Carbon Material and Cobalt-Substitution Effects in the Electrochemical Behavior of LaMnO3 for ORR and OER
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Synthesis of Catalysts
2.3. Characterization Techniques and Electrode Preparation
3. Results and Discussion
3.1. Perovskite Materials
3.1.1. Electrochemical Characterization
3.1.2. Analysis of the Electrocatalytic Activity towards ORR
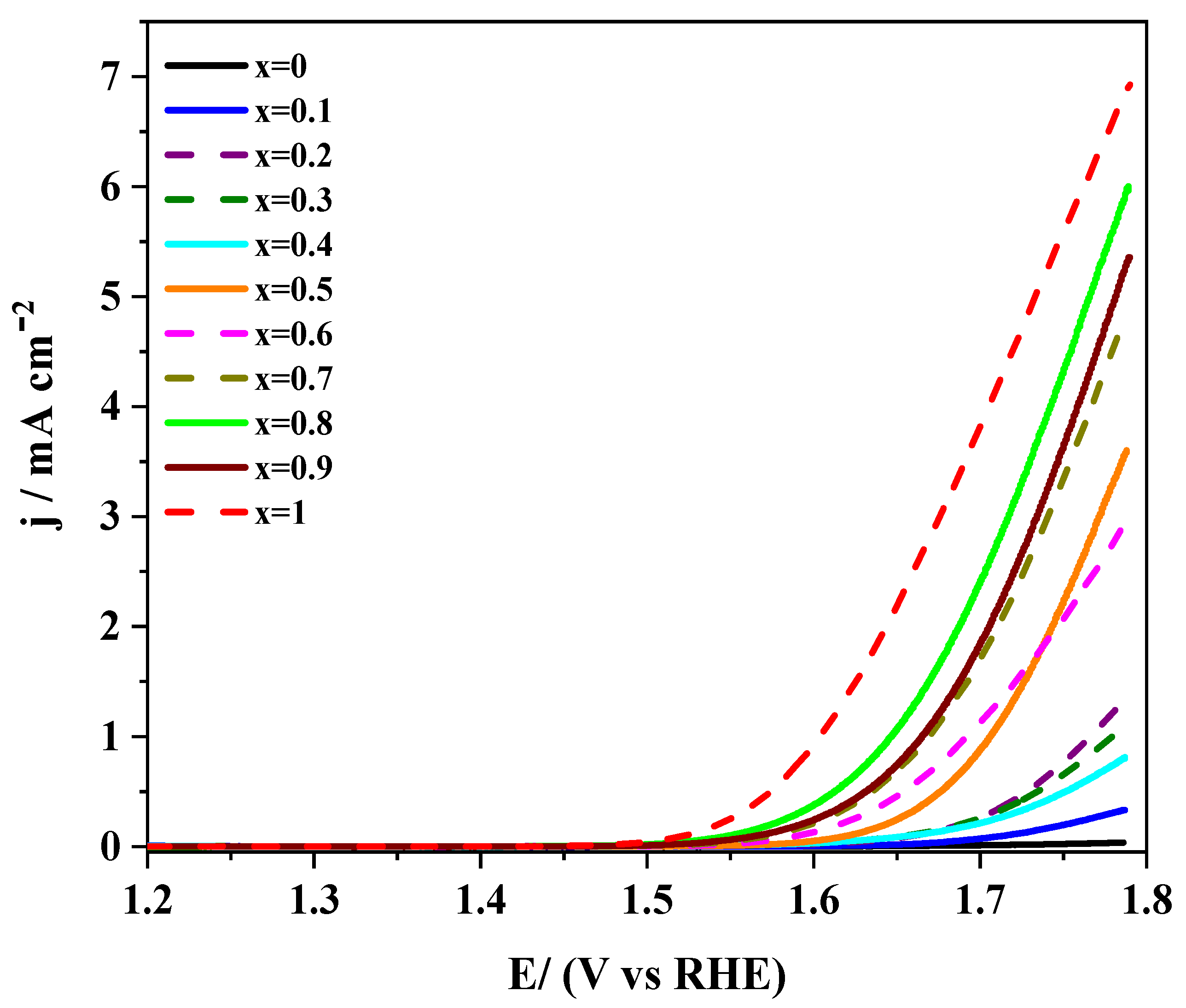
3.1.3. Analysis of the Electrocatalytic Activity towards OER
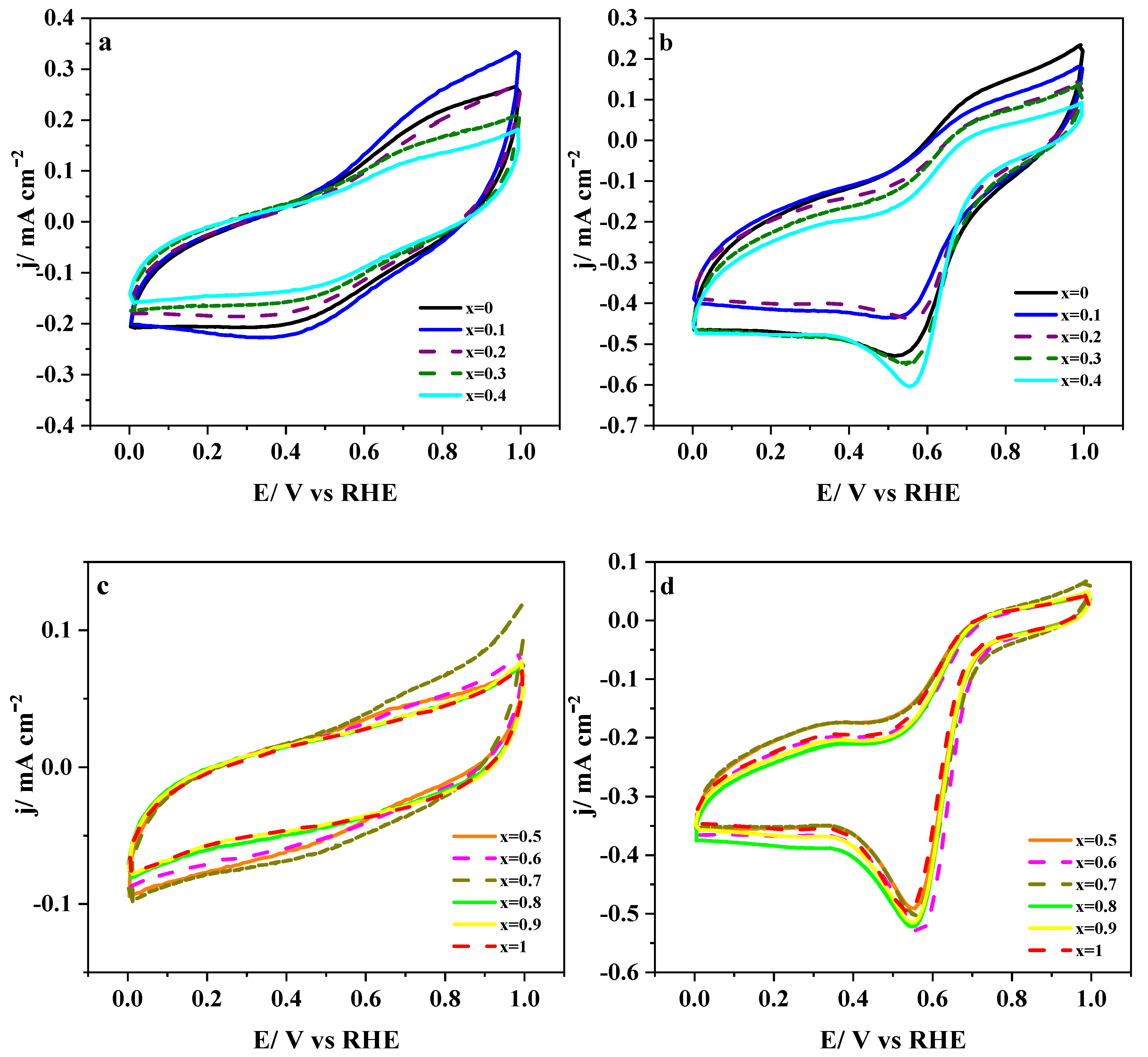
3.2. Perovskite Materials Mixed with Carbon Materials
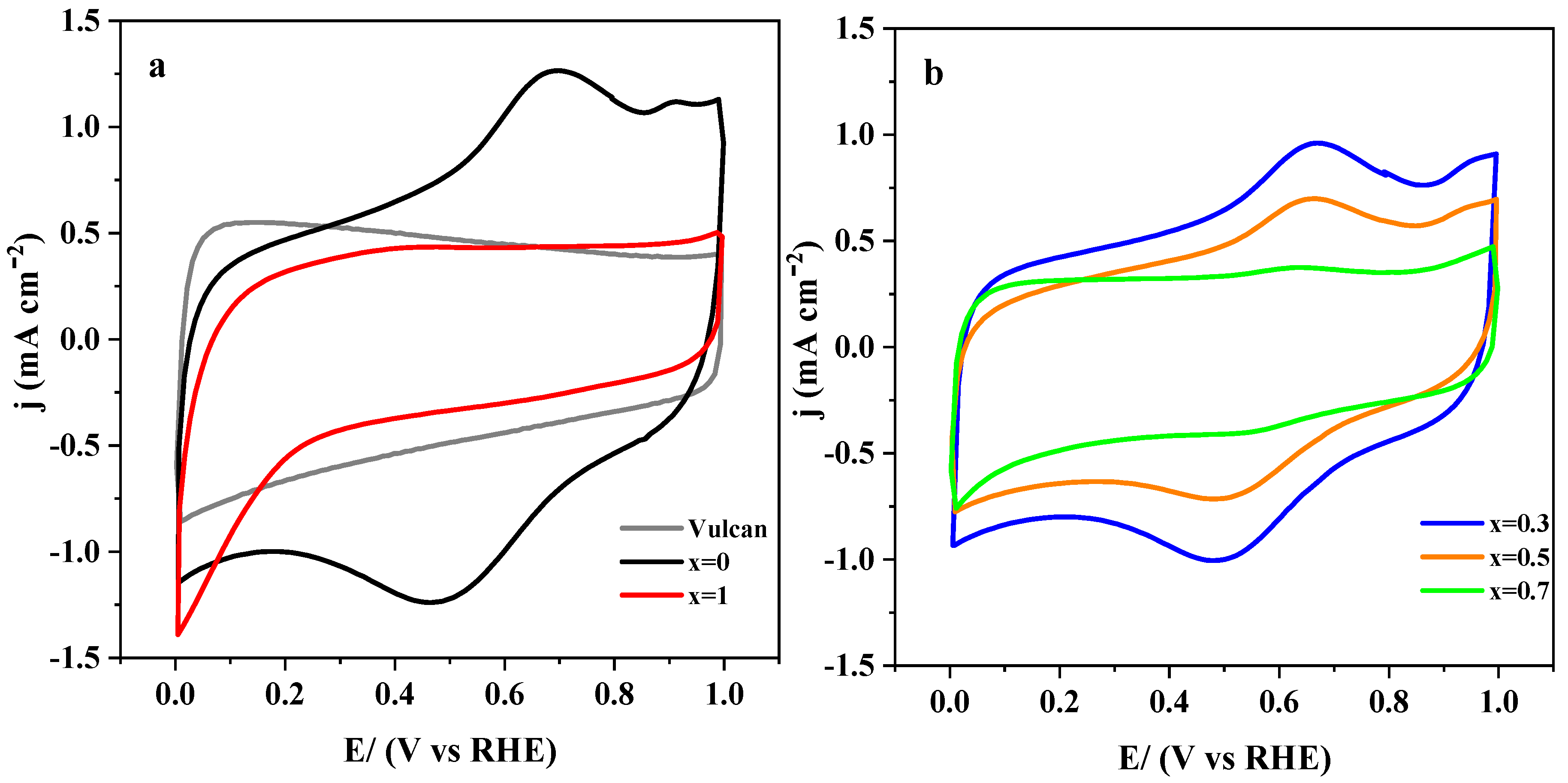
3.2.1. Electrochemical Characterization
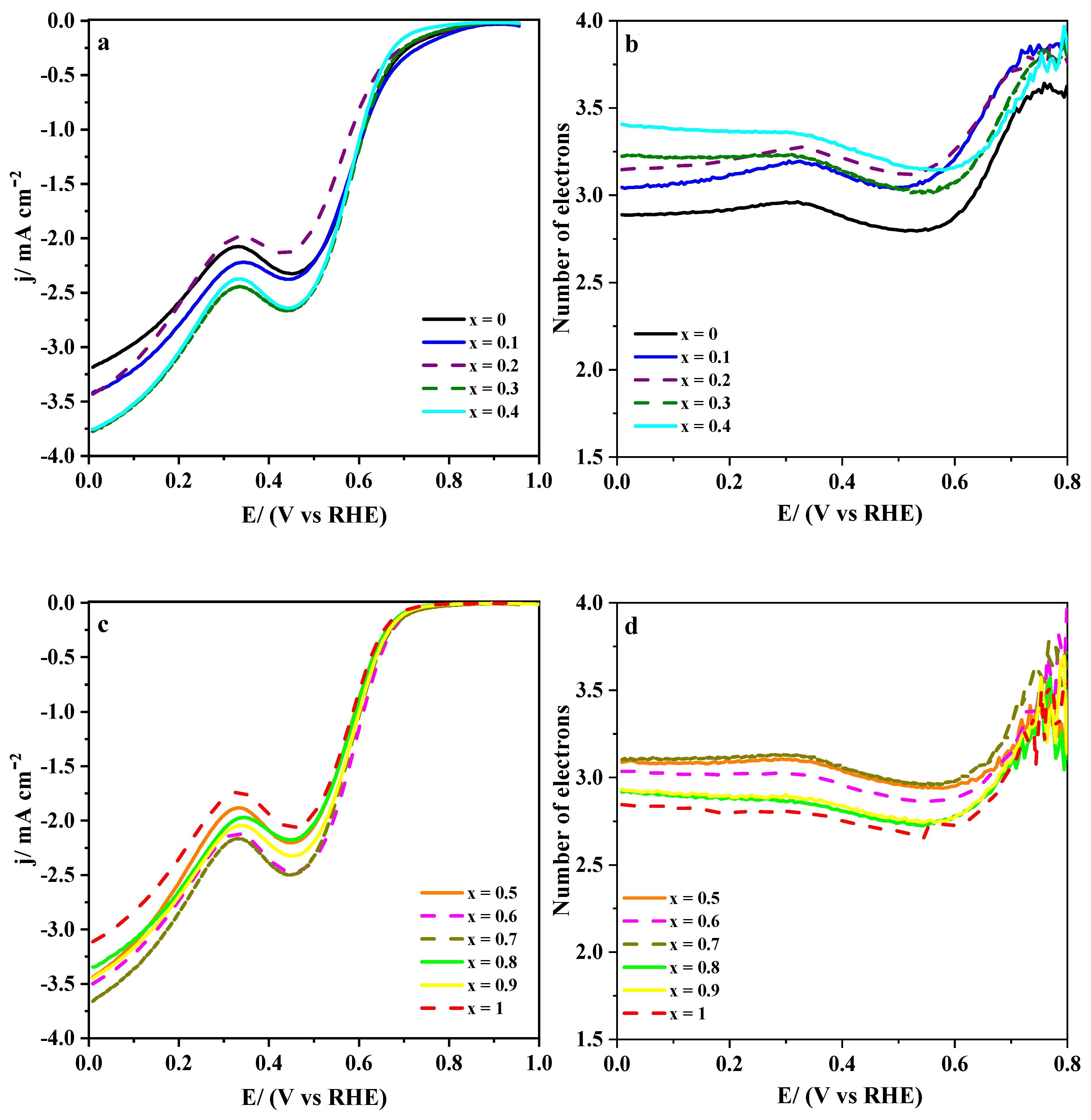
3.2.2. Electro-catalytic Activity towards ORR and OER
3.2.3. Effect of the Carbon Materials on the ORR Electrocatalysis
3.2.4. XPS Characterization
3.2.5. TPR Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Marković, N.M.; Schmidt, T.J.; Stamenković, V.; Ross, P.N. Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Baiyee, Z.M.; Shao, Z.; Ciucci, F. Nonstoichiometric Oxides as Low-Cost and Highly-Efficient Oxygen Reduction/Evolution Catalysts for Low-Temperature Electrochemical Devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Longhi, M.; Cova, C.; Pargoletti, E.; Coduri, M.; Santangelo, S.; Patanè, S.; Ditaranto, N.; Cioffi, N.; Facibeni, A.; Scavini, M. Synergistic effects of active sites’ nature and hydrophilicity on the oxygen reduction reaction activity of Pt-free catalysts. Nanomaterials 2018, 8, 643. [Google Scholar] [CrossRef]
- Minguzzi, A.; Longoni, G.; Cappelletti, G.; Pargoletti, E.; Di Bari, C.; Locatelli, C.; Marelli, M.; Rondinini, S.; Vertova, A. The influence of carbonaceous matrices and electro-catalytic MnO2 nanopowders on lithium-air battery performances. Nanomaterials 2016, 6, 10. [Google Scholar] [CrossRef]
- Han, X.; He, G.; He, Y.; Zhang, J.; Zheng, X.; Li, L.; Zhong, C.; Hu, W.; Deng, Y.; Ma, T.-Y. Engineering Catalytic Active Sites on Cobalt Oxide Surface for Enhanced Oxygen Electrocatalysis. Adv. Energy Mater. 2018, 8, 1702222. [Google Scholar] [CrossRef]
- Gao, S.; Geng, K. Facile construction of Mn3O4 nanorods coated by a layer of nitrogen-doped carbon with high activity for oxygen reduction reaction. Nano Energy 2014, 6, 44–50. [Google Scholar] [CrossRef]
- Han, X.; Zhang, W.; Ma, X.; Zhong, C.; Zhao, N.; Hu, W.; Deng, Y. Identifying the Activation of Bimetallic Sites in NiCo2S4@g-C 3N4-CNT Hybrid Electrocatalysts for Synergistic Oxygen Reduction and Evolution. Adv. Mater. 2019, 31, 1808281. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wu, X.; Deng, Y.; Liu, J.; Lu, J.; Zhong, C.; Hu, W. Ultrafine Pt Nanoparticle-Decorated Pyrite-Type CoS 2 Nanosheet Arrays Coated on Carbon Cloth as a Bifunctional Electrode for Overall Water Splitting. Adv. Energy Mater. 2018, 8, 1800935. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Zhong, C.; Zhao, N.; Deng, Y.; Han, X.; Hu, W. Spontaneous Synthesis of Silver-Nanoparticle-Decorated Transition-Metal Hydroxides for Enhanced Oxygen Evolution Reaction. Angew. Chem. 2020, 132, 7312–7317. [Google Scholar] [CrossRef]
- Han, X.; Ling, X.; Yu, D.; Xie, D.; Li, L.; Peng, S.; Zhong, C.; Zhao, N.; Deng, Y.; Hu, W. Atomically Dispersed Binary Co-Ni Sites in Nitrogen-Doped Hollow Carbon Nanocubes for Reversible Oxygen Reduction and Evolution. Adv. Mater. 2019, 31, 1905622. [Google Scholar] [CrossRef]
- Gupta, S.; Kellogg, W.; Xu, H.; Liu, X.; Cho, J.; Wu, G. Bifunctional Perovskite Oxide Catalysts for Oxygen Reduction and Evolution in Alkaline Media. Chem. Asian J. 2016, 11, 10–21. [Google Scholar] [CrossRef]
- Celorrio, V.; Dann, E.; Calvillo, L.; Morgan, D.J.; Hall, S.R.; Fermin, D.J. Oxygen Reduction at Carbon-Supported Lanthanides: The Role of the B-Site. ChemElectroChem 2016, 3, 283–291. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Almomani, F.; Malik, S.S.; Suslov, S.; Tarlochan, F. Combustion synthesis of bifunctional LaMO3 (M = Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 22–30. [Google Scholar] [CrossRef]
- Sunarso, J.; Torriero, A.A.J.; Zhou, W.; Howlett, P.C.; Forsyth, M. Oxygen reduction reaction activity of La-based perovskite oxides in alkaline medium: A thin-film rotating ring-disk electrode study. J. Phys. Chem. C 2012, 116, 5827–5834. [Google Scholar] [CrossRef]
- Sun, J.; Du, L.; Sun, B.; Han, G.; Ma, Y.; Wang, J.; Huo, H.; Zuo, P.; Du, C.; Yin, G. A bifunctional perovskite oxide catalyst: The triggered oxygen reduction/evolution electrocatalysis by moderated Mn-Ni co-doping. J. Energy Chem. 2020, 54, 217–224. [Google Scholar] [CrossRef]
- Liu, X.; Gong, H.; Wang, T.; Guo, H.; Song, L.; Xia, W.; Gao, B.; Jiang, Z.; Feng, L.; He, J. Cobalt-Doped Perovskite-Type Oxide LaMnO3 as Bifunctional Oxygen Catalysts for Hybrid Lithium-Oxygen Batteries. Chem. Asian J. 2018, 13, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, L.; Shi, L.; Huang, H. Oxygen reduction reaction activity of LaMn1−xCoxO3-graphene nanocomposite for zinc-air battery. Electrochim. Acta 2015, 161, 115–123. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, M.G.; Park, H.W.; Seo, M.H.; Ismayilov, V.; Ahmed, R.; Chen, Z. Highly active Co-doped LaMnO3 perovskite oxide and N-doped carbon nanotube hybrid bi-functional catalyst for rechargeable zinc–air batteries. Electrochem. Commun. 2015, 60, 38–41. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Structural and morphological alterations induced by cobalt substitution in LaMnO3 perovskites. J. Colloid Interface Sci. 2019, 556, 658–666. [Google Scholar] [CrossRef]
- Pecchi, G.; Campos, C.; Peña, O. Thermal stability against reduction of LaMn1−yCoyO3 perovskites. Mater. Res. Bull. 2009, 44, 846–853. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Safakas, A.; Bampos, G.; Bebelis, S. Oxygen reduction reaction on La0.8Sr0.2CoxFe1−xO3-δ perovskite/carbon black electrocatalysts in alkaline medium. Appl. Catal. B Environ. 2019, 244, 225–232. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, T.; Shi, Q.; Yang, Q.; Li, C.; Zhang, D.; Zhang, C. Perovskite oxides La0.4Sr0.6CoxMn1−xO3 (x = 0, 0.2, 0.4) as an effective electrocatalyst for lithium—Air batteries. Green Energy Environ. 2018, 3, 78–85. [Google Scholar] [CrossRef]
- Xu, Y.; Tsou, A.; Fu, Y.; Wang, J.; Tian, J.-H.; Yang, R. Carbon-Coated Perovskite BaMnO3 Porous Nanorods with Enhanced Electrocatalytic Perporites for Oxygen Reduction and Oxygen Evolution. Electrochim. Acta 2015, 174, 551–556. [Google Scholar] [CrossRef]
- Alegre, C.; Modica, E.; Aricò, A.S.; Baglio, V. Bifunctional oxygen electrode based on a perovskite/carbon composite for electrochemical devices. J. Electroanal. Chem. 2018, 808, 412–419. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Q.; Shi, Z.; Zhang, L.; Huang, H. LaNiO3-nanorod/graphene composite as an efficient bi-functional catalyst for zinc–air batteries. RSC Adv. 2016, 6, 86386–86394. [Google Scholar] [CrossRef]
- Mattick, V.F.; Jin, X.; White, R.E.; Huang, K. Understanding the role of carbon in alkaline oxygen electrocatalysis: A case study on La0.6Sr0.4CoO3-δ/Vulcan carbon composite electrocatalyst. Int. J. Hydrogen Energy 2019, 44, 2760–2769. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Wang, Q.; Wang, X.; Qian, D.; Jiang, J.; Li, J.; Chen, Z. Designed synthesis of LaCoO3/N-doped reduced graphene oxide nanohybrid as an efficient bifunctional electrocatalyst for ORR and OER in alkaline medium. J. Alloys Compd. 2017, 725, 260–269. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, D.U.; Park, M.G.; Ahmed, R.; Seo, M.H.; Nazar, L.F.; Chen, Z. Perovskite-Nitrogen-Doped Carbon Nanotube Composite as Bifunctional Catalysts for Rechargeable Lithium-Air Batteries. ChemSusChem 2015, 8, 1058–1065. [Google Scholar] [CrossRef]
- Poux, T.; Napolskiy, F.S.; Dintzer, T.; Kéranguéven, G.; Istomin, S.Y.; Tsirlina, G.A.; Antipov, E.V.; Savinova, E.R. Dual role of carbon in the catalytic layers of perovskite/carbon composites for the electro-catalytic oxygen reduction reaction. Catal. Today 2012, 189, 83–92. [Google Scholar] [CrossRef]
- Kéranguéven, G.; Ulhaq-Bouillet, C.; Papaefthimiou, V.; Royer, S.; Savinova, E. Perovskite-carbon composites synthesized through in situ autocombustion for the oxygen reduction reaction: The carbon effect. Electrochim. Acta 2017, 245, 156–164. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Jin, X.; Wang, F.; Song, Y. Composition-dependent electro-catalytic activities of covalent carbon-LaMnO3 hybrids as synergistic catalysts for oxygen reduction reaction. Electrochim. Acta 2016, 198, 115–126. [Google Scholar] [CrossRef]
- Liu, J.; Jin, X.; Song, W.; Wang, F.; Wang, N.; Song, Y. Facile preparation of modified carbon black-LaMnO3 hybrids and the effect of covalent coupling on the catalytic activity for oxygen reduction reaction. Chin. J. Catal. 2014, 35, 1173–1188. [Google Scholar] [CrossRef]
- Alexander, C.T.; Abakumov, A.M.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Role of the Carbon Support on the Oxygen Reduction and Evolution Activities in LaNiO3 Composite Electrodes in Alkaline Solution. ACS Appl. Energy Mater. 2018, 1, 1549–1558. [Google Scholar] [CrossRef]
- Gabe, A.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Understanding of oxygen reduction reaction by examining carbon-oxygen gasification reaction and carbon active sites on metal and heteroatoms free carbon materials of different porosities and structures. Carbon N. Y. 2019, 148, 430–440. [Google Scholar] [CrossRef]
- Ryabova, A.S.; Bonnefont, A.; Simonov, P.A.; Dintzer, T.; Ulhaq-Bouillet, C.; Bogdanova, Y.G.; Tsirlina, G.A.; Savinova, E.R. Further insights into the role of carbon in manganese oxide/carbon composites in the oxygen reduction reaction in alkaline media. Electrochim. Acta 2017, 246, 643–653. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry of inorganic solids. Thermochim. Acta 1987, 110, 303–317. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, H.; Sun, S.; Wang, Q.; Li, S.; Liu, Z. (La1−xSrx)0.98MnO3 perovskite with A-site deficiencies toward oxygen reduction reaction in aluminum-air batteries. J. Power Sources 2017, 342, 192–201. [Google Scholar] [CrossRef]
- Celorrio, V.; Calvillo, L.; Granozzi, G.; Russell, A.E.; Fermin, D.J. AMnO3 (A = Sr, La, Ca, Y) Perovskite Oxides as Oxygen Reduction Electrocatalysts. Top. Catal. 2018, 61, 154–161. [Google Scholar] [CrossRef]
- La Rosa-Toro, A.; Berenguer, R.; Quijada, C.; Montilla, F.; Morallón, E.; Vázquez, J.L. Preparation and Characterization of Copper-Doped Cobalt Oxide Electrodes. J. Phys. Chem. B 2006, 110, 24021–24029. [Google Scholar] [CrossRef]
- Pawar, S.M.; Pawar, B.S.; Babar, P.T.; Ahmed, A.T.A.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Hou, B.; Inamdar, A.I.; et al. Nanoporous CuCo2O4 nanosheets as a highly efficient bifunctional electrode for supercapacitors and water oxidation catalysis. Appl. Surf. Sci. 2019, 470, 360–367. [Google Scholar] [CrossRef]
- Palikundwar, U.A.; Sapre, V.B.; Moharil, S.V.; Priolkar, K.R. Local structure around Mn and Co in LaMn1−x CoxO3±δ: An EXAFS study. J. Phys. Condens. Matter 2009, 21, 235405. [Google Scholar] [CrossRef]
- Mattick, V.F.; Jin, X.; Yang, T.; White, R.E.; Huang, K. Unraveling Oxygen Electrocatalysis Mechanisms on a Thin-Film Oxygen-Deficient Perovskite La0.6Sr0.4CoO3−δ. ACS Appl. Energy Mater. 2018, 1, 3937–3946. [Google Scholar] [CrossRef]
- Zhang, T.; Anderson, A.B. Oxygen reduction on platinum electrodes in base: Theoretical study. Electrochim. Acta 2007, 53, 982–989. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H. Oxygen Reduction Activity on Perovskite Oxide Surfaces: A Comparative First-Principles Study of LaMnO3, LaFeO3, and LaCrO3. J. Phys. Chem. C 2013, 117, 2106–2112. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Risch, M.; Han, B.; Shao-Horn, Y. Recent Insights into Manganese Oxides in Catalyzing Oxygen Reduction Kinetics. ACS Catal. 2015, 5, 6021–6031. [Google Scholar] [CrossRef]
- Bockris, J.O.; Otagawa, T. The Electrocatalysis of Oxygen Evolution on Perovskites. J. Electrochem. Soc. 1984, 131, 290. [Google Scholar] [CrossRef]
- Zhao, Y.; Hang, Y.; Zhang, Y.; Wang, Z.; Yao, Y.; He, X.; Zhang, C.; Zhang, D. Strontium-doped perovskite oxide La1−xSrxMnO3 (x = 0, 0.2, 0.6) as a highly efficient electrocatalyst for nonaqueous Li-O2 batteries. Electrochim. Acta 2017, 232, 296–302. [Google Scholar] [CrossRef]
- Yamada, I.; Fujii, H.; Takamatsu, A.; Ikeno, H.; Wada, K.; Tsukasaki, H.; Kawaguchi, S.; Mori, S.; Yagi, S. Bifunctional Oxygen Reaction Catalysis of Quadruple Manganese Perovskites. Adv. Mater. 2017, 29, 1603004. [Google Scholar] [CrossRef]
- Malkhandi, S.; Trinh, P.; Manohar, A.K.; Manivannan, A.; Balasubramanian, M.; Prakash, G.K.S.; Narayanan, S.R. Design Insights for Tuning the Electrocatalytic Activity of Perovskite Oxides for the Oxygen Evolution Reaction. J. Phys. Chem. C 2015, 119, 8004–8013. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Shao, Z. Perovskite/Carbon Composites: Applications in Oxygen Electrocatalysis. Small 2017, 13, 1603793. [Google Scholar] [CrossRef]
- Mefford, J.T.; Kurilovich, A.A.; Saunders, J.; Hardin, W.G.; Abakumov, A.M.; Forslund, R.P.; Bonnefont, A.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Decoupling the roles of carbon and metal oxides on the electro-catalytic reduction of oxygen on La1−xSr x CoO3−δ perovskite composite electrodes. Phys. Chem. Chem. Phys. 2019, 21, 3327–3338. [Google Scholar] [CrossRef]
- Falcón, H.; Carbonio, R.; Fierro, J.L. Correlation of Oxidation States in LaFexNi1−xO3+δ Oxides with Catalytic Activity for H2O2 Decomposition. J. Catal. 2001, 203, 264–272. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and Recent Development of Polymer Electrolyte Membranes for Fuel Cells Operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef]
- Zhou, J.; Song, H.; Ma, L.; Chen, X. Magnetite/graphene nanosheet composites: Interfacial interaction and its impact on the durable high-rate performance in lithium-ion batteries. RSC Adv. 2011, 1, 782. [Google Scholar] [CrossRef]
- Ge, X.; Goh, F.W.T.; Li, B.; Hor, T.S.A.; Zhang, J.; Xiao, P.; Wang, X.; Zong, Y.; Liu, Z. Efficient and durable oxygen reduction and evolution of a hydrothermally synthesized La(Co0.55Mn0.45)0.99O3−δ nanorod/graphene hybrid in alkaline media. Nanoscale 2015, 7, 9046–9054. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G.; Baylet, A.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emission over LaMnO3 and LaB0.2Mn0.8O3 (B = Co, Ni, Fe) catalysts. Appl. Catal. B Environ. 2013, 129, 509–516. [Google Scholar] [CrossRef]
- Pargoletti, E.; Salvi, A.; Giordana, A.; Cerrato, G.; Longhi, M.; Minguzzi, A.; Cappelletti, G.; Vertova, A. ORR in Non-Aqueous Solvent for Li-Air Batteries: The Influence of Doped MnO2-Nanoelectrocatalyst. Nanomaterials 2020, 10, 1735. [Google Scholar] [CrossRef]
- Assumpção, M.H.M.T.; De Souza, R.F.B.; Rascio, D.C.; Silva, J.C.M.; Calegaro, M.L.; Gaubeur, I.; Paixão, T.R.L.C.; Hammer, P.; Lanza, M.R.V.; Santos, M.C. A comparative study of the electrogeneration of hydrogen peroxide using Vulcan and Printex carbon supports. Carbon N. Y. 2011, 49, 2842–2851. [Google Scholar] [CrossRef]
- Hyrve, S.M.; Regli, S.K.; Zubair, M.; Enger, B.C.; Lødeng, R.; Waller, D.; Rønning, M. Catalytic Oxidation of NO over LaCo1−xBxO3 (B = Mn, Ni) Perovskites for Nitric Acid Production. Catalysts 2019, 9, 429. [Google Scholar]
- Román-Martínez, M.C.; Cazorla-Amorós, D.; Linares-Solano, A.; de Lecea, C.S.M. TPD and TPR characterization of carbonaceous supports and Pt/C catalysts. Carbon 1993, 31, 895–902. [Google Scholar]

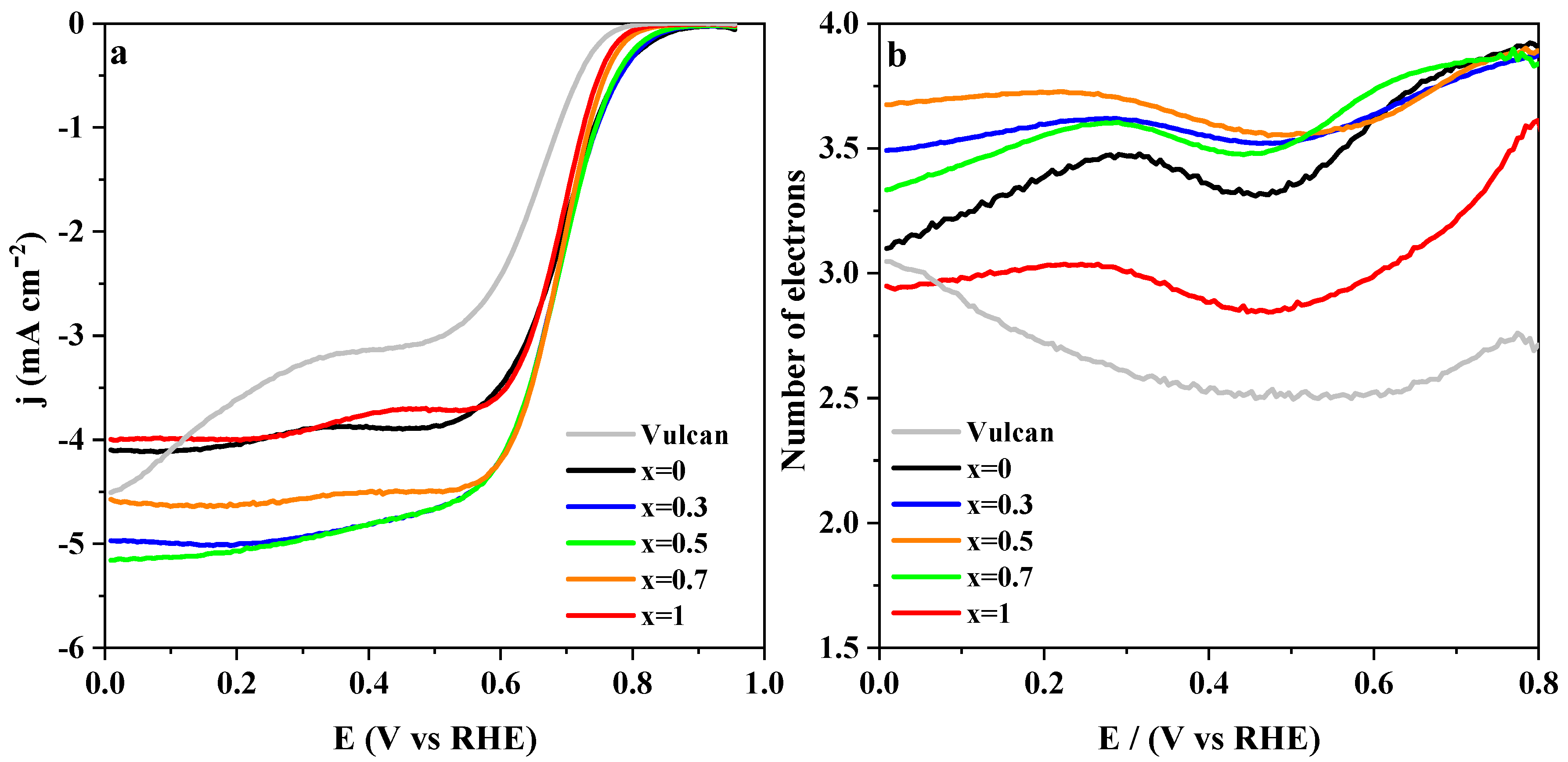
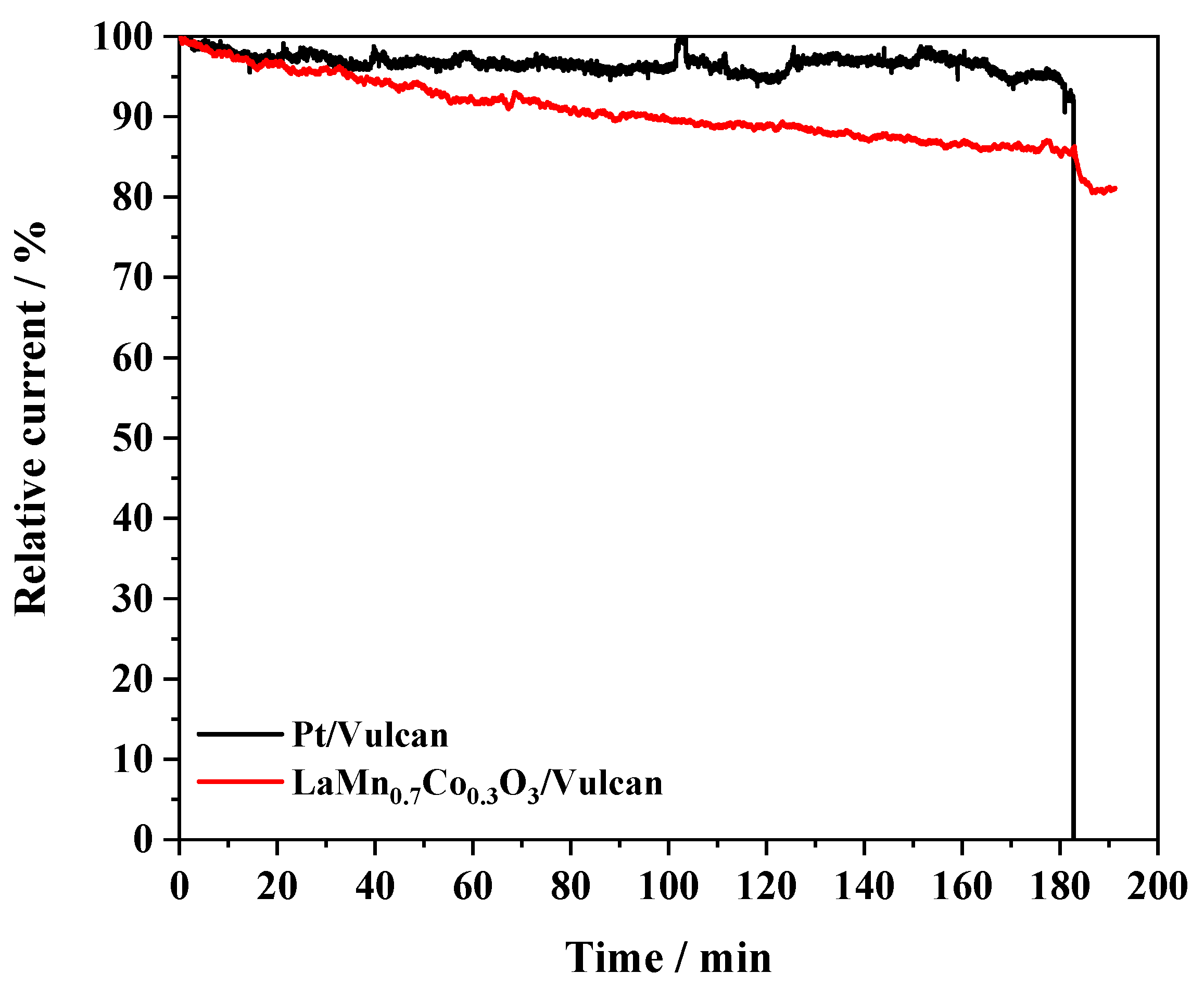
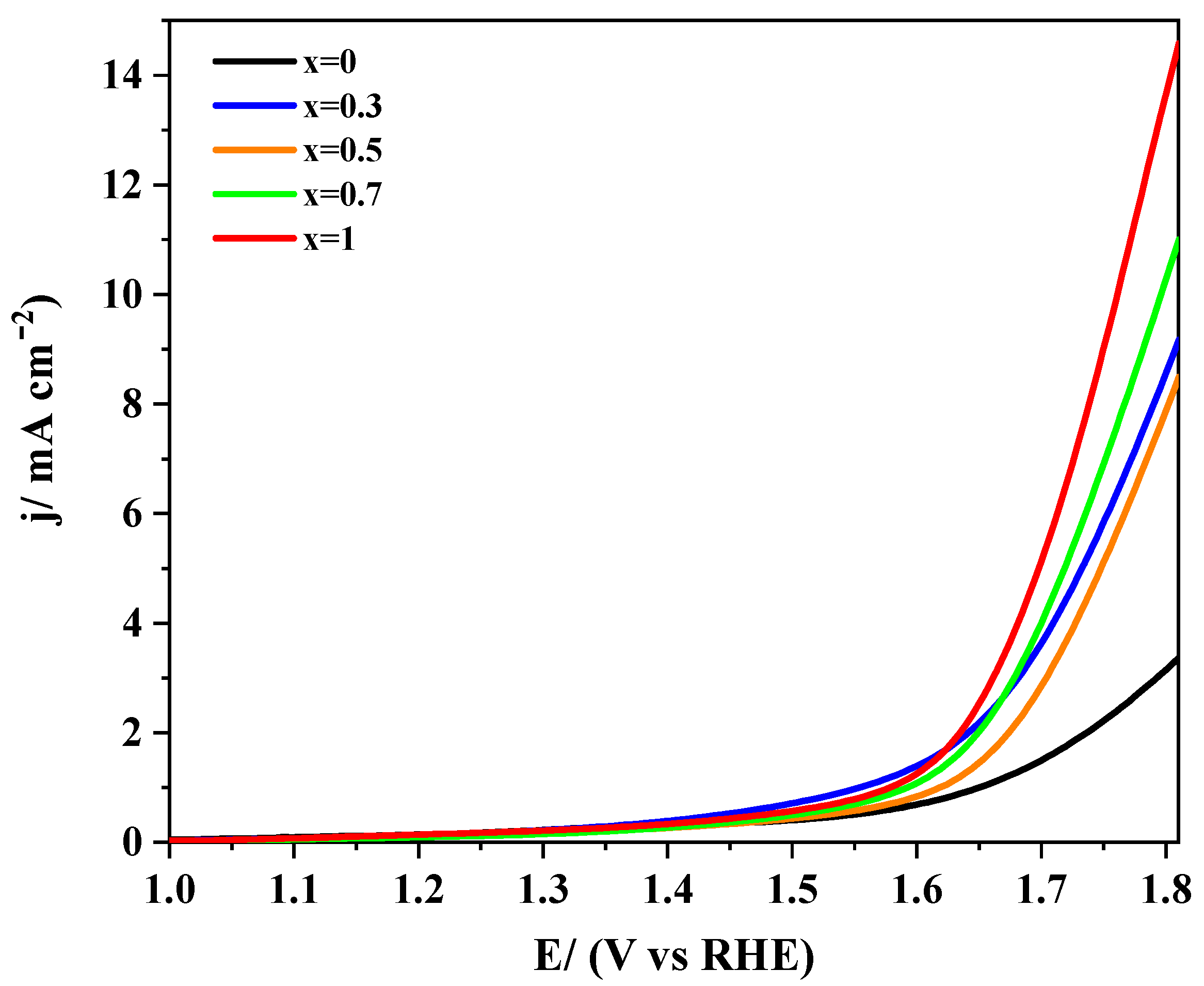

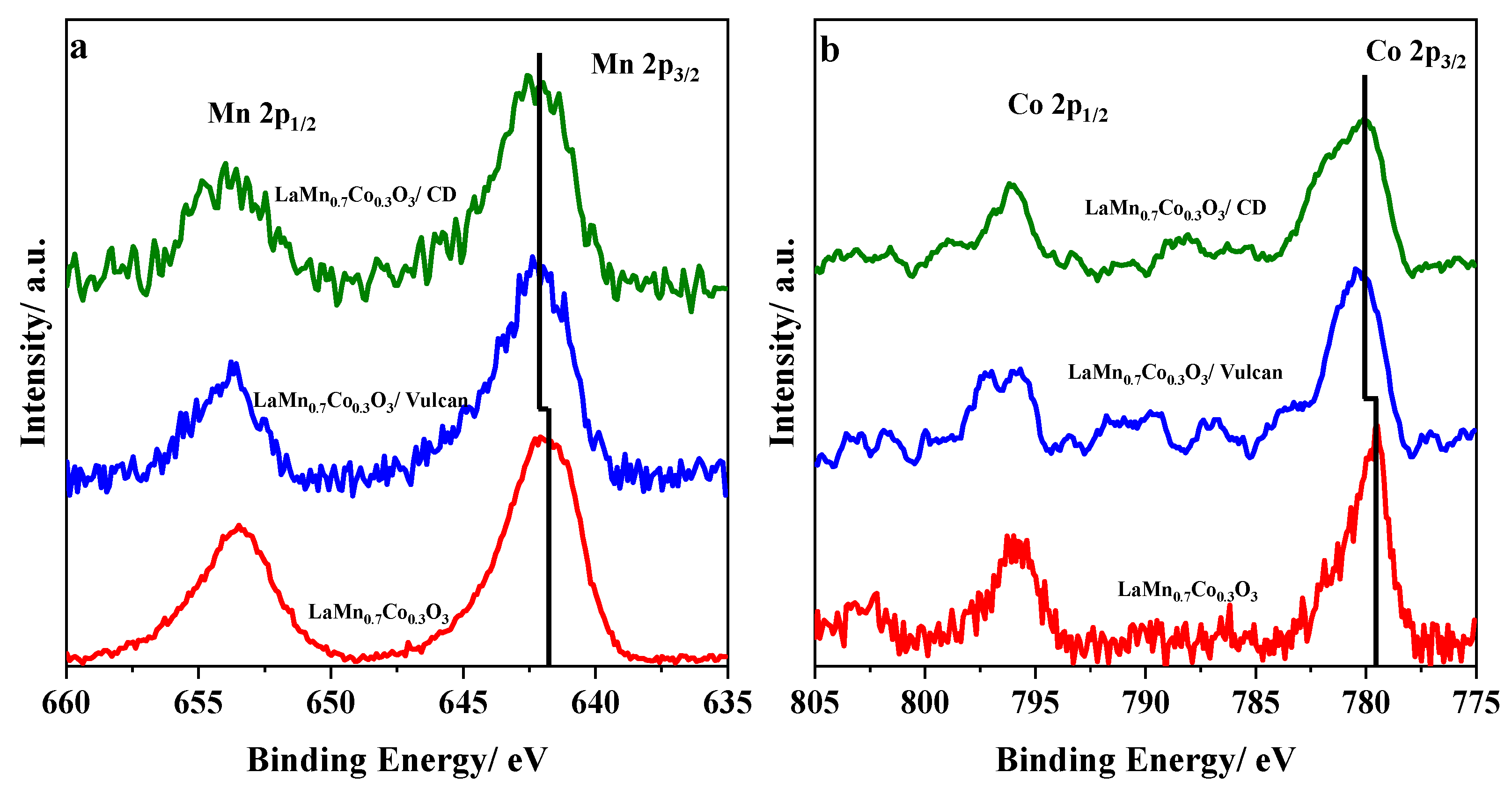

| Sample | (at 0.7 V vs. RHE) | j/mA cm−2 (at 0.4 V) | j/A g−1 (at 0.4 V) | BET/m2 g−1 | Tafel Slope/mV dec−1 | |
|---|---|---|---|---|---|---|
| LaMnO3 | 0.79 | 3.45 | −2.2 | −5.44 | 14 | 178 |
| LaMn0.9Co0.1O3 | 0.81 | 3.73 | −2.3 | −5.69 | 19 | 214 |
| LaMn0.8Co0.2O3 | 0.78 | 3.73 | −2.1 | −5.19 | 18 | 213 |
| LaMn0.7Co0.3O3 | 0.78 | 3.58 | −2.6 | −6.43 | 14 | 151 |
| LaMn0.6Co0.4O3 | 0.73 | 3.48 | −2.5 | −6.18 | 13 | 114 |
| LaMn0.5Co0.5O3 | 0.70 | 3.18 | −2.1 | −5.19 | 11 | 90 |
| LaMn0.4Co0.6O3 | 0.71 | 3.16 | −2.3 | −5.69 | 12 | 87 |
| LaMn0.3Co0.7O3 | 0.71 | 3.33 | −2.4 | −5.93 | 14 | 103 |
| LaMn0.2Co0.8O3 | 0.70 | 3.10 | −2.0 | −4.94 | 14 | 87 |
| LaMn0.1Co0.9O3 | 0.70 | 3.10 | −2.2 | −5.44 | 12 | 88 |
| LaCoO3 | 0.69 | 3.06 | −1.9 | −4.70 | 13 | 86 |
| Sample | Potential/V (at 1 mA cm−2) | Potential/V (at 5 mA cm−2) | Tafel Slope/mV dec−1 |
|---|---|---|---|
| LaMnO3 | - | - | 486 |
| LaMn0.9Co0.1O3 | - | - | 200 |
| LaMn0.8Co0.2O3 | 1.77 | - | 111 |
| LaMn0.7Co0.3O3 | 1.78 | - | 139 |
| LaMn0.6Co0.4O3 | - | - | 156 |
| LaMn0.5Co0.5O3 | 1.71 | - | 87 |
| LaMn0.4Co0.6O3 | 1.69 | - | 93 |
| LaMn0.3Co0.7O3 | 1.67 | - | 97 |
| LaMn0.2Co0.8O3 | 1.65 | 1.77 | 100 |
| LaMn0.1Co0.9O3 | 1.66 | 1.78 | 100 |
| LaCoO3 | 1.60 | 1.73 | 94 |
| LaMnO3/Vulcan | 1.65 | - | 365 |
| LaMn0.7Co0.3O3/Vulcan | 1.56 | 1.73 | 332 |
| LaMn0.5Co0.5O3/Vulcan | 1.62 | 1.75 | 312 |
| LaMn0.3Co0.7O3/Vulcan | 1.59 | 1.72 | 266 |
| LaCoO3/Vulcan | 1.58 | 1.70 | 240 |
| Sample | Tafel Slope/mV dec−1 | ||||
|---|---|---|---|---|---|
| LaMnO3/Vulcan | 0.84 | 3.83 | −3.87 | −7.97 | 101 |
| LaMn0.7Co0.3O3/Vulcan | 0.84 | 3.78 | −4.82 | −9.93 | 81 |
| LaMn0.5Co0.5O3/Vulcan | 0.83 | 3.80 | −4.82 | −9.93 | 80 |
| LaMn0.3Co0.7O3/Vulcan | 0.80 | 3.84 | −4.50 | −9.27 | 60 |
| LaCoO3/Vulcan | 0.79 | 3.23 | −3.76 | −7.75 | 57 |
| Vulcan support | 0.77 | 2.63 | −3.14 | −6.47 | 62 |
| Sample | Tafel Slope/mV dec−1 | ||||
|---|---|---|---|---|---|
| Vulcan | 0.77 | 2.63 | −3.14 | −6.47 | 62 |
| CD | 0.79 | 2.70 | −2.87 | −5.91 | 63 |
| LaMn0.7Co0.3O3/Vulcan | 0.84 | 3.78 | −4.82 | −9.93 | 81 |
| LaMn0.7Co0.3O3/CD | 0.85 | 3.63 | −4.74 | −9.76 | 79 |
| Pt/Vulcan | 0.98 | 3.99 | −5.51 | −11.35 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallon, E. Carbon Material and Cobalt-Substitution Effects in the Electrochemical Behavior of LaMnO3 for ORR and OER. Nanomaterials 2020, 10, 2394. https://doi.org/10.3390/nano10122394
Flores-Lasluisa JX, Huerta F, Cazorla-Amorós D, Morallon E. Carbon Material and Cobalt-Substitution Effects in the Electrochemical Behavior of LaMnO3 for ORR and OER. Nanomaterials. 2020; 10(12):2394. https://doi.org/10.3390/nano10122394
Chicago/Turabian StyleFlores-Lasluisa, Jhony X., Francisco Huerta, Diego Cazorla-Amorós, and Emilia Morallon. 2020. "Carbon Material and Cobalt-Substitution Effects in the Electrochemical Behavior of LaMnO3 for ORR and OER" Nanomaterials 10, no. 12: 2394. https://doi.org/10.3390/nano10122394
APA StyleFlores-Lasluisa, J. X., Huerta, F., Cazorla-Amorós, D., & Morallon, E. (2020). Carbon Material and Cobalt-Substitution Effects in the Electrochemical Behavior of LaMnO3 for ORR and OER. Nanomaterials, 10(12), 2394. https://doi.org/10.3390/nano10122394








