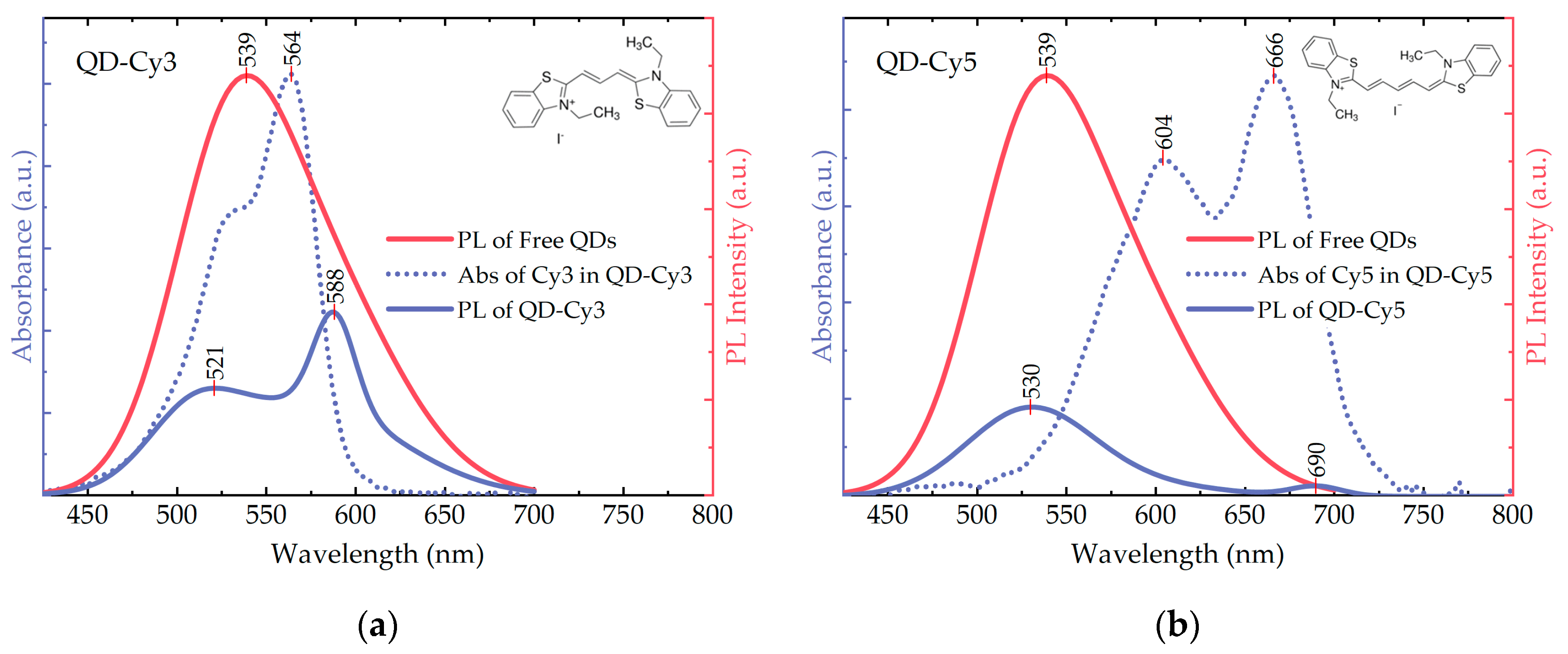
3.1. Spectroscopy of the Free AIS/ZAIS/ZnS QDs and the QD-Dye Complexes
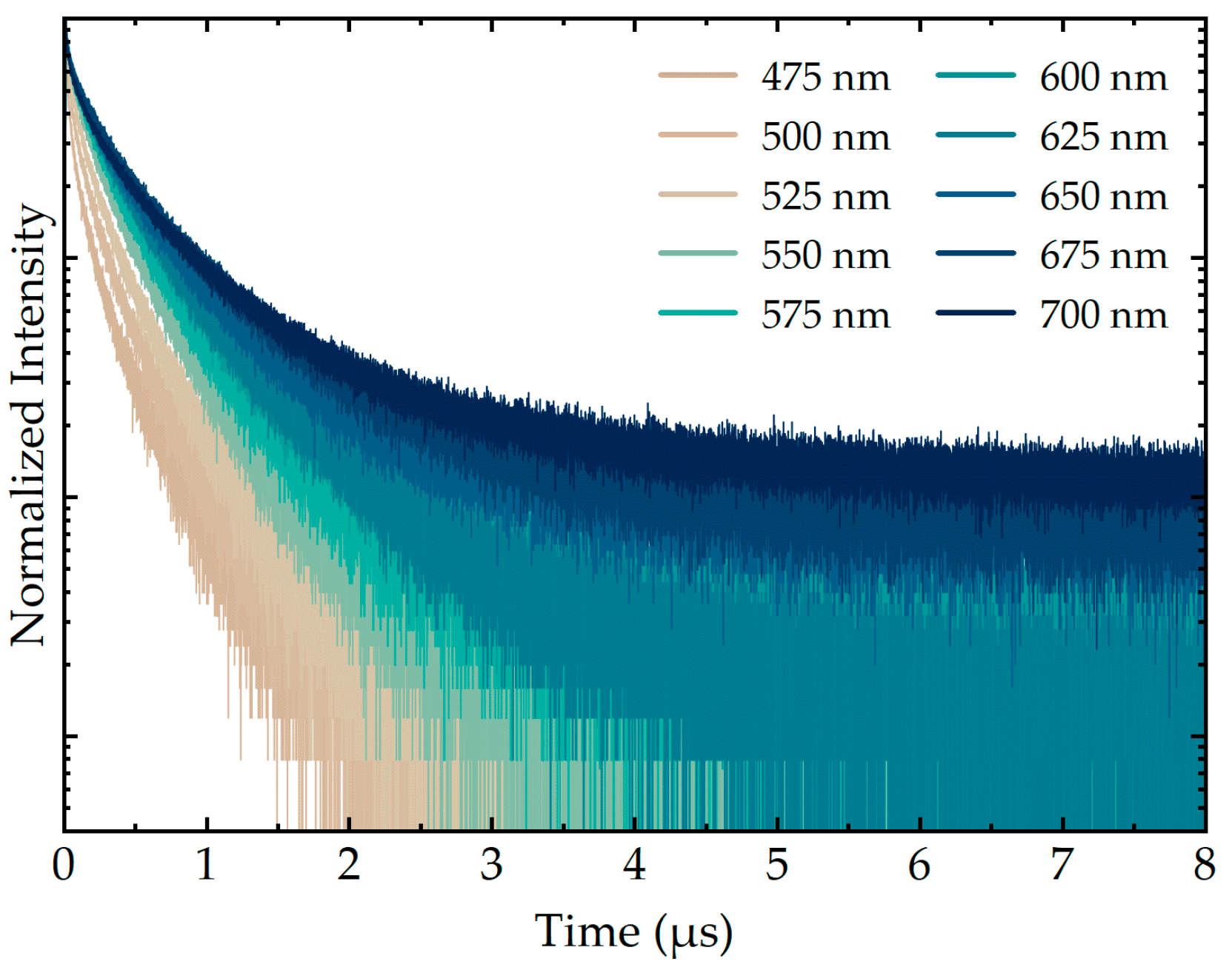
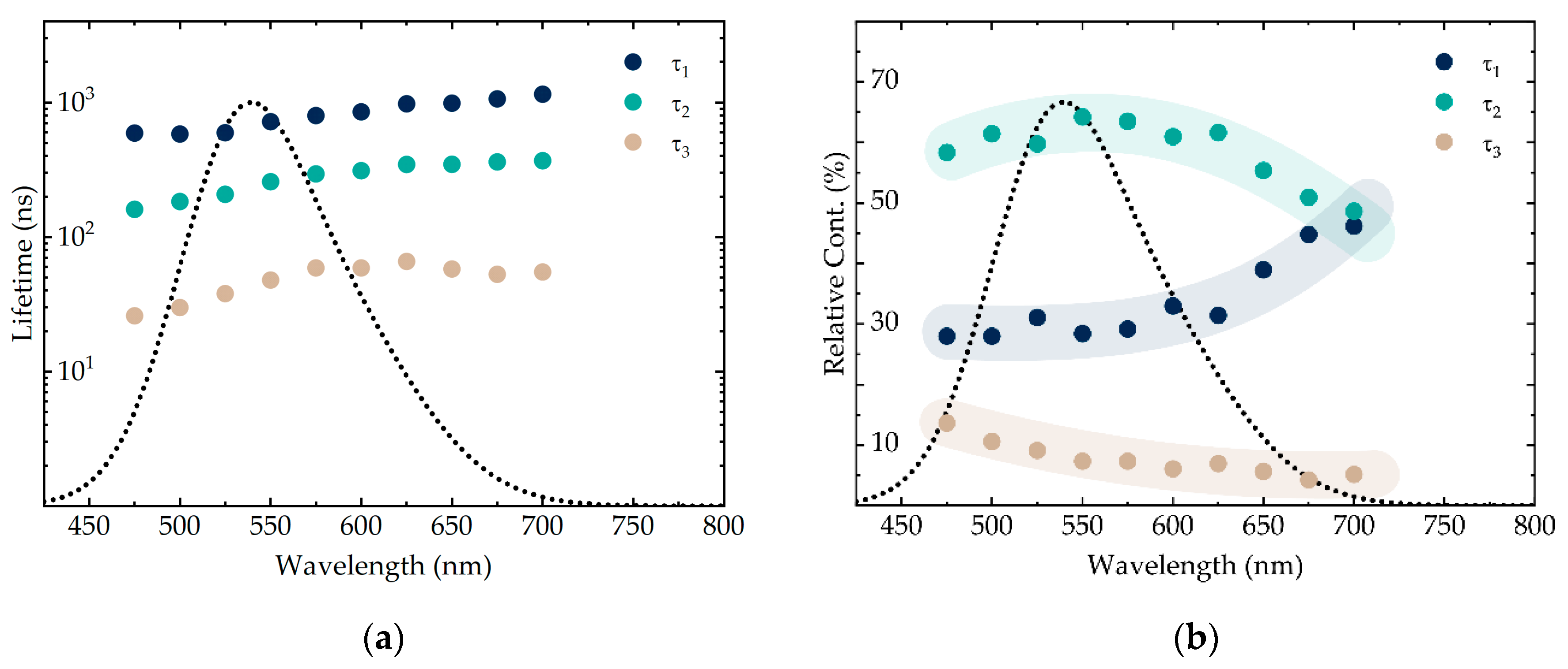
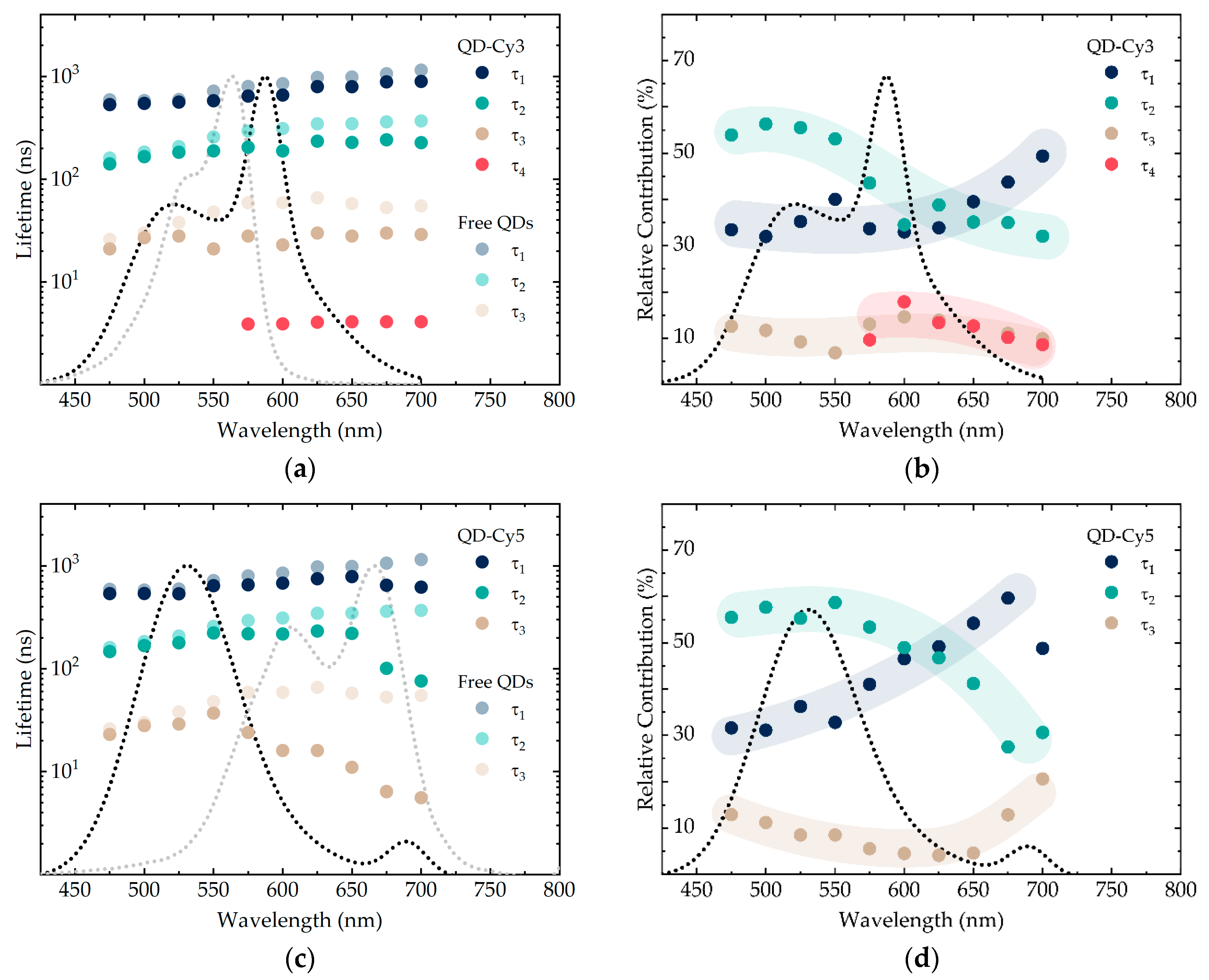
3.2. Analysis of AIS/ZAIS/ZnS QD Recombination Dynamics
3.3. FRET from AIS/ZAIS/ZnS QDs to the Dyes in the QD-Dye Complexes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leach, A.D.P.; Macdonald, J.E. Optoelectronic Properties of CuInS2 Nanocrystals and Their Origin. J. Phys. Chem. Lett. 2016, 7, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Van Der Stam, W.; Berends, A.C.; Donega, C.D.M. Prospects of Colloidal Copper Chalcogenide Nanocrystals. Chem. Phys. Chem. 2016, 17, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.Y. Synthetic strategies and biomedical applications of I–III–VI ternary quantum dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Parani, S.; Matoetoe, M.C.; Songca, S.P.; Oluwafemi, O.S. Evolution of ternary I–III–VI QDs: Synthesis, characterization and application. Nano-Struct. Nano-Obj. 2017, 12, 46–56. [Google Scholar] [CrossRef]
- Bai, X.; Purcell-Milton, F.; Gun’Ko, Y.K. Optical Properties, Synthesis, and Potential Applications of Cu-Based Ternary or Quaternary Anisotropic Quantum Dots, Polytypic Nanocrystals, and Core/Shell Heterostructures. Nanomaterials 2019, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Berends, A.C.; Mangnus, M.J.J.; Xia, C.; Rabouw, F.T.; Donega, C.D.M. Optoelectronic Properties of Ternary I–III–VI2 Semiconductor Nanocrystals: Bright Prospects with Elusive Origins. J. Phys. Chem. Lett. 2019, 10, 1600–1616. [Google Scholar] [CrossRef]
- Moodelly, D.; Kowalik, P.; Bujak, P.; Pron, A.; Reiss, P. Synthesis, photophysical properties and surface chemistry of chalcopyrite-type semiconductor nanocrystals. J. Mater. Chem. C 2019, 7, 11665–11709. [Google Scholar] [CrossRef]
- Ogawa, T.; Kuzuya, T.; Hamanaka, Y.; Sumiyama, K. Synthesis of Ag–In binary sulfide nanoparticles—structural tuning and their photoluminescence properties. J. Mater. Chem. 2010, 20, 2226. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Ogawa, T.; Tsuzuki, M.; Kuzuya, T. Photoluminescence Properties and Its Origin of AgInS2 Quantum Dots with Chalcopyrite Structure. J. Phys. Chem. C 2011, 115, 1786–1792. [Google Scholar] [CrossRef]
- Mao, B.; Chuang, C.-H.; Wang, J.; Burda, C. Synthesis and Photophysical Properties of Ternary I–III–VI AgInS2 Nanocrystals: Intrinsic versus Surface States. J. Phys. Chem. C 2011, 115, 8945–8954. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Ozawa, K.; Kuzuya, T. Enhancement of Donor–Acceptor Pair Emissions in Colloidal AgInS2 Quantum Dots with High Concentrations of Defects. J. Phys. Chem. C 2014, 118, 14562–14568. [Google Scholar] [CrossRef]
- Chevallier, T.; Le Blevennec, G.; Chandezon, F. Photoluminescence properties of AgInS2–ZnS nanocrystals: The critical role of the surface. Nanoscale 2016, 8, 7612–7620. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, T.; Benayad, A.; Le Blevennec, G.; Chandezon, F. Method to determine radiative and non-radiative defects applied to AgInS2–ZnS luminescent nanocrystals. Phys. Chem. Chem. Phys. 2017, 19, 2359–2363. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-E.; Song, W.-S.; Yang, H. Noninjection, one-pot synthesis of Cu-deficient CuInS2/ZnS core/shell quantum dots and their fluorescent properties. J. Colloid Interface Sci. 2011, 361, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Park, H.K.; Oh, J.H.; Yang, H.; Do, Y.R. Comparisons of the structural and optical properties of o-AgInS2, t-AgInS2, and c-AgIn5S8 nanocrystals and their solid-solution nanocrystals with ZnS. J. Mater. Chem. 2012, 22, 18939–18949. [Google Scholar] [CrossRef]
- Mao, B.; Chuang, C.-H.; Lu, F.; Sang, L.; Zhu, J.; Burda, C. Study of the Partial Ag-to-Zn Cation Exchange in AgInS2/ZnS Nanocrystals. J. Phys. Chem. C 2012, 117, 648–656. [Google Scholar] [CrossRef]
- Hughes, K.E.; Ostheller, S.R.; Nelson, H.D.; Gamelin, D.R. Copper’s Role in the Photoluminescence of Ag1–xCuxInS2 Nanocrystals, from Copper-Doped AgInS2 (x ∼ 0) to CuInS2 (x = 1). Nano Lett. 2019, 19, 1318–1325. [Google Scholar] [CrossRef]
- Jara, D.H.; Stamplecoskie, K.G.; Kamat, P. Two Distinct Transitions in CuxInS2 Quantum Dots. Bandgap versus Sub-Bandgap Excitations in Copper-Deficient Structures. J. Phys. Chem. Lett. 2016, 7, 1452–1459. [Google Scholar] [CrossRef]
- Torimoto, T.; Ogawa, S.; Adachi, T.; Kameyama, T.; Okazaki, K.-I.; Shibayama, T.; Kudo, A.; Kuwabata, S. Remarkable photoluminescence enhancement of ZnS–AgInS2 solid solution nanoparticles by post-synthesis treatment. Chem. Commun. 2010, 46, 2082. [Google Scholar] [CrossRef]
- Dai, M.; Ogawa, S.; Kameyama, T.; Okazaki, K.-I.; Kudo, A.; Kuwabata, S.; Tsuboi, Y.; Torimoto, T. Tunable photoluminescence from the visible to near-infrared wavelength region of non-stoichiometric AgInS2 nanoparticles. J. Mater. Chem. 2012, 22, 12851–12858. [Google Scholar] [CrossRef]
- Zang, H.; Li, H.; Makarov, N.S.; Velizhanin, K.A.; Wu, K.; Park, Y.-S.; Klimov, V.I. Thick-Shell CuInS2/ZnS Quantum Dots with Suppressed “Blinking” and Narrow Single-Particle Emission Line Widths. Nano Lett. 2017, 17, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Vokhmintcev, K.V.; A Linkov, P.; Samokhvalov, P.; Nabiev, I.R. Two-stage ZnS Shell Coating on the CuInS2 Quantum Dots for Their Effective Solubilization. KnE Energy 2018, 3, 535–540. [Google Scholar] [CrossRef]
- Sandroni, M.; Wegner, K.D.; Aldakov, D.; Reiss, P. Prospects of Chalcopyrite-Type Nanocrystals for Energy Applications. ACS Energy Lett. 2017, 2, 1076–1088. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N. Solar light harvesting with multinary metal chalcogenide nanocrystals. Chem. Soc. Rev. 2018, 47, 5354–5422. [Google Scholar] [CrossRef]
- Bai, B.; Kou, D.-X.; Zhou, W.; Zhou, Z.; Tian, Q.-W.; Meng, Y.; Wu, S. Quaternary Cu2ZnSnS4 quantum dot-sensitized solar cells: Synthesis, passivation and ligand exchange. J. Power Sources 2016, 318, 35–40. [Google Scholar] [CrossRef]
- Cai, C.; Zhai, L.; Ma, Y.; Zou, C.; Zhang, L.; Yang, Y.; Huang, S. Synthesis of AgInS2 quantum dots with tunable photoluminescence for sensitized solar cells. J. Power Sources 2017, 341, 11–18. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.; Zhong, Y.; Zhao, Q. Zn-Ag-In-S quantum dot sensitized solar cells with enhanced efficiency by tuning defects. J. Colloid Interface Sci. 2019, 547, 267–274. [Google Scholar] [CrossRef]
- Santos, C.I.; S Machado, W.; Wegner, K.D.; Gontijo, L.A.; Bettini, J.; Schiavon, M.A.; Aldakov, D. Hydrothermal Synthesis of Aqueous-Soluble Copper Indium Sulfide Nanocrystals and Their Use in Quantum Dot Sensitized Solar Cells. Nanomaterials 2020, 10, 1252. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, H.S.; Tabare, C.; Paiva, A.; Capanema, N.S.V. Eco-friendly AgInS2/ZnS quantum dot nanohybrids with tunable luminescent properties modulated by pH-sensitive biopolymer for potential solar energy harvesting applications. J. Mater. Sci. Mater. Electron. 2019, 30, 16702–16717. [Google Scholar] [CrossRef]
- Ko, M.; Yoon, H.C.; Yoo, H.; Oh, J.H.; Yang, H.; Do, Y.R. Highly Efficient Green Zn–Ag–In–S/Zn–In–S/ZnS QDs by a Strong Exothermic Reaction for Down-Converted Green and Tripackage White LEDs. Adv. Funct. Mater. 2017, 27, 1602638. [Google Scholar] [CrossRef]
- Chen, B.; Pradhan, N.; Zhong, H. From Large-Scale Synthesis to Lighting Device Applications of Ternary I–III–VI Semiconductor Nanocrystals: Inspiring Greener Material Emitters. J. Phys. Chem. Lett. 2018, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Wang, L.; Li, M.; Mei, S.; Wei, X.; Dai, H.; Hu, Z.; Xie, F.; Guo, R. Highly luminescent water-soluble AgInS2/ZnS quantum dots-hydrogel composites for warm white LEDs. J. Alloy. Compd. 2020, 824, 153896. [Google Scholar] [CrossRef]
- Guijarro, N.; Prévot, M.S.; Yu, X.; Jeanbourquin, X.A.; Bornoz, P.; Bourée, W.; Johnson, M.; Le Formal, F.; Sivula, K. A Bottom-Up Approach toward All-Solution-Processed High-Efficiency Cu(In,Ga)S2 Photocathodes for Solar Water Splitting. Adv. Energy Mater. 2016, 6, 1501949. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Mao, B.; Luo, B.; Zhang, K.; Wei, W.; Yuan, S. Facile Surface Engineering of Ag–In–Zn–S Quantum Dot Photocatalysts by Mixed-Ligand Passivation with Improved Charge Carrier Lifetime. Catal. Lett. 2019, 149, 1800–1812. [Google Scholar] [CrossRef]
- Hsieh, P.-Y.; Kameyama, T.; Takiyama, T.; Masuoka, K.; Yamamoto, T.; Hsu, Y.-J.; Torimoto, T. Controlling the visible-light driven photocatalytic activity of alloyed ZnSe–AgInSe2 quantum dots for hydrogen production. J. Mater. Chem. A 2020, 8, 13142–13149. [Google Scholar] [CrossRef]
- Deng, D.; Cao, J.; Qu, L.; Achilefu, S.; Gu, Y. Highly luminescent water-soluble quaternary Zn–Ag–In–S quantum dots for tumor cell-targeted imaging. Phys. Chem. Chem. Phys. 2013, 15, 5078. [Google Scholar] [CrossRef]
- Deng, D.; Qu, L.; Gu, Y. Near-infrared broadly emissive AgInSe2/ZnS quantum dots for biomedical optical imaging. J. Mater. Chem. C 2014, 2, 7077–7085. [Google Scholar] [CrossRef]
- Shamirian, A.; Appelbe, O.; Zhang, Q.; Ganesh, B.B.; Kron, S.J.; Snee, P.T. A toolkit for bioimaging using near-infrared AgInS2/ZnS quantum dots. J. Mater. Chem. B 2015, 3, 8188–8196. [Google Scholar] [CrossRef]
- Zhao, C.; Bai, Z.; Liu, X.; Zhang, Y.; Zou, B.; Zhong, H. Small GSH-Capped CuInS2 Quantum Dots: MPA-Assisted Aqueous Phase Transfer and Bioimaging Applications. ACS Appl. Mater. Interfaces 2015, 7, 17623–17629. [Google Scholar] [CrossRef]
- Deng, T.; Peng, Y.; Zhang, R.; Wang, J.; Zhang, J.; Gu, Y.; Huang, D.; Deng, D. Water-Solubilizing Hydrophobic ZnAgInSe/ZnS QDs with Tumor-Targeted cRGD-Sulfobetaine-PIMA-Histamine Ligands via a Self-Assembly Strategy for Bioimaging. ACS Appl. Mater. Interfaces 2017, 9, 11405–11414. [Google Scholar] [CrossRef]
- Preeyanka, N.; Dey, H.; Seth, S.; Rahaman, A.; Sarkar, M. Highly efficient energy transfer from a water soluble zinc silver indium sulphide quantum dot to organic J-aggregates. Phys. Chem. Chem. Phys. 2020, 22, 12772–12784. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wang, W.; Du, L.; Rabouw, F.T.; Heuvel, D.J.V.D.; Gerritsen, H.C.; Mattoussi, H.; Donega, C.D.M. Förster Resonance Energy Transfer between Colloidal CuInS2/ZnS Quantum Dots and Dark Quenchers. J. Phys. Chem. C 2020, 124, 1717–1731. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Hu, S.; Liu, Y.; Ren, Y.; Zhang, X. Förster resonance energy transfer properties of a new type of near-infrared excitation PDT photosensitizer: CuInS2/ZnS quantum dots-5-aminolevulinic acid conjugates. RSC Adv. 2016, 6, 55568–55576. [Google Scholar] [CrossRef]
- Tsolekile, N.; Ncapayi, V.; Parani, S.; Sakho, E.H.M.; Matoetoe, M.C.; Songca, S.P.; Oluwafemi, O.S. Synthesis of fluorescent CuInS2/ZnS quantum dots—Porphyrin conjugates for photodynamic therapy. MRS Commun. 2018, 8, 398–403. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Kuznetsova, V.A.; Orlova, A.; Kanaev, P.; Gromova, Y.A.; Maslov, V.; Baranov, A.; Fedorov, A.V. ZnSe/ZnS quantum dots-photosensitizer complexes: Optical properties and cancer cell photodynamic destruction effect. Photonics Eur. 2014, 9126. [Google Scholar] [CrossRef]
- Evstigneev, R.V.; Parfenov, P.S.; Dubavik, A.; Cherevkov, S.A.; Fedorov, A.V.; Martynenko, I.V.; Baranov, A.V. Time-resolved FRET in AgInS2/ZnS-CdSe/ZnS quantum dot systems. Nanotechnology 2019, 30, 195501. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Kusić, D.; Weigert, F.; Stafford, S.; Donnelly, F.C.; Evstigneev, R.; Gromova, Y.; Baranov, A.V.; Rühle, B.; Kunte, H.-J.; et al. Magneto-Fluorescent Microbeads for Bacteria Detection Constructed from Superparamagnetic Fe3O4 Nanoparticles and AIS/ZnS Quantum Dots. Anal. Chem. 2019, 91, 12661–12669. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Tkach, A.; Cherevkov, S.A.; Sokolova, A.; Gromova, Y.; Osipova, V.; Baranov, M.A.; Ugolkov, V.; Fedorov, A.; Baranov, A.V. Spectral-Time Multiplexing in FRET Complexes of AgInS2/ZnS Quantum Dot and Organic Dyes. Nanomaterials 2020, 10, 1569. [Google Scholar] [CrossRef]
- Knowles, K.E.; Nelson, H.D.; Kilburn, T.B.; Gamelin, D.R. Singlet–Triplet Splittings in the Luminescent Excited States of Colloidal Cu+:CdSe, Cu+:InP, and CuInS2 Nanocrystals: Charge-Transfer Configurations and Self-Trapped Excitons. J. Am. Chem. Soc. 2015, 137, 13138–13147. [Google Scholar] [CrossRef]
- Berends, A.C.; Rabouw, F.T.; Spoor, F.C.M.; Bladt, E.; Grozema, F.C.; Houtepen, A.J.; Siebbeles, L.D.A.; Donega, C.D.M. Radiative and Nonradiative Recombination in CuInS2 Nanocrystals and CuInS2-Based Core/Shell Nanocrystals. J. Phys. Chem. Lett. 2016, 7, 3503–3509. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Spranger, F.; Selyshchev, O.; Dzhagan, V.; Schulze, S.; Zahn, D.R.T.; Eychmüller, A. Origin and Dynamics of Highly Efficient Broadband Photoluminescence of Aqueous Glutathione-Capped Size-Selected Ag–In–S Quantum Dots. J. Phys. Chem. C 2018, 122, 13648–13658. [Google Scholar] [CrossRef]
- Hoffmann, K.; Behnke, T.; Grabolle, M.; Resch-Genger, U. Nanoparticle-encapsulated vis- and NIR-emissive fluorophores with different fluorescence decay kinetics for lifetime multiplexing. Anal. Bioanal. Chem. 2014, 406, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Kage, D.; Hoffmann, K.; Wittkamp, M.; Ameskamp, J.; Göhde, W.; Resch-Genger, U. Luminescence lifetime encoding in time-domain flow cytometry. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kage, D.; Hoffmann, K.; Nifontova, G.; Krivenkov, V.; Sukhanova, A.; Nabiev, I.; Resch-Genger, U. Tempo-spectral multiplexing in flow cytometry with lifetime detection using QD-encoded polymer beads. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Li, L.; Pandey, A.; Werder, D.J.; Khanal, B.P.; Pietryga, J.M.; Klimov, V.I. Efficient Synthesis of Highly Luminescent Copper Indium Sulfide-Based Core/Shell Nanocrystals with Surprisingly Long-Lived Emission. J. Am. Chem. Soc. 2011, 133, 1176–1179. [Google Scholar] [CrossRef]
- Kraatz, I.T.; Booth, M.; Whitaker, B.J.; Nix, M.G.D.; Critchley, K. Sub-Bandgap Emission and Intraband Defect-Related Excited-State Dynamics in Colloidal CuInS2/ZnS Quantum Dots Revealed by Femtosecond Pump–Dump–Probe Spectroscopy. J. Phys. Chem. C 2014, 118, 24102–24109. [Google Scholar] [CrossRef]
- Omata, T.; Nose, K.; Kurimoto, K.; Kita, M. Electronic transition responsible for size-dependent photoluminescence of colloidal CuInS2 quantum dots. J. Mater. Chem. C 2014, 2, 6867–6872. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, D.; Zhao, J.; Ikezawa, M.; Wang, X.; Masumoto, Y. Ultrafast carrier dynamics in CuInS2 quantum dots. Appl. Phys. Lett. 2014, 104, 23118. [Google Scholar] [CrossRef]
- Sharma, D.K.; Hirata, S.; Bujak, Ł.; Biju, V.; Kameyama, T.; Kishi, M.; Torimoto, T.; Vacha, M. Single-particle spectroscopy of I–III–VI semiconductor nanocrystals: Spectral diffusion and suppression of blinking by two-color excitation. Nanoscale 2016, 8, 13687–13694. [Google Scholar] [CrossRef]
- Fuhr, A.S.; Yun, H.J.; Makarov, N.S.; Li, H.; McDaniel, H.; Klimov, V.I. Light Emission Mechanisms in CuInS2 Quantum Dots Evaluated by Spectral Electrochemistry. ACS Photon. 2017, 4, 2425–2435. [Google Scholar] [CrossRef]
- Pinchetti, V.; Lorenzon, M.; McDaniel, H.; Lorenzi, R.; Meinardi, F.; Klimov, V.I.; Brovelli, S. Spectro-electrochemical Probing of Intrinsic and Extrinsic Processes in Exciton Recombination in I–III–VI2 Nanocrystals. Nano Lett. 2017, 17, 4508–4517. [Google Scholar] [CrossRef] [PubMed]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N.; Selyshchev, O.; Dzhagan, V.; Schulze, S.; Zahn, D.R. Origin of the Broadband Photoluminescence of Pristine and Cu+/Ag+-Doped Ultrasmall CdS and CdSe/CdS Quantum Dots. J. Phys. Chem. C 2018, 122, 10267–10277. [Google Scholar] [CrossRef]
- Stroyuk, O.; Dzhagan, V.; Raevskaya, A.; Spranger, F.; Gaponik, N.; Zahn, D.R. Insights into different photoluminescence mechanisms of binary and ternary aqueous nanocrystals from the temperature dependence: A case study of CdSe and Ag-In-S. J. Lumin. 2019, 215, 116630. [Google Scholar] [CrossRef]
- Stroyuk, O.; Weigert, F.; Raevskaya, A.; Spranger, F.; Würth, C.; Resch-Genger, U.; Gaponik, N.; Zahn, D.R.T.; E Raevskaya, A. Inherently Broadband Photoluminescence in Ag–In–S/ZnS Quantum Dots Observed in Ensemble and Single-Particle Studies. J. Phys. Chem. C 2019, 123, 2632–2641. [Google Scholar] [CrossRef]
- Komarala, V.K.; Xie, C.; Wang, Y.; Xu, J.; Xiao, M. Time-resolved photoluminescence properties of CuInS2/ZnS nanocrystals: Influence of intrinsic defects and external impurities. J. Appl. Phys. 2012, 111, 124314. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Zhai, W.; Wang, Y.; Zhang, T.; Gu, P.; Chu, H.; Zhang, H.; Cui, T.; Wang, Y.; et al. Temperature-Dependent Photoluminescence of ZnCuInS/ZnSe/ZnS Quantum Dots. J. Phys. Chem. C 2013, 117, 19288–19294. [Google Scholar] [CrossRef]
- Seo, F.J.; Raut, S.; Abdel-Fattah, M.; Rice, Q.; Tabibi, B.; Rich, R.; Fudala, R.; Gryczynski, I.; Gryczynski, Z.; Kim, W.-J.; et al. Time-resolved and temperature-dependent photoluminescence of ternary and quaternary nanocrystals of CuInS2 with ZnS capping and cation exchange. J. Appl. Phys. 2013, 114, 094310. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Z.; Xu, X.; Wang, N.; Fang, J.; Wang, J.; Xu, G. A high efficient photoluminescence Zn–Cu–In–S/ZnS quantum dots with long lifetime. J. Alloys Compd. 2015, 640, 134–140. [Google Scholar] [CrossRef]
- Chen, S.; Ahmadiantehrani, M.; Zhao, J.; Zhu, S.; Mamalis, A.G.; Zhu, X. Heat-up synthesis of Ag–In–S and Ag–In–S/ZnS nanocrystals: Effect of indium precursors on their optical properties. J. Alloys Compd. 2016, 665, 137–143. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Chun, S.Y.; Yoon, H.C.; Han, N.S.; Oh, J.H.; Park, S.M.; Do, Y.R.; Song, J.K. Enhancement Mechanism of the Photoluminescence Quantum Yield in Highly Efficient ZnS–AgIn5S8 Quantum Dots with Core/Shell Structures. J. Phys. Chem. C 2018, 122, 10125–10132. [Google Scholar] [CrossRef]
- Orlova, A.O.; Gromova, Y.A.; Maslov, V.G.; Prudnikau, A.V.; Artemyev, M.; Fedorov, A.V.; Baranov, A.V. Formation of structures based on semiconductor quantum dots and organic molecules in track pore membranes. J. Appl. Phys. 2013, 113, 214305. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Baimuratov, A.S.; Weigert, F.; Soares, J.X.; Dhamo, L.; Nickl, P.; Resch-Genger, U. Photoluminescence of Ag-In-S/ZnS quantum dots: Excitation energy dependence and low-energy electronic structure. Nano Res. 2019, 12, 1595–1603. [Google Scholar] [CrossRef]
- Jiao, M.; Huang, X.; Ma, L.; Li, Y.; Zhang, P.; Wei, X.; Jing, L.; Luo, X.; Rogach, A.L.; Gao, M. Biocompatible off-stoichiometric copper indium sulfide quantum dots with tunable near-infrared emission via aqueous based synthesis. Chem. Commun. 2019, 55, 15053–15056. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Xie, C.; Wang, J.; Zhong, J.; Liang, X.; Yang, H.; Chen, Z. Studies on highly luminescent AgInS2 and Ag–Zn–In–S quantum dots. J. Alloys Compd. 2014, 588, 114–121. [Google Scholar] [CrossRef]
- Chong, E.Z.; Matthews, D.R.; Summers, H.D.; Njoh, K.L.; Errington, R.J.; Smith, P.J. Development of FRET-Based Assays in the Far-Red Using CdTe Quantum Dots. J. Biomed. Biotechnol. 2007, 2007, 1–7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miropoltsev, M.; Kuznetsova, V.; Tkach, A.; Cherevkov, S.; Sokolova, A.; Osipova, V.; Gromova, Y.; Baranov, M.; Fedorov, A.; Gun'ko, Y.; et al. FRET-Based Analysis of AgInS2/ZnAgInS/ZnS Quantum Dot Recombination Dynamics. Nanomaterials 2020, 10, 2455. https://doi.org/10.3390/nano10122455
Miropoltsev M, Kuznetsova V, Tkach A, Cherevkov S, Sokolova A, Osipova V, Gromova Y, Baranov M, Fedorov A, Gun'ko Y, et al. FRET-Based Analysis of AgInS2/ZnAgInS/ZnS Quantum Dot Recombination Dynamics. Nanomaterials. 2020; 10(12):2455. https://doi.org/10.3390/nano10122455
Chicago/Turabian StyleMiropoltsev, Maksim, Vera Kuznetsova, Anton Tkach, Sergei Cherevkov, Anastasiia Sokolova, Viktoria Osipova, Yulia Gromova, Mikhail Baranov, Anatoly Fedorov, Yurii Gun'ko, and et al. 2020. "FRET-Based Analysis of AgInS2/ZnAgInS/ZnS Quantum Dot Recombination Dynamics" Nanomaterials 10, no. 12: 2455. https://doi.org/10.3390/nano10122455
APA StyleMiropoltsev, M., Kuznetsova, V., Tkach, A., Cherevkov, S., Sokolova, A., Osipova, V., Gromova, Y., Baranov, M., Fedorov, A., Gun'ko, Y., & Baranov, A. (2020). FRET-Based Analysis of AgInS2/ZnAgInS/ZnS Quantum Dot Recombination Dynamics. Nanomaterials, 10(12), 2455. https://doi.org/10.3390/nano10122455







