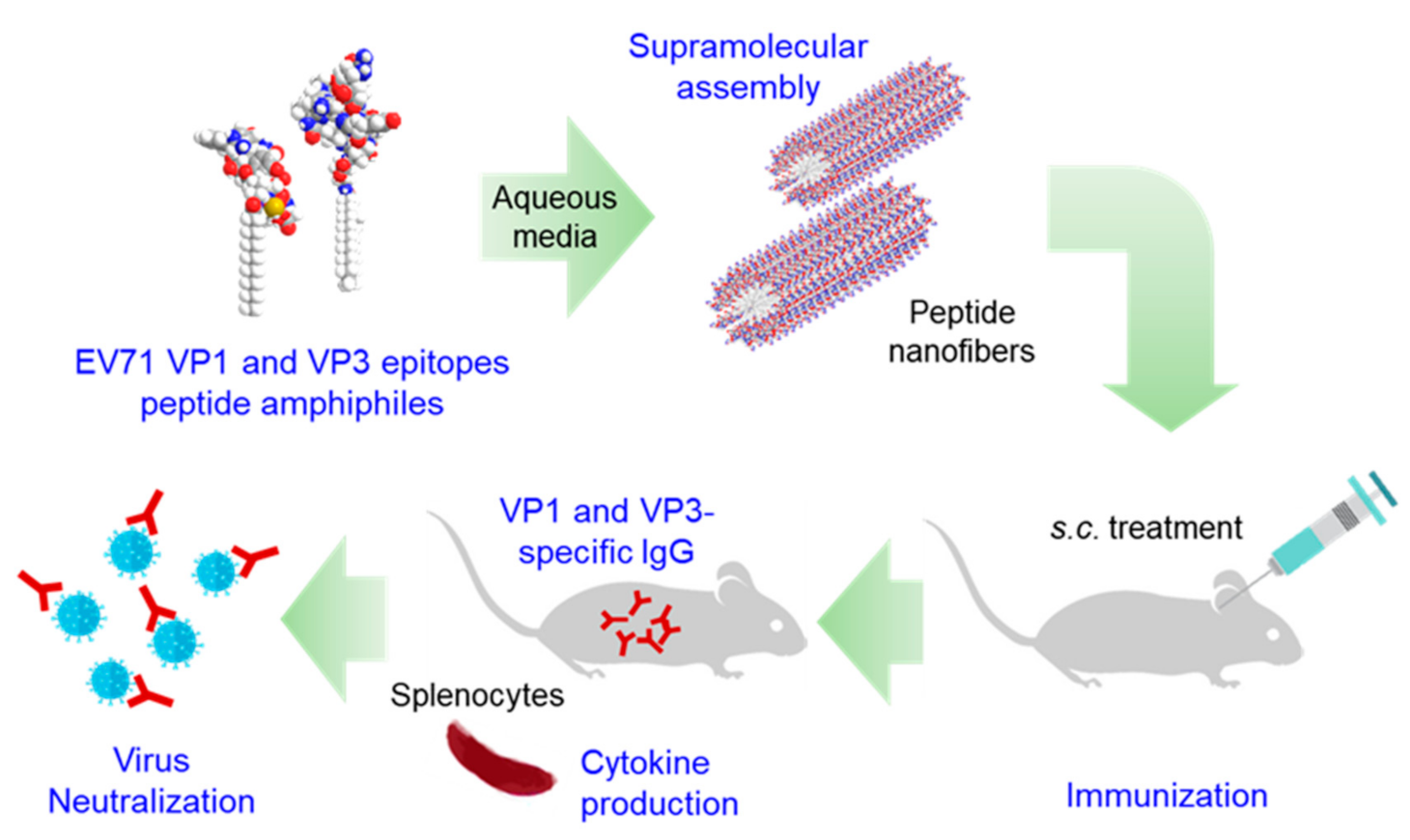
Self-Assembled Multi-Epitope Peptide Amphiphiles Enhance the Immune Response against Enterovirus 71
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
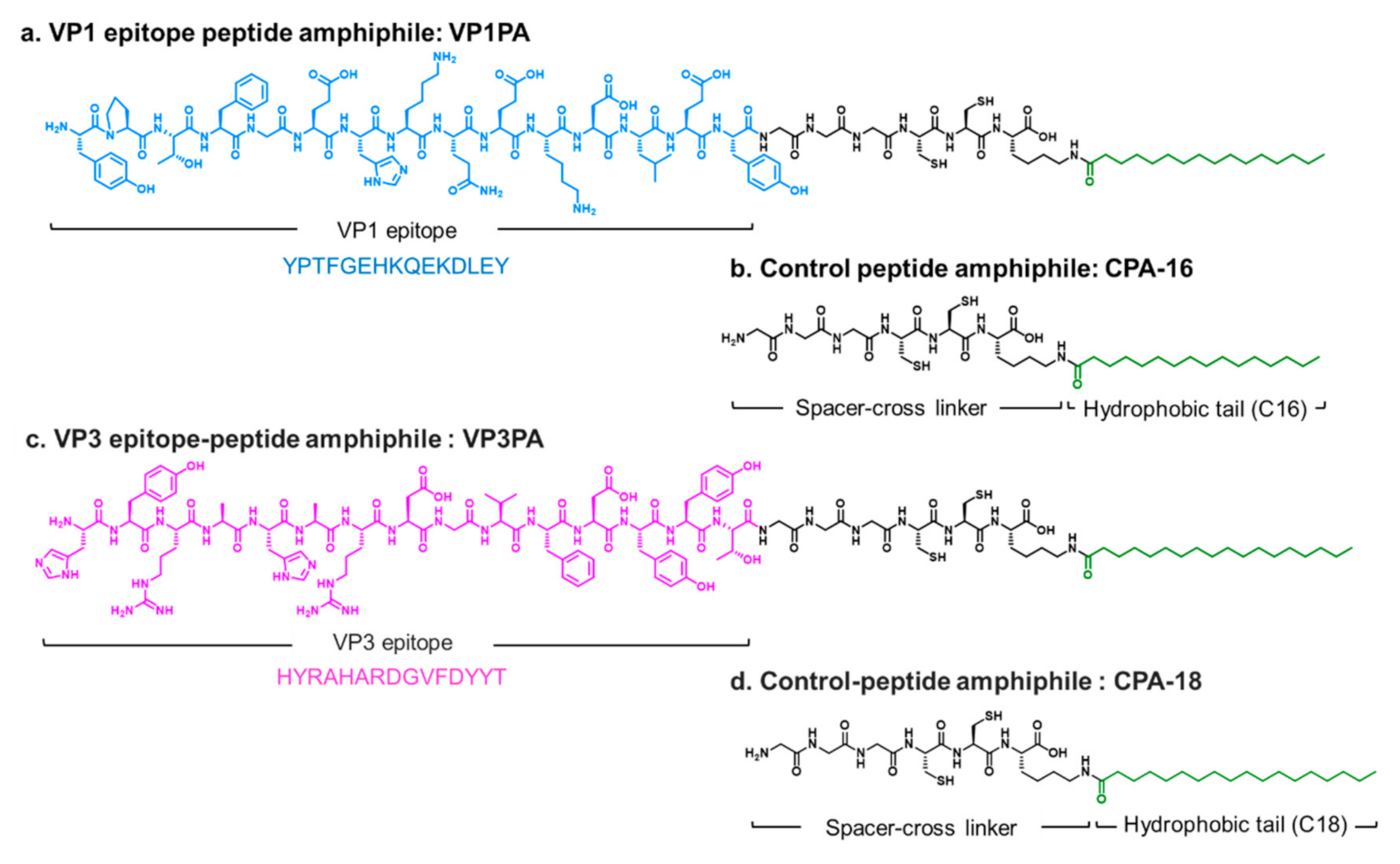
2.2.1. Design and Synthesis of Virus Epitope-PAs
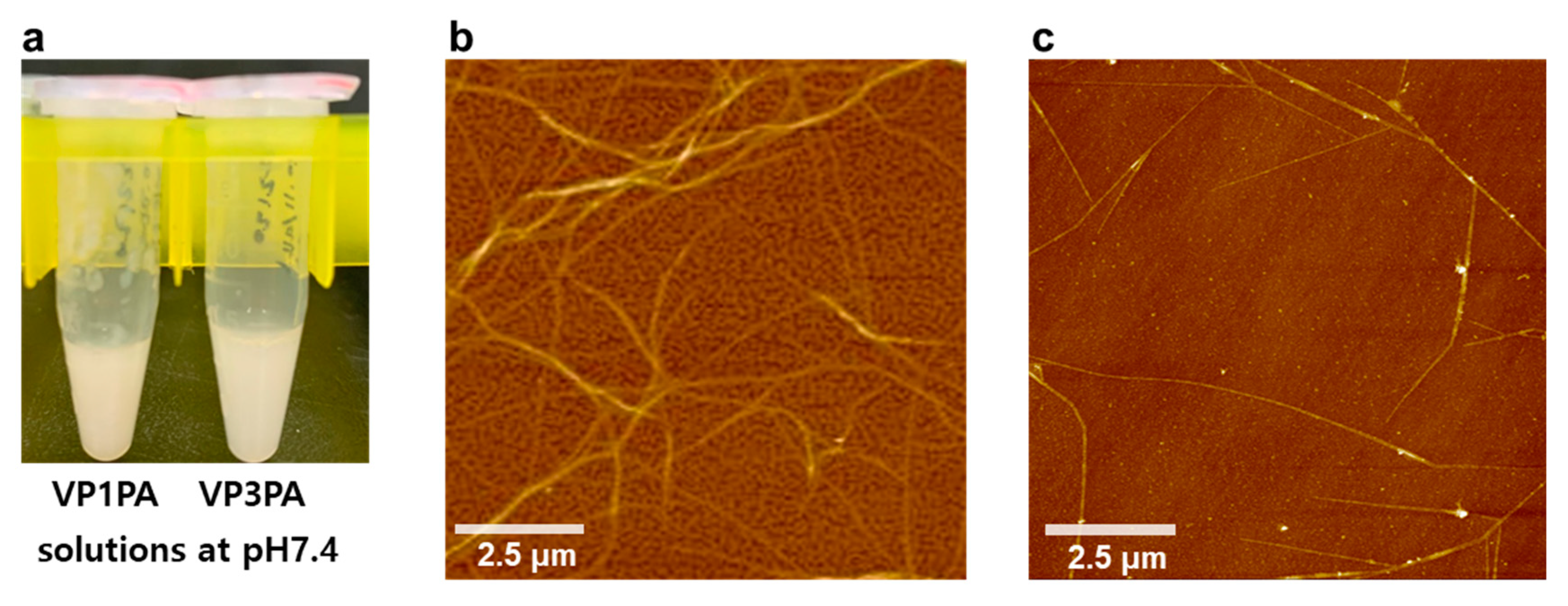
2.2.2. Self-Assembly of PAs for Supramolecular Structures
2.2.3. Supramolecular Structures of PAs Analyzed Using AFM
2.2.4. Purification of EV71 Capsid Proteins
2.2.5. Immunization
2.2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
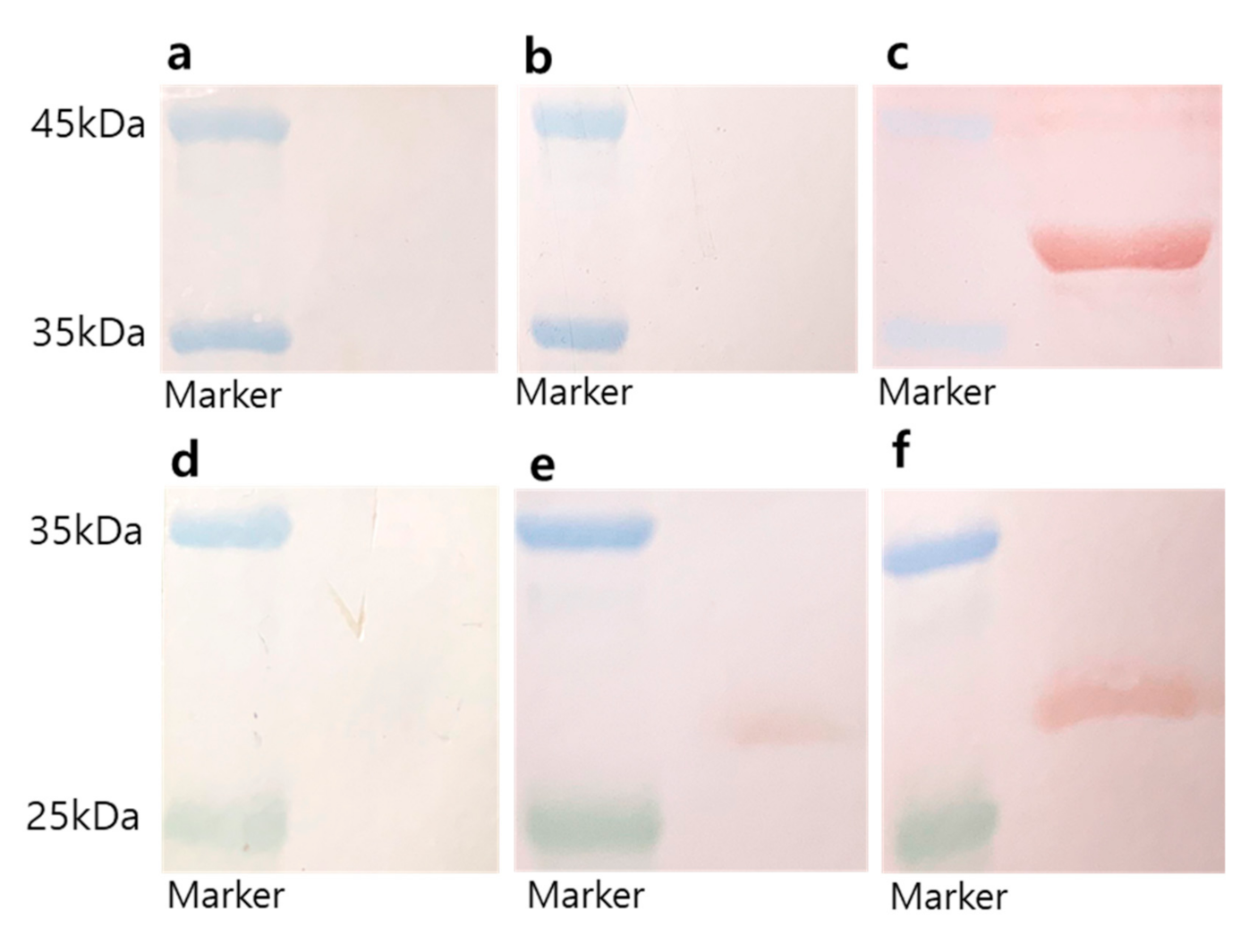
2.2.7. Western Blot Analysis
2.2.8. Cytokine Assay
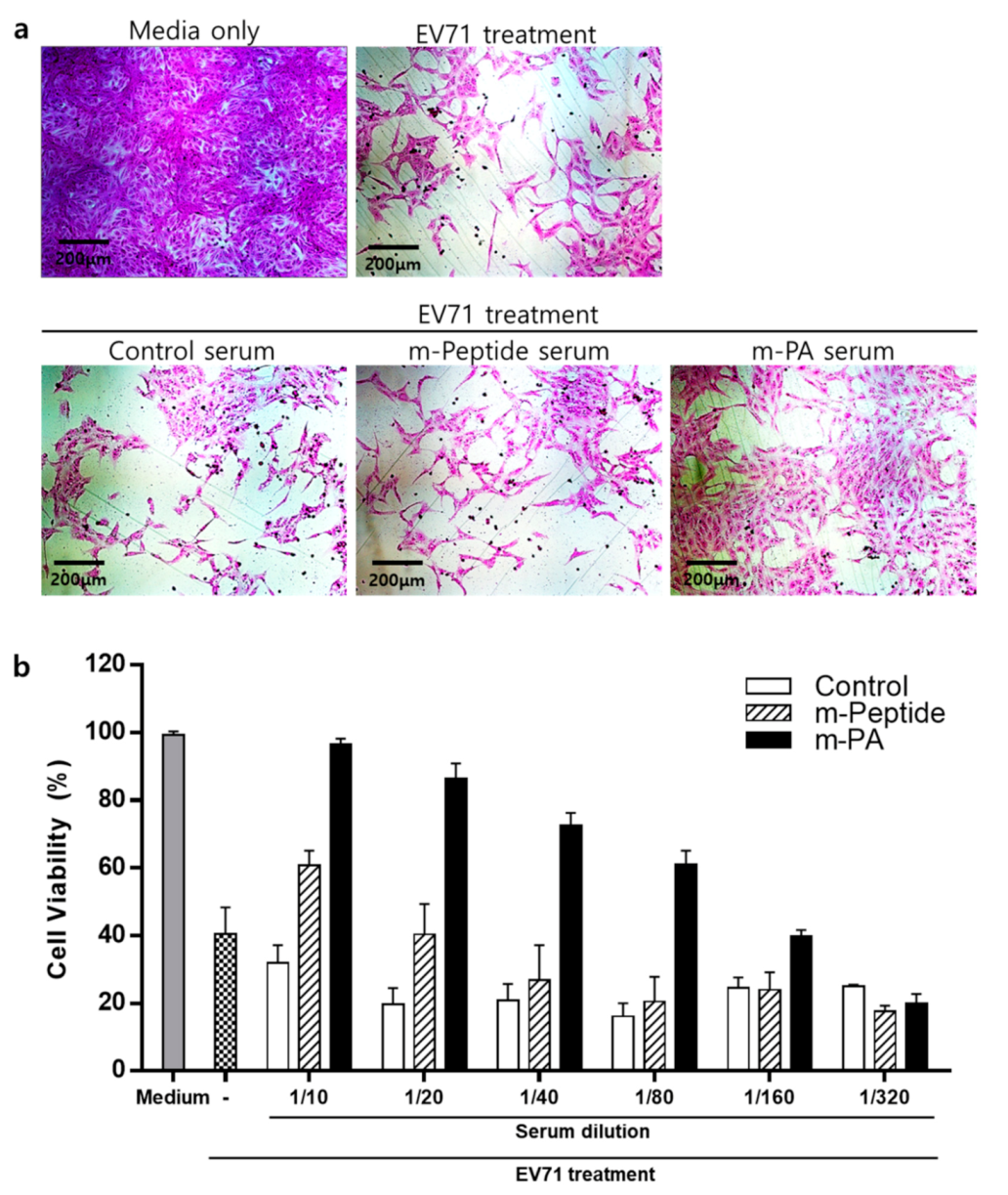
2.2.9. Neutralization Assay
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Design and Self-Assembly Properties of Epitope-PAs
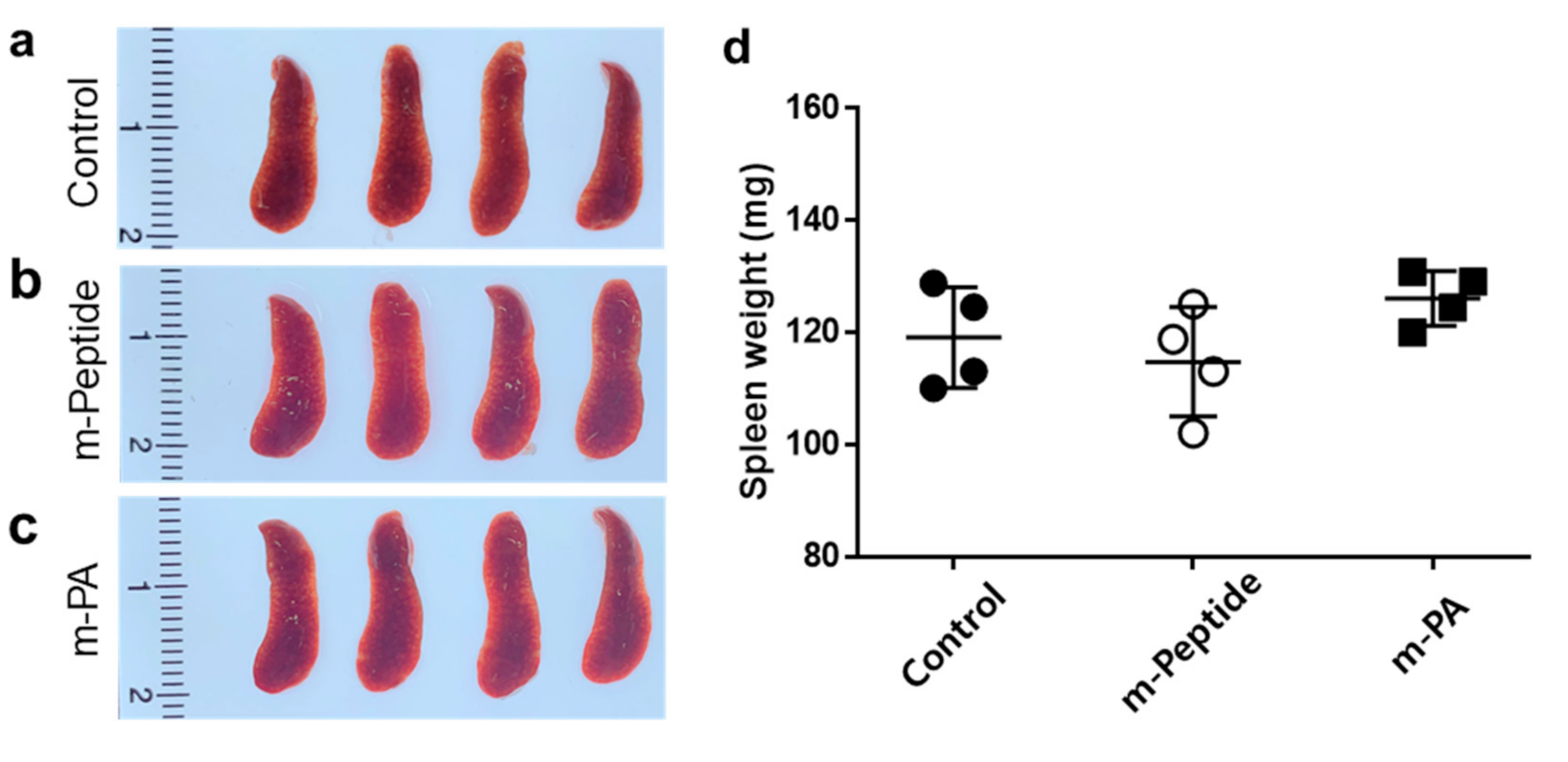
3.2. Spleen Morphology by the Immunization with EV71 Epitope m-PA
3.3. Immunization with EV71 Epitope m-PA Elicits Anti-EV71 Antibody Production
3.4. m-PA Conjugation Improved Humoral Immune Response to EV71 Epitopes
3.5. EV71 Epitope m-PA Induces a Cell-Mediated Immune Response
3.6. EV71 Epitope m-PA-Treated Sera Protects Cells from EV71 Infection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Vaccine Action Plan 2011–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Baxby, D. Edward Jenner’s inquiry after 200 years. BMJ 1999, 318, 390. [Google Scholar] [CrossRef] [PubMed]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. (Lond.) 2007, 57, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Scott, C. Classifying vaccines. BioProcesses Int. 2004, 14–23. Available online: https://bioprocessintl.com/manufacturing/monoclonal-antibodies/chapter-2-classifying-vaccines-41200452/ (accessed on 24 November 2020).
- Vartak, A.; Sucheck, S.J. Recent advances in subunit vaccine carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Rudra, J.S.; Tian, Y.F.; Jung, J.P.; Collier, J.H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. USA 2010, 107, 622–627. [Google Scholar] [CrossRef]
- Schroeder, U.; Graff, A.; Buchmeier, S.; Rigler, P.; Silvan, U.; Tropel, D.; Jockusch, B.M.; Aebi, U.; Burkhard, P.; Schoenenberger, C.-A. Peptide nanoparticles serve as a powerful platform for the immunogenic display of poorly antigenic actin determinants. J. Mol. Biol. 2009, 386, 1368–1381. [Google Scholar] [CrossRef]
- Braun, M.; Jandus, C.; Maurer, P.; Hammann-Haenni, A.; Schwarz, K.; Bachmann, M.F.; Speiser, D.E.; Romero, P. Virus-like particles induce robust human T-helper cell responses. Eur. J. Immunol. 2012, 42, 330–340. [Google Scholar] [CrossRef]
- Fujita, Y.; Taguchi, H. Nanoparticle-Based Peptide Vaccines. In Micro and Nanotechnology in Vaccine Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 149–170. [Google Scholar]
- Jin, H.E.; Jang, J.; Chung, J.; Lee, H.J.; Wang, E.; Lee, S.W.; Chung, W.J. Biomimetic Self-Templated Hierarchical Structures of Collagen-Like Peptide Amphiphiles. Nano Lett. 2015, 15, 7138–7145. [Google Scholar] [CrossRef]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-based drug-delivery systems in biotechnological applications: Recent advances and perspectives. Molecules 2019, 24, 351. [Google Scholar] [CrossRef]
- Zhang, R.; Smith, J.D.; Allen, B.N.; Kramer, J.S.; Schauflinger, M.; Ulery, B.D. Peptide amphiphile micelle vaccine size and charge influence the host antibody response. ACS Biomater. Sci. Eng. 2018, 4, 2463–2472. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles self-assembly: Basic concepts and future perspectives of supramolecular approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, Y.; Jung, J.-W.; Jin, H.-E. Epitope Peptide Amphiphile-Based Nanofiber as an Effective Vaccine for Viral Infectious Diseases. J. Nanosci. Nanotechnol. 2020, 20, 5329–5332. [Google Scholar] [CrossRef] [PubMed]
- Rudra, J.S.; Sun, T.; Bird, K.C.; Daniels, M.D.; Gasiorowski, J.Z.; Chong, A.S.; Collier, J.H. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano 2012, 6, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gao, M.; Cui, D. Molecular Characteristics of VP1 Region of Enterovirus 71 Strains in China. Gut Pathog. 2020, 12, 38. [Google Scholar] [CrossRef]
- Ye, X.; Ku, Z.; Liu, Q.; Wang, X.; Shi, J.; Zhang, Y.; Kong, L.; Cong, Y.; Huang, Z. Chimeric virus-like particle vaccines displaying conserved enterovirus 71 epitopes elicit protective neutralizing antibodies in mice through divergent mechanisms. J. Virol. 2014, 88, 72–81. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, R.; Sun, S.; Fan, P.; Su, W.; Zhou, Y.; Gao, F.; Xu, F.; Kong, W.; Jiang, C. A new EV71 VP3 epitope in norovirus P particle vector displays neutralizing activity and protection in vivo in mice. Vaccine 2015, 33, 6596–6603. [Google Scholar] [CrossRef]
- Yi, E.J.; Shin, Y.J.; Kim, J.H.; Kim, T.G.; Chang, S.Y. Enterovirus 71 infection and vaccines. Clin. Exp. Vaccine Res. 2017, 6, 4–14. [Google Scholar] [CrossRef]
- Kim, Y.I.; Song, J.H.; Kwon, B.E.; Kim, H.N.; Seo, M.D.; Park, K.; Lee, S.; Yeo, S.G.; Kweon, M.N.; Ko, H.J.; et al. Pros and cons of VP1-specific maternal IgG for the protection of Enterovirus 71 infection. Vaccine 2015, 33, 6604–6610. [Google Scholar] [CrossRef] [PubMed]
- Al-azzawi, S.; Masheta, D. Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. J. Pharm. Investig. 2019, 49, 643–654. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, C.; Zhang, S.; Li, A.; Zhang, Q.; Wu, W.; Liu, L.; Li, C.; Liang, M.; Li, X.; et al. Oral immunization with recombinant enterovirus 71 VP1 formulated with chitosan protects mice against lethal challenge. Virol. J. 2014, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Okur, Z.; Senturk, O.I.; Yilmaz, C.; Gulseren, G.; Mammadov, B.; Guler, M.O.; Tekinay, A.B. Promotion of neurite outgrowth by rationally designed NGF-beta binding peptide nanofibers. Biomater. Sci. 2018, 6, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, J.H.; Lee, C.H.; Ahn, Y.J.; Song, J.H.; Baek, S.H.; Kwon, D.H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral. Res. 2009, 81, 77–81. [Google Scholar] [CrossRef]
- Nagarajan, R. Molecular packing parameter and surfactant self-assembly: The neglected role of the surfactant tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Cooper, N.R.; Nemerow, G.R. The role of antibody and complement in the control of viral infections. J. Investig. Dermatol. 1984, 83, 121s–127s. [Google Scholar] [CrossRef]
- Cao, R.Y.; Dong, D.Y.; Liu, R.J.; Han, J.F.; Wang, G.C.; Zhao, H.; Li, X.F.; Deng, Y.Q.; Zhu, S.Y.; Wang, X.Y.; et al. Human IgG subclasses against enterovirus Type 71: Neutralization versus antibody dependent enhancement of infection. PLoS ONE 2013, 8, e64024. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garcon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G.D. The history of MF59((R)) adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Ott, G.; Barchfeld, G.L.; Chernoff, D.; Radhakrishnan, R.; van Hoogevest, P.; Van Nest, G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm. Biotechnol. 1995, 6, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.F.; Abubakar, S. Enterovirus 71 infection induces apoptosis in Vero cells. Malays J. Pathol. 2003, 25, 29–35. [Google Scholar] [PubMed]
- Fujii, K.; Nagata, N.; Sato, Y.; Ong, K.C.; Wong, K.T.; Yamayoshi, S.; Shimanuki, M.; Shitara, H.; Taya, C.; Koike, S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 14753–14758. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Yu, S.-L.; Shao, H.-Y.; Lin, H.-Y.; Liu, C.-C.; Hsiao, K.-N.; Chitra, E.; Tsou, Y.-L.; Chang, H.-W.; Sia, C. Human SCARB2 transgenic mice as an infectious animal model for enterovirus 71. PLoS ONE 2013, 8, e57591. [Google Scholar] [CrossRef]
- Xu, F.; Yao, P.-P.; Xia, Y.; Qian, L.; Yang, Z.-N.; Xie, R.-H.; Sun, Y.-S.; Lu, H.-J.; Miao, Z.-P.; Li, C. Enterovirus 71 infection causes severe pulmonary lesions in gerbils, meriones unguiculatus, which can be prevented by passive immunization with specific antisera. PLoS ONE 2015, 10, e0119173. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Li, Y.; Xia, Y.; Xu, F.; Wang, W.; Yang, Z.-N.; Lu, H.-J.; Chen, Z.-P.; Miao, Z.-P.; Liang, W.-F. Coxsackievirus A16 induced neurological disorders in young gerbils which could serve as a new animal model for vaccine evaluation. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]

| Cytokine (pg/mL) | Control | m-Peptide | m-PA |
|---|---|---|---|
| INF-γ | 29.54 ± 10.38 | 29.74 ± 19.87 | 1353.9 ± 463.9 *,† |
| IL-2 | 19.97 ± 4.39 | 18.93 ± 3.46 | 59.29 ± 11.63 **,†† |
| IL-10 | 5.94 ± 0.79 | 7.49 ± 1.67 | 198.6 ± 119.5 |
| IL-17A | 2.07 ± 0.93 | 3.75 ± 1.30 | 74.75 ± 25.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-G.; Lee, Y.; Kim, J.H.; Chang, S.-Y.; Jung, J.-W.; Chung, W.-J.; Jin, H.-E. Self-Assembled Multi-Epitope Peptide Amphiphiles Enhance the Immune Response against Enterovirus 71. Nanomaterials 2020, 10, 2342. https://doi.org/10.3390/nano10122342
Kim Y-G, Lee Y, Kim JH, Chang S-Y, Jung J-W, Chung W-J, Jin H-E. Self-Assembled Multi-Epitope Peptide Amphiphiles Enhance the Immune Response against Enterovirus 71. Nanomaterials. 2020; 10(12):2342. https://doi.org/10.3390/nano10122342
Chicago/Turabian StyleKim, Yu-Gyeong, Yunsu Lee, Joo Hee Kim, Sun-Young Chang, Jong-Wha Jung, Woo-Jae Chung, and Hyo-Eon Jin. 2020. "Self-Assembled Multi-Epitope Peptide Amphiphiles Enhance the Immune Response against Enterovirus 71" Nanomaterials 10, no. 12: 2342. https://doi.org/10.3390/nano10122342
APA StyleKim, Y.-G., Lee, Y., Kim, J. H., Chang, S.-Y., Jung, J.-W., Chung, W.-J., & Jin, H.-E. (2020). Self-Assembled Multi-Epitope Peptide Amphiphiles Enhance the Immune Response against Enterovirus 71. Nanomaterials, 10(12), 2342. https://doi.org/10.3390/nano10122342





