Biomimetic Nanoparticles Potentiate the Anti-Inflammatory Properties of Dexamethasone and Reduce the Cytokine Storm Syndrome: An Additional Weapon against COVID-19?
Abstract
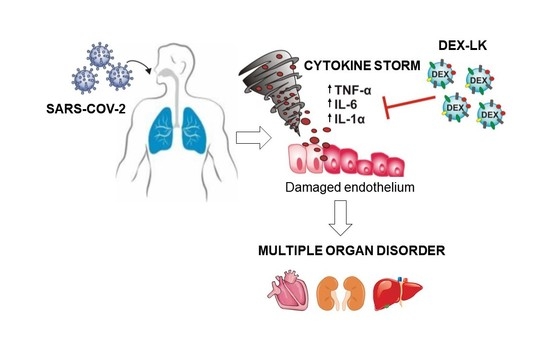
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Nanoparticles
2.2. Cells
2.3. RNA Extraction and qRT-PCR Analysis
2.4. In Vivo Study
2.5. Cytokine Protein Array
3. Results
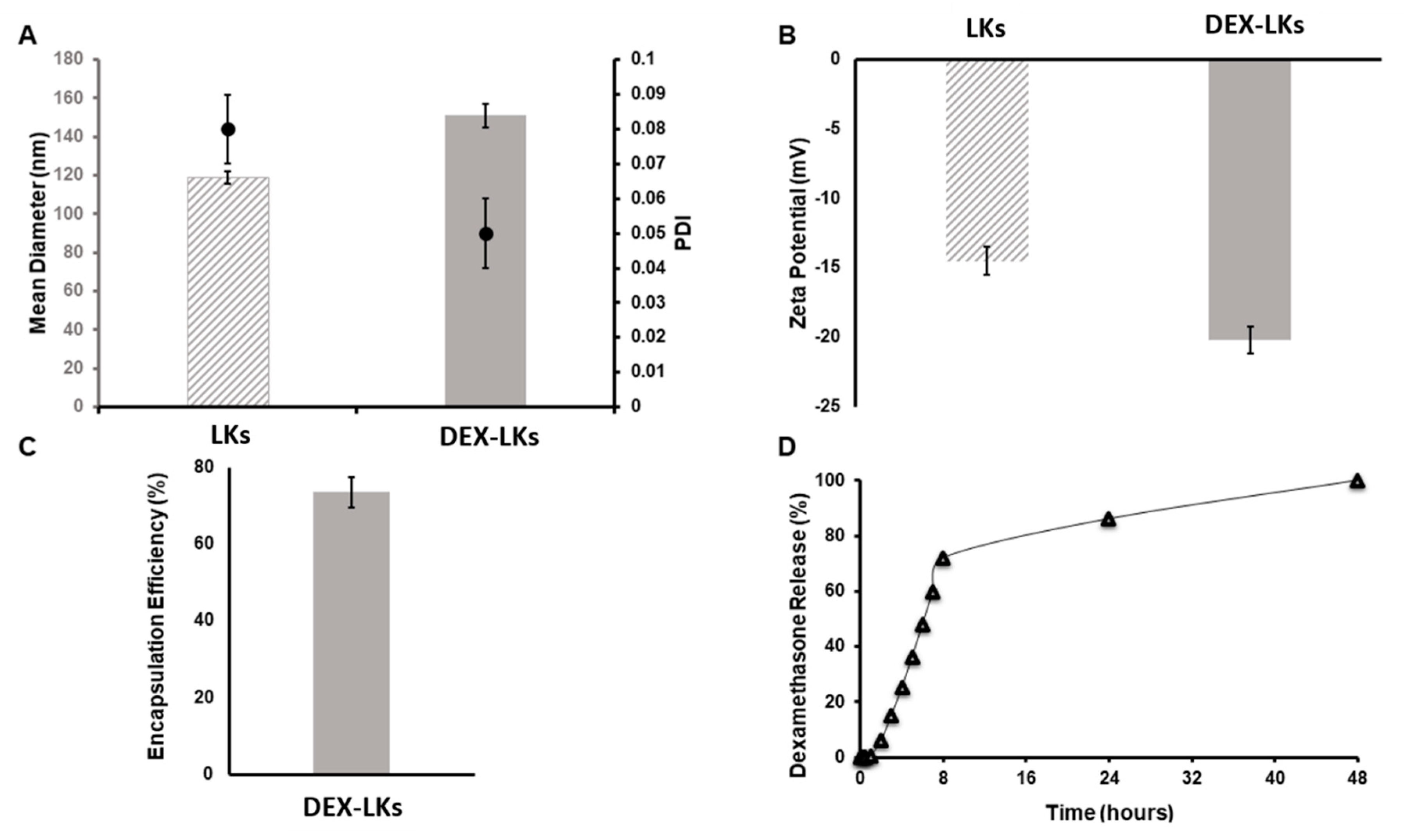
3.1. Preparation and Characterization of Nanoparticles
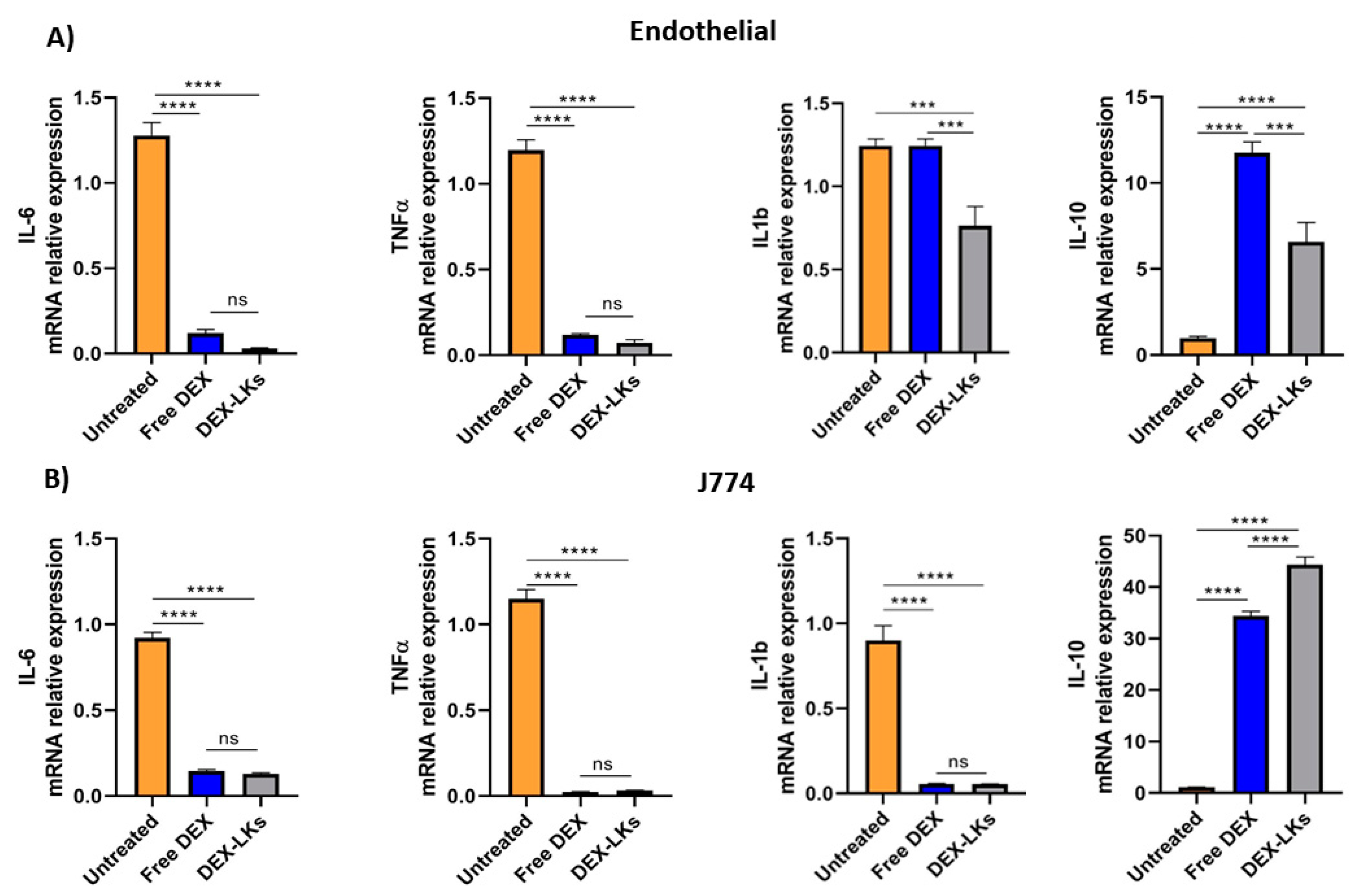
3.2. Free and Leukosome-Loaded Dexamethasone Reduces the Expression of Anti-Inflammatory Genes Both in Endothelial and Macrophage Cell Lines
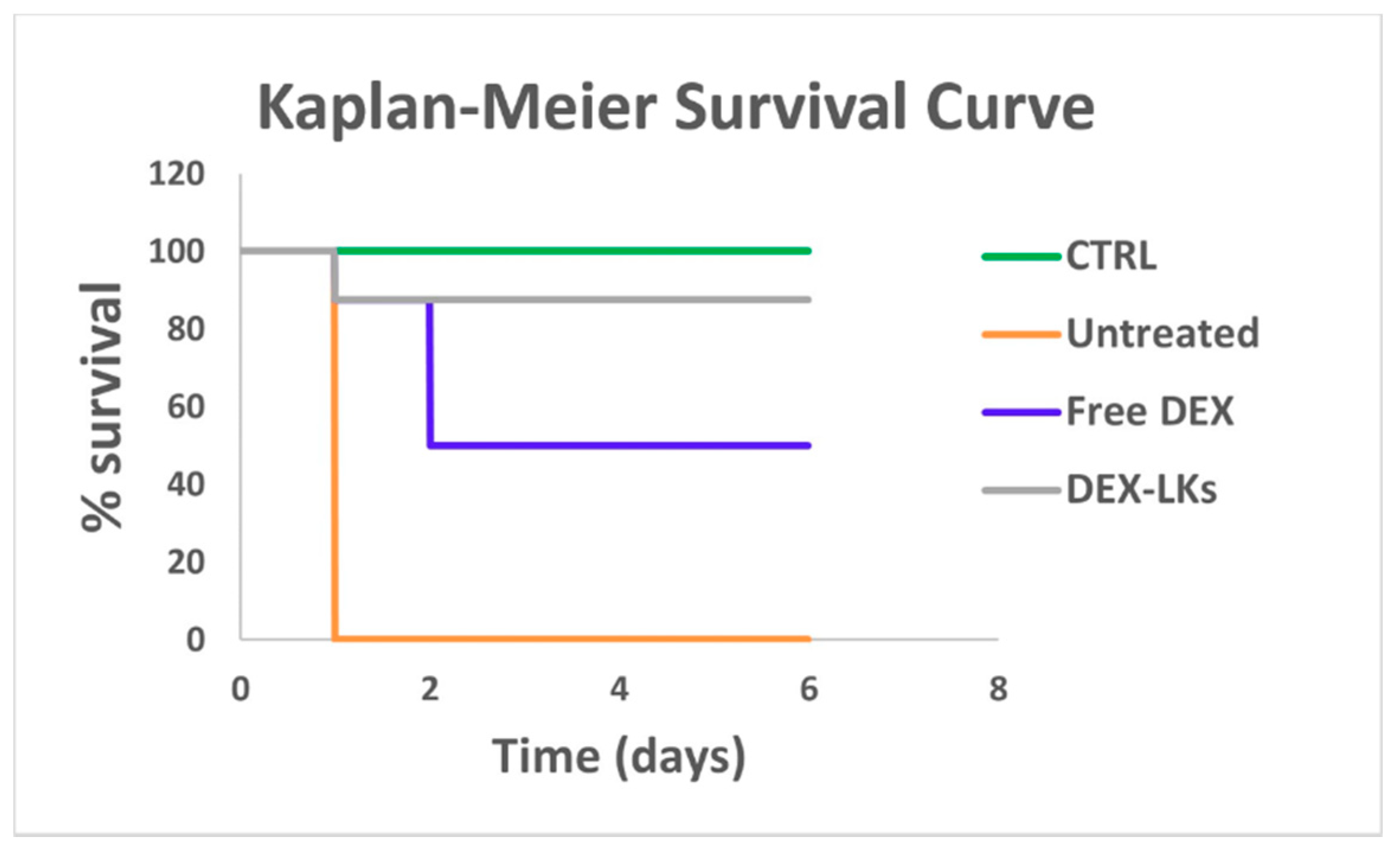
3.3. Leukosomes Potentiate Dexamethasone Activity and Prolong Mouse Survival in an LPS-Induced Endotoxemia Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat. Rev. Immunol. 2020, 20, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Corbo, C.; Cevenini, A.; Molinaro, R.; Palomba, R.; Pandolfi, L.; Agostini, M.; Salvatore, F.; Tasciotti, E. Enabling cytoplasmic delivery and organelle targeting by surface modification of nanocarriers. Nanomedicine 2015, 10, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Molinaro, R.; Sushnitha, M.; Evangelopoulos, M.; Martinez, J.O.; Arrighetti, N.; Corbo, C.; Tasciotti, E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials 2017, 147, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Arrighetti, N.; Corbo, C.; Evangelopoulos, M.; Pastò, A.; Zuco, V.; Tasciotti, E. Exosome-like nanovectors for drug delivery in cancer. Curr. Med. Chem. 2018, 26, 6132–6148. [Google Scholar] [CrossRef]
- Palomba, R.; Parodi, A.; Evangelopoulos, M.; Acciardo, S.; Corbo, C.; de Rosa, E.; Yazdi, I.K.; Scaria, S.; Molinaro, R.; Furman, N.E.T.; et al. Biomimetic carriers mimicking leukocyte plasma membrane to increase tumor vasculature permeability. Sci. Rep. 2016, 6, 34422. [Google Scholar] [CrossRef]
- Corbo, C.; Parodi, A.; Evangelopoulos, M.; Engler, D.A.; Matsunami, R.K.; Engler, A.C.; Molinaro, R.; Scaria, S.; Salvatore, F.; Tasciotti, E. Proteomic Profiling of a Biomimetic Drug Delivery Platform. Curr. Drug Targets 2015, 16, 1540–1547. [Google Scholar] [CrossRef]
- Wang, T.; Wang, D.; Yu, H.; Feng, B.; Zhou, F.; Zhang, H.; Zhou, L.; Jiao, S.; Li, Y. A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nat. Commun. 2018, 9, 1532. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Copp, J.; Luk, B.T.; Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Molinaro, R.; Corbo, C.; Martinez, J.O.; Taraballi, F.; Evangelopoulos, M.; Minardi, S.; Yazdi, I.K.; Zhao, P.; de Rosa, E.; Sherman, M.B.; et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 2016, 15, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.O.; Molinaro, R.; Hartman, K.A.; Boada, C.; Sukhovershin, R.; De Rosa, E.; Kirui, D.; Zhang, S.; Evangelopoulos, M.; Carter, A.M.; et al. Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics 2018, 8, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Thamphiwatana, S.; Angsantikul, P.; Escajadillo, T.; Zhang, Q.; Olson, J.; Luk, B.T.; Zhang, S.; Fang, R.H.; Gao, W.; Nizet, V.; et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. USA 2017, 114, 11488–11493. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Han, F.; Yuan, A.; Wu, L.; Cao, J.; Qian, J.; Qi, X.; Yan, Y.; Ge, Y. Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials 2019, 189, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Toledano Furman, N.E.; Hartman, K.A.; Sherman, M.B.; de Rosa, E.; Kirui, D.K.; Salvatore, F.; Tasciotti, E. Unveiling the in Vivo Protein Corona of Circulating Leukocyte-like Carriers. ACS Nano 2017, 11, 3262–3273. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Corbo, C.; Molinaro, R.; Lakey, J.R.T. Biohybrid Nanoparticles to Negotiate with Biological Barriers. Small 2019, 15, e1902333. [Google Scholar] [CrossRef]
- Boada, C.; Zinger, A.; Tsao, C.; Zhao, P.; Martinez, J.O.; Hartman, K.; Naoi, T.; Sukhovershin, R.; Sushnitha, M.; Molinaro, R.; et al. Rapamycin-Loaded Biomimetic Nanoparticles Reverse Vascular Inflammation. Circ. Res. 2020, 126, 25–37. [Google Scholar] [CrossRef]
- Molinaro, R.; Martinez, J.O.; Zinger, A.; De Vita, A.; Storci, G.; Arrighetti, N.; De Rosa, E.; Hartman, K.A.; Basu, N.; Taghipour, N.; et al. Leukocyte-mimicking nanovesicles for effective doxorubicin delivery to treat breast cancer and melanoma. Biomater. Sci. 2020, 8, 333–341. [Google Scholar] [CrossRef]
- Molinaro, R.; Corbo, C.; Livingston, M.; Evangelopoulos, M.; Parodi, A.; Boada, C.; Agostini, M.; Tasciotti, E. Inflammation and Cancer: In Medio Stat Nano. Curr. Med. Chem. 2018, 25, 4208–4223. [Google Scholar] [CrossRef]
- Molinaro, R.; Pastò, A.; Corbo, C.; Taraballi, F.; Giordano, F.; Martinez, J.O.; Zhao, P.; Wang, X.; Zinger, A.; Boada, C.; et al. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale 2019, 11, 13576–13586. [Google Scholar] [CrossRef]
- Corbo, C.; Cromer, W.E.; Molinaro, R.; Toledano Furman, N.E.; Hartman, K.A.; De Rosa, E.; Boada, C.; Wang, X.; Zawieja, D.C.; Agostini, M.; et al. Engineered biomimetic nanovesicles show intrinsic anti-inflammatory properties for the treatment of inflammatory bowel diseases. Nanoscale 2017, 9, 14581–14591. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, R.; Evangelopoulos, M.; Hoffman, J.R.; Corbo, C.; Taraballi, F.; Martinez, J.O.; Hartman, K.A.; Cosco, D.; Costa, G.; Romeo, I.; et al. Design and Development of Biomimetic Nanovesicles Using a Microfluidic Approach. Adv. Mater. 2018, 30, e1702749. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Slutsky, A.S. Sepsis and endothelial permeability. N. Engl. J. Med. 2010, 363, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Simpson, L.J.; Ferreira, A.M.; Rustagi, A.; Roque, J.; Asuni, A.; Ranganath, T.; Grant, P.M.; Subramanian, A.; Rosenberg-Hasson, Y.; et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight 2020, e140289. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bellamri, N.; Viel, R.; Morzadec, C.; Lecureur, V.; Joannes, A.; De Latour, B.; Llamas-Gutierrez, F.; Wollin, L.; Jouneau, S.; Vernhet, L. TNF-α and IL-10 Control CXCL13 Expression in Human Macrophages. J. Immunol. 2020, 204, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, J.; Demaria, O.; Vély, F.; Batista, L.; Benmansour, N.C.; Fares, J.; Carpentier, S.; Thibult, M.-L.; Morel, A.; Remark, R.; et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020. [Google Scholar] [CrossRef]
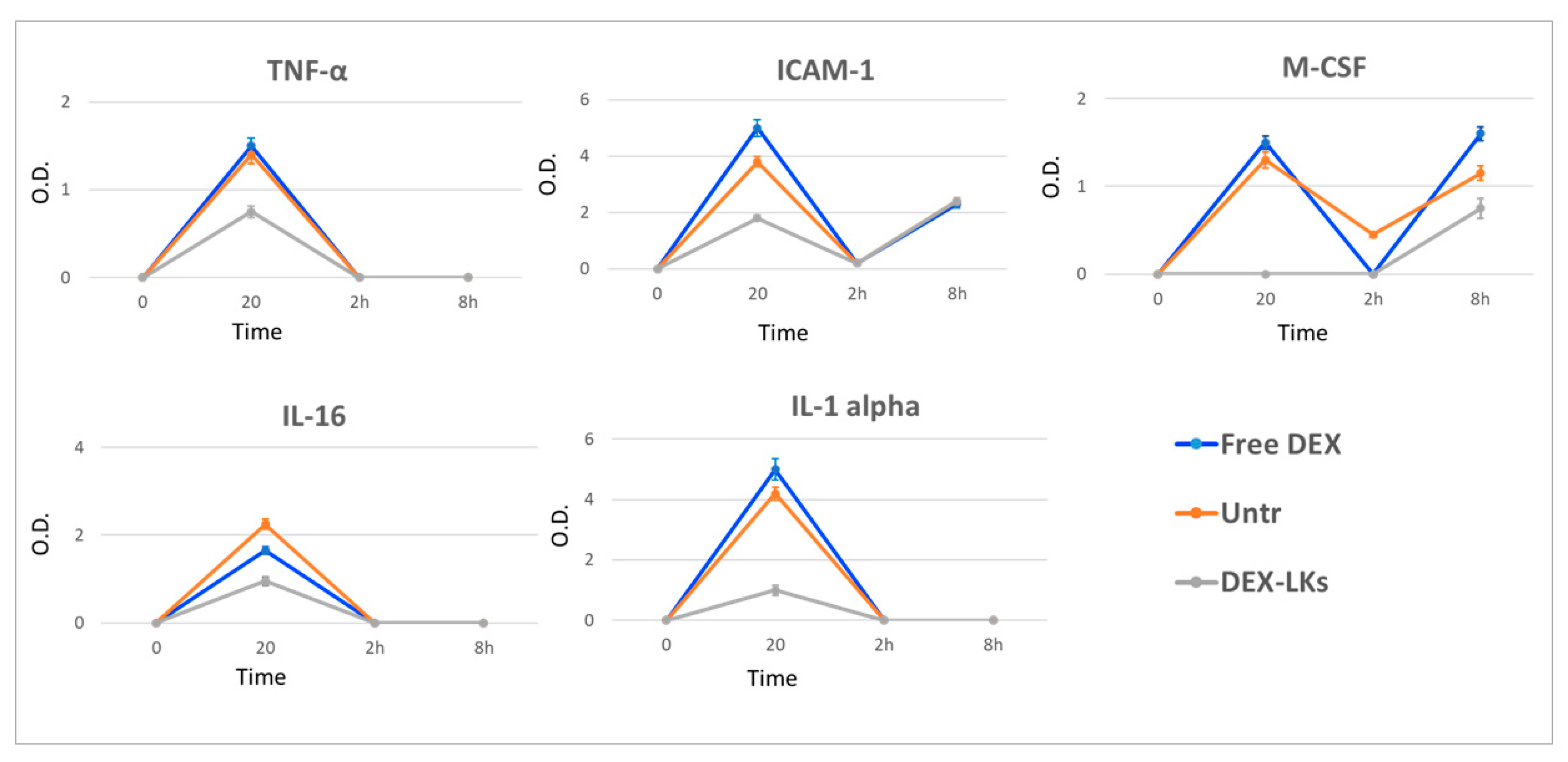
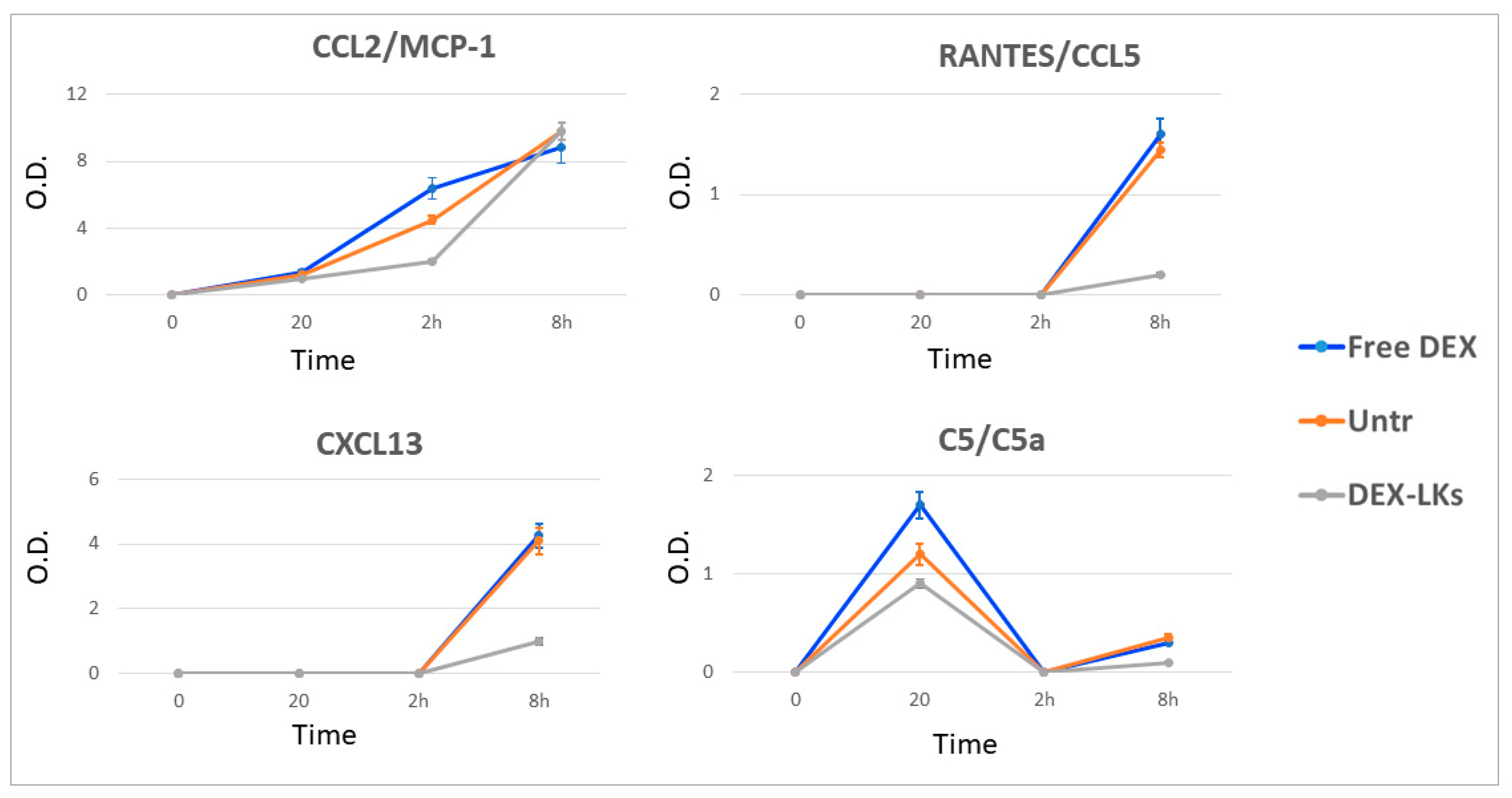
| Name/Abbreviation | Class | Produced by | Action | Following Treatment with DEX-LKs | |
|---|---|---|---|---|---|
| Interleukin 1 alpha/IL-1α | Cytokine | Macrophages and lymphocytes | Pro-inflammatory, promotes activation and secretion of cytokines and other acute-phase proteins | 20 min |  |
| Interleukin 6/IL-6 | Cytokine | Macrophages, lymphocytes, fibroblast, and others | Pro-inflammatory properties | 2 h and 8 h |  |
| Interleukin 16/IL-16 | Cytokine | T-cells, eosinophils, and mast cells | Chemoattractant, modulator of T-cell activation | 20 min |  |
| Tumor necrosis factor alpha/TNFα | Cytokine | Macrophages and lymphocytes | Differentiation and activation of cells of the immune system | 20 min |  |
| circulating adhesion molecule-1/cICAM-1 | Adhesion molecule | Endothelial cells | Involved in the interaction of circulating neutrophils with vascular endothelium during inflammation. | 20 min |  |
| B lymphocyte chemoattractant/BLC (CXCL13) | Chemokine | B cell follicles of secondary lymphoid organs | Attracts B and T-cells to sites of infection and inflammation | 8 h |  |
| RANTES (CCL5) | Chemokine | Circulating T-cells | Pro-inflammatory | 8 h |  |
| Macrophage inflammatory protein 2-alpha/MIP-2 (CXCL2) | Chemokine | Macrophages and monocytes | Chemotactic for human polymorphonuclear leukocytes | 20 min |  |
| Complement component 5/C5-C5a | Complement protein | Macrophages, hepatocytes | C5a is a protein fragment highly inflammatory | 20 min |  |
| Macrophage colony-stimulating factor/M-CSF | Cytokine | Monocytes, fibroblast, others | Induces the differentiation of hematopoietic stem cells into macrophages | 20 min and 8 h |  |
| Monocyte chemotactic protein 1/(MCP1) CCL2 | Chemokine | Monocytes, macrophages, and dendritic cells | Recruits monocytes, T-cells, and dendritic cells to the sites of inflammation | 2 h |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinaro, R.; Pasto, A.; Taraballi, F.; Giordano, F.; Azzi, J.A.; Tasciotti, E.; Corbo, C. Biomimetic Nanoparticles Potentiate the Anti-Inflammatory Properties of Dexamethasone and Reduce the Cytokine Storm Syndrome: An Additional Weapon against COVID-19? Nanomaterials 2020, 10, 2301. https://doi.org/10.3390/nano10112301
Molinaro R, Pasto A, Taraballi F, Giordano F, Azzi JA, Tasciotti E, Corbo C. Biomimetic Nanoparticles Potentiate the Anti-Inflammatory Properties of Dexamethasone and Reduce the Cytokine Storm Syndrome: An Additional Weapon against COVID-19? Nanomaterials. 2020; 10(11):2301. https://doi.org/10.3390/nano10112301
Chicago/Turabian StyleMolinaro, Roberto, Anna Pasto, Francesca Taraballi, Federica Giordano, Jamil A. Azzi, Ennio Tasciotti, and Claudia Corbo. 2020. "Biomimetic Nanoparticles Potentiate the Anti-Inflammatory Properties of Dexamethasone and Reduce the Cytokine Storm Syndrome: An Additional Weapon against COVID-19?" Nanomaterials 10, no. 11: 2301. https://doi.org/10.3390/nano10112301
APA StyleMolinaro, R., Pasto, A., Taraballi, F., Giordano, F., Azzi, J. A., Tasciotti, E., & Corbo, C. (2020). Biomimetic Nanoparticles Potentiate the Anti-Inflammatory Properties of Dexamethasone and Reduce the Cytokine Storm Syndrome: An Additional Weapon against COVID-19? Nanomaterials, 10(11), 2301. https://doi.org/10.3390/nano10112301