Barium Titanate (BaTiO3) Nanoparticles Exert Cytotoxicity through Oxidative Stress in Human Lung Carcinoma (A549) Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of BaTiO3 Nanoparticles
2.2. Characterization of BaTiO3 Nanoparticles
2.3. Cell Culture and Exposure of BaTiO3 Nanoparticles
2.4. Cytotoxicity Study
2.5. Oxidative Stress Study
2.6. Statistical Analysis
3. Results and Discussion
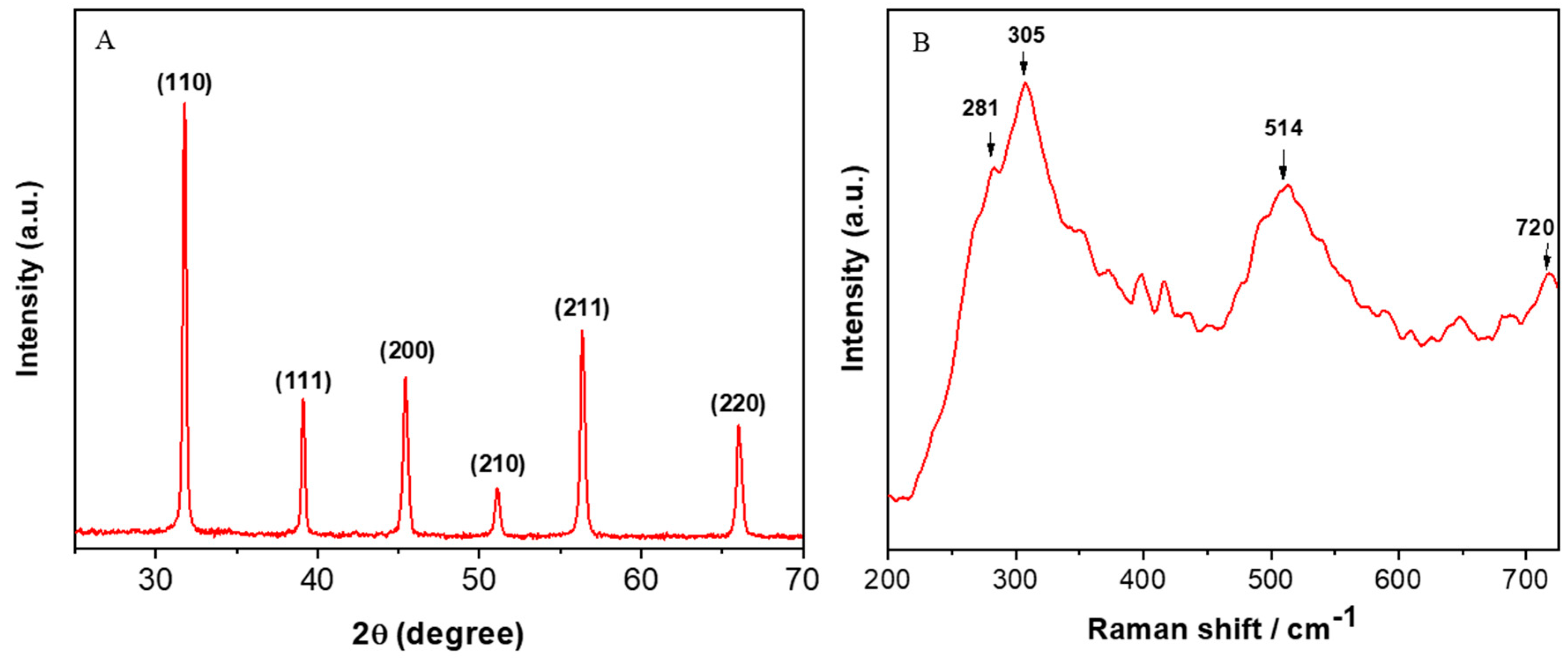
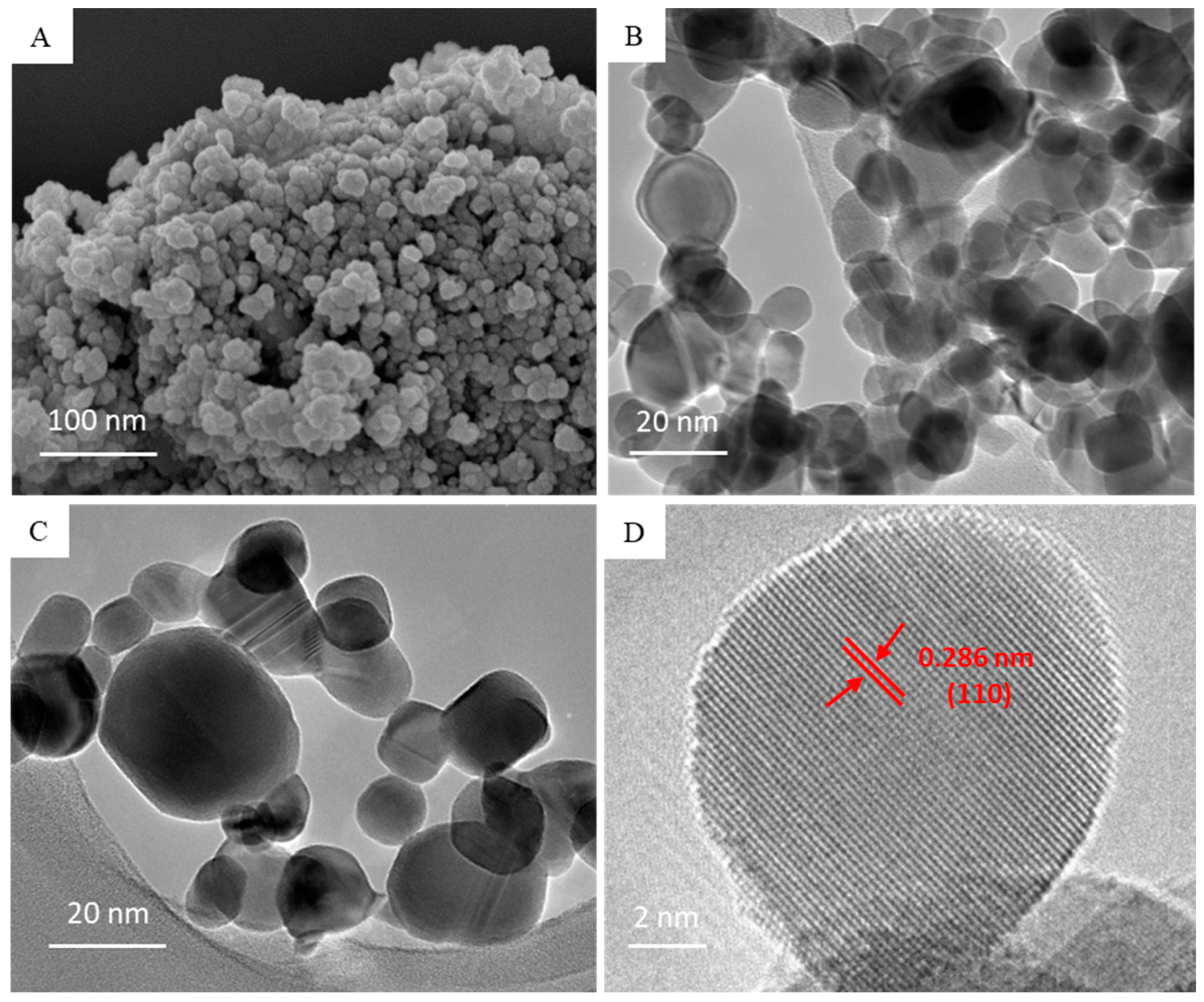
3.1. Characterization of BaTiO3 Nanoparticles
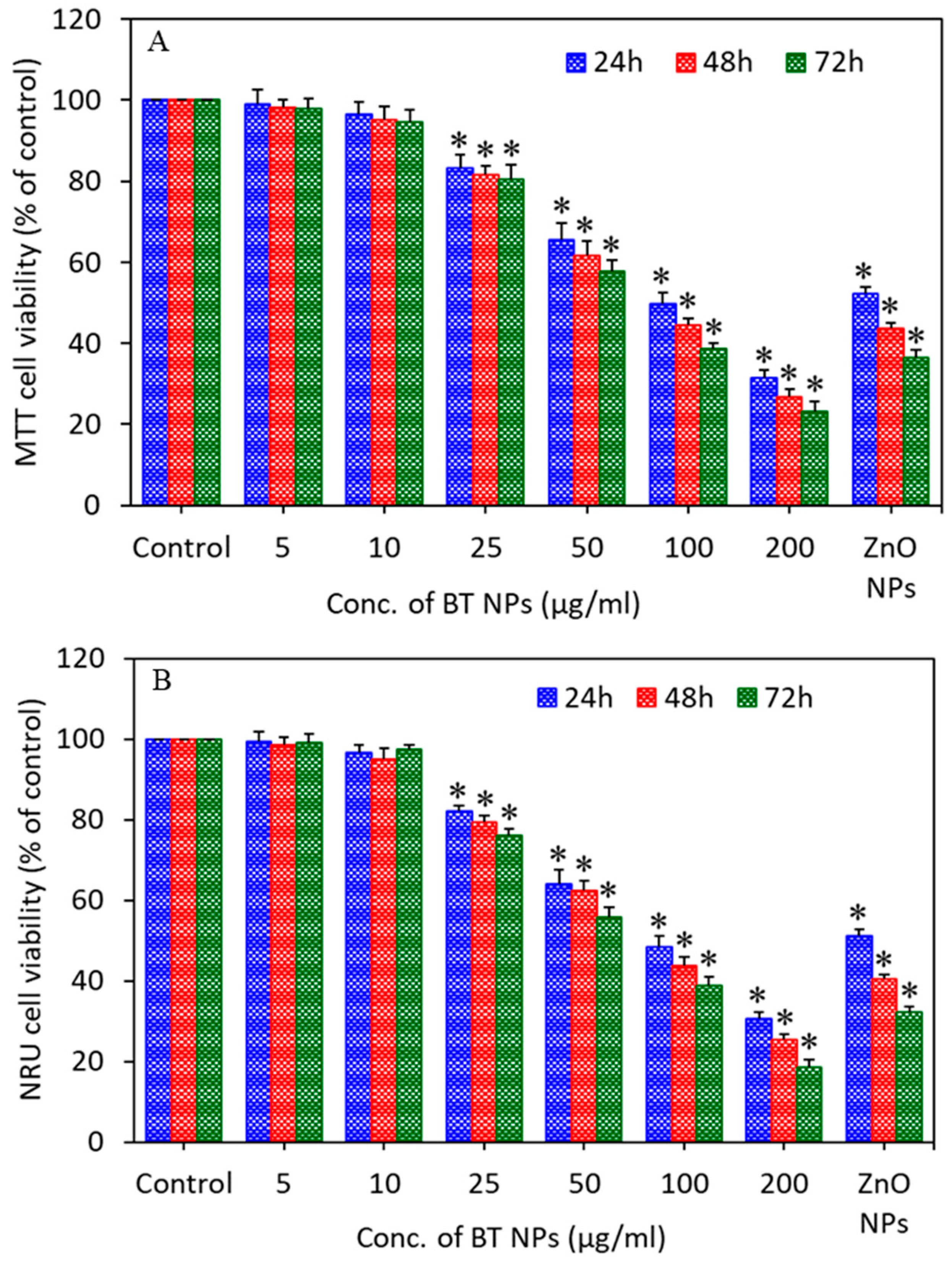
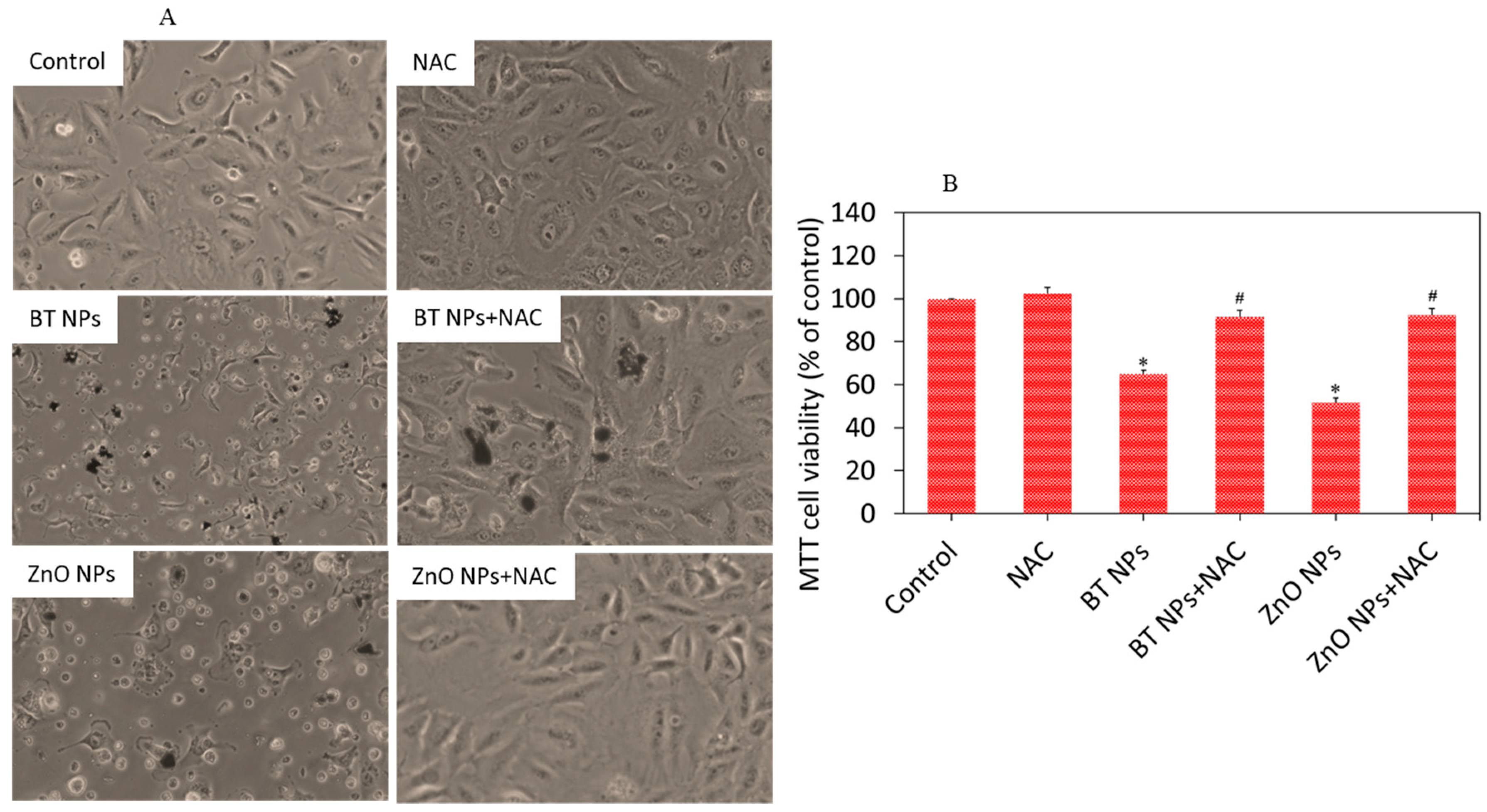
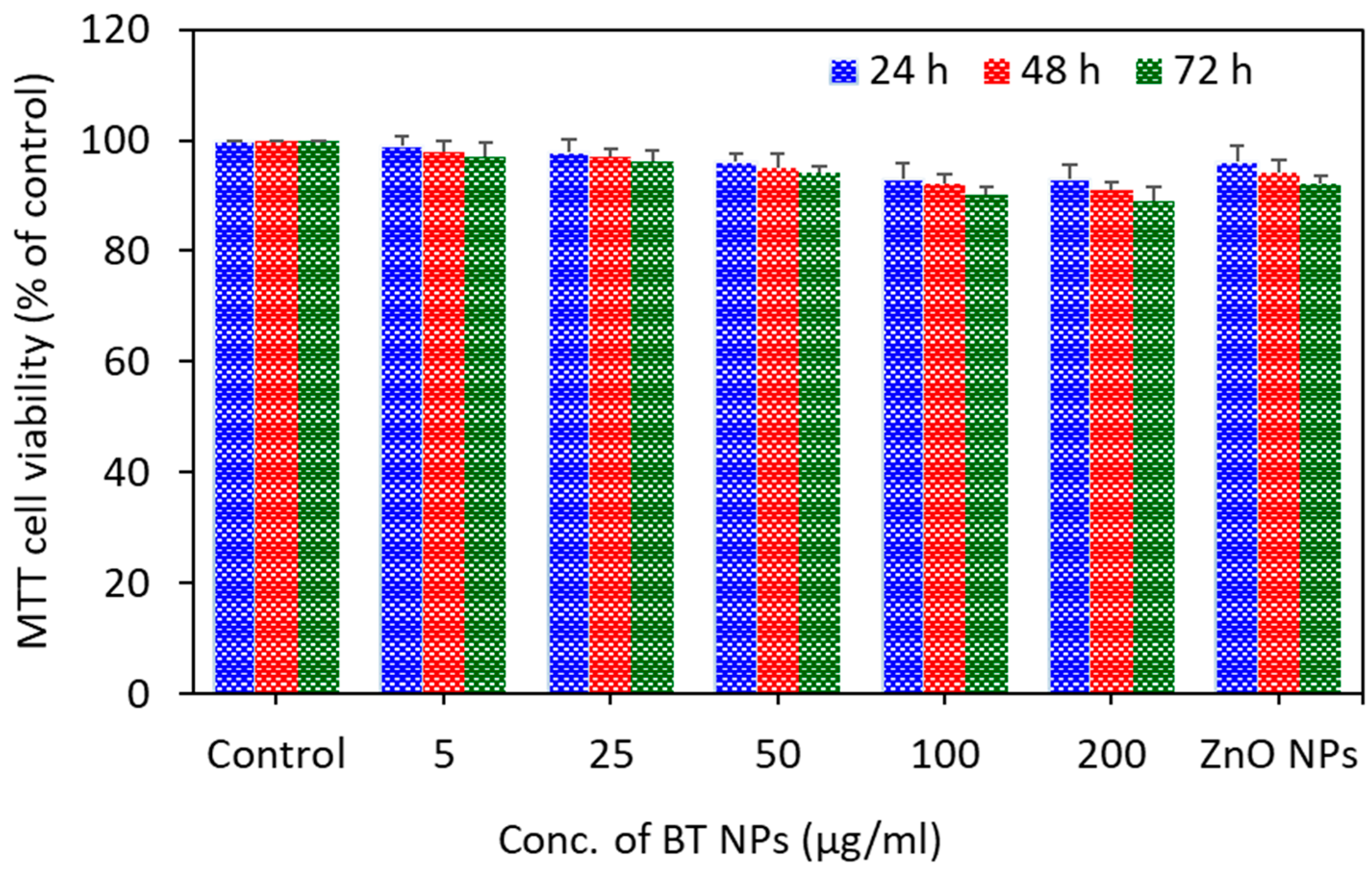
3.2. Cytotoxic Response of BaTiO3 Nanoparticles
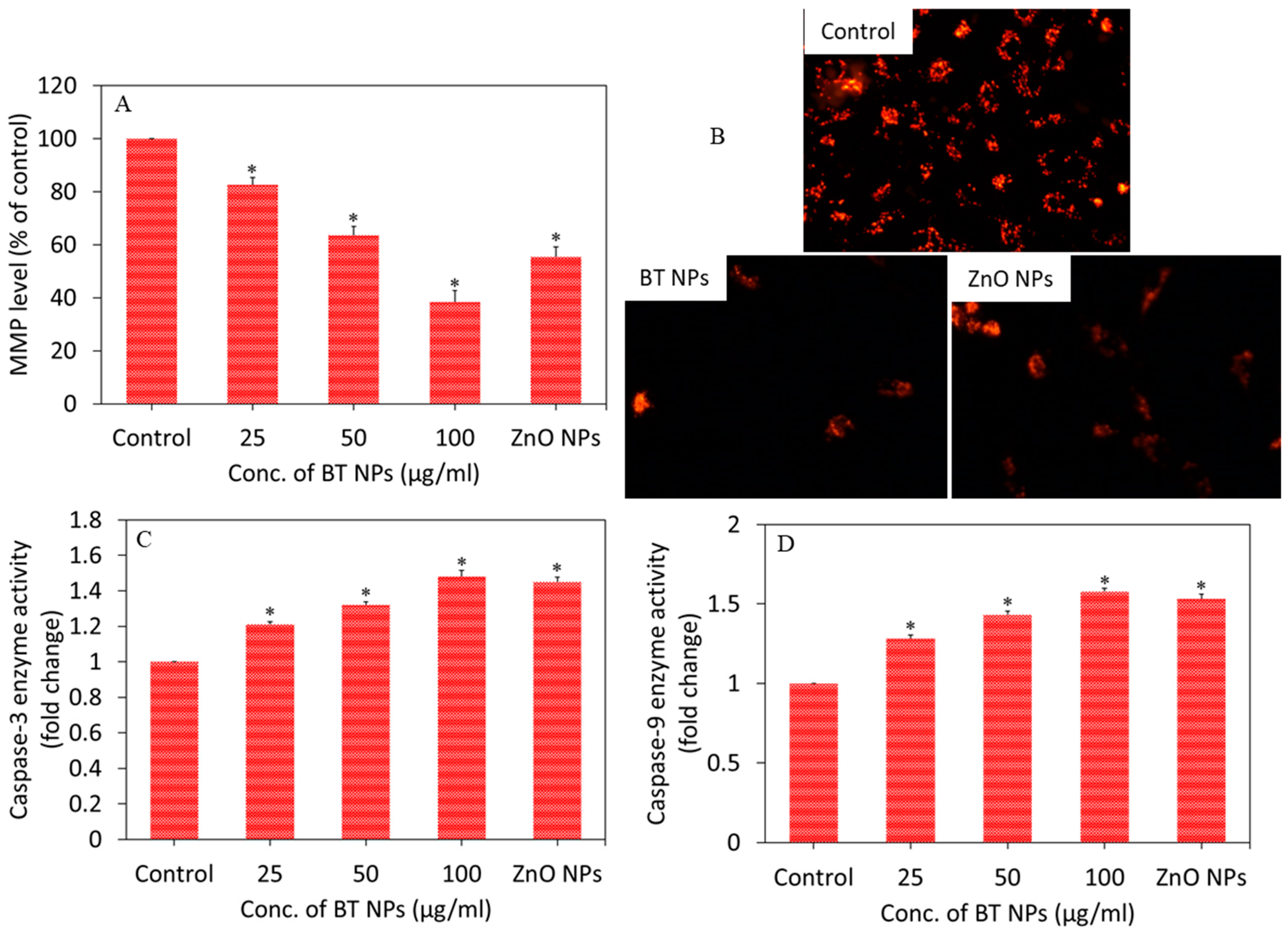
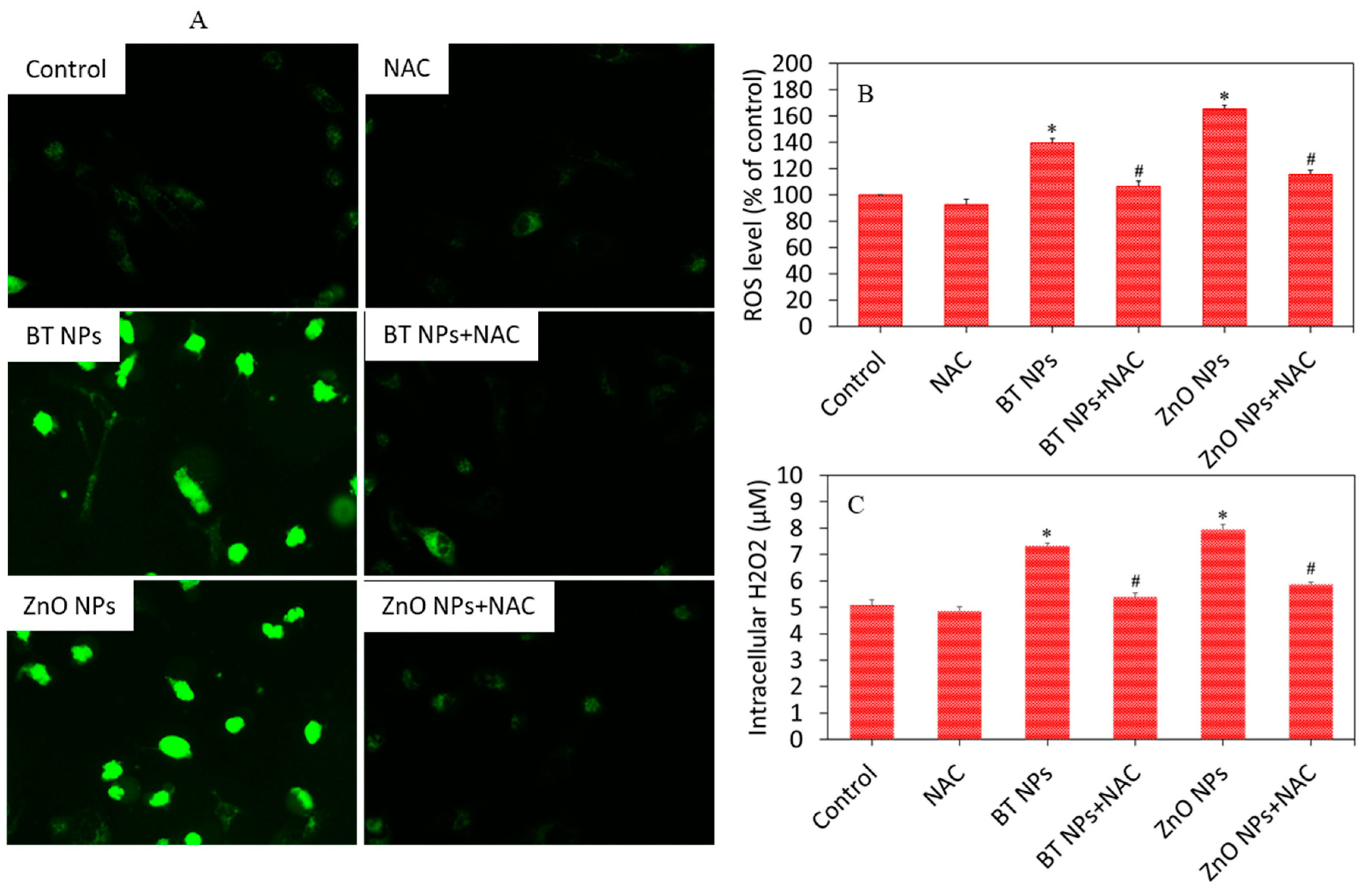
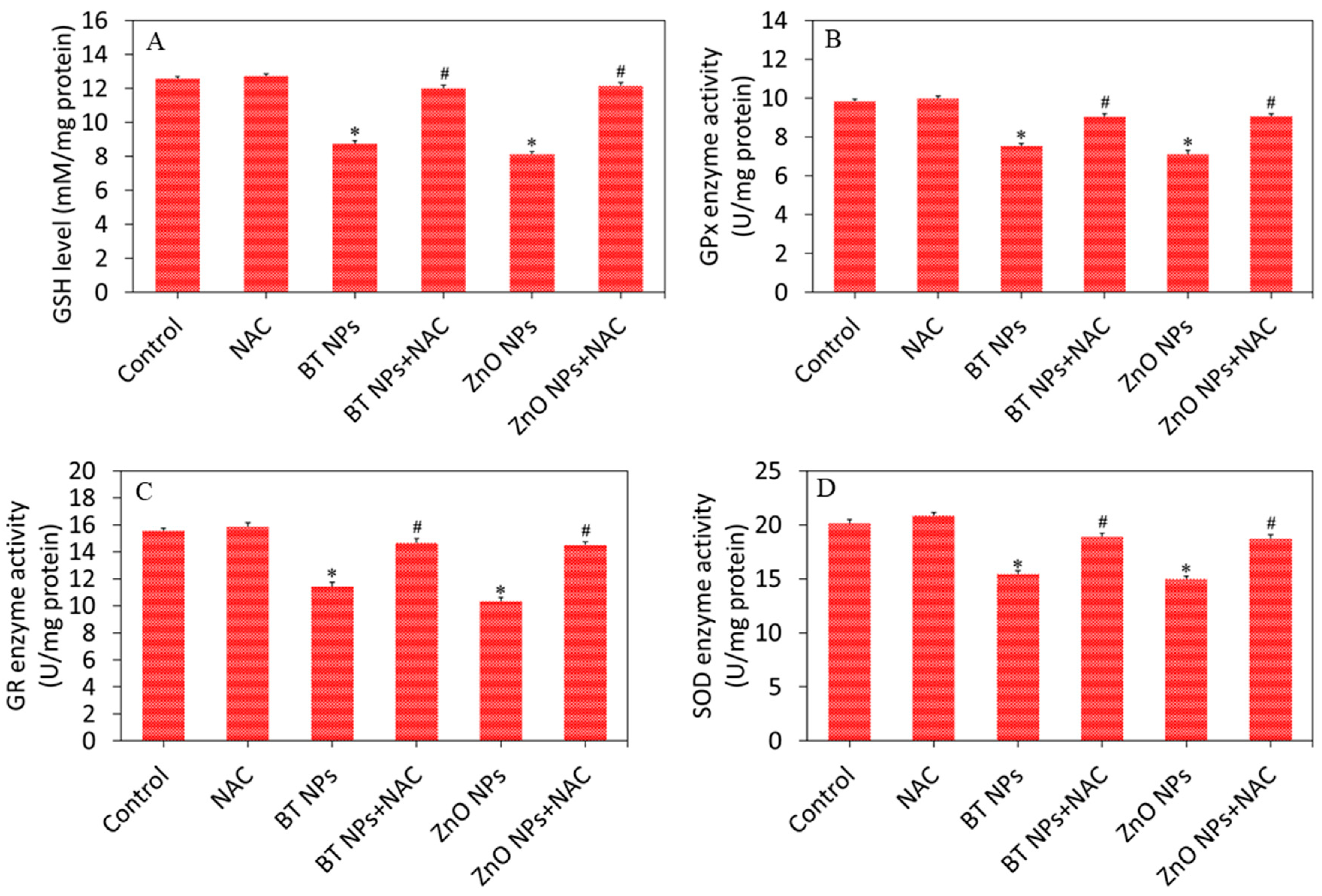
3.3. Oxidative Stress Response of BaTiO3 Nanoparticles
3.4. BaTiO3 Nanoparticles Induced Cytotoxicity via Oxidative Stress
3.5. Effect of BaTiO3 Nanoparticles on Non-Cancerous Normal Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, B.; Iocozzia, J.; Zhao, L.; Zhang, H.; Harn, Y.-W.; Chen, Y.; Lin, Z. Barium titanate at the nanoscale: Controlled synthesis and dielectric and ferroelectric properties. Chem. Soc. Rev. 2019, 48, 1194–1228. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.M.; Chu, N.C.; Luu, V.B.; Xuan, H.N.; Pham, D.T.; Martin, I.; Carriere, P. Enhancement of polarization property of silane-modified BaTiO3 nanoparticles and its effect in increasing dielectric property of epoxy/BaTiO3 nanocomposites. J. Sci. Adv. Mater. Devices 2016, 1, 90–97. [Google Scholar] [CrossRef]
- Na Yoon, Y.; Lee, D.-S.; Park, H.J.; Kim, J.-S. Barium Titanate Nanoparticles Sensitise Treatment-Resistant Breast Cancer Cells to the Antitumor Action of Tumour-Treating Fields. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Marino, A.; Almici, E.; Migliorin, S.; Tapeinos, C.; Battaglini, M.; Cappello, V.; Marchetti, M.; De Vito, G.; Cicchi, R.; Pavone, F.S.; et al. Piezoelectric barium titanate nanostimulators for the treatment of glioblastoma multiforme. J. Colloid Interface Sci. 2019, 538, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Qian, J.; He, S. Polyelectrolyte coated BaTiO3 nanoparticles for second harmonic generation imaging-guided photodynamic therapy with improved stability and enhanced cellular uptake. RSC Adv. 2016, 6, 40615–40625. [Google Scholar] [CrossRef]
- Bai, Y.; Dai, X.; Yin, Y.; Wang, J.; Sun, X.; Liang, W.; Li, Y.; Deng, X.; Zhang, X. Biomimetic piezoelectric nanocomposite membranes synergistically enhance osteogenesis of deproteinized bovine bone grafts. Int. J. Nanomed. 2019, 14, 3015–3026. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Moscato, S.; Petrini, M.; Menciassi, A. Barium Titanate Nanoparticles: Highly Cytocompatible Dispersions in Glycol-chitosan and Doxorubicin Complexes for Cancer Therapy. Nanoscale Res. Lett. 2010, 5, 1093–1101. [Google Scholar] [CrossRef]
- Li, Y.; Dai, X.; Bai, Y.; Liu, Y.; Wang, Y.; Liu, O.; Yan, F.; Tang, Z.; Zhang, X.; Deng, X. Electroactive BaTiO3 nanoparticle-functionalized fibrous scaffolds enhance osteogenic differentiation of mesenchymal stem cells. Int. J. Nanomed. 2017, 12, 4007–4018. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Ansari, M.O.; Parveen, N.; Ahmad, F.; Wani, A.L.; Afrin, S.; Rahman, Y.; Jameel, S.; Khan, Y.A.; Siddique, H.R.; Tabish, M.; et al. Evaluation of DNA interaction, genotoxicity and oxidative stress induced by iron oxide nanoparticles both in vitro and in vivo: Attenuation by thymoquinone. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, F.; Raimondi, V.; Sharova, E.; Silic-Benussi, M.; Ciminale, V. Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells. Antioxidants 2020, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Mu, Q.; Yan, B. Editorial: Nanoparticles in Cancer Therapy-Novel Concepts, Mechanisms, and Applications. Front. Pharmacol. 2019, 9, 1552. [Google Scholar] [CrossRef] [PubMed]
- Bor, G.; Azmi, I.D.M.; Yaghmur, A. Nanomedicines for cancer therapy: Current status, challenges and future prospects. Ther. Deliv. 2019, 10, 113–132. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Ostrovsky, S.; Kazimirsky, G.; Gedanken, A.; Brodie, C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009, 2, 882–890. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Ag-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cells. Sci. Rep. 2017, 7, 17662. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A.; Khan, M.M.; Ahamed, M. Aluminum doping tunes band gap energy level as well as oxidative stress-mediated cytotoxicity of ZnO nanoparticles in MCF-7 cells. Sci. Rep. 2015, 5, 13876. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alhadlaq, H.A. Oxidative stress mediated cytotoxicity of tin (IV) oxide (SnO2) nanoparticles in human breast cancer (MCF-7) cells. Colloids Surfaces B Biointerfaces 2018, 172, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Role of Zn doping in oxidative stress mediated cytotoxicity of TiO2 nanoparticles in human breast cancer MCF-7 cells. Sci. Rep. 2016, 6, 30196. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Heal. 2019, 85. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alhadlaq, H.A.; Aldalbahi, A. Nanocubes of indium oxide induce cytotoxicity and apoptosis through oxidative stress in human lung epithelial cells. Colloids Surf B Biointerfaces 2017, 156, 157–164. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). J. Tissue Cult. Methods 1985, 9, 7–9. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; AlSalhi, M.S.; Alrokayan, S.A. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 2011, 283, 101–108. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper Oxide Nanoparticles Induced Mitochondria Mediated Apoptosis in Human Hepatocarcinoma Cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alrokayan, S.A.; Alhadlaq, H.A. Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere 2019, 216, 823–831. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. [59] Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [PubMed]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singh, M.; Yadav, B.; Ranjan, A.; Kaur, M.; Gupta, S. Synthesis and characterization of perovskite barium titanate thin film and its application as LPG sensor. Sensors Actuators B Chem. 2017, 241, 1170–1178. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Z.; Cui, Z. Low temperature synthesis of cubic BaTiO3 nanoparticles. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013; pp. 399–402. [Google Scholar]
- Qi, J.Q.; Li, Y.; Zhang, X.; Wang, J.; Zhang, Q.; Xue, Y.; Cheng, J.; Li, M.; Han, X.; Ma, Z.; et al. Solid-state synthesis semiconducting BaTiO3 nanoparticles at low temperature. Mater. Chem. Phys. 2020, 242, 122496. [Google Scholar] [CrossRef]
- Galata, E.; Georgakopoulou, E.A.; Kassalia, M.-E.; Papadopoulou-Fermeli, N.; Pavlatou, E. Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties. Materials 2019, 12, 2589. [Google Scholar] [CrossRef]
- Zhuang, C.; She, Y.; Zhang, H.; Song, M.; Han, Y.; Li, Y.; Zhu, Y. Cytoprotective effect of deferiprone against aluminum chloride-induced oxidative stress and apoptosis in lymphocytes. Toxicol. Lett. 2018, 285, 132–138. [Google Scholar] [CrossRef]
- Balakireva, A.V.; Zamyatnin, A.A.J. Cutting Out the Gaps Between Proteases and Programmed Cell Death. Front. Plant Sci. 2019, 10, 704. [Google Scholar] [CrossRef]
- Hu, L.; Chen, M.; Chen, X.; Zhao, C.; Fang, Z.; Wang, H.; Dai, H. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gào, X.; Holleczek, B.; Cuk, K.; Zhang, Y.; Anusruti, A.; Xuan, Y.; Xu, Y.; Brenner, H.; Schöttker, B. Investigation on potential associations of oxidatively generated DNA/RNA damage with lung, colorectal, breast, prostate and total cancer incidence. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Woo, S.M.; Kim, M.W.; Lee, H.-S.; Kim, S.H.; Kang, S.C.; Lee, E.-W.; Min, K.-J.; Kwon, T.K. Cathepsin K inhibition-induced mitochondrial ROS enhances sensitivity of cancer cells to anti-cancer drugs through USP27x-mediated Bim protein stabilization. Redox Biol. 2020, 30, 101422. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Alarifi, S.; Bishari, W.; Al-Bishri, W. In vitro apoptotic and DNA damaging potential of nanobarium oxide. Int. J. Nanomed. 2016, 11, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free. Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Ahmad, J.; Siddiqui, M.A.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A.; Khan, S.; Wahab, R.; Al-Khedhairy, A.A.A.; Al-Salim, A.; Musarrat, J.; et al. Copper doping enhanced the oxidative stress–mediated cytotoxicity of TiO2 nanoparticles in A549 cells. Hum. Exp. Toxicol. 2017, 37, 496–507. [Google Scholar] [CrossRef]
- Smulders, S.; Kaiser, J.-P.; Zuin, S.; Van Landuyt, K.L.; Golanski, L.; Vanoirbeek, J.; Wick, P.; Hoet, P.H.M. Contamination of nanoparticles by endotoxin: Evaluation of different test methods. Part. Fibre Toxicol. 2012, 9, 41. [Google Scholar] [CrossRef]
- Dubey, A.K.; Thrivikraman, G.; Basu, B. Absence of systemic toxicity in mouse model towards BaTiO3 nanoparticulate based eluate treatment. J. Mater. Sci. Mater. Electron. 2015, 26, 1–11. [Google Scholar] [CrossRef]
- Taylor, Z.; Marucho, M. The Self-Adaptation Ability of Zinc Oxide Nanoparticles Enables Reliable Cancer Treatments. Nanomaterials 2020, 10, 269. [Google Scholar] [CrossRef]
- Wiesmann, N.; Tremel, W.; Brieger, J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B 2020, 8, 4973–4989. [Google Scholar] [CrossRef]
| BaTiO3 NPs | ZnO NPs [25] | ||
|---|---|---|---|
| Parameters | Mean Value | Parameters | Mean Value |
| Primary size | Primary size | ||
| TEM | 16 nm | TEM | 41 nm |
| XRD | 15 nm | XRD | 43 nm |
| Hydrodynamic size # | Hydrodynamic size # | ||
| Distilled water | 105 ± 5 nm | Distilled water | 113 ± 7 nm |
| Culture medium | 114 ± 3 nm | Culture medium | 155 ± 6 nm |
| Zeta potential # | Zeta potential # | ||
| Distilled water | 23 ± 2 eV | Distilled water | 17 ± 3 eV |
| Culture medium | −27 ± 3 eV | Culture medium | −19 ± 2 eV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A.; Alshamsan, A. Barium Titanate (BaTiO3) Nanoparticles Exert Cytotoxicity through Oxidative Stress in Human Lung Carcinoma (A549) Cells. Nanomaterials 2020, 10, 2309. https://doi.org/10.3390/nano10112309
Ahamed M, Akhtar MJ, Khan MAM, Alhadlaq HA, Alshamsan A. Barium Titanate (BaTiO3) Nanoparticles Exert Cytotoxicity through Oxidative Stress in Human Lung Carcinoma (A549) Cells. Nanomaterials. 2020; 10(11):2309. https://doi.org/10.3390/nano10112309
Chicago/Turabian StyleAhamed, Maqusood, Mohd Javed Akhtar, M.A. Majeed Khan, Hisham A. Alhadlaq, and Aws Alshamsan. 2020. "Barium Titanate (BaTiO3) Nanoparticles Exert Cytotoxicity through Oxidative Stress in Human Lung Carcinoma (A549) Cells" Nanomaterials 10, no. 11: 2309. https://doi.org/10.3390/nano10112309
APA StyleAhamed, M., Akhtar, M. J., Khan, M. A. M., Alhadlaq, H. A., & Alshamsan, A. (2020). Barium Titanate (BaTiO3) Nanoparticles Exert Cytotoxicity through Oxidative Stress in Human Lung Carcinoma (A549) Cells. Nanomaterials, 10(11), 2309. https://doi.org/10.3390/nano10112309









