Flexible Transparent Electrode Characteristics of Graphene Oxide/Cysteamine/AgNP/AgNW Structure
Abstract
1. Introduction
2. Materials and Methods
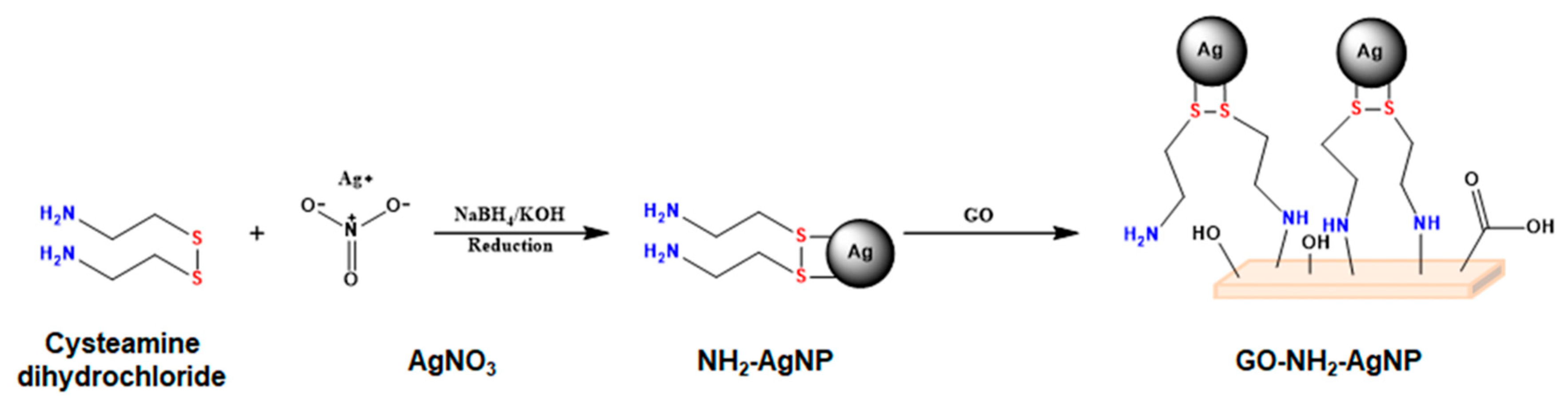
2.1. Material Preparation
2.2. Analytical Methods
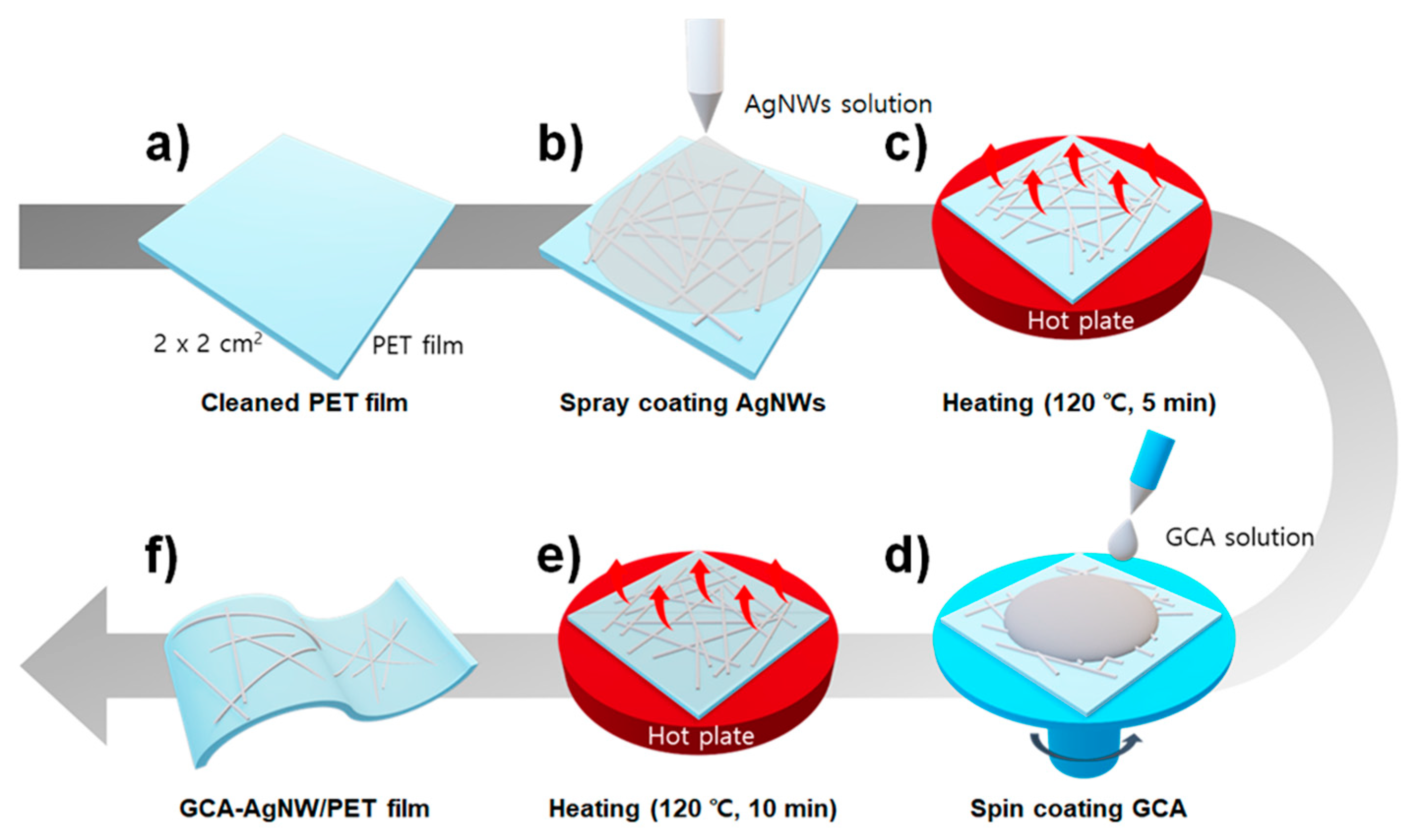
2.3. GCA-AgNW Electrode Fabrication Process
2.4. Pt/GO/PEDOT:PSS/GCA-AgNW/PET Memory Fabrication Process
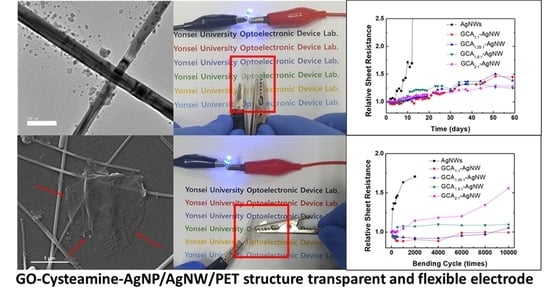
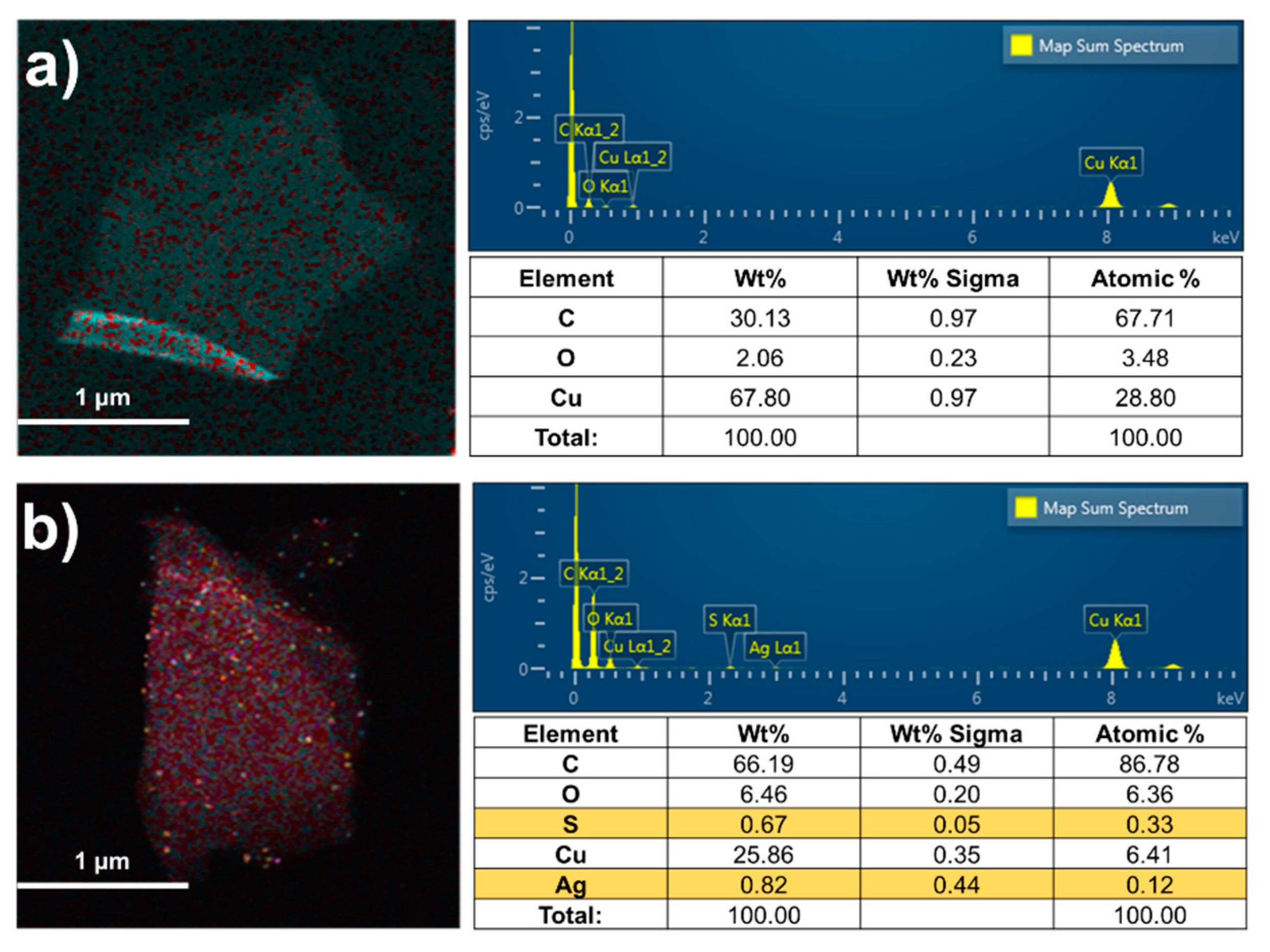
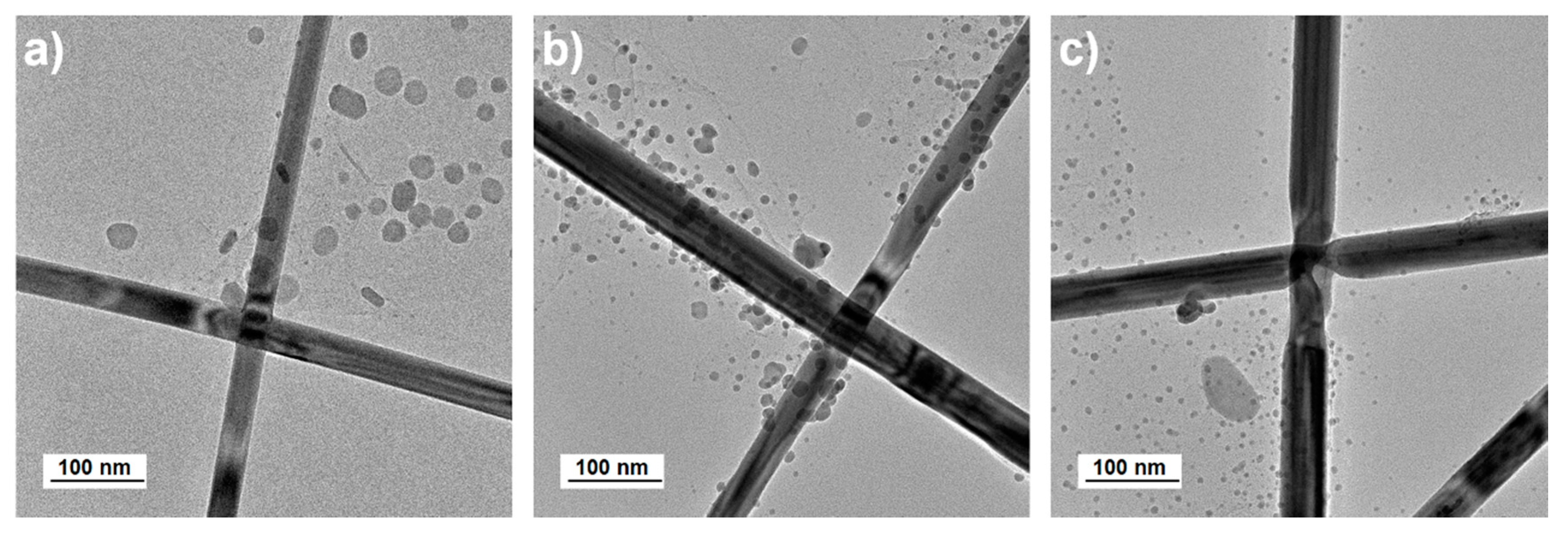
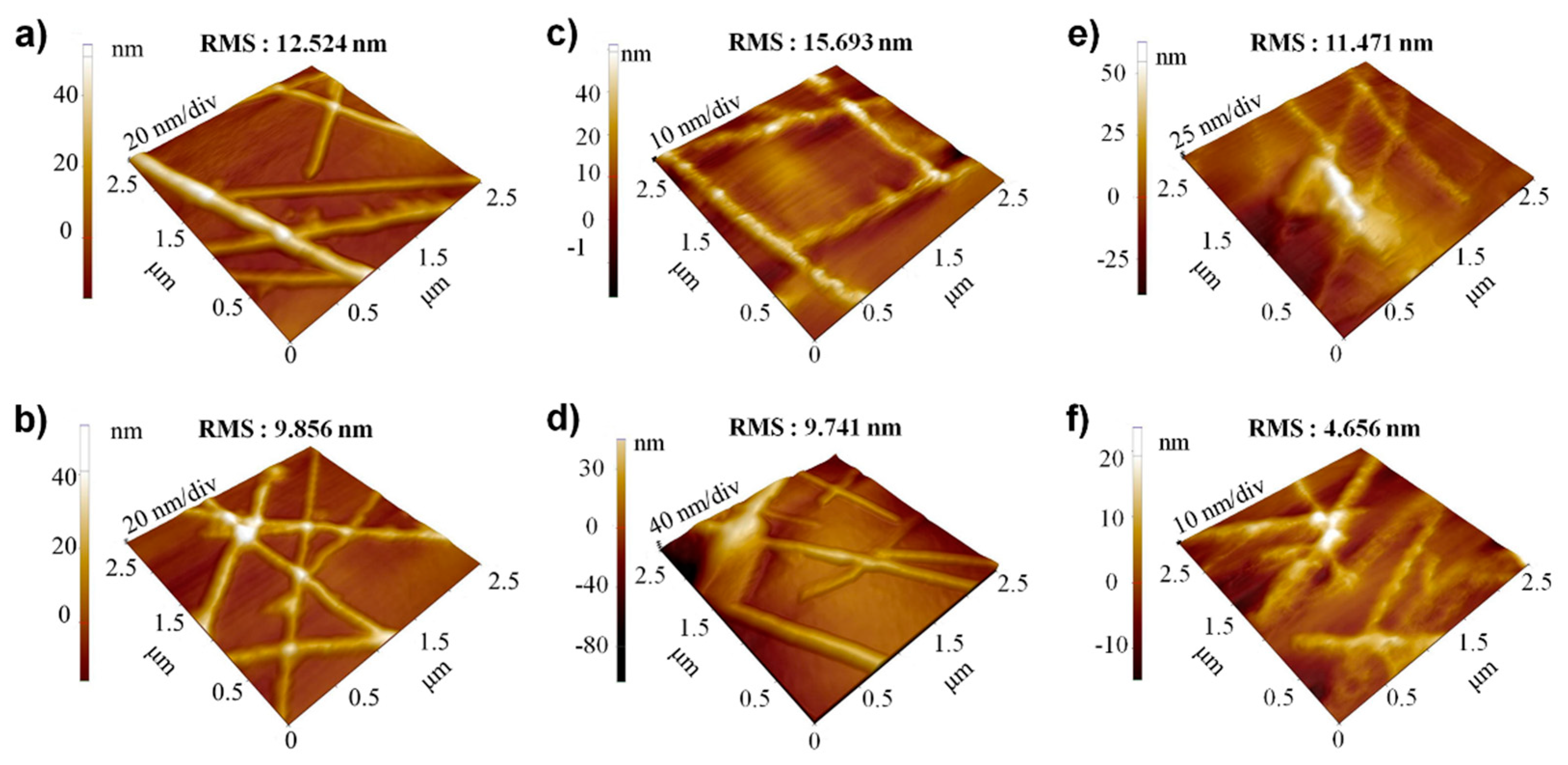
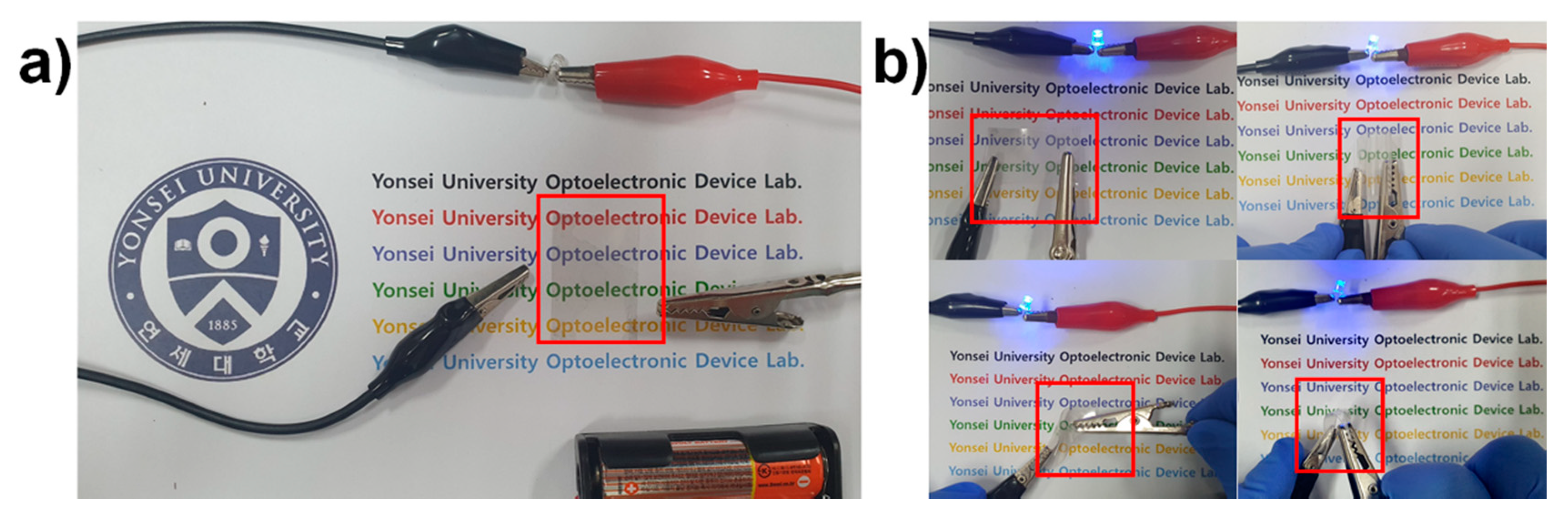
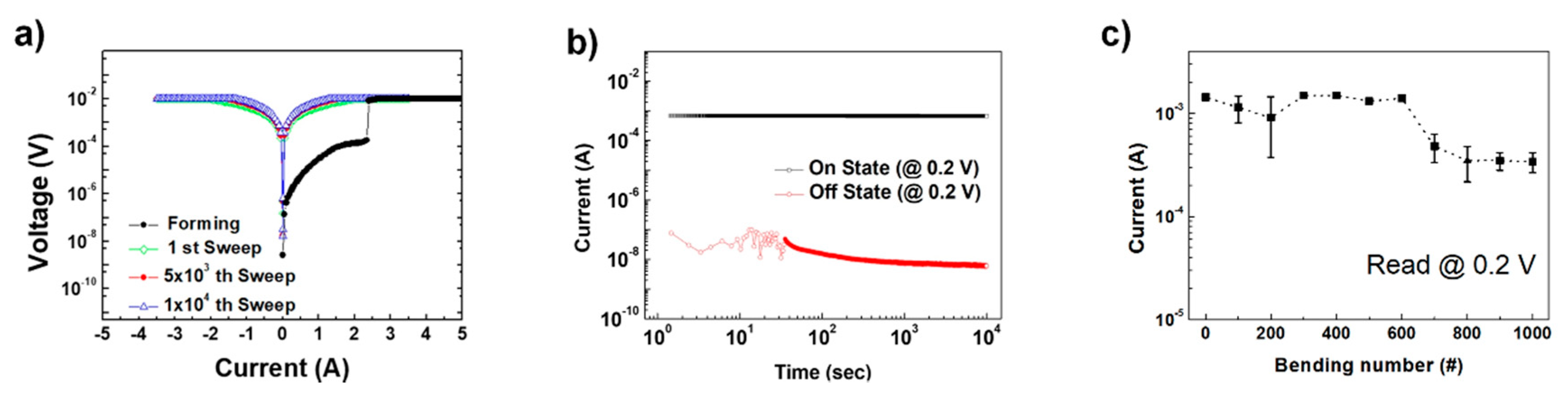
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Helander, M.G.; Wang, Z.B.; Qiu, J.; Greiner, M.T.; Puzzo, D.P.; Liu, Z.W.; Lu, Z.H. Chlorinated indium tin oxide electrodes with high work function for organic device compatibility. Science 2011, 332, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Vossen, J.L.; Chaudhari, P.; Raether, H. Physics of Thin Films; Academic Press: New York, NY, USA, 1977; Volume 9. [Google Scholar]
- Alzoubi, K.; Hamasha, M.M.; Lu, S.; Sammakia, B. Bending fatigue study of sputtered ITO on flexible substrate. J. Disp. Technol. 2011, 7, 593–600. [Google Scholar] [CrossRef]
- Sierros, K.A.; Morris, N.J.; Ramji, K.; Cairns, D.R. Stress–corrosion cracking of indium tin oxide coated polyethylene terephthalate for flexible optoelectronic devices. Thin Solid Film. 2009, 517, 2590–2595. [Google Scholar] [CrossRef]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.M.; Wu, X.; Zhou, J.; Duda, A.; van de Lagemaat, J.; Coutts, T.J.; Weeks, C.L.; Britz, D.A.; Glatkowski, P. Single-wall carbon nanotube networks as a transparent back contact in CdTe solar cells. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Rowell, M.W.; Topinka, M.A.; McGehee, M.D.; Prall, H.-J.; Dennler, G.; Sariciftci, N.S.; Hu, L.; Gruner, G. Organic solar cells with carbon nanotube network electrodes. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Kang, M.G.; Guo, L.J. Nanoimprinted Semitransparent Metal Electrodes and Their Application in Organic Light-Emitting Diodes. Adv. Mater. 2007, 19, 1391–1396. [Google Scholar] [CrossRef]
- Gomez De Arco, L.; Zhang, Y.; Schlenker, C.W.; Ryu, K.; Thompson, M.E.; Zhou, C. Continuous, highly flexible, and transparent graphene films by chemical vapor deposition for organic photovoltaics. ACS Nano 2010, 4, 2865–2873. [Google Scholar] [CrossRef]
- Cho, W.; Sarada, G.; Lee, H.; Song, M.; Gal, Y.-S.; Lee, Y.; Jin, S.-H. Highly efficient, conventional and flexible deep-red phosphorescent OLEDs using ambipolar thiophene/selenophene-phenylquinoline ligand-based Ir(III) complexes. Dyes Pigment. 2017, 136, 390–397. [Google Scholar] [CrossRef]
- Kang, T.W.; Kim, S.H.; Kim, C.H.; Lee, S.M.; Kim, H.K.; Park, J.S.; Lee, J.H.; Yang, Y.S.; Lee, S.J. Flexible polymer/metal/polymer and polymer/metal/inorganic trilayer transparent conducting thin film heaters with highly hydrophobic surface. ACS Appl. Mater. Interfaces 2017, 9, 33129–33136. [Google Scholar] [CrossRef]
- Choi, D.Y.; Kang, H.W.; Sung, H.J.; Kim, S.S. Annealing-free, flexible silver nanowire-polymer composite electrodes via a continuous two-step spray-coating method. Nanoscale 2013, 5, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yuan, S.; Liu, L.; Qiu, X.; Gong, H.; Yang, X.; Li, C.; Hao, Y.; Cao, B. Fully indium-free flexible Ag nanowires/ZnO:F composite transparent conductive electrodes with high haze. J. Mater. Chem. A 2015, 3, 5375–5384. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, S.; Ren, J.; Khalid, B.; Peng, H.; Wu, H. A transparent, conducting tape for flexible electronics. Nano Res. 2016, 9, 917–924. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.Y.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Yu, X. Silver nanowire-based transparent, flexible, and conductive thin film. Nanoscale Res. Lett. 2011, 6, 75. [Google Scholar] [CrossRef]
- Scardaci, V.; Coull, R.; Lyons, P.E.; Rickard, D.; Coleman, J.N. Spray deposition of highly transparent, low-resistance networks of silver nanowires over large areas. Small 2011, 7, 2621–2628. [Google Scholar] [CrossRef]
- Huang, J.; Fan, R.; Connor, S.; Yang, P. One-step patterning of aligned nanowire arrays by programmed dip coating. Angew. Chem. Int. Ed. Engl. 2007, 46, 2414–2417. [Google Scholar] [CrossRef]
- Chou, T.-H.; Yang, W.-H.; Cheng, K.-Y.; Chang, Y.-C.; Luo, T. The simulation and inspection for the starting phenomenon of slit coating process on glass substrate. Int. J. Autom. Technol. 2011, 5, 190–194. [Google Scholar] [CrossRef]
- Ouyang, J.Y.; Guo, T.F.; Yang, Y.; Higuchi, H.; Yoshioka, M.; Nagatsuka, T. High-performance, flexible polymer light-emitting diodes fabricated by a continuous polymer coating process. Adv. Mater. 2002, 14, 915–918. [Google Scholar] [CrossRef]
- Hu, S.; Han, T.; Lin, C.; Xiang, W.; Zhao, Y.; Gao, P.; Du, F.; Li, X.; Sun, Y. Enhanced electrocatalysis via 3D graphene aerogel engineered with a silver nanowire network for ultrahigh-rate zinc-air batteries. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Butwong, N.; Srijaranai, S.; Glennon, J.D.; Luong, J.H.T. Cysteamine capped silver nanoparticles and single-walled carbon nanotubes composite coated on glassy carbon electrode for simultaneous analysis of hydroquinone and catechol. Electroanalysis 2018, 30, 962–968. [Google Scholar] [CrossRef]
- Jilani, S.M.; Gamot, T.D.; Banerji, P.; Chakraborty, S. Studies on resistive switching characteristics of aluminum/graphene oxide/semiconductor nonvolatile memory cells. Carbon 2013, 64, 187–196. [Google Scholar] [CrossRef]
- Sung, S.; Kim, T.W. Flexible nonvolatile memory devices based on Au/PMMA nanocomposites deposited on PEDOT:PSS/Ag nanowire hybrid electrodes. Appl. Surf. Sci. 2017, 411, 67–72. [Google Scholar] [CrossRef]
- De, S.; Higgins, T.M.; Lyons, P.E.; Doherty, E.M.; Nirmalraj, P.N.; Blau, W.J.; Boland, J.J.; Coleman, J.N. Silver nanowire networks as flexible, transparent, conducting films: Extremely high DC to optical conductivity ratios. ACS Nano 2009, 3, 1767–1774. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Liu, H.S.; Pan, B.C.; Liou, G.S. Highly transparent AgNW/PDMS stretchable electrodes for elastomeric electrochromic devices. Nanoscale 2017, 9, 2633–2639. [Google Scholar] [CrossRef]
- Liang, J.J.; Li, L.; Tong, K.; Ren, Z.; Hu, W.; Niu, X.F.; Chen, Y.S.; Pei, Q.B. Silver nanowire percolation network soldered with graphene oxide at room temperature and its application for fully stretchable polymer light-emitting diodes. ACS Nano 2014, 8, 1590–1600. [Google Scholar] [CrossRef]
- Moon, I.K.; Kim, J.I.; Lee, H.; Hur, K.; Kim, W.C.; Lee, H. 2D graphene oxide nanosheets as an adhesive over-coating layer for flexible transparent conductive electrodes. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.; Choi, J.-Y.; Jeon, J.; Lee, J.; Im, J.; Lee, J.; Jin, S.-W.; Park, H.-J.; Lee, S.-H.; Kim, D.-B.; et al. Flexible Transparent Electrode Characteristics of Graphene Oxide/Cysteamine/AgNP/AgNW Structure. Nanomaterials 2020, 10, 2352. https://doi.org/10.3390/nano10122352
Jang J, Choi J-Y, Jeon J, Lee J, Im J, Lee J, Jin S-W, Park H-J, Lee S-H, Kim D-B, et al. Flexible Transparent Electrode Characteristics of Graphene Oxide/Cysteamine/AgNP/AgNW Structure. Nanomaterials. 2020; 10(12):2352. https://doi.org/10.3390/nano10122352
Chicago/Turabian StyleJang, Junhwan, Ju-Young Choi, Jihyun Jeon, Jeongjun Lee, Jaehyuk Im, Jaegun Lee, Seung-Won Jin, Hyeong-Joo Park, Seung-Hyun Lee, Dam-Bi Kim, and et al. 2020. "Flexible Transparent Electrode Characteristics of Graphene Oxide/Cysteamine/AgNP/AgNW Structure" Nanomaterials 10, no. 12: 2352. https://doi.org/10.3390/nano10122352
APA StyleJang, J., Choi, J.-Y., Jeon, J., Lee, J., Im, J., Lee, J., Jin, S.-W., Park, H.-J., Lee, S.-H., Kim, D.-B., Chung, C.-M., & Cho, S. (2020). Flexible Transparent Electrode Characteristics of Graphene Oxide/Cysteamine/AgNP/AgNW Structure. Nanomaterials, 10(12), 2352. https://doi.org/10.3390/nano10122352