Abstract
Defect-rich photocatalytic materials with excellent charge transfer properties are very popular. Herein, Sm-doped CeO2 nanorods were annealed in a N2 atmosphere to obtain the defective Sm-doped CeO2 photocatalysts (Vo–Sm–CeO2). The morphology and structure of Vo–Sm–CeO2 were systematically characterized. The Vo–Sm–CeO2 nanorods demonstrated an excellent photodegradation performance of methyl blue under visible light irradiation compared to CeO2 nanorods and Sm–CeO2. Reactive oxygen species including OH, ·O2−, and h+ were confirmed to play a pivotal role in the removal of pollutants via electron spin resonance spectroscopy. Doping Sm enhances the conductivity of CeO2 nanorods, benefiting photogenerated electrons being removed from the surface reactive sites, resulting in the superior performance.
1. Introduction
Semiconductor-based photocatalytic oxidation reactions have been extensively considered as promising advanced oxidation processes (AOPS) technology for the removal of pollutants in the air and water that have a negative impact on environmental quality, ecosystem safety, and human health [1,2,3,4,5,6,7,8,9,10,11]. However, low photocatalytic efficiency limits its practical application. Thus, various works have been developed to improve its photocatalytic performance via enhanced light absorption and increased the separation efficiency of photogenerated charge carriers [12,13,14,15]. Among them, defect engineering is an efficient method to prepare the ideal photocatalysts [16,17].
Crystallographic defects are generated in materials when the neat arrangement of atoms is broken [18,19,20,21]. At present, defects are mainly prepared by the following methods: hydrogen reduction, calcining under nitrogen atmosphere, strong reducing agent reduction, electric reduction, etc. [22,23,24]. The defects in photocatalysts not only act as recombination centers for free electrons and holes, but also scattering centers for electron and hole travelling, which is not conducive to the diffusion of charge carriers [25,26,27,28]. Defects on the surface of semiconductors can be reactive sites where photoelectrons reduce dissolved O2 to O2− [29]. Electron trapping sites can consume the photoelectrons, thus preventing the recombination of charge carriers, which can enhance the photocatalytic performance [30,31]. However, the instability of defective materials limits its application because O2 gases will refill the defect sites. Therefore, how to use defects effectively to improve the performance and stability of catalysts is one of the research hotspots at present.
Cerium oxide (CeO2) has attracted attention in many research fields such as photocatalysis, thermo-catalysis, and electro-catalysis due to its remarkable oxygen-storage ability and redox properties (Ce4+/Ce3+) [32,33,34,35,36,37]. These properties enhance the release of active oxygen species, balancing the nascent electric charges spontaneously, resulting in defects in CeO2 forming and being eliminated quickly [38,39]. Aslam et al. prepared CeO2-x surface defects and used them for the degradation of phenol and its derivatives. Defects serve as the traps and transfer centers to enhance the generation of reactive oxygen species [40]. Jiang and coworkers confirmed the surface-defect dependence of photo-performance [39]. Furthermore, doping a trivalent element into CeO2 introduces defects such as Eu-doped CeO2 and Yb-CeO2 [41,42,43,44]. Our reported work demonstrated that Eu doping can introduce oxygen vacancies into CeO2 nanosheets, enhancing the charge transfer. This phenomenon has inspired us to propose enhancing the oxidation and reduction properties and introducing surface defects to improve the photocatalytic performance of CeO2.
In this work, we prepared the defective Ce-based photocatalysts (Vo–Sm–CeO2) where Sm-doped CeO2 nanorods were annealed in a N2 atmosphere (Figure 1a). The defects in CeO2 were systematically characterized by electron spin resonance (EPR), X-ray photoelectron spectroscopy (XPS), and Raman. The Vo–Sm–CeO2 nanorods were tested for the photodegradation of methyl blue (MB) and the results revealed that the photocatalytic activity of Vo–Sm–CeO2 was higher than those of pristine CeO2 and Sm-doped CeO2, which can be attributed to the existence of defects in CeO2. Defects in CeO2 nanorods enhanced the electrical conduction and promoted charge transfer dynamics. Moreover, the role of defects in producing reactive oxygen species (ROS) was also studied by electron spin resonance spectroscopy.
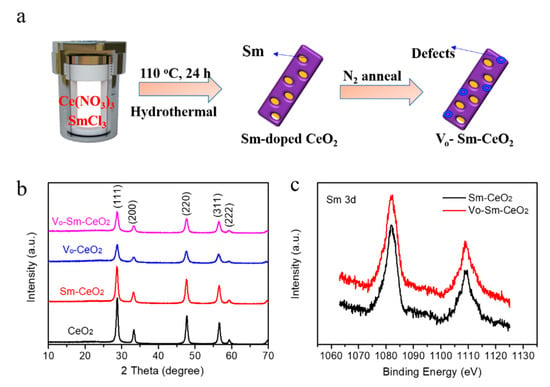
Figure 1.
(a) Sketch map for preparing Vo–Sm–CeO2 sample. (b) XRD spectra of CeO2, Sm–CeO2, Vo–CeO2 and Vo–Sm–CeO2. (c) Sm 3d XPS spectra of Sm–CeO2 and Vo–Sm–CeO2.
2. Experimental Section
2.1. Preparation of Photocatalysts
CeO2 was obtained by the following method. Ce(NO3)3 6H2O was dissolved in distilled water (5 mL). Then, 10 mL of 14 M NaOH was added into the above solution. Finally, the solution was transferred into a Teflon-lined stainless-steel autoclave and it was kept at 110 °C for 24 h. The obtained solid was washed with water and calcined in air at 200 °C for 1 h. Sm doped-CeO2 was obtained with the same CeO2 nanorods by adding 0.13 g, 0.26 g, and 0.39 g SmCl3, respectively. CeO2 and Sm–CeO2 were annealed in N2 gas at 600 °C for 4 h with a ramp rate of 10 °C min−1 to obtain Vo–CeO2 and Vo–Sm–CeO2, respectively. The 2.0 g prepared samples were put into a tube furnace (OTF-1500X-II corundum tube ø 60 mm by Hefei Kejing Materials Technology Co. Ltd., Hefei, China), and the flow rate of N2 was continuously pumped into the tube for 30 min at a flow rate of 300 mL min−1. The purpose was to drain the air out of the tube and form a high concentration of N2 atmosphere in the tube. A flow controller (Beijing Sevenstar Flow Co. Ltd., Beijing, China) was used to maintain the flow stability of N2.
2.2. Characterization of Photocatalysts
The main instruments used in the experiment are listed as follows: transmission electron microscope (JEM2010-HR, Tokyo, Japan), scanning electron microscope (Gemini SEM 500, Jena, Germany), X-ray diffractometer (D8 ADVANCE, NASDAQ, Billerica, MA, USA), UV−Vis−NIR spectrophotometer (UV-2450, Shimadzu, China), X-ray photoelectron spectroscope (ESCALAB250, Waltham, MA, USA), specific surface area measurements (ASAP 2020V3.03H, Waltham, MA, USA), Raman (Nicolet NXR 9650, Waltham, MA, USA), and a room-temperature photoluminescence spectroscope (FLS920, Edinburgh, UK). The electrochemical tests were carried out with a CHI 660C electrochemical station in a standard three electrode configuration. The illumination source was an AM 1.5 G solar simulator (Newport, LCS 100 94011A (class A), Waltham, MA, USA) directed at the quartz PEC cell (100 mW cm2). The working electrode (photoanode) was as follows: 20 mg of the sample was mixed with 2 mL ethyl alcohol to form a slurry and then coated onto a 1 cm × 1 cm fluorine-doped tin oxide (FTO) glass substrate and dried. The reactive species in the photocatalysis were investigated by the electron spin resonance test using the X-band (9.45 GHZ) with 5.00 G modulation amplitude and a magnetic field modulation of 100 kHz. The contact angles of H2O drops deposited on the surface of the film were measured at 25 °C using a contact angle meter (SL150, Kino Industrial Co., Ltd., Shanghai, China).
2.3. Photocatalytic Performance
In a typical process, 20 mg photocatalyst and 100 mL MB solution (10 mg L−1) as a standard pollutant were mixed in a 250 mL reaction vessel with a recirculating cooling water system at 25 °C under simulated solar light irradiation. Prior to the photocatalysis experiment, the sample solution was stirred for 60 min in the dark. The suspension was then exposed to a 300 W xenon lamp light equipped with a UV cutoff filter (λ > 420 nm) under continuous magnetic stirring. At given time intervals, 3 mL suspension from the reaction vessel was pipetted and centrifuged to separate the photocatalyst powder and MB solution. Finally, the absorption spectrum of the supernatant was determined by a UV–Vis spectrophotometer and the absorbance of MB was measured at 665 nm. The degradation efficiency of MB was calculated by the following equation:
where C0 and Ct represent the initial concentration of MB before irradiation and the residual concentration of MB in solution at irradiation time t, respectively.
Degradation efficiency of MB (%) = (C0 − Ct)/C0 × 100%.
3. Results and Discussion
3.1. The Morphology and Structure Characterization of the Catalysts
The crystal structures of CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2 were first identified by X-ray diffraction (XRD). As described in Figure 1b, the peaks of all the prepared samples could be indexed to the (111), (200), (220), (311), (222) planes of the typical cubic structure of CeO2 (JCPD#34-0394) [34]. No other new peaks appeared for Sm–CeO2 and the Vo–Sm–CeO2 samples, suggesting that Sm2O3 was not produced, while the footprints of Sm could be detected by the X-ray photoelectron spectroscopy (XPS) (Figure 1c). The Sm 3d peaks were located at 1084 eV and 1110 eV, suggesting the existent of Sm3+ [45]. Combined with the XRD results, it confirms that Sm was doped into the Sm–CeO2 and Vo–Sm–CeO2 samples.
The morphology of the prepared samples was observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Figure S1 demonstrates the SEM images of CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2. Four samples demonstrated almost the same morphology of nanorods, suggesting that doping and introduction of defects did not change the morphology and that the specific surface areas did not have much difference (Figure S2). Moreover, Figure 2a shows the TEM image of Vo–Sm–CeO2. Nanorod morphology could be seen and the inset selected area electron diffraction (SAED) pattern demonstrated that it was polycrystalline. High resolution transmission electron microscopy (HRTEM) of pristine CeO2 and Vo–Sm–CeO2 is provided for contrast (Figure 2b,c). Pristine CeO2 demonstrates the well-lined 0.156 nm lattice spacing, which is in accordance with (222) CeO2 [34]. Significantly, the inside lattice spacing of Vo–Sm–CeO2 became disordered, suggesting that numerous defects are generated after doping Sm. Closer inspection using high-angle annular dark-field scanning TEM (HAADF-TEM) showed the nanorod morphology of Vo–Sm–CeO2 (Figure 2d). High multiples of the images of Vo–Sm–CeO2 demonstrated that there was an obvious distortion atom, which may originate from Sm doping [46]. Furthermore, the corresponding EDX mapping (Figure 2g) indicated that Ce and Sm were homogeneously distributed in the Vo–Sm–CeO2 nanorods.
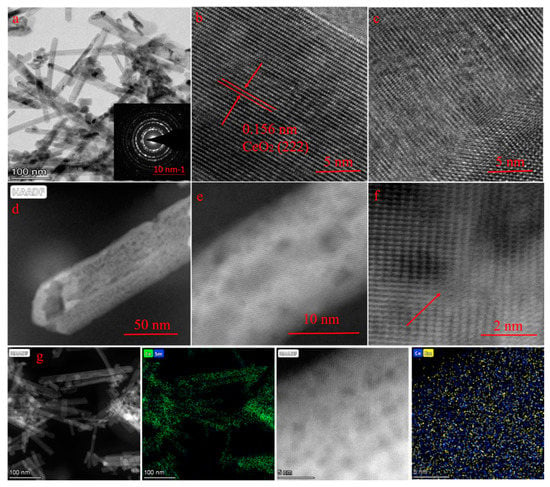
Figure 2.
(a) Transmission electron microscope (TEM) images of Vo–Sm–CeO2, high resolution transmission electron microscopy (HR-TEM) of (b) CeO2, and (c) Vo–Sm–CeO2, (d–f) high-angle annular dark-field scanning TEM (HAADF-STEM) mages of Vo–Sm–CeO2, (g) energy dispersive X-ray spectroscopy (EDS) images of Vo–Sm–CeO2.
The existence of defects was confirmed by XPS, Raman, and electron spin resonance spectroscopy [47,48]. Figure 3a shows the O 1s XPS of all samples. Two strong peaks appeared at 529.1 eV and 531.3 eV for pristine CeO2 nanorods, which were indexed to lattice oxygen and surface active oxygen [49]. The lattice oxygen peak of Vo–Sm–CeO2 shifted 0.3 eV toward low energy due to the effect of Sm doping and the intensity of the surface active oxygen peak increased, suggesting the existence of more surface defects. The surface property of the four samples was detected by the contact angles with the water droplet (Figure 3b). The contact angles decreased after Sm doping and introducing defects, suggesting that Vo–Sm–CeO2 was in better contact with water. Furthermore, the Raman spectra of the four samples demonstrated the existence of defects (Figure 3c). The peak at 465 cm−1 can be indexed to the vibrational mode of fluorite-type CeO2 and the peak at 600 cm−1 was attributed to the defects [50]. Figure 3d describes the EPR image of CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2. No obvious signal was detected in the pristine CeO2 nanorods, while Sm–CeO2 and Vo–Sm–CeO2 demonstrated a characteristic peak of defect, confirming the approach to creating defective materials [47].
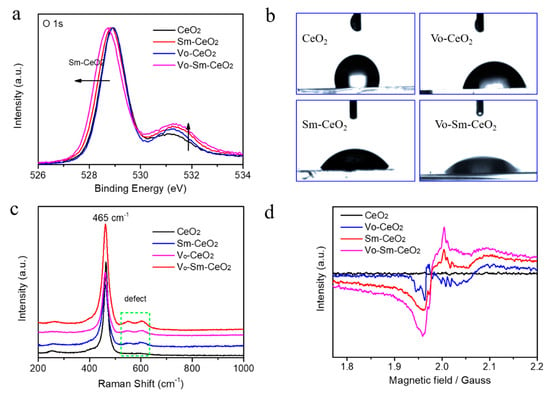
Figure 3.
(a) O 1s XPS spectra. (b) Contact angles with water droplet, (c) Raman spectra, and (d) EPR spectra of CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2.
3.2. Photocatalytic Performance of the Catalysts
To study the relationship between Sm doping and defects with photocatalytic performance, CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2 were used to remove the MB with a 300 W xenon lamp irradiation. Figure 4a shows the photodegradation efficiencies of the four samples. Obviously, the photolysis of MB without photocatalysts can be ignored under our experimental conditions. Pristine CeO2 nanorods had 40% photodegradation efficiency after 2 h irradiation. Significantly, Sm doping and defects can enhance the photocatalytic performance of CeO2. Sm–CeO2 and Vo–CeO2 possessed 85% and 80% photodegradation efficiencies, respectively. The Vo–Sm–CeO2 sample demonstrated the best degradation performance among the four samples, which could almost entirely remove the MB at 90 min irradiation. The doping amount of Sm was also optimized, which is shown in Figure S3. This result suggests that Sm-doping and surface defects co-promote the photocatalytic activity of CeO2. Furthermore, Figure 4b displays the reaction kinetic of photodegradation MB based on Figure 4a, which can be indexed to the Langmuir–Hinshelwood first-order kinetics model. The rate constant value of the Vo–Sm–CeO2 sample was 0.012 min−1, which was much higher than those of CeO2 (0.003 min−1), Sm-CeO2 (0.007 min−1), and Vo-CeO2 (0.007 min−1). The performance of Vo–Sm–CeO2 was also compared with other reported Ce based photocatalysts (Table S1), suggesting that the Vo–Sm–CeO2 sample had a superior photocatalytic performance. Furthermore, the total organic carbon (TOC) removal was performed to identify that the MB removal could be attributed to mineralization. The TOC removal efficiencies (120 min) for MB of CeO2, Vo–CeO2, Sm–CeO2, and Vo–Sm–CeO2 samples were 35%, 79%, 75%, and 98%, respectively (Figure S4). This result revealed that most of the MB were mineralized to H2O and CO2 during our degradation condition.
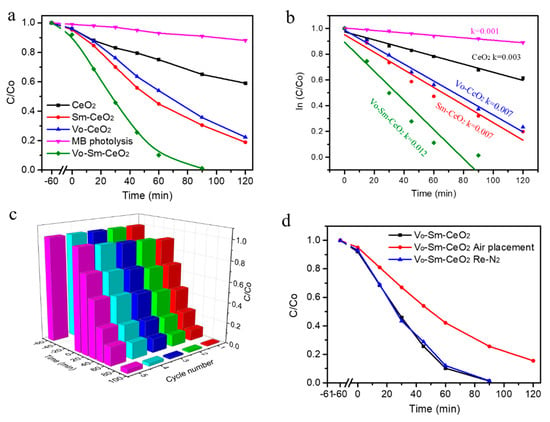
Figure 4.
(a) Photocatalytic performance of the degradation of MB, (b) reaction kinetic, (c) stability test toward CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2. (d) Photocatalytic performance of Vo-Sm-CeO2 after treatment.
Photocatalytic stability is an important factor for the application of photocatalysts [51]. Figure 4c depicts the cycling stability of Vo–Sm–CeO2 nanorods under visible light irradiation. After five cycles of testing, the photodegradation efficiency of Vo–Sm–CeO2 nanorods reduced to 85% and the morphology and the crystal structures remained the same (Figure S4). Defects on the Vo–Sm–CeO2 surface of the nanorods can be refilled with oxygen gas, which then affects its stability, as can be observed in other reports. Therefore, we used Vo–Sm–CeO2 nanorods to anneal in air and test its performance. Just as we expected, the performance decreased, similar to the performance of the Sm–CeO2 nanorods. Interestingly, the degradation efficiency could reach 99% after re-calcining in nitrogen, suggesting that defects can also regenerate on the surface of the Vo–Sm–CeO2 nanorods (Figure 4d).
3.3. Active Species Trapping Experiments
Active species such as superoxide radicals, hydroxyl radicals, and h+ play an important role in advanced oxidation processes (AOPS) technology [52]. To understand which active species generated during the photocatalytic oxidation reaction, we performed active species trapping experiments and electron spin resonance (ESR) measurements [4,34]. Figure 5a shows the photodegradation efficiency of Vo–Sm–CeO2 nanorods with different scavengers after 90 min irradiation with visible light (benzoquinone for O2−, ter-butyl alcohol for OH, and methanol for h+). Obviously, the photodegradation efficiencies decreased after adding three scavengers, suggesting that O2−, OH, and h+ are generated during photocatalysis. The active species have a high oxidizing ability to degrade MB into small molecules. Furthermore, Figure 5b,c displays the ESR results of DMPO-·OH and DMPO-O2− for Vo–Sm–CeO2 nanorods using 5,5-dimethyl-1-pyrriline noxide (DMPO) as a spin trap. No signal corresponding to DMPO-·OH and DMPO-·O2− were detected for Vo–Sm–CeO2 nanorods in the dark, suggesting ROS did not generated without light irradiation. Four strong peaks were observed when the light was turned on, which was indexed to DMPO-·OH [4,34] and six peaks corresponding to DMPO-O2− appeared, suggesting that ·O2− was produced during the photocatalysis [30]. The ESR results were consistent with the active species trapping experiments, suggesting that O2−, OH, and H+ play an extremely important role in photocatalysis.
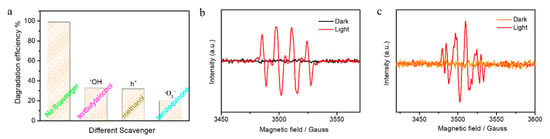
Figure 5.
(a) Photodegradation efficiency of Vo–Sm–CeO2 nanorods with different scavengers after 90 min. The ESR results of the Vo–Sm–CeO2 nanorods: (b) DMPO-OH and (c) DMPO-O2−.
3.4. Charge Transfer Analysis
To study the effect of defects and Sm doping in CeO2 nanorods, several characterizations were performed including transient photocurrent responses and electrochemical impedance spectra (ZIS) [53,54,55]. Figure 6a shows the transient photocurrent responses of the four samples. The current density value of Vo–Sm–CeO2 was higher than those of CeO2, Sm–CeO2, and Vo–CeO2 in the same window, suggesting that Vo–Sm–CeO2 had the fast charge transfer. Note that the values of Vo–CeO2 and Vo–Sm–CeO2 decreased after several ON–OFF cycles, which was in accordance with the stability. Furthermore, the charge behaviors were analyzed by ZIS spectra under visible light irradiation (Figure 6b) [56]. Vo–Sm–CeO2 displayed the smallest dimeter among the four samples, suggesting that it had a small charge transfer resistance [57]. The charge transfer resistance value of Sm–CeO2 was smaller than that of Vo–CeO2, suggesting that Sm doping mainly improved the conductivity of CeO2 [58]. Therefore, Sm doping and defects can improve the charge transfer of CeO2.
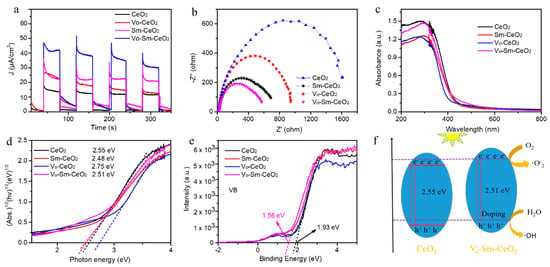
Figure 6.
(a) Transient photocurrent responses, (b) electrochemical impedance spectra (applied potential 0.1 eV), (c) UV–Vis spectra, (d) optical band gaps, (e) VB spectra from XPS of CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2. (f) Schematic for the proposed mechanism.
The light absorption range of CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2 was observed by UV-Vis spectra (Figure 6c). It can be seen that pristine CeO2 nanorods had an absorption region at 420 nm, suggesting that it responds to UV light. After Sm doping and introducing defects into CeO2, the absorption band gap displayed little blue shift, suggesting that light absorption was not the main effect for the photocatalytic performance. The bandgaps of four samples could be calculated based on the following formula: a = A(hv − Eg)2/hv (a is the absorption coefficient and A is the absorption constant for indirect transition) [59,60]. Therefore, the bandgap values of CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2 were 2.55 eV, 2.48 eV. 2.75 eV, and 2.51 eV, respectively. Interestingly, Sm doping could narrow the bandgap while defects increased the bandgap [61,62,63]. The relative valence band maximum (VBM) value could be obtained by the XPS valence spectra (Figure 6e). The VBM values of pristine CeO2 and Vo–Sm–CeO2 were about 1.93 and 1.56 eV, respectively. The schematic for the proposed mechanism is shown in Figure 6f. Doping Sm into the CeO2 nanorods changed the location of VBM and CBM, and enhanced the electrical conductivity. Photoelectrons in the CBM of Vo–Sm–CeO2 are more conductive to producing reactive oxygen species due to its high potential of CBM. Furthermore, defects on the surface of the Vo–Sm–CeO2 nanorods can act as reactor sites for O2 reduction. All these results enable Vo–Sm–CeO2 nanorods to have better photocatalytic performance.
4. Conclusions
In this work, we report on a defective Sm–CeO2 nanorod photocatalyst that had a superior photodegradation performance of MB (almost 100%, 90 min) under visible light irradiation. Such performance was achieved due to the synergistic effect of defects and Sm doping, which enhanced the separation of the photogenerated holes and electrons. Sm doping can effectively improve the conductivity of CeO2 nanorods and the surface defects can act as reactive sites for photogenerated electrons to reduce O2 into ·O2−. This work not only provides a better understanding of the photocatalytic mechanism, but also offers some guidance for designing a Ce based photocatalyst with high efficient performance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-4991/10/11/2307/s1, Figure S1: SEM images of CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2; Figure S2: The N2 adsorption spectra of all samples; Figure S3: Photocatalytic performance of Sm doping CeO2; Figure S4: TOC removal efficiency CeO2, Sm–CeO2, Vo–CeO2, and Vo–Sm–CeO2 at 120 min; Figure S5: XRD spectra and SEM image of Vo–Sm–CeO2 after the five cycles test; Table S1: The performance of Vo–Sm–CeO2 was also compared with other reported Ce based photocatalysts.
Author Contributions
J.Y. performed the experiments and wrote the paper; N.X., J.Z. and W.F. partly performed the experiments; Y.H. designed and reviewed the manuscript; Y.T. conceived, designed, and provided the equipment. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (21706295 and 21773315), the Natural Science Foundation of Guangdong Province (2020A1515010798), the Pearl River S & T Nova Program of Guangzhou (201906010024), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01C102), the Science and Technology Research Project of Guangzhou (202002010007), the Special Fund for Science and Technology Innovation Strategy of Guangdong Province (pdjh2020b0468), and the Guangzhou University national college students’ innovation and entrepreneurship training program (202011078020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yin, H.; Li, G.; Chen, X.; Wang, W.; Wong, P.K.; Zhao, H.; An, T. Accelerated evolution of bacterial antibiotic resistance through early emerged stress responses driven by photocatalytic oxidation. Appl. Catal. B Environ. 2020, 269, 118829. [Google Scholar] [CrossRef]
- Martin, D.J.; Liu, G.; Moniz, S.J.A.; Bi, Y.; Beale, A.M.; Ye, J.; Tang, J. Efficient visible driven photocatalyst, silver phosphate: Performance, understanding and perspective. Chem. Soc. Rev. 2015, 44, 7808–7828. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Xiong, J.; Li, H.; Liu, Z. Ultrathin 2D photocatalysts: Electronic-structure tailoring, hybridization, and applications. Adv. Mater. 2018, 30, 1704548. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Z.; Liu, H.; Zhang, S.; Wang, P.; Lu, J.; Tong, Y. Heterojunction architecture of N-Doped WO3 nanobundles with Ce2S3 nanodots hybridized on a carbon textile enables a highly efficient flexible photocatalyst. Adv. Funct. Mater. 2019, 29, 1903490. [Google Scholar] [CrossRef]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, G.; Xia, D.; An, T.; Zhao, H.; Wong, P.K. Photocatalytic nanomaterials for solar-driven bacterial inactivation: Recent progress and challenges. Environ. Sci. Nano 2017, 4, 782–799. [Google Scholar] [CrossRef]
- Bertagna Silva, D.; Cruz-Alcalde, A.; Sans, C.; Giménez, J.; Esplugas, S. Performance and kinetic modelling of photolytic and photocatalytic ozonation for enhanced micropollutants removal in municipal wastewaters. Appl. Catal. B Environ. 2019, 249, 211–217. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interface Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Liu, H.; Chen, J.; Ma, S.; Wen, M.; Kong, J.; An, T. Photocatalytic degradation mechanism of gaseous styrene over Au/TiO2@CNTs: Relevance of superficial state with deactivation mechanism. Appl. Catal. B Environ. 2020, 272, 118969. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.; Abu Hanif, M.; Islam, M.A.; Hahn, J.R. Solar-light-driven efficient ZnO-single-Walled carbon nanotube photocatalyst for the degradation of a persistent water pollutant organic dye. Catalysts 2019, 9, 498. [Google Scholar] [CrossRef]
- Cahino, A.M.; Loureiro, R.G.; Dantas, J.; Madeira, V.S.; Ribeiro Fernandes, P.C. Characterization and evaluation of ZnO/CuO catalyst in the degradation of methylene blue using solar radiation. Ceram. Int. 2019, 45, 13628–13636. [Google Scholar] [CrossRef]
- Li, K.; Lu, X.; Zhang, Y.; Liu, K.; Huang, Y.; Liu, H. Bi3TaO7/Ti3C2 heterojunctions for enhanced photocatalytic removal of water-borne contaminants. Environ. Res. 2020, 185, 109409. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of interfaces in two-dimensional photocatalyst for water splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.; Qin, Z.; Wu, Z. Monolayer Ti3C2Tx as an effective co-catalyst for enhanced photocatalytic hydrogen production over TiO2. ACS Appl. Energy Mater. 2019, 2, 4640–4651. [Google Scholar] [CrossRef]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-driven metal halide perovskite photocatalysis: Design, stability, and performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Y.; Zhu, Y.; Zhu, Y. Surface oxygen vacancy induced photocatalytic performance enhancement of a BiPO4 nanorod. J. Mater. Chem. A 2014, 2, 1174–1182. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, Q.; Liu, Y.; Wang, H.; Zhu, Y. Photocatalytic performance of BiPO4 nanorods adjusted via defects. Appl. Catal. B Environ. 2016, 187, 204–211. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Ye, H.; Chen, S.; Ju, H.; Liu, D.; Lin, Y.; Ye, W.; Wang, C.; Xu, Q.; et al. Oxide defect engineering enables to couple solar energy into oxygen activation. J. Am. Chem. Soc. 2016, 138, 8928–8935. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Shi, W.; Ling, P.; Sun, Y.; Jiao, X.; Gao, S.; Liang, L.; Xu, J.; Yan, W.; et al. Efficient visible-light-driven CO2 reduction mediated by defect-engineered BiOBr atomic layers. Angew. Chem. Int. Edit. 2018, 57, 8719–8723. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Yang, N.; Zhou, W.; Wang, P.; Jiang, K.; Li, S.; Song, H.; Ding, X.; Chen, H.; et al. Oxygen vacancies induced special CO2 adsorption modes on Bi2MoO6 for highly selective conversion to CH4. Appl. Catal. B Environ. 2019, 259, 118088. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Li, H.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Chen, R.; Wang, S. Defect chemistry in heterogeneous catalysis: Recognition, understanding and utilization. ACS Catal. 2020, 10, 11082–11098. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Li, X.; Li, J.; Cen, W.; Li, Q.; Dong, F. Highly enhanced visible light photocatalysis and in situ FT-IR studies on Bi metal@defective BiOCl hierarchical microspheres. Appl. Catal. B Environ. 2018, 225, 218–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Li, G.; Huang, X.; Hao, W.; Bi, Y. Direct observation of oxygen vacancy self-healing on TiO2 photocatalysts for solar water splitting. Angew. Chem. 2019, 58, 14229–14233. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Chen, P.; Du, A.; Ostrikov, K.; Huang, W.; Wang, L. In situ formation of oxygen vacancies achieving near-complete charge separation in planar BiVO4 photoanodes. Adv. Mater. 2020, 32, 2001385. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Xia, J.; Chisholm, M.F.; Zhong, J.; Chen, C.; Cao, X.; Dong, F.; Chi, Z.; Chen, H.; Weng, Y.-X.; et al. Defect-tailoring mediated electron–hole separation in single-unit-cell Bi3O4Br nanosheets for boosting photocatalytic hydrogen evolution and nitrogen fixation. Adv. Mater. 2019, 31, 1807576. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Zhang, Y.; Yang, S.; Han, K.; Dong, F.; Ma, T. Three-in-one oxygen vacancies: Whole visible-spectrum absorption, efficient charge separation, and surface site activation for robust CO2 photoreduction. Angew. Chem. 2019, 58, 3880–3884. [Google Scholar] [CrossRef]
- Selim, S.; Pastor, E.; García-Tecedor, M.; Morris, M.R.; Francàs, L.; Sachs, M.; Moss, B.; Corby, S.; Mesa, C.A.; Gimenez, S.; et al. Impact of oxygen vacancy occupancy on charge carrier dynamics in BiVO4 photoanodes. J. Am. Chem. Soc. 2019, 141, 18791–18798. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Yu, Y.; Zhao, B.-H.; Wang, W.; Du, Y.; Zhang, B. Understanding the nature of ammonia treatment to synthesize oxygen vacancy-enriched transition metal oxides. Chem 2019, 5, 376–389. [Google Scholar] [CrossRef]
- Glass, D.; Cortés, E.; Ben-Jaber, S.; Brick, T.; Peveler, W.J.; Blackman, C.S.; Howle, C.R.; Quesada-Cabrera, R.; Parkin, I.P.; Maier, S.A. Dynamics of photo-induced surface oxygen vacancies in metal-oxide semiconductors studied under ambient conditions. Adv. Sci. 2019, 6, 1901841. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Sun, Y.; Huang, H.; Wu, Z. Synergistic integration of thermocatalysis and photocatalysis on black defective (BiO)2CO3 microspheres. J. Mater. Chem. A 2015, 3, 18466–18474. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Dong, F.; Li, M.; Zhang, T.; Huang, H. Readily achieving concentration-tunable oxygen vacancies in Bi2O2CO3: Triple-functional role for efficient visible-light photocatalytic redox performance. Appl. Catal. B Environ. 2018, 226, 441–450. [Google Scholar] [CrossRef]
- Kong, J.; Xiang, Z.; Li, G.; An, T. Introduce oxygen vacancies into CeO2 catalyst for enhanced coke resistance during photothermocatalytic oxidation of typical VOCs. Appl. Catal. B Environ. 2020, 269, 118755. [Google Scholar] [CrossRef]
- Ye, K.; Li, Y.; Yang, H.; Li, M.; Huang, Y.; Zhang, S.; Ji, H. An ultrathin carbon layer activated CeO2 heterojunction nanorods for photocatalytic degradation of organic pollutants. Appl. Catal. B Environ. 2019, 259, 118085. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Lin, Y.; Mao, Y.; Ouyang, G.; Liu, H.; Zhang, S.; Tong, Y. Cerium-based hybrid nanorods for synergetic photo-thermocatalytic degradation of organic pollutants. J. Mater. Chem. A 2018, 6, 24740–24747. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Yu, J.; Jaroniec, M. Enhanced formaldehyde oxidation on CeO2/AlOOH-supported Pt catalyst at room temperature. Appl. Catal. B Environ. 2016, 199, 458–465. [Google Scholar] [CrossRef]
- Yang, H.; Xu, B.; Yuan, S.; Zhang, Q.; Zhang, M.; Ohno, T. Synthesis of Y-doped CeO2/PCN nanocomposited photocatalyst with promoted photoredox performance. Appl. Catal. B Environ. 2019, 243, 513–521. [Google Scholar] [CrossRef]
- Ma, Y.; Ou, P.; Wang, Z.; Zhu, A.; Lu, L.; Zhang, Y.; Zeng, W.; Song, J.; Pan, J. Interface engineering in CeO2 (111) facets decorated with CdSe quantum dots for photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 579, 707–713. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, C.; Zhang, N.; Cai, L.; Xiong, Y.; Chai, Y. CeO2-induced interfacial Co2+ octahedral sites and oxygen vacancies for water oxidation. ACS Catal. 2019, 9, 6484–6490. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, W.; Zhang, L.; Zheng, Y.; Wang, Z. Insights into the surface-defect dependence of photoreactivity over CeO2 nanocrystals with well-defined crystal facets. ACS Catal. 2015, 5, 4851–4858. [Google Scholar] [CrossRef]
- Aslam, M.; Qamar, M.T.; Soomro, M.T.; Ismail, I.M.I.; Salah, N.; Almeelbi, T.; Gondal, M.A.; Hameed, A. The effect of sunlight induced surface defects on the photocatalytic activity of nanosized CeO2 for the degradation of phenol and its derivatives. Appl. Catal. B Environ. 2016, 180, 391–402. [Google Scholar] [CrossRef]
- Huang, Y.; Long, B.; Tang, M.; Rui, Z.; Balogun, M.-S.; Tong, Y.; Ji, H. Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B Environ. 2016, 181, 779–787. [Google Scholar] [CrossRef]
- Subramanyam, K.; Sreelekha, N.; Reddy, D.A.; Ramanadha, M.; Poornaprakash, B.; Reddy, K.C.; Vijayalakshmi, R.P. Influence of transition metals co-doping on CeO2 magnetic and photocatalytic activities. Ceram. Int. 2020, 46, 5086–5097. [Google Scholar] [CrossRef]
- Zheng, N.-C.; Ouyang, T.; Chen, Y.; Wang, Z.; Chen, D.-Y.; Liu, Z.-Q. Ultrathin CdS shell-sensitized hollow S-doped CeO2 spheres for efficient visible-light photocatalysis. Catal. Sci. Technol. 2019, 9, 1357–1364. [Google Scholar] [CrossRef]
- Laberty-Robert, C.; Long, J.W.; Pettigrew, K.A.; Stroud, R.M.; Rolison, D.R. Ionic nanowires at 600 °C: Using nanoarchitecture to optimize electrical transport in nanocrystalline gadolinium-doped ceria. Adv. Mater. 2007, 19, 1734–1739. [Google Scholar] [CrossRef]
- Cheng, Y.; Nan, H.; Li, Q.; Luo, Y.; Chu, K. A rare-earth samarium oxide catalyst for electrocatalytic nitrogen reduction to ammonia. ACS Sustain. Chem. Eng. 2020, 8, 13908–13914. [Google Scholar] [CrossRef]
- Liyanage, A.D.; Perera, S.D.; Tan, K.; Chabal, Y.; Balkus, K.J. Synthesis, characterization, and photocatalytic activity of Y-doped CeO2 nanorods. ACS Catal. 2014, 4, 577–584. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Liang, M.; Borjigin, T.; Zhang, Y.; Liu, B.; Liu, H.; Guo, H. Controlled assemble of hollow heterostructured g-C3N4@CeO2 with rich oxygen vacancies for enhanced photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 243, 566–575. [Google Scholar] [CrossRef]
- Huang, Y.; Li, K.; Lin, Y.; Tong, Y.; Liu, H. Enhanced efficiency of electron–hole separation in Bi2O2CO3 for photocatalysis via acid treatment. ChemCatChem 2018, 10, 1982–1987. [Google Scholar] [CrossRef]
- O’Neill, D.B.; Prezgot, D.; Ianoul, A.; Otto, C.; Mul, G.; Huijser, A. Silver nanocubes coated in ceria: Core/shell size effects on light-induced charge transfer. ACS Appl. Mater. Interfaces 2020, 12, 1905–1912. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, X.; Gao, B.; Zou, W.; Zheng, Y.; Yang, Y.; Zhang, Y.; Tong, Q.; Dong, L. Synergistic adsorption-photocatalysis processes of graphitic carbon nitrate (g-C3N4) for contaminant removal: Kinetics, models, and mechanisms. Chem. Eng. J. 2019, 375, 122019. [Google Scholar] [CrossRef]
- Verma, N.; Ananthakrishnan, R. Riboflavin-immobilized CeO2–RGO nanohybrid as a potential photoredox catalyst for enhanced removal of organic pollutants under visible light. J. Phys. Chem. C 2020, 124, 404–415. [Google Scholar] [CrossRef]
- Shang, H.; Huang, S.; Li, H.; Li, M.; Zhao, S.; Wang, J.; Ai, Z.; Zhang, L. Dual-site activation enhanced photocatalytic removal of no with Au/CeO2. Chem. Eng. J. 2020, 386, 124047. [Google Scholar] [CrossRef]
- Wang, B.; Yang, S.-Z.; Chen, H.; Gao, Q.; Weng, Y.-X.; Zhu, W.; Liu, G.; Zhang, Y.; Ye, Y.; Zhu, H.; et al. Revealing the role of oxygen vacancies in bimetallic PbBiO2Br atomic layers for boosting photocatalytic CO2 conversion. Appl. Catal. B Environ. 2020, 277, 119170. [Google Scholar] [CrossRef]
- Balapure, A.; Ganesan, R. Anatase versus Triphasic TiO2: Near-identical synthesis and comparative structure-sensitive photocatalytic degradation of methylene blue and 4-chlorophenol. J. Colloid Interface Sci. 2021, 581, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiong, T.; Balogun, M.S.J.T.; Huang, Y.; Adekoya, D.; Zhang, S.; Tong, Y. Enhanced metallicity boosts hydrogen evolution capability of dual-bimetallic Ni–Fe nitride nanoparticles. Mater. Today Phys. 2020, 15, 100267. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Jiang, Z.; Xu, F.; Xian, Q.; Sun, C.; Tong, Q.; Zou, W.; Duan, X.; Wang, S. CeO2 nanocrystal-modified layered MoS2/g-C3N4 as 0D/2D ternary composite for visible-light photocatalytic hydrogen evolution: Interfacial consecutive multi-step electron transfer and enhanced H2O reactant adsorption. Appl. Catal. B Environ. 2019, 259, 118072. [Google Scholar] [CrossRef]
- Ye, K.-H.; Wang, Z.; Gu, J.; Xiao, S.; Yuan, Y.; Zhu, Y.; Zhang, Y.; Mai, W.; Yang, S. Carbon quantum dots as a visible light sensitizer to significantly increase the solar water splitting performance of bismuth vanadate photoanodes. Energy Environ. Sci. 2017, 10, 772–779. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.; Li, K.; Yang, G.; Yin, S. One-step low-temperature synthesis of 0D CeO2 quantum dots/2D BiOX (X = Cl, Br) nanoplates heterojunctions for highly boosting photo-oxidation and reduction ability. Appl. Catal. B Environ. 2019, 250, 17–30. [Google Scholar] [CrossRef]
- Song, H.; Wu, R.; Yang, J.; Dong, J.; Ji, G. Fabrication of CeO2 nanoparticles decorated three-dimensional flower-like BiOI composites to build p-n heterojunction with highly enhanced visible-light photocatalytic performance. J. Colloid Interface Sci. 2018, 512, 325–334. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, H.; Yang, H.; Lin, Y.; Liu, H.; Tong, Y. Efficient charges separation using advanced BiOI-based hollow spheres decorated with palladium and manganese dioxide nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 2751–2757. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Zhang, J.; Yang, J.; Huang, Y.; Tong, Y. Engineering the band-edge of Fe2O3/ZnO nanoplates via separate dual cation incorporation for efficient photocatalytic performance. Ind. Eng. Chem. Res. 2020, 59, 18865–18872. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Liu, K.; Ye, K.; Wang, Q.; Zhang, S.; Huang, Y.; Liu, H. Constructing Fe-MOF-derived Z-scheme photocatalysts with enhanced charge transport: Nanointerface and carbon sheath synergistic effect. ACS Appl. Mater. Interfaces 2020, 12, 25494–25502. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).