Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
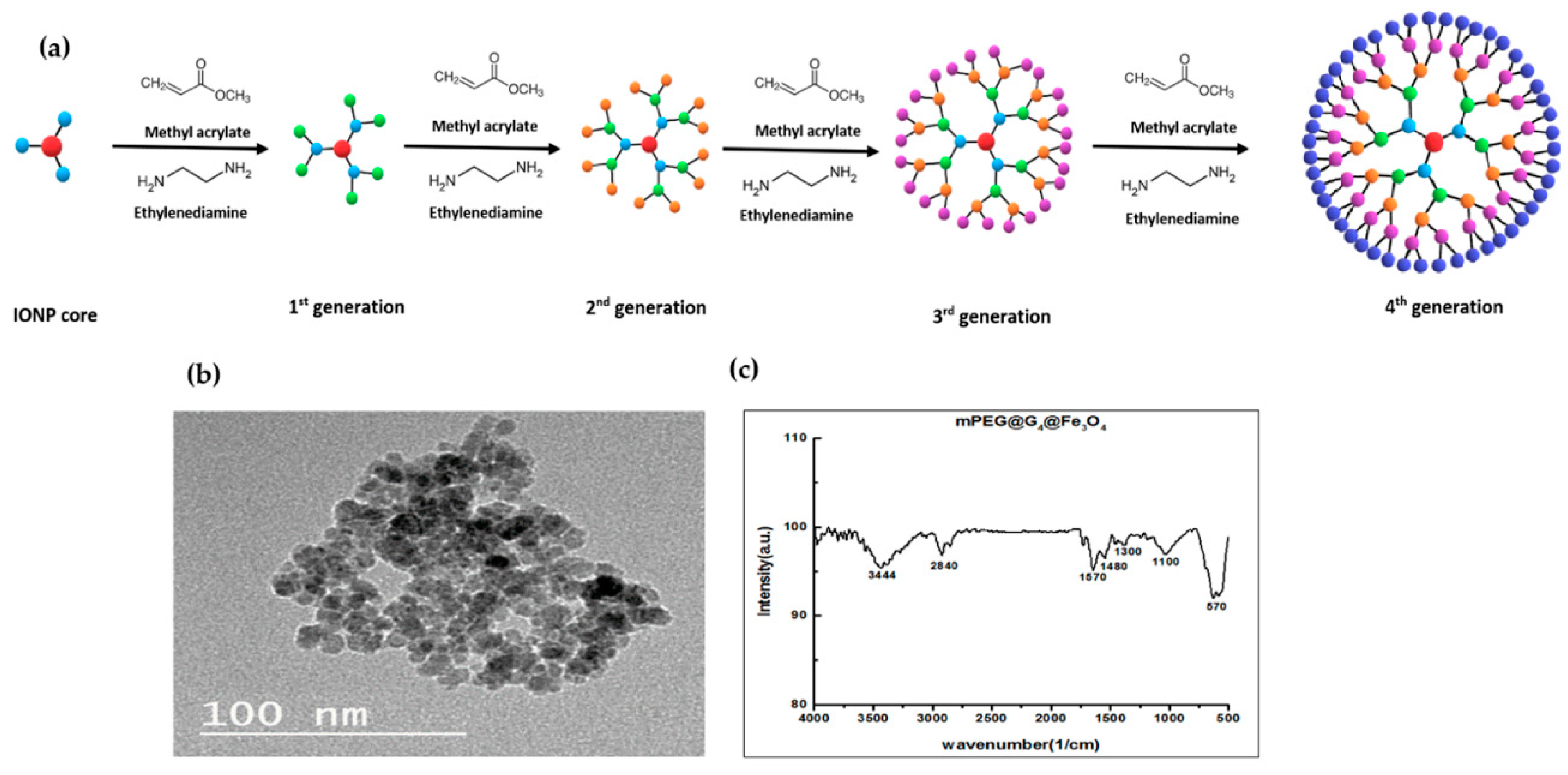
2.2. Magnetic Nanoparticles Synthesis
2.3. Cell Culture
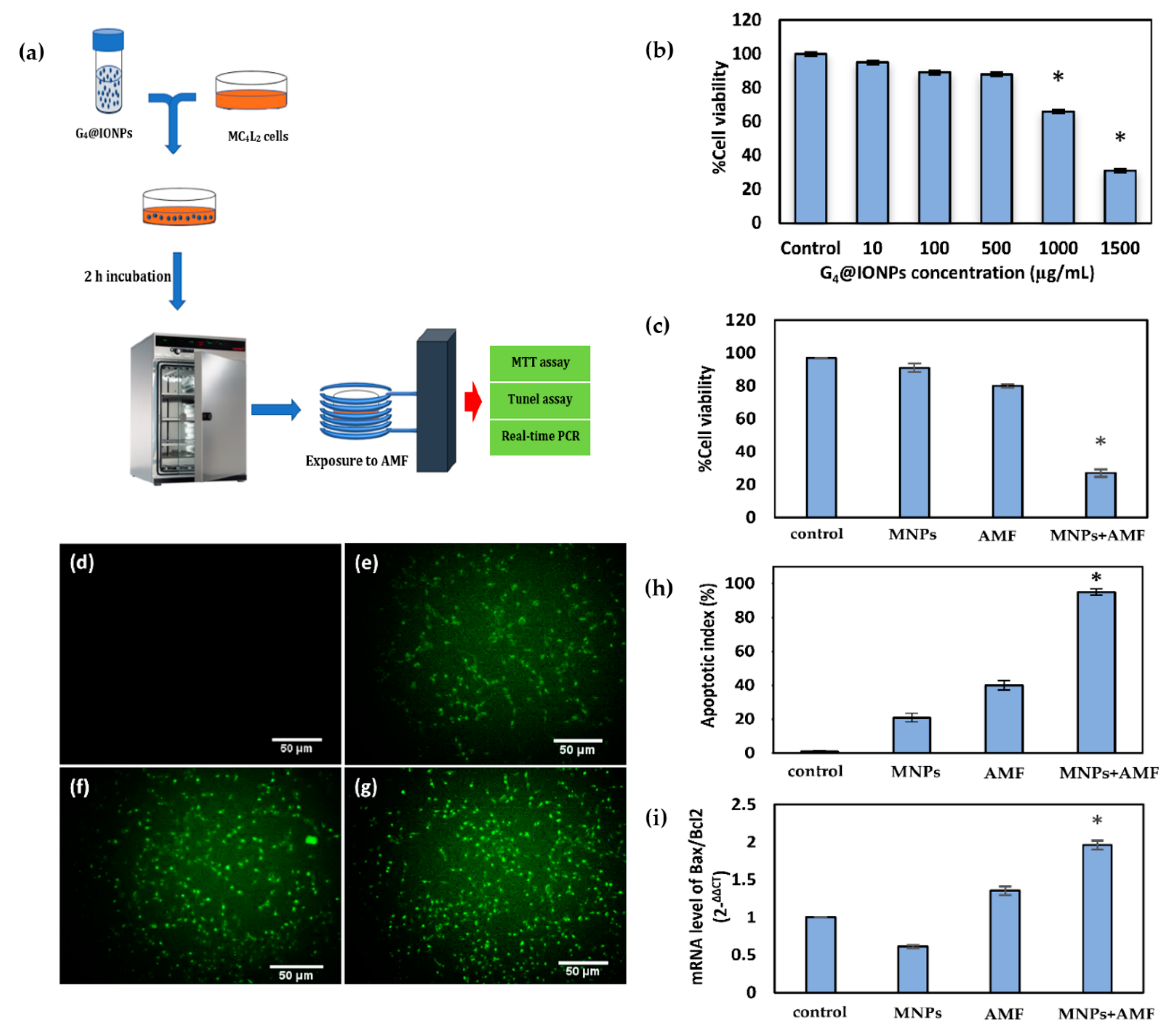
2.4. Magnetic Hyperthermia Treatment in Cancer Cells
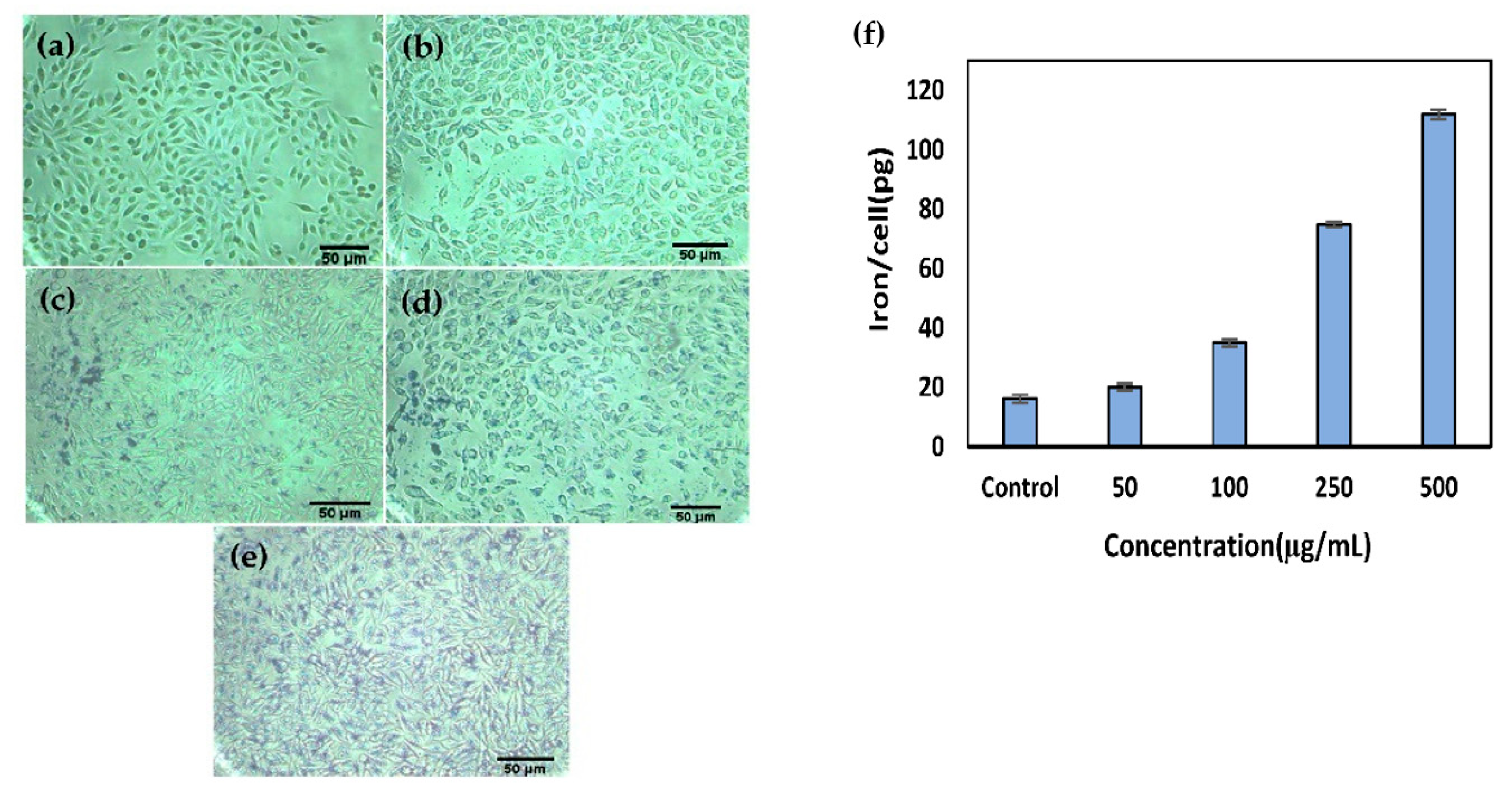
2.5. G4@IONPs Cellular Uptake and Localization
2.6. Apoptotic Cell Death Assessment
2.7. Effect of Magnetic Hyperthermia Treatment on the Expression of Apoptosis-related Genes
2.8. Ethical Statement and Animal Welfare
2.9. Breast Tumor Induction in BALB/c Mice
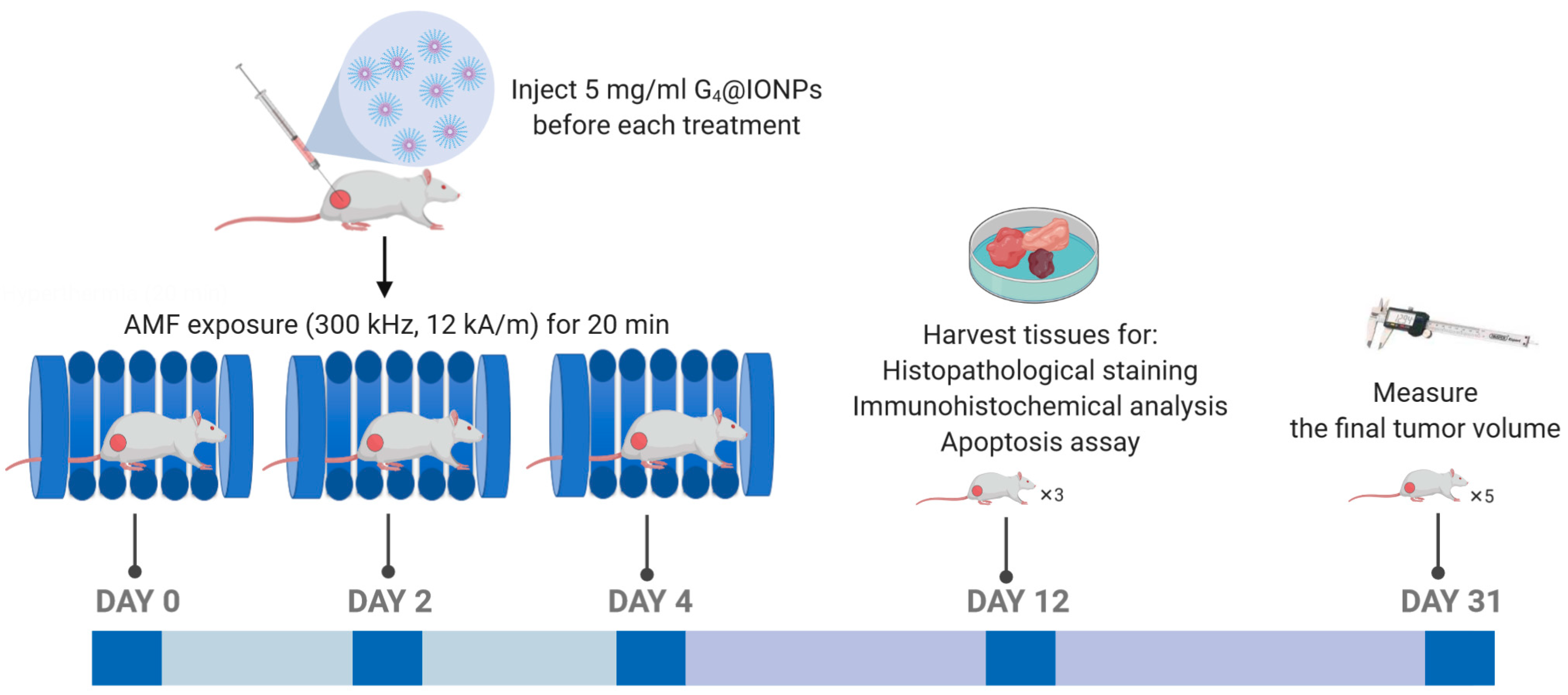
2.10. Magnetic Hyperthermia Treatment in BALB/c Mice
2.11. Histopathological Studies in Liver, Lung, and Tumor Tissues
2.12. Immunohistochemistry (IHC) Assay in Tumor Tissues
2.13. Apoptosis in the Tumor Tissues (TUNEL Assay)
2.14. Statistical Analysis
3. Results
3.1. Characterization of G4@IONPs
3.2. Cytotoxicity of G4@IONPs in Cancer Cells (MTT Assay)
3.3. Effect of Magnetic Hyperthermia Treatment on the Viability of Cancer Cells
3.4. Cellular Apoptosis and Expression of Apoptosis-Related Genes after Magnetic Hyperthermia Treatment
3.5. Cellular Uptake and Localization of G4@IONPs
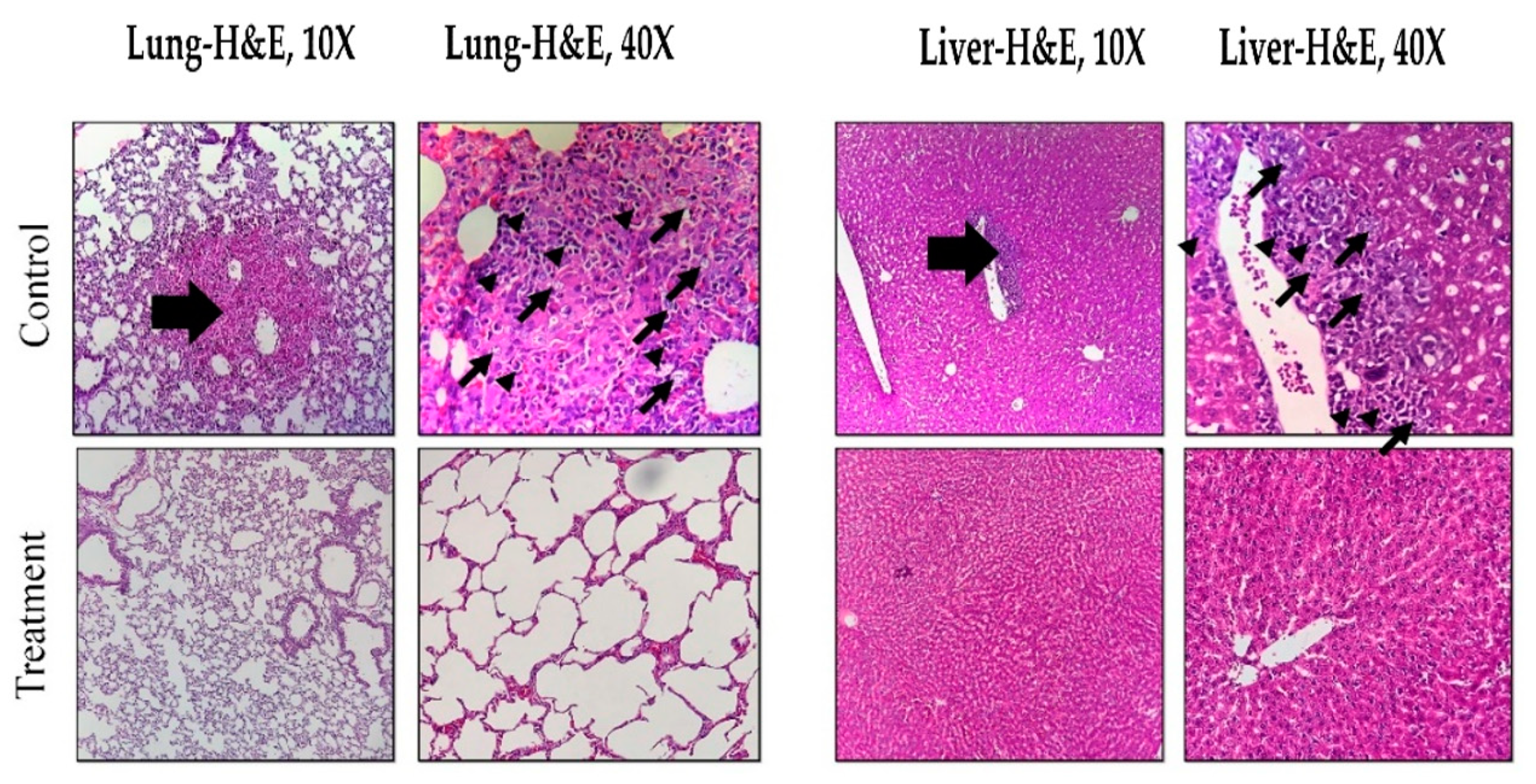
3.6. Histopathological Effects of Magnetic Hyperthermia Treatment on Liver and Lung Tissues
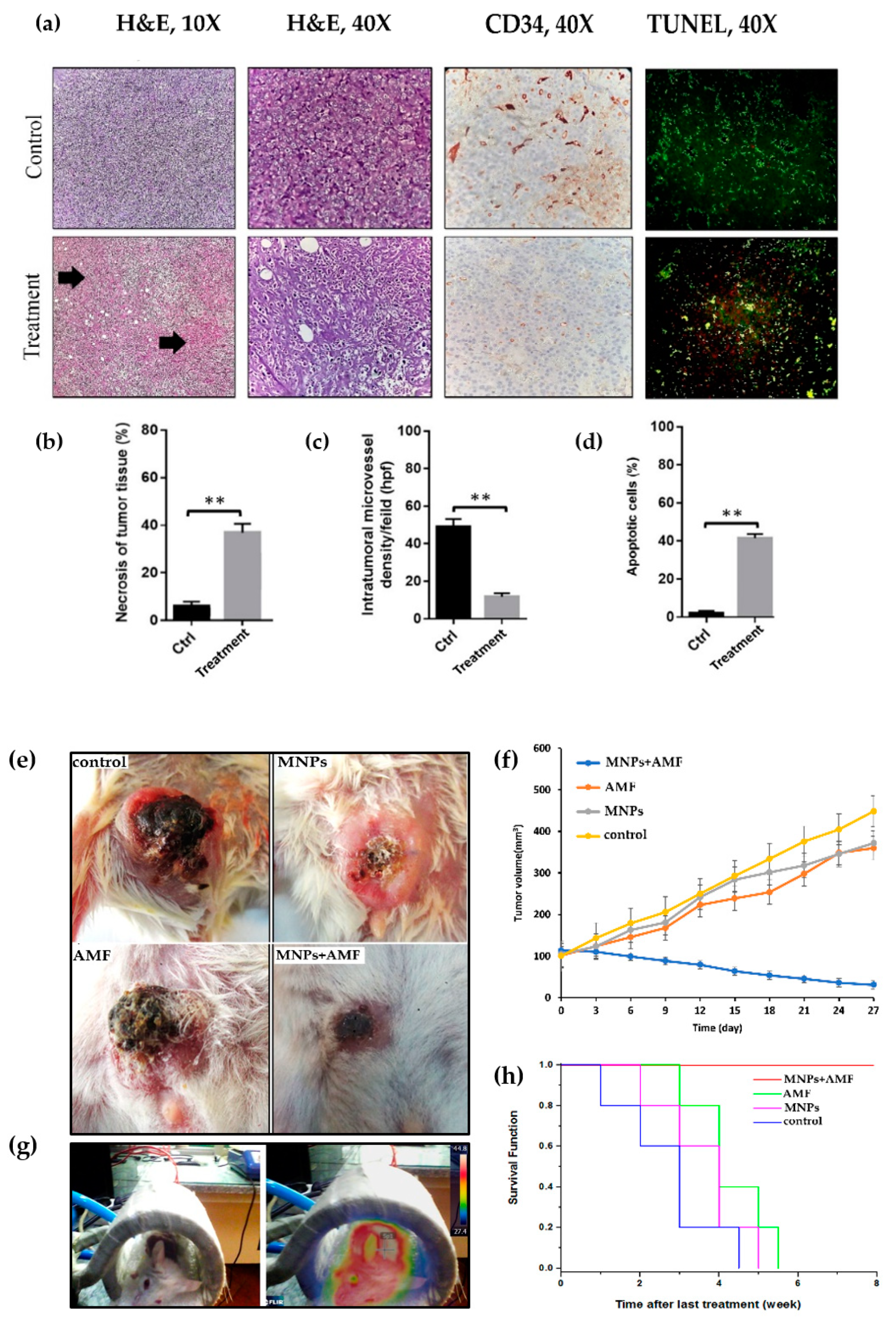
3.7. Histopathological Effects of Magnetic Hyperthermia Treatment on Tumor Tissue
3.8. Angiogenesis and Apoptosis in Tumor Tissue after Magnetic Hyperthermia Treatment
3.9. Effect of Magnetic Hyperthermia Treatment on Tumor Volume
3.10. Kaplan–Meier Curve and Survival Rate of BALB/c Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carioli, G.; Malvezzi, M.; Rodríguez, T.; Bertuccio, P.; Negri, E.; La Vecchia, C. Trends and predictions to 2020 in breast cancer mortality: Americas and Australasia. Breast 2018, 37, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Hu, P.; Guo, Y.; Hao, J.; Ni, D.; Xu, Y.; Bao, Q.; Yao, H.; Wei, C.; Wu, Q.; et al. Combined Magnetic Hyperthermia and Immune Therapy for Primary and Metastatic Tumor Treatments. ACS Nano 2020, 14, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Gawęda, W.; Osial, M.; Żuk, M.; Pękała, M.; Bilewicz, A.; Krysinski, P. Lanthanide-Doped SPIONs Bioconjugation with Trastuzumab for Potential Multimodal Anticancer Activity and Magnetic Hyperthermia. Nanomaterials 2020, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Nguyen, V.T.; Kim, H.H.; Nam, S.Y.; Lee, K.D.; Oh, J. Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment. Nanomaterials 2017, 7, 426. [Google Scholar] [CrossRef]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice. Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef]
- Brero, F.; Albino, M.; Antoccia, A.; Arosio, P.; Avolio, M.; Berardinelli, F.; Bettega, D.; Calzolari, P.; Ciocca, M.; Corti, M.; et al. Hadron Therapy, Magnetic Nanoparticles and Hyperthermia: A Promising Combined Tool for Pancreatic Cancer Treatment. Nanomaterials 2020, 10, 1919. [Google Scholar] [CrossRef]
- Lin, T.-C.; Lin, F.-H.; Lin, J.-C. In vitro feasibility study of the use of a magnetic electrospun chitosan nanofiber composite for hyperthermia treatment of tumor cells. Acta Biomater. 2012, 8, 2704–2711. [Google Scholar] [CrossRef]
- Sugahara, T.; Van Der Zee, J.; Kampinga, H.H.; Vujaskovic, Z.; Kondo, M.; Ohnishi, T.; Li, G.; Park, H.J.; Leeper, D.B.; Ostapenko, V.; et al. Kadota Fund International Forum 2004. Application of thermal stress for the improvement of health, 15–18 June 2004, Awaji Yumebutai International Conference Center, Awaji Island, Hyogo, Japan. Final Report. Int. J. Hyperth. 2008, 24, 123–140. [Google Scholar] [CrossRef]
- Takada, T.; Yamashita, T.; Sato, M.; Sato, A.; Ono, I.; Tamura, Y.; Sato, N.; Miyamoto, A.; Ito, A.; Honda, H.; et al. Growth Inhibition of Re-Challenge B16 Melanoma Transplant by Conjugates of Melanogenesis Substrate and Magnetite Nanoparticles as the Basis for Developing Melanoma-Targeted Chemo-Thermo-Immunotherapy. J. Biomed. Biotechnol. 2009, 2009, 1–13. [Google Scholar] [CrossRef]
- Etheridge, M.; Manuchehrabadi, N.; Franklin, R.; Bischof, J.; Minkowycz, W.; Sparrow, E.; Abraham, J. Superparamagnetic Iron Oxide Nanoparticle Heating. Comput. Phys. Process. Mech. Therm. Sci. 2012, 97–122. [Google Scholar] [CrossRef]
- Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials 2019, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Dufes, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent Folate-Dendrimer-Coated Iron Oxide Theranostic Nanoparticles for Simultaneous Magnetic Resonance Imaging and Precise Cancer Cell Targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Uzun, K.; Çevik, E.; Şenel, M.; Sözeri, H.; Baykal, A.; Abasıyanık, M.F.; Toprak, M.S. Covalent immobilization of invertase on PAMAM-dendrimer modified superparamagnetic iron oxide nanoparticles. J. Nanoparticle Res. 2010, 12, 3057–3067. [Google Scholar] [CrossRef]
- Salimi, M.; Sarkar, S.; Saber, R.; Delavari, H.; Alizadeh, A.M.; Mulder, H.T. Magnetic hyperthermia of breast cancer cells and MRI relaxometry with dendrimer-coated iron-oxide nanoparticles. Cancer Nanotechnol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakamura, M.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Sakamoto, W.; Yogo, T.; Ishimura, K. Magnetically Responsive Smart Nanoparticles for Cancer Treatment with a Combination of Magnetic Hyperthermia and Remote-Control Drug Release. Theranostics 2014, 4, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dähring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Paknikar, K.M.; Haghniaz, R.; Umrani, R.D. Hyperthermia mediated by dextran-coated La0.7Sr0.3MnO3 nanoparticles: In vivo studies. Int. J. Nanomed. 2016, 11, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jang, J.-T.; Choi, J.-S.; Moon, S.H.; Noh, S.-H.; Kim, J.-W.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Huang, C.-C.; Ruan, P.-W.; Huang, K.-J.; Shieh, D.-B.; Yeh, C.-S. In vivo anti-cancer efficacy of magnetite nanocrystal—based system using locoregional hyperthermia combined with 5-fluorouracil chemotherapy. Biomaterials 2013, 34, 7873–7883. [Google Scholar] [CrossRef] [PubMed]
- Rabias, I.; Tsitrouli, D.; Karakosta, E.; Kehagias, T.; Diamantopoulos, G.; Fardis, M.; Stamopoulos, D.; Maris, T.G.; Falaras, P.; Zouridakis, N.; et al. Rapid magnetic heating treatment by highly charged maghemite nanoparticles on Wistar rats exocranial glioma tumors at microliter volume. Biomicrofluidics 2010, 4, 024111. [Google Scholar] [CrossRef]
- Bae, K.H.; Park, M.; Do, M.J.; Lee, N.; Ryu, J.H.; Kim, G.W.; Kim, C.; Park, T.G.; Hyeon, T. Chitosan Oligosaccharide-Stabilized Ferrimagnetic Iron Oxide Nanocubes for Magnetically Modulated Cancer Hyperthermia. ACS Nano 2012, 6, 5266–5273. [Google Scholar] [CrossRef]
- Ling, Y.; Tang, X.; Wang, F.; Zhou, X.; Wang, R.; Deng, L.; Shang, T.; Liang, B.; Li, P.; Ran, H.; et al. Highly efficient magnetic hyperthermia ablation of tumors using injectable polymethylmethacrylate–Fe3O4. RSC Adv. 2017, 7, 2913–2918. [Google Scholar] [CrossRef]
- Arriortua, O.K.; Garaio, E.; De La Parte, B.H.; Insausti, M.; Lezama, L.; Plazaola, F.; García, J.A.; Aizpurua, J.M.; Sagartzazu, M.; Irazola, M.; et al. Antitumor magnetic hyperthermia induced by RGD-functionalized Fe3O4 nanoparticles, in an experimental model of colorectal liver metastases. Beilstein J. Nanotechnol. 2016, 7, 1532–1542. [Google Scholar] [CrossRef]
- Ohtake, M.; Umemura, M.; Sato, I.; Akimoto, T.; Oda, K.; Nagasako, A.; Kim, J.-H.; Fujita, T.; Yokoyama, U.; Nakayama, T.; et al. Hyperthermia and chemotherapy using Fe(Salen) nanoparticles might impact glioblastoma treatment. Sci. Rep. 2017, 7, 42783. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Umemura, M.; Mitsudo, K.; Fukumura, H.; Kim, J.-H.; Hoshino, Y.; Nakashima, H.; Kioi, M.; Nakakaji, R.; Sato, M.; et al. Simultaneous hyperthermia-chemotherapy with controlled drug delivery using single-drug nanoparticles. Sci. Rep. 2016, 6, 24629. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, F.; Zheng, K.; Deng, L.; Yang, L.; Zhang, N.; Xu, C.; Ran, H.; Wang, Z.; Wang, Z.; et al. Injectable PLGA/Fe3O4 implants carrying cisplatin for synergistic magnetic hyperthermal ablation of rabbit VX2 tumor. PLoS ONE 2017, 12, e0177049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Song, S.-C. Multiple hyperthermia-mediated release of TRAIL/SPION nanocomplex from thermosensitive polymeric hydrogels for combination cancer therapy. Biomaterials 2017, 132, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic Nanoparticle Clusters for Cancer Theranostics Combining Magnetic Resonance Imaging and Hyperthermia Treatment. Theranostics 2013, 3, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Sarkar, S.; Fathi, S.; Alizadeh, A.M.; Saber, R.; Moradi, F.; Delavari, H. Biodistribution, pharmacokinetics, and toxicity of dendrimer-coated iron oxide nanoparticles in BALB/c mice. Int. J. Nanomed. 2018, 13, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Shirshahi, V.; Hatamie, S.; Tabatabaei, S.N.; Salimi, M.; Saber, R. Enhanced Thermal Stability and Biocompatibility of Gold Nanorods by Graphene Oxide. Plasmonics 2017, 13, 1585–1594. [Google Scholar] [CrossRef]
- Hsiao, I.-L.; Bierkandt, F.S.; Reichardt, P.; Luch, A.; Huang, Y.-J.; Jakubowski, N.; Tentschert, J.; Haase, A. Quantification and visualization of cellular uptake of TiO2 and Ag nanoparticles: Comparison of different ICP-MS techniques. J. Nanobiotechnol. 2016, 14, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Cuschieri, A. Tumour Cell Labelling by Magnetic Nanoparticles with Determination of Intracellular Iron Content and Spatial Distribution of the Intracellular Iron. Int. J. Mol. Sci. 2013, 14, 9111–9125. [Google Scholar] [CrossRef]
- Hashemi, M.; Fallah, A.; Aghayan, H.R.; Arjmand, B.; Yazdani, N.; Verdi, J.; Ghodsi, S.M.; Miri, S.M.; Hadjighassem, M. A New Approach in Gene Therapy of Glioblastoma Multiforme: Human Olfactory Ensheathing Cells as a Novel Carrier for Suicide Gene Delivery. Mol. Neurobiol. 2015, 53, 5118–5128. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.; Ellis, I. pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ufuk, G.; Unsoy, G.; Yalcın, S.; Gunduz, G.; Gunduz, U. PAMAM dendrimer-coated iron oxide nanoparticles: Synthesis and characterization of different generations. J. Nanoparticle Res. 2013, 15, 1–13. [Google Scholar] [CrossRef]
- Yamaura, M.; Camilo, R.; Sampaio, L.; Macêdo, M.; Nakamura, M.; Toma, H. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Takayama, T. Surface modification of chitosan powder by grafting of ‘dendrimer-like’ hyperbranched polymer onto the surface. React. Funct. Polym. 2000, 43, 341–350. [Google Scholar] [CrossRef]
- Julian, J.M.; Brezinski, D.R. An Infrared Spectroscopy Atlas for the Coatings Industry; Federation of Societies for Paint Technology: Plymouth, PA, USA, 1991. [Google Scholar]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Kojima, C.; Turkbey, B.; Ogawa, M.; Bernardo, M.; Regino, C.A.S.; Bryant, L.H.; Choyke, P.L.; Kono, K.; Kobayashi, H. Dendrimer-based MRI contrast agents: The effects of PEGylation on relaxivity and pharmacokinetics. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 1001–1008. [Google Scholar] [CrossRef]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Mecke, A.; Majoros, I.J.; Patri, A.K.; Baker, J.J.R.; Holl, M.M.B.; Orr, B.G. Lipid Bilayer Disruption by Polycationic Polymers: The Roles of Size and Chemical Functional Group. Langmuir 2005, 21, 10348–10354. [Google Scholar] [CrossRef]
- Wood, A.J.; Alexanian, R.; Dimopoulos, M. The Treatment of Multiple Myeloma. N. Engl. J. Med. 1994, 330, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Walther, M.M.; Zbar, B. The Genetic Basis of Cancer of the Kidney. J. Urol. 2003, 170, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Cheng, A.; Wang, M.-S.; Jia, R.-Y.; Sun, K.-F.; Yang, Q.; Wu, Y.; Zhu, D.; Chen, S.; Liu, M.-F.; et al. The suppression of apoptosis by α-herpesvirus. Cell Death Dis. 2017, 8, e2749. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anti-Cancer Drugs 1999, 10, 767–776. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Liang, Q.; Liang, X.-J.; Tian, J. Optimization and Design of Magnetic Ferrite Nanoparticles with Uniform Tumor Distribution for Highly Sensitive MRI/MPI Performance and Improved Magnetic Hyperthermia Therapy. Nano Lett. 2019, 19, 3618–3626. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Singletary, S.E.; Allred, C.; Ashley, P.; Bassett, L.W.; Berry, D.; Bland, K.I.; Borgen, P.I.; Clark, G.; Edge, S.B.; Hayes, D.F.; et al. Revision of the American Joint Committee on Cancer Staging System for Breast Cancer. J. Clin. Oncol. 2002, 20, 3628–3636. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Zhang, A.; He, K.; Liu, P.; Xu, L.X. Cryo-thermal therapy elicits potent anti-tumor immunity by inducing extracellular Hsp70-dependent MDSC differentiation. Sci. Rep. 2016, 6, 27136. [Google Scholar] [CrossRef]
| Study | MNPs Core | MNPs Size | Coating | Treatment Time | MNPs Concentration/Type of Injection | Tumor Model | AMF | Results |
|---|---|---|---|---|---|---|---|---|
| Hayashi et al. [22] | Fe3O4 | 10.5 nm | PPy-PEG-FA Dox | 20 min | 5 mg/kg; intratumoral injection | Multiple myeloma | 8 kA/m 230 kHz | The combination of magnetic hyperthermia treatment and chemotherapy completely cured the tumor without any recurrence. |
| Kossatz et al. [23] | SPIONs | 12 ± 3 nm | N6L or/and DOX | 60 min | 0.25 mg Fe/100 mm3; intratumoral injection | Breast | 15.4 kA/m 435 kHz | Substantial tumor growth inhibition up to 40% and complete tumor regression were seen after magnetic hyperthermia treatment. |
| Haghniaz et al. [24] | La0.7sr0.3MnO3 | 25–50 nm | dextran | 20 min | 5 mg/100 μL saline; intratumoral injection | Melanoma | 700 A 8000 W 365 kHz | Treatment inhibited tumor growth (84%) and increased animal survival (50%). In addition, levels of caspase-3 and caspase-6 also increased after treatment. |
| Lee et al. [25] | CoFe2O4 | 15 nm | MnFe2O4 | 10 min | 75 mg; intratumoral injection | Glioblastoma | 37.3 kA/m 500 kHz | The tumor was clearly eliminated in 18 days after treatment. |
| Li et al. [26] | Fe3O4 | 22 nm | anti-HER2, 5-FU and PEG | 15 min | 500 mg/mL iron; systematic injection | Bladder carcinoma | 33 kA/m 1.3 MHz | Prominent tumor remission was seen after hyperthermia and chemotherapy. |
| Rabias et al. [27] | Fe2O3 | 10–12 nm | dextran | 20 min | 150 μL; intratumoral injection | Glioma | 11 kA/m 150 kHz | Significant tumor tissue damage and dissolution were seen after treatment. |
| Bae et al. [28] | Fe3O4 | 30 nm | Chitosan-DOPA | 20 min | 375 μg Fe/kg; Intratumoral injection | Lung carcinoma | 660 A/m 1 MHz | The tumor volume decreased substantially by about 70%. |
| Ling et al. [29] | Fe3O4 | 20–50 nm | PMMA | 3 min | 0.1 mL; intratumoral injection | Breast | 28.6 A 626 kHz | Tumor volume decreased within 15 days after treatment. |
| Arriortua et al. [30] | Fe3O4 | 19 ± 2 nm | RGD peptide | >21 min | 1–1.5 mg Fe/mL; systemic injection | Colon adenocarcinoma | 14 kA/m 606 kHz | Tumor necrosis was observed. Approximately whole tumor tissue was demolished in some animals, others showed very low damage in tumor tissue. |
| Ohtake et al. [31] | Fe(Salen) | 200 nm | Salen | 60 min | 0.12–0.60 mg/body; intratumoral injection | Glioblastoma | 335. 4 A 280 kHz | The tumor size was decreased, by 80–90%, in treatment group after 4 weeks. |
| Sato et al. [32] | Fe(Salen) | 200 nm | - | 30 min | 50 mM; intratumoral injection | Tongue | 250 A 308 kHz | The tumor volume significantly decreased (223 ± 80.6%). The tumor almost completely disappeared after one week. |
| Yang et al. [33] | Fe3O4 | - | PLGA | 3 min | 100 μL; intratumoral injection | Hepatic carcinoma | 28.6 A 626 kHz | Coagulative necrosis was seen in cancer tissues after treatment. In addition, anti-tumor immune system was activated in treated mice and promoted apoptosis in tumor cells. |
| Zhang et al. [34] | Fe3O4 | 18 nm | PPZ polymer | 60 min | 0.8 µL/mm3; intratumoral injection | Glioblastoma | 13.3 kA/m 366 kHz | The tumor size was significantly smaller than the control 25 days after the last treatment. Pyknosis, karyorrhexis, and apoptosis were seen in treated tumor tissues. |
| Hayashi et al. [35] | SPIONs | 7−9 nm | PEG and FA | 20 min | 48 μmol Fe/kg; systematic injection | Multiple myeloma | 8 kA/m 230 kHz | Tumor volume in treated mice was one-tenth of control in 35 days after treatment |
| Gene | Sequences (5′ → 3′) | Product Size, bp |
|---|---|---|
| GAPDH-F | AAGTTCAACGGCACAGTCAAGG | 22 |
| GAPDH-R | CATACTCAGCACCAGCATCACC | 22 |
| Bax-F | AGGGTGGCTGGGAAGGC | 17 |
| Bax-R | TGAGCGAGGCGGTGAGG | 17 |
| Bcl2-F | ATCGCTCTGTGGATGACTGAGTAC | 24 |
| Bcl2-R | AGAGACAGCCAGGAGAAATCAAAC | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salimi, M.; Sarkar, S.; Hashemi, M.; Saber, R. Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles. Nanomaterials 2020, 10, 2310. https://doi.org/10.3390/nano10112310
Salimi M, Sarkar S, Hashemi M, Saber R. Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles. Nanomaterials. 2020; 10(11):2310. https://doi.org/10.3390/nano10112310
Chicago/Turabian StyleSalimi, Marzieh, Saeed Sarkar, Mansoureh Hashemi, and Reza Saber. 2020. "Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles" Nanomaterials 10, no. 11: 2310. https://doi.org/10.3390/nano10112310
APA StyleSalimi, M., Sarkar, S., Hashemi, M., & Saber, R. (2020). Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles. Nanomaterials, 10(11), 2310. https://doi.org/10.3390/nano10112310






