1. Introduction
Increasing demand for electronic appliances in daily life results in a quest for new energy materials for secondary batteries. In this era, the Lithium-ion battery (LIB) satisfies energy storage system requirements with low energy density [
1]. Various materials, including carbonaceous, alloying, and transition metal oxides (TMOs), are studied as an anode of LIBs to improve energy and power densities [
2,
3]. Carbonaceous materials including graphite, carbon nanotubes, single-walled carbon nanotube (SWCNTs), and graphene oxides exhibiting intercalation chemistry are used as anodes of LIBs [
4]. These materials are cost-effective and eco-friendly, but they deliver low energy density for LIBs. Graphite is commercially used as an anode material for LIBs and delivers almost 370 mAh g
−1 of specific capacity. Conversely, alloying materials such as Si, Sn, Zn, etc., are abundant, eco-friendly, cost-effective, and provide high specific capacities. However, structural degradation because of extra volume expansion restricts their applicability. TMOs, such as Ni
IIO, Fe
III2O
3, Co
II/III3O
4, and Mn
II/III3O
4, are currently studied as replacements to carbonaceous materials with low energy density [
5,
6,
7]. TMOs are low cost, environmentally friendly, and can deliver high energy density with optimal working potential. However, they still they have low electronic conductivity and Li
+ ion diffusivity [
6,
8].
M
3V
2O
8 (M = Ni
II, Co
II/III, Mn
II/III, Zn
II and V
V/IV) are new electroactive vanadate materials containing various oxidation states applied in energy storage systems like super capacitors, fuel cells, batteries, etc. These mixed TMOs have two distinct metal oxides, which can activate better redox reactions with superior specific capacities [
9]. It is very hard to synthesize these kinds of mixed metal oxides because of their multiple oxidation states, anion role (O
2−), and co-ordination chemistry. Also, it is challenging to control the related synthesis conditions of temperature, pressure, and time. There are very few reports on the synthesis of these materials and their applications [
10,
11,
12,
13,
14]. Electronic conductivity and ionic transportation are the two main parameters that address the electrochemical performance of battery materials. These parameters can be enhanced through controlling the particle size, shape, phase, and porosity. To achieve these peculiar properties is a very challenging task and can be attained through different synthesis techniques [
15,
16]. These specific properties are obtained through various synthesis routes, such as co-precipitation, spray pyrolysis technique, and hydrothermal methods [
10,
17,
18]. The hydrothermal process is already quite notorious for facile growth of different nanomaterials [
19,
20]. Hollow microspheres, rods, or tubular shape particles can modify surface properties and speculate porous nature to promote electrical and ionic conductivities. Ni
3V
2O
8, comprised of Nickel oxide and a complex Vanadate structure, provides high specific capacities and is an appropriate choice of anode material for LIBs. However, fast structural degradation and sluggish diffusion kinetics hinder its practical application in energy storage systems [
21,
22]. Thus, maintaining structural integrity during the cycling process for high-capacity retention and columbic efficiency is a big challenge. As we know, coating carbonaceous materials or constructing composites can facilitate electronic conductivity and withstand volume changes during the charge/discharge of LIBs [
23,
24,
25,
26]. Reduced graphene oxide has a highly disordered surface, high specific surface area and better electronic conductivity, making it a suitable choice for constructing composites with Ni
3V
2O
8 [
24].
The objective of this work is to report a simple, facile one-step hydrothermal synthesis of the reduced graphene oxide interconnected hollow microspheres composite with Ni3V2O8. We synthesized a rGO@Ni3V2O8 hollow microspheres composite without the addition of any surfactant. This facile approach can enhance the specific surface area and porosity of the rGO@Ni3V2O8 microspheres composite, which effectively supports ionic transportation. The electrochemical performance of rGO@Ni3V2O8 was analyzed as anode material for LIBs. Discussions about electrochemical performance and rate capabilities are underlying.
2. Materials and Methods
All the materials were in analytical grades and used as received. NiII (CH3COO)2·4H2O was purchased from Kanto Chemical Industries, Tokyo, Japan. NH4VVO3 was purchased from Sigma-Aldrich (Saint Louis, MO, USA). Ethylene Glycol was purchased from Sigma-Aldrich, Daejung Chemical Industries, Busan, South Korea. The stoichiometric amounts of NH4VVO3 and NiII (CH3COO)2·4H2O were mixed with 10 mL of H2O each, separately, before being mixed together. After 1 h of mixing, 2 mL of ethylene glycol was added to the solution and stirred at room temperature for a further 4 h. The reduced graphene oxide was mixed into the precursor solution with rGO to a total cations ratio of 0.5:1 by weight, which was then stirred and sonicated for an hour. The solution was heated at 180 °C for 24 h in a 50 mL Teflon lined stainless steel autoclave (KISTEC, KIST, Seoul, Korea). Precipitates were collected, centrifuged, and washed several times with water and ethanol, respectively. They were then vacuum dried at 80 °C for 8 h to get the final rGO@Ni3V2O8 interconnected hollow microspheres composite. For comparison, the Ni3V2O8 interconnected hollow microspheres were also prepared through the same procedure without the addition of reduced graphene oxide.
The crystal phase, morphology, and topographical characteristics were measured using powder X-ray diffraction analysis (XRD; Rigaku D/MAX–2500 V/PC, Tokyo, Japan), scanning electron microscopy (FE–SEM; S–4200, Hitachi, High Technologies, Tokyo, Japan), and energy-filtered transmission electron microscopy (EF–TEM, Titan FEI, Corp., Hillsboro, OR, USA). N2 adsorption/desorption isotherm was studied via the BJH N2 adsorption instrument (Belsorp II mini BEL, Tokyo, Japan Inc.). The percentage weight loss of the rGO@Ni3V2O8 interconnected hollow microspheres composite was investigated through Thermogravimetric (TGA) analysis (TGA, Q-50, TA instruments Inc., New Castle, PA, USA) at 10 °C min−1 under an N2 atmosphere. Raman Spectroscopy (Renishaw, Seoul, Korea, Model at 532 nm) analysis was performed to study the molecular structure of the rGO@Ni3V2O8 interconnected hollow microspheres composite. The working electrode was prepared by mixing 70% rGO@Ni3V2O8, 20% Super P carbon additive, and 10% of 5% wt. poly (vinyl difluoride) binder in N–methyl pyrrolidone (NMP) solvent. The mass loading amount used around 0.8–1 mg cm−3 with 24 µm electrode thickness. The coin size (CR2032) electrodes were assembled in 1 M LiPF6 in an EC/DMC (1:1, v/v) electrolyte using polypropylene separators in an Ar filled glove box (Glove Box System (KK–011–AS), Korea KIYON, Seoul, Korea) with Li as a counter electrode. Electrochemical performance was studied via a galvanostatic/potentiostat system (Multi–Cycling Battery & Capacitor Test System, Series 4000, Thermo–Tech Co., Ltd., Seoul, Korea) in a voltage range of 3.0 V~0.01 V at a current density of 200 mA g−1. Cyclic Voltammometery (CV) and Electrochemical impedance spectroscopy (EIS) analysis were performed using a Biologic potentiostat/galvanostat Model VMP3 (BioLab, Inc. Pariset, France) at a scan rate of 0.1 mV s−1 and within the frequency range of 10 mHz−100 KHz.
3. Results and Discussion
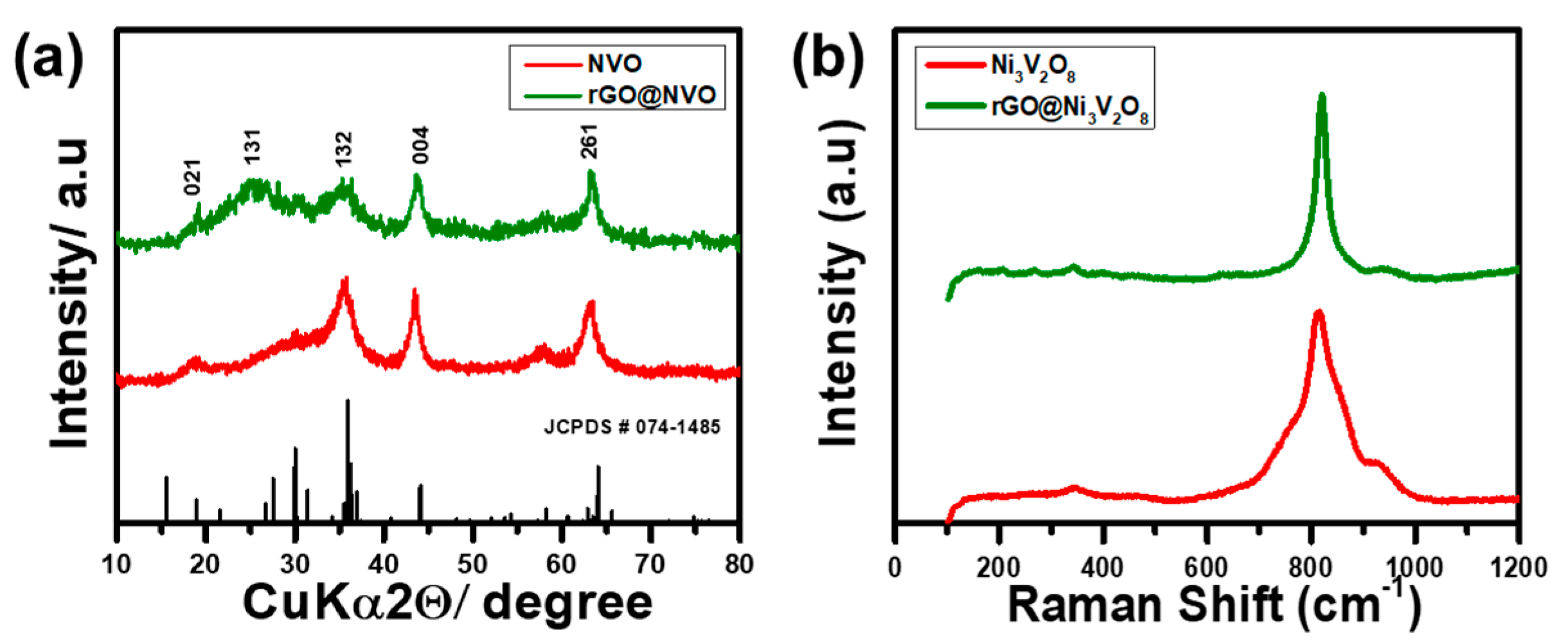
Figure 1 shows the synthesis schematic of the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite. Initially, metal ions Ni
II and V
VO
3− were produced when hydrolyzed for a certain time period. These ions, acting as electron donor and acceptor, chelated into one another to form a compact ligand. The insertion of ethylene glycol reduced the compact ligands and started nucleating nickel vanadate with a spherical shape. When reduced graphene oxide was added into the solution mixture, these microspheres entangled with one another on account of hydrothermal treatment for a specific time period. This entanglement generated interconnected hollow microspheres with a porous structure. The XRD patterns of the Ni
3V
2O
8 and rGO@Ni
3V
2O
8 composites are shown in
Figure 2a, which shows that all the diffraction peaks match well with the orthorhombic crystal phase of JCPDS card # 074-1485. The broad peak around 25.34° is because of the incorporation of rGO into Ni
3V
2O
8, which is confirmed in
Figure S1. Rietveld refinement XRD analysis was performed using FullProf2000 software separately to the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 interconnected hollow microspheres composites, and refined XRD results are shown in the
Figure S4. Rietveld refinement analysis shows that the lattice parameters vary slightly from the standard unit cell data of Ni
3V
2O
8. The increased unit cell volume for the rGO@Ni
3V
2O
8 interconnected hollow microspheres is due to the presence of reduced graphene oxide. The crystallite sizes (calculated using the Scherrer equation), lattice parameters, and crystal volumes of the Ni
3V
2O
8 and rGO@Ni
3V
2O
8 composites are shown in
Table 1.
Raman spectroscopy is a highly sensitive technique for studying amorphous phases and molecular infringements.
Figure 2b shows the Raman spectrum of the Ni
3V
2O
8 and rGO@Ni
3V
2O
8 composite with two obvious peaks around 380 cm
−1 and 800 cm
−1. These peaks correspond to the bending vibration mode of V-O-V and symmetric stretching mode of V-O for Ni
3V
2O
8. The existence of these two peaks confirms the formation of Ni
3V
2O
8 microspheres and a rGO@Ni
3V
2O
8 composite. Furthermore, the Raman spectrum of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite are shown in
Figure S2, which indicates two clear peaks consistent with the D band and G band at 1420.89 cm
−1, 1618.87 cm
−1 and 1349.52 cm
−1, 1582.76 cm
−1, respectively. The D band describes the defects induced in the graphene structure, whereas the G band explains the graphitic nature of reduced graphene oxide [
27]. Ethylene glycol not only chelated the metal ions (Ni
II and V
VO
3−) but also reduced the carbon source during hydrothermal treatment and corresponded to the appearance of D and G bands in the Ni
3V
2O
8 microspheres [
28,
29]. The high intensity ratio (I
D/I
G = 1.07) of the rGO@Ni
3V
2O
8 composite compared with I
D/I
G = 0.66 of the Ni
3V
2O
8 microspheres confirmed that there were large amounts of defects and grain boundaries on the surface. The high degree of disorder in the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite promoted Li
+ ion diffusion and acted as a buffer to volume changes that occurred during the charge/discharge process. The N
2 adsorption/desorption isotherms of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite were measured at 77 K and shown in
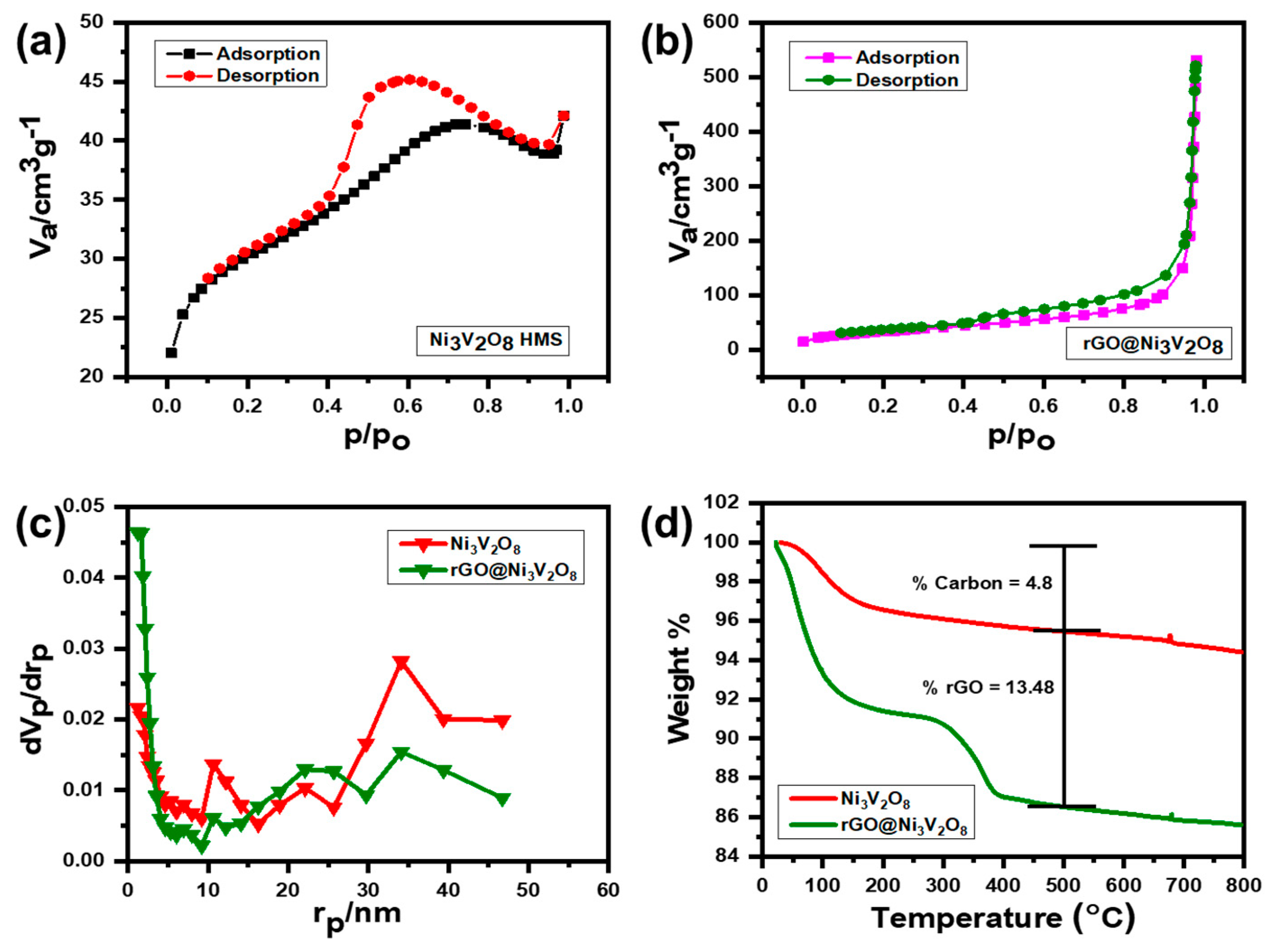
Figure 3. The N
2 adsorption/desorption isotherm of the Ni
3V
2O
8 microspheres shown in the
Figure 3a indicates that type IV isotherm with monolayer-multilayer adsorption proceeds at low pressure following the capillary condensation process at high pressure. This isotherm explains that the capillary condensation process occurs in mesopores with a limiting uptake up to high P/P0 pressure. The N
2 adsorption/desorption isotherm of the rGO@Ni
3V
2O
8 composite, shown in
Figure 3b, conversely depicts the type III isotherm. This means that weak van der Waal interactions start to accumulate the adsorbate at low pressure, which continues to adsorb the lateral layers because of their strong interactions, leading to an unrestricted multilayer formation process in the filling of mesopores. The pore size distribution curves of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite are shown in
Figure 3c. The pore size of electrode materials is an important parameter to control the diffusion kinetics of the Li+ ion within the electrode material. Increased pore size decreases the diffusion transportation length and reduces ionic diffusion resistance, which results in enhanced Li
+ ion diffusivity. High mass transports conductivity results in a high specific capacity and cycle stability. Therefore, pore size is an effective parameter to control the rate performance of battery materials.
The specific surface area, pore sizes, and pore volumes of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite are shown in
Table 2. Thermogravimetric (TGA) analysis of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite was performed under air atmosphere to measure the carbon content of rGO@Ni
3V
2O
8 interconnected hollow microspheres composite, and the results are shown in
Figure 3d. The TGA curves indicated that the moisture content evaporated when the temperature was increased up to 200 °C and the burning of carbon occurred between 400–500 °C. The percentage weight loss of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite was estimated at 4.80% and 13.48%, respectively. The presence of a small amount of carbon in the Ni
3V
2O
8 microspheres was due to the addition of ethylene glycol, which reduced the precursors to nickel vanadate with a spherical shape. The addition of reduced graphene oxide to the solution resulted in increased amounts of carbon existence in the rGO@Ni
3V
2O
8 composite, as is evident in
Figure 3d. Therefore, it was inferred that the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite was successfully synthesized by the facile one-step hydrothermal technique, which has a high specific surface area and a porous nature to enhance electrical and ionic conductivities.
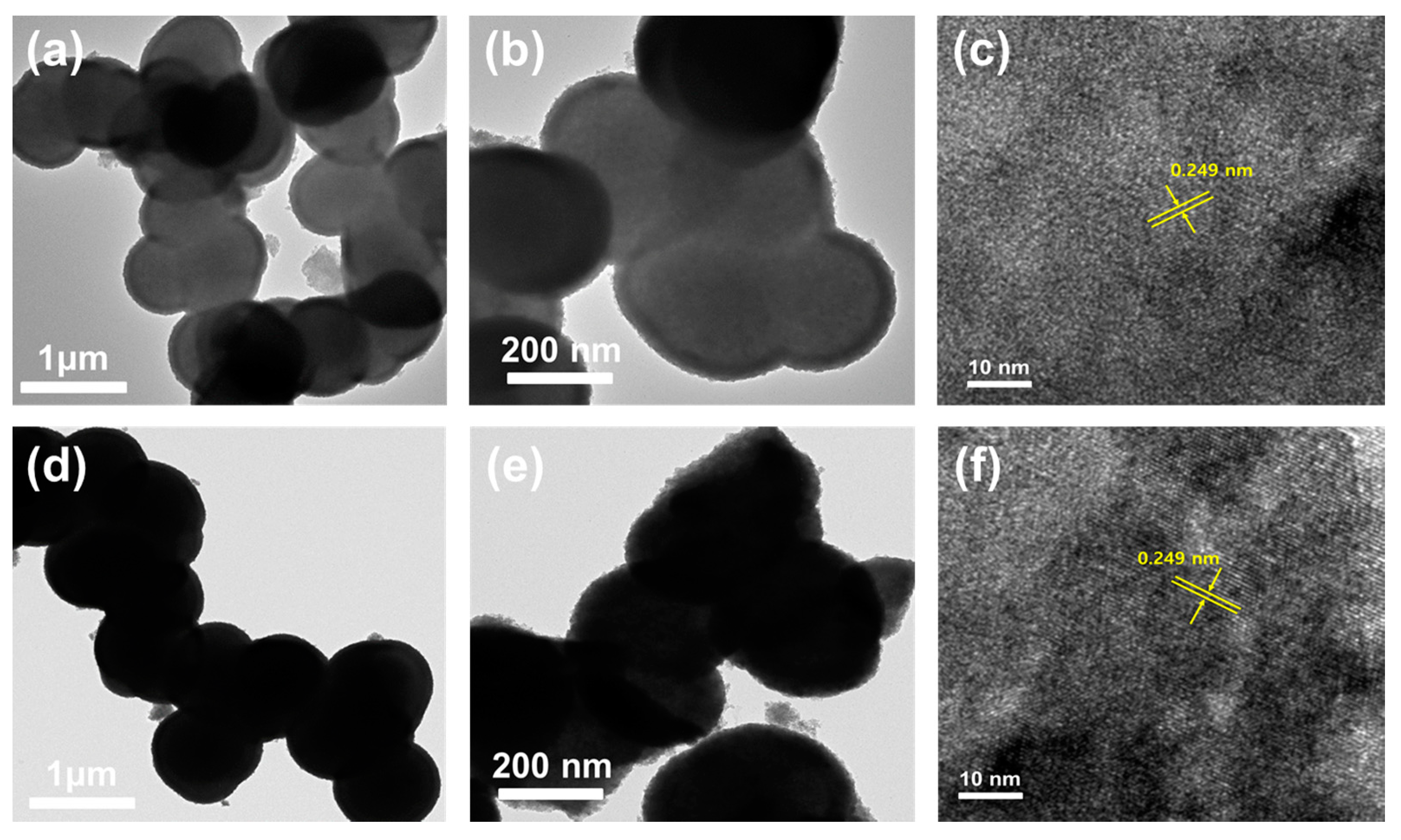
Figure 4 depicts the morphological characteristics of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite. Scanning electron microscopy (FESEM) images of the Ni
3V
2O
8 microspheres, taken at various magnifications, are displayed in
Figure 4a–c. The microspheres’ morphology shows that they were prominently interconnected with one another. The densification of the microspheres’ morphology is because of their amorphous nature. The microspheres’ sizes are estimated through ImageJ software and found to be 650 nm. Similarly, the SEM images of the rGO@Ni
3V
2O
8 microspheres composite are shown in
Figure 4d–f. The FESEM images depict the reduced graphene oxide sheets covered with microspheres. The presence of rGO and their amorphous nature result in their densification. The microspheres’ size was calculated as 675 nm. The energy dispersive spectroscopy (EDS) elemental mapping performed to investigate the existence of carbon in the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite and its corresponding results are shown in
Figures S5 and S6, respectively. The
Figure S5 indicated a small amount of carbon presence in homogenously mixed Ni, V, and O elements. However, the EDS elemental mapping of the rGO@Ni
3V
2O
8 composite showed that reduced graphene oxide was homogenously coated over the Ni
3V
2O
8 microspheres. The amount of reduced graphene oxide was high when compared with the Ni
3V
2O
8 microspheres. Besides, the topological features of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite were investigated through transmission electron microscopy (TEM) analyses and are shown in
Figure 5. TEM images of Ni
3V
2O
8 microspheres are shown in
Figure 5a,b and confirm the formation of clusters of interconnected hollow microspheres.
The particle size calculated via TEM analyses was in accordance with the one calculated using FESEM analyses. The TEM images of the rGO@Ni
3V
2O
8 microspheres composite are shown in
Figure 5d,e and show rGO covering over the Ni
3V
2O
8 interconnected hollow microspheres, confirming the rGO@Ni
3V
2O
8 microspheres composite formation. The high resolution transmission electron microscopy (HRTEM) image of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composites are shown in
Figure 5c,f. The images explain the lattice fringe patterns with d spacing of 0.249 nm. The observed d spacing at the crystal plane of (122) agreed well with the JCPDS card # 074-1485 for the XRD pattern of
Figure 2a. Hollow microspheres predominantly promoted Li
+ ion diffusion and enhanced the penetration of electrolytes within the rGO@Ni
3V
2O
8 microspheres composite, acting as a buffer against volume changes during the cycling process. Moreover, reduced graphene oxide facilitated the electron transfer from the composite to the current collector and also acted as a buffer against volume changes during the charge/discharge process.
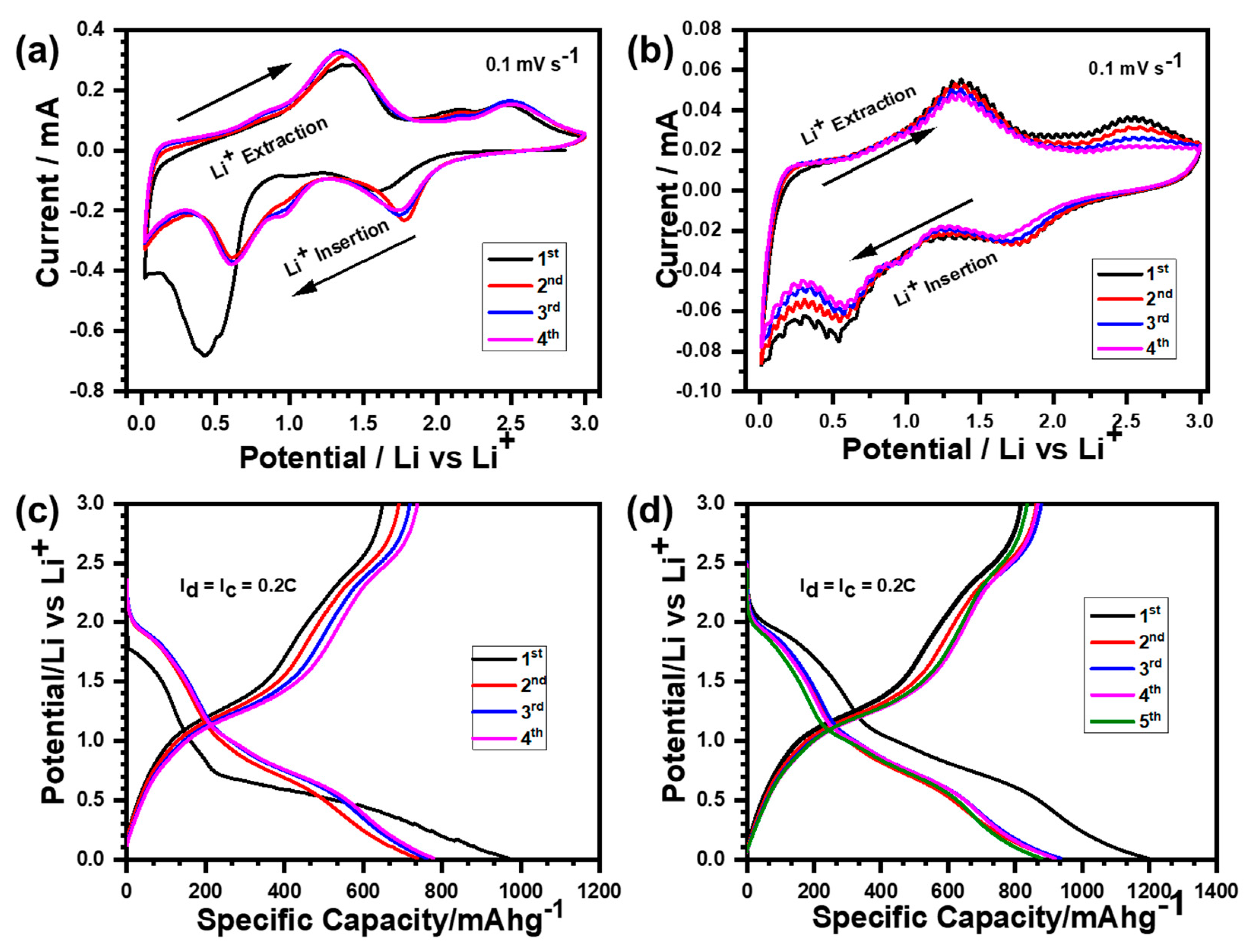
The cyclic voltammetry (CV) of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite, investigated at 0.1 mV s
−1 in a voltage range of 3.0 V~0.01 V for four cycles, is shown in
Figure 6a,b. In the first cathodic sweep, lithiation started at around 2.0 V until the Ni
3V
2O
8 microspheres were converted to NiO and Li
x+yV
2O
5 at 1.55 V, with a change in the oxidation state of V
V to V
IV. The solid electrolyte interface (SEI) layer started nucleating at around 0.8 V and shifted to 1.0 V in successive cycles, as shown in
Figure 6a. A very clear reduction peak, observed at 0.45 V, was associated with the conversion of NiO to pure metallic Ni and Li
2O, with some smaller reduction peaks linked to the lithiation of Li
x+yV
2O
5. The cathodic peak at 0.45 V shifted to 0.55 V, and the conversion of the Ni
3V
2O
8 microspheres to NiO and Li
x+yV
2O
5 at 1.55 V moved to 1.78 V in successive cycles, with a change in the oxidation state of V
V to V
IV. However, the broad peak observed at 1.3 V during the first anodic scan was for the pure metallic Ni oxidation to NiO, with a change in the V
II oxidation state to V
IV. The second oxidation peak was found at 2.5 V and linked with the oxidation of Li
x+yV
2O
5, with a change in the oxidation state of V
IV to V
V. The overlapping of subsequent cycles indicated that the Ni
3V
2O
8 microspheres had a similar electrochemical reaction and were suited to structural stability [
24]. Consequently, the rGO@Ni
3V
2O
8 composite reduced to NiO and Li
x+yV
2O
5 at 1.78 V during the first cathodic scan, with a change in the oxidation state of V
V to V
IV, as shown in
Figure 6b. The solid electrolyte interface (SEI) layer was formed at around 0.9 V. The first cathodic scan indicated that there was a clear and wide peak at around 0.54 V, consistent with the conversion of NiO to pure metallic Ni and Li
2O, which moved to 0.58 in successive cycles. While the broad oxidation peak observed at 1.3 V correspond to the oxidation of pure metallic Ni to NiO during the anodic scan, the peak observed at 2.5 V was associated with Li
x+yV
2O
5 oxidation in the V
V oxidation state. Successive CV scans overlapped, demonstrating that the rGO@Ni
3V
2O
8 composite followed a similar electrochemical reaction mechanism to the Ni
3V
2O
8 microspheres. The presence of Li
x+yV
2O
5 acted as a buffer for NiO to retain the structural integrity of Ni
3V
2O
8 during the cycling process. Moreover, reduced graphene oxide also buffered the volume changes during the charging process, hence facilitating the structural integrity of the rGO@Ni
3V
2O
8 composite. The discharge/charge voltage profiles of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite were investigated at 200 mA g
−1 within the voltage window of 3.0 V~0.01 V and presented in
Figure 6c,d. The first discharge curve shows that lithiation starts around 2.0 V with two distinct slopes at 1.5 V and 0.5 V, analogous to the reduction of the Ni
3V
2O
8 microspheres to NiO and Li
x+yV
2O
5 and then into pure metallic Ni and Li
2O, as shown in
Figure 6c. SEI layer formation was clearly observed at around 1.0 V. Similarly, two distinct charge slopes were observed at 1.3 V and 2.5 V, which were associated with the oxidation of pure metallic Ni into NiO and Li
x+yV
2O
5.
The discharge/charge profiles of the following cycles indicated the same voltage plateaus as observed in the first cycle. These discharge/charge plateaus agreed well with the reduction/oxidation peaks of CV analysis, confirming a similar electrochemical mechanism throughout the cycling process. Likewise, the discharge/charge profile of the rGO@Ni
3V
2O
8 composite was investigated at the current density of 200 mA g
−1 within the voltage window of 3.0 V~0.01 V, as shown in
Figure 6d. The first discharge curve indicated that lithiation was initiated at around 2.0 V, with two obvious slopes at 1.78 V and 0.55 V analogous to the conversion of the rGO@Ni
3V
2O
8 composite to NiO, Li
x+yV
2O
5 and then NiO into pure metallic Ni and Li
2O. The formation of a solid electrolyte SEI layer was confirmed at around 1.0 V. The charging profile exhibited two discrete plateau formations at 1.3 V and 2.5 V, which correspond to the oxidation of pure metallic Ni to NiO and Li
x+yV
2O
5. The subsequent cycles displayed similar voltage plateaus during the charge/discharge process, exhibiting the same electrochemical reaction chemistry. Furthermore, the voltage plateaus of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite shown in
Figure 6c,d matched with the reduction/oxidation peaks of the respective CV analysis of
Figure 6a,b. The discharge/charge plateaus matched with the reduction/oxidation peaks of the CV analysis, confirming the presence of reversible electrochemical reaction chemistry during the discharge/charge process.
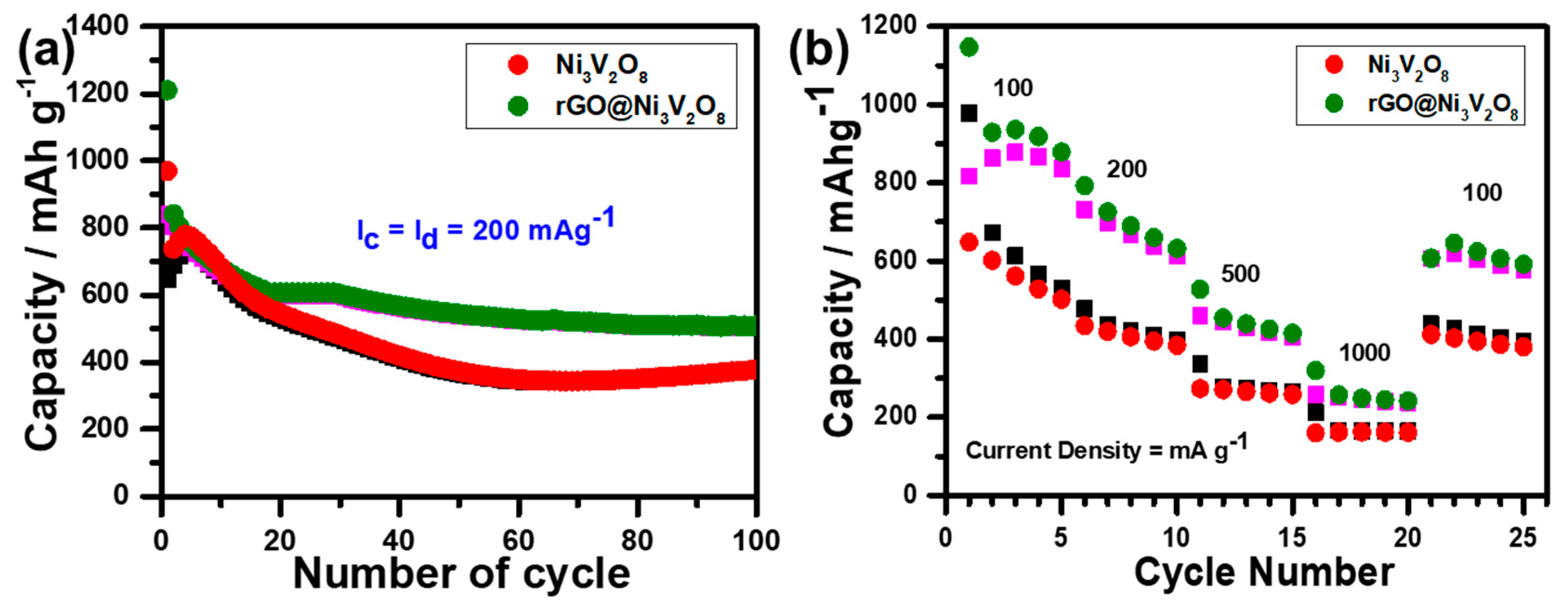
The cyclic performance of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite was investigated at the current density of 200 mA g
−1, and the respective results are shown in
Figure 7a. It is evident that the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite deliver the initial discharge/charge capacities of 969.52/648.86 mAh g
−1 and 1209.76/839.85 mAh g
−1 with a columbic efficiency of 66.92% and 69.42%, respectively, which approaches 99% after 100 cycles. The stability of the columbic efficiency of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite up to 100 cycles is shown in
Figure S3. The initial low columbic efficiency of the rGO@Ni
3V
2O
8 composite is due to the presence of amorphous graphene oxide [
29,
30,
31]. The capacity fading in the initial 15 cycles is associate with unstable SEI layer formation because of the complex lithiation chemistry of Li
x+yV
2O
5. Cycle stability was induced because of rGO. The incorporation of rGO facilitated the electronic conductivity to the rGO@Ni
3V
2O
8 composite and buffered the volume changes during the cycling process, thus retaining structural integrity. The rate capability of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite were measured at the current densities of 100, 200, 500, and 1000 mA g
−1 for 5 cycles each and are shown in
Figure 7b. The discharge capacities of the Ni
3V
2O
8 microspheres were investigated at 978.09 mAh g
−1, 478.10 mAh g
−1, 336.15 mAh g
−1, and 212.06 mAh g
−1, respectively. However, the discharge capacities of the rGO@Ni
3V
2O
8 composite were measured as 1147.24 mAh g
−1, 792.63 mAh g
−1, 527.49 mAh g
−1, and 319.33 mAh g
−1, respectively.
Figure 7b demonstrates the specific discharge/charge capacities of 527.49/505.25 mAh g
−1, even at a high current density of 500 mA g
−1, which is much higher than that of commercial graphite anode material (370 mAh g
−1). High rate performance and cycle life make the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite an appropriate choice of anode material for LIBs. The high rate and cyclic performance are connected with higher surface area and pore size, which enhance the contact area between electrode and electrolyte and allow more electrolytes to penetrate the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite, sufficiently improving its ionic conductivity. Furthermore, the inclusion of reduced graphene oxide in the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite imparts electronic conductivity and buffers the volume changes during the cycling process, which retains its structural integrity.
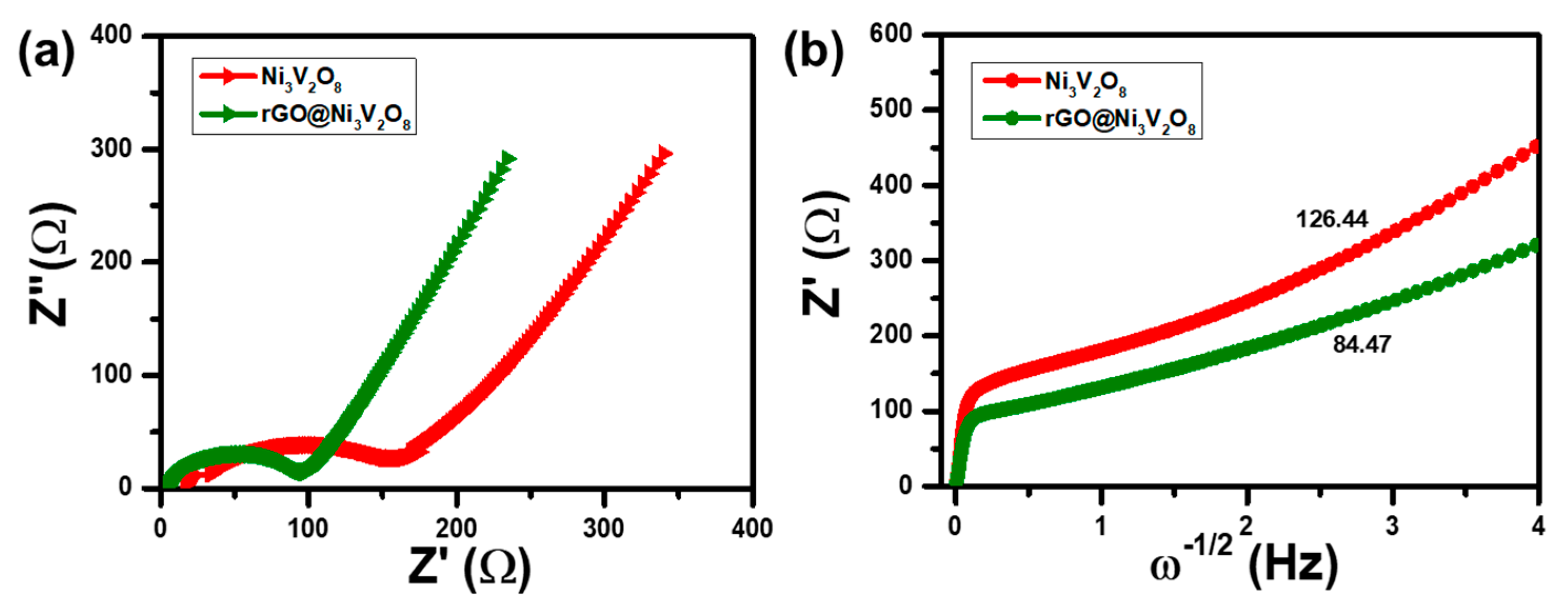
In addition, the electrochemical reaction kinetic was investigated through impedance spectroscopy analysis, and results are shown in
Figure 8. The Nyquist plot shown in
Figure 8a indicates the internal solution resistance (R
s) of the Ni
3V
2O
8 microspheres and rGO@Ni
3V
2O
8 composite as 17.35 Ω and 4.76 Ω after 100 cycles, respectively. The charge transfer resistance (R
ct) corresponds to the semi-circle in the medium frequency range of the Ni
3V
2O
8 microspheres and the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite and is measured as 175 Ω and 100 Ω, respectively. The charge transfer resistance demonstrated that electronic conductivity increased in the case of the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite because of the existence of reduced graphene oxide. Furthermore, the Warburg impedance was consistent with the sloping line in the low frequency region and confirmed Li
+ ion diffusion.
The letter σ is the slope of linear fit of Z
real vs. ω
−1/2, which is evident in
Figure 8b and is consistent with the Warburg impedance. This indicates that the rGO@Ni
3V
2O
8 interconnected hollow microspheres composite exhibits higher diffusivity due to rGO incorporation, which shortens the Li
+ diffusion length.