Long-Term Toxicity of ZnO Nanoparticles on Scenedesmus rubescens Cultivated in Semi-Batch Mode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Microalgae
2.3. Microalgae Cultures and ZnO NPs Exposure
2.4. Analytical Methods
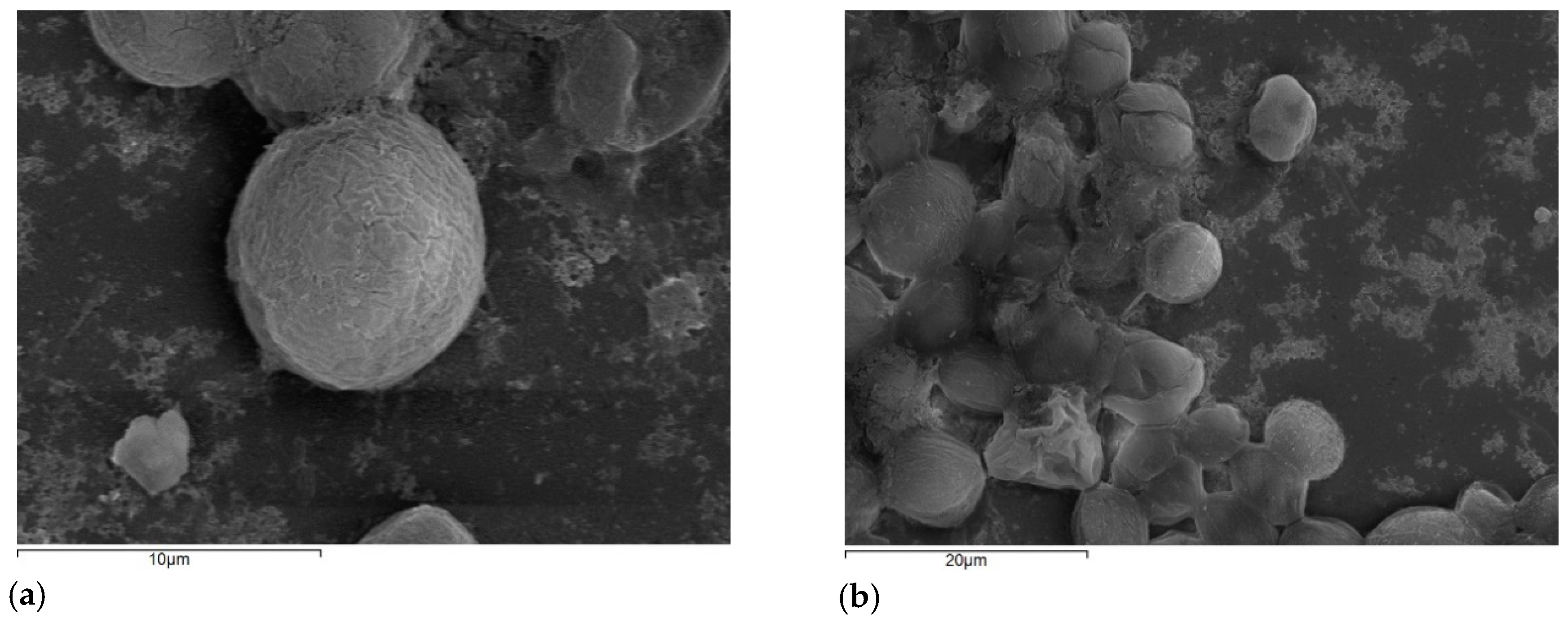
2.5. Microscopy Monitoring
2.6. Data Analysis
3. Results
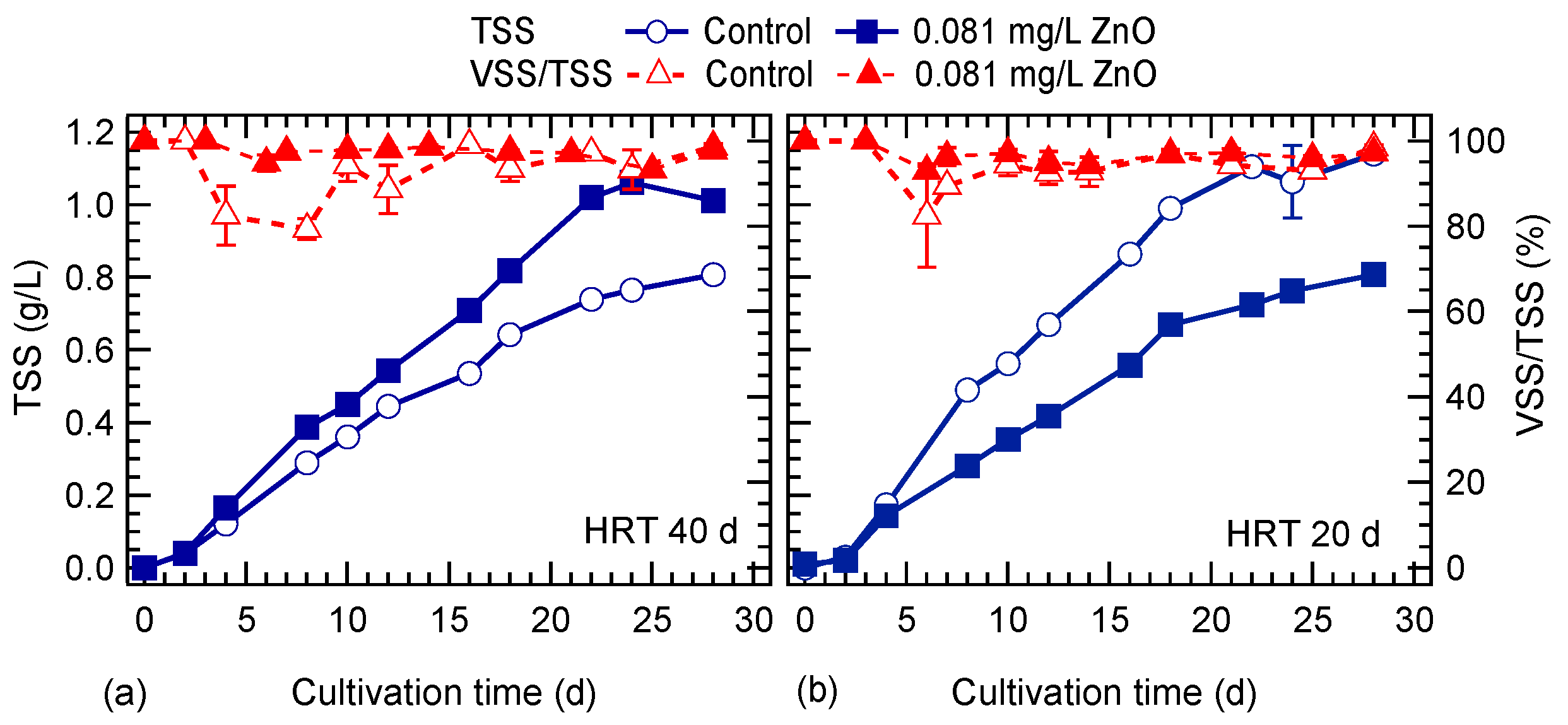
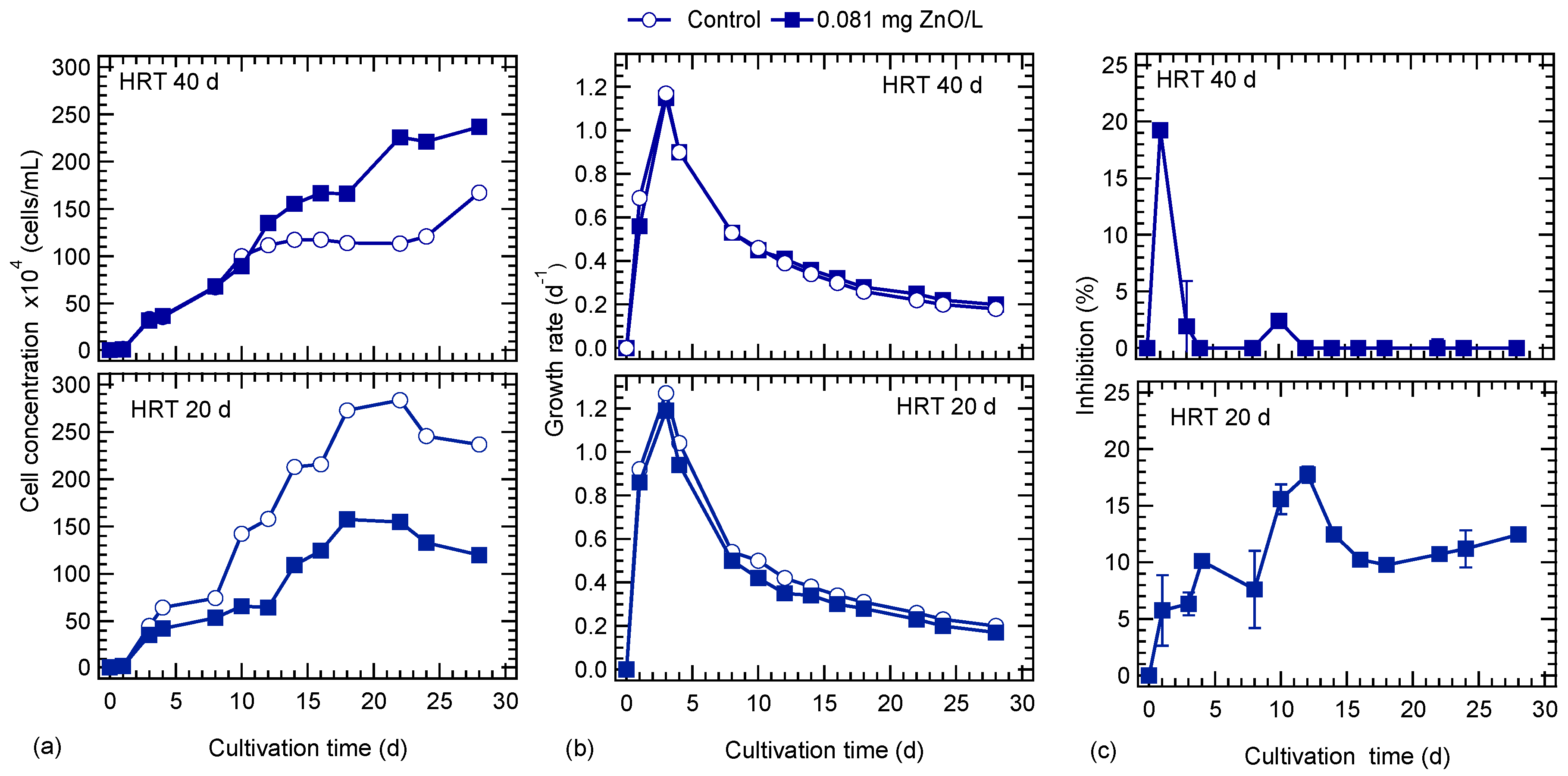
3.1. HRT and ZnO NPs Effect on Algal Growth
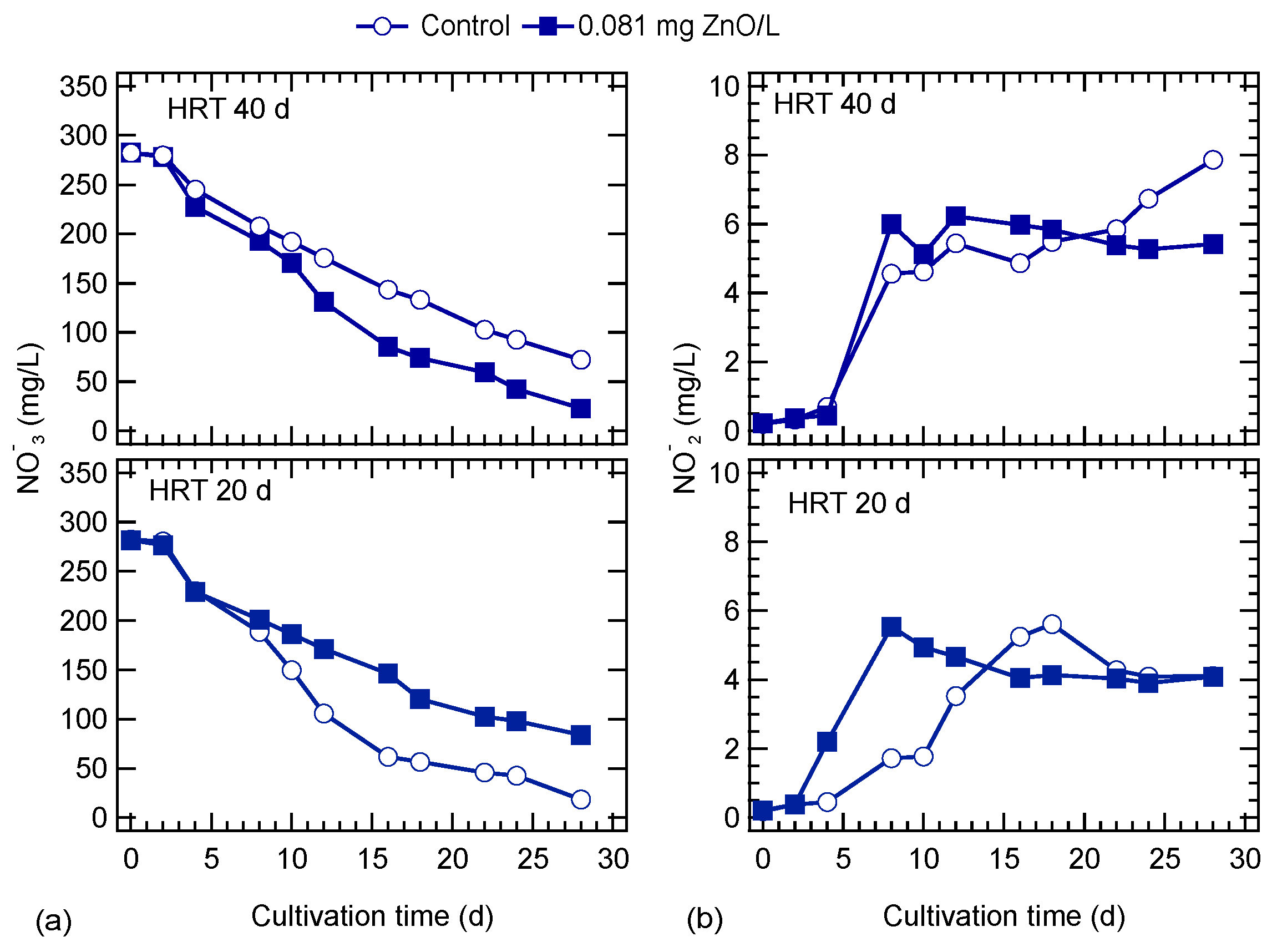
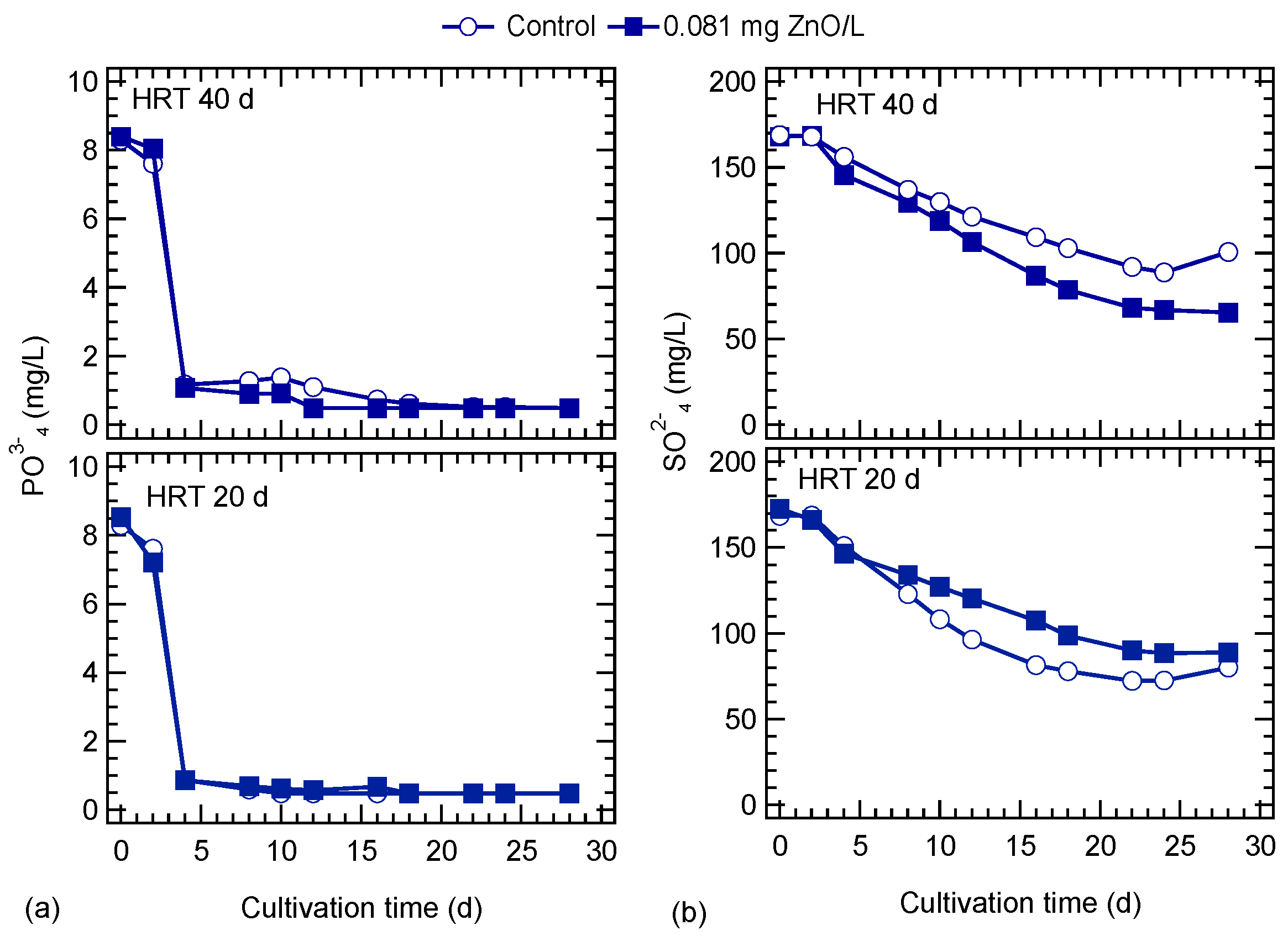
3.2. ZnO NPs Effects on Nutrients Uptake
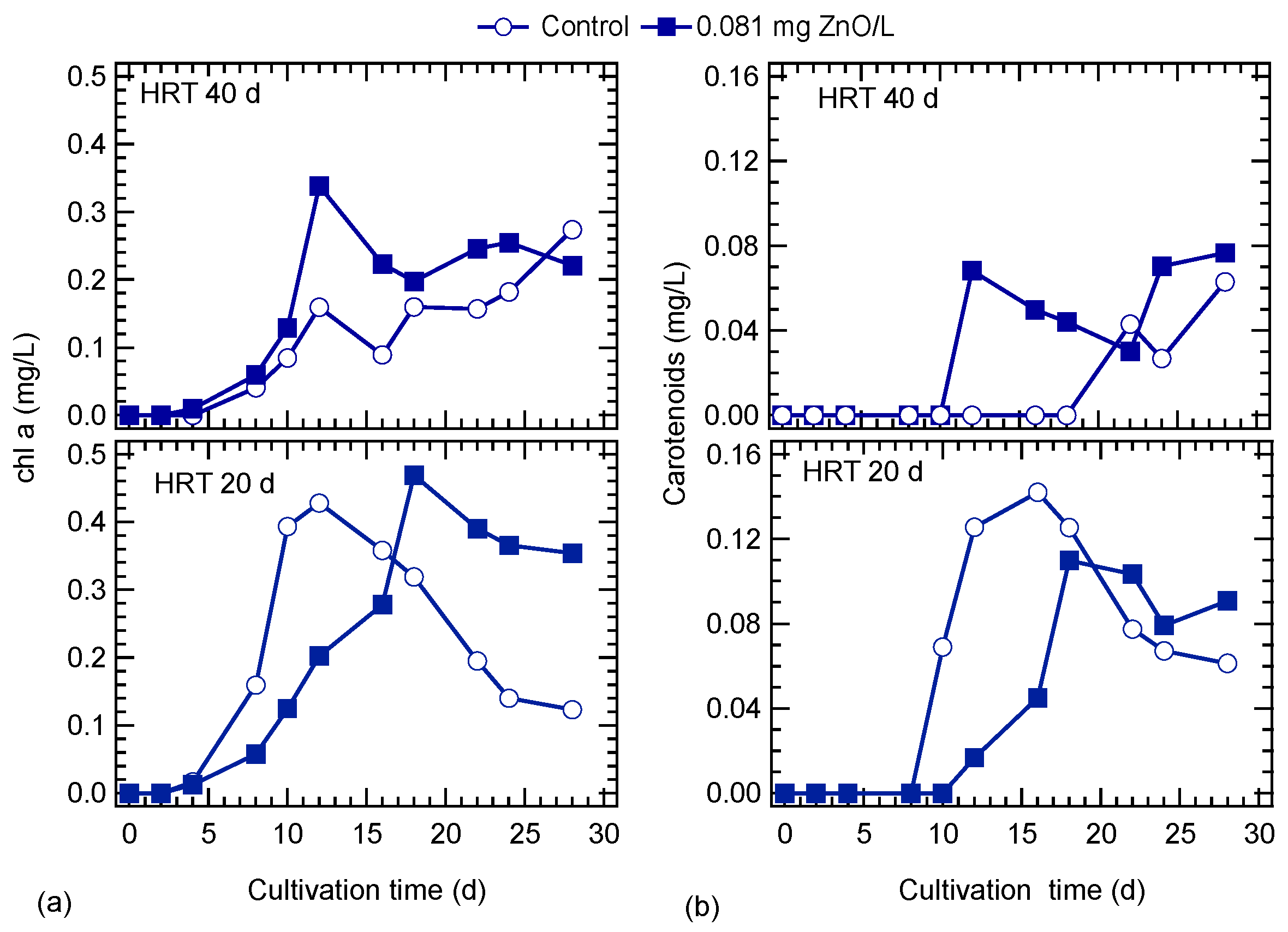
3.3. ZnO NPs Effect on Lipid Content
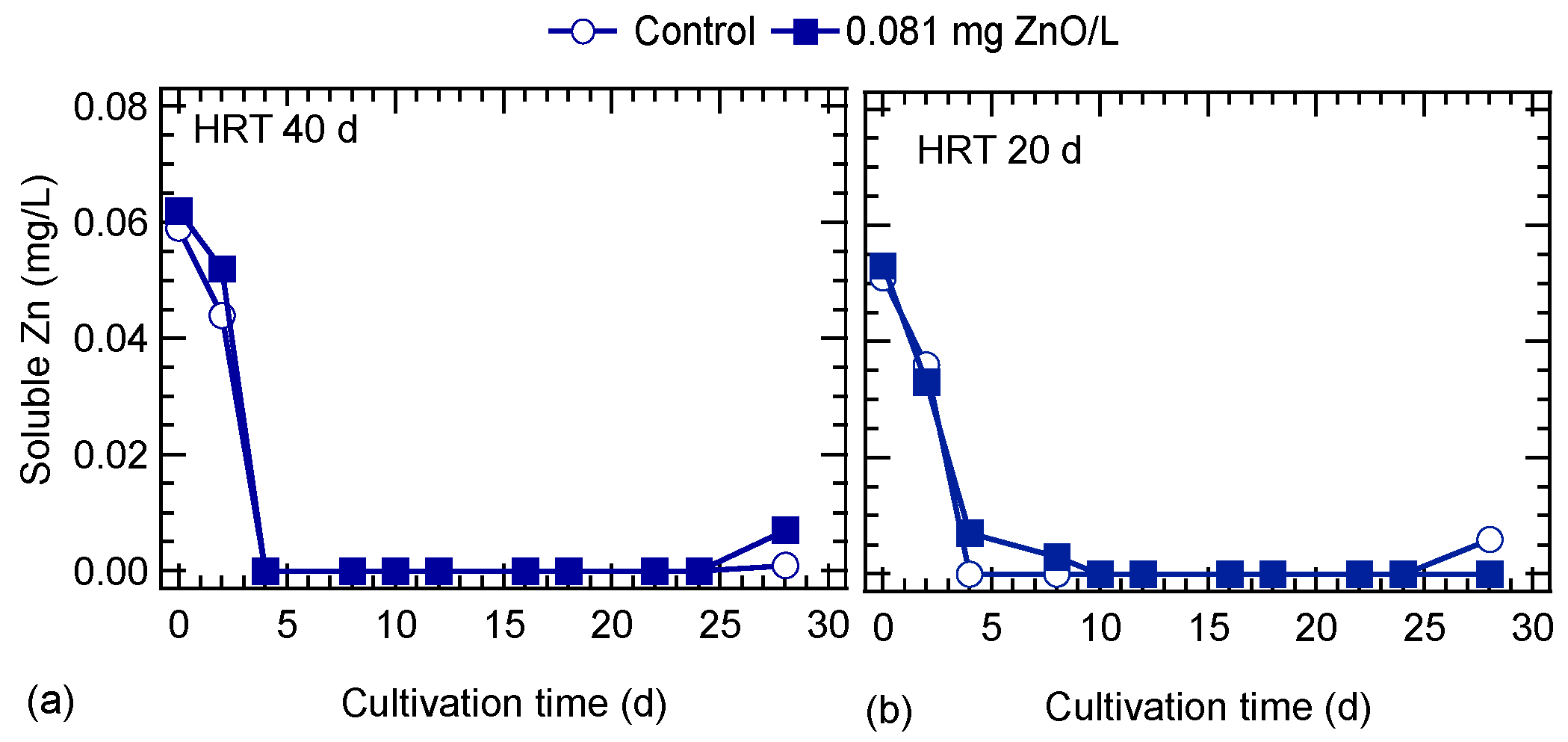
3.4. ZnO NPs Behavior
4. Discussion
4.1. HRT and ZnO NPs Effect on Algal Growth
4.2. ZnO NPs Effects on Algal Nutrients Uptake and Lipid Accumulation
4.3. Evaluation of Toxicity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health Part. C 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, S.H.; Zhao, D. Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: A review. J. Hazard. Mater. 2017, 322, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Y.; Guo, R.; Meng, F.; Gao, Y.; Ma, C.; Zhang, H. Harmful effect of nanoparticles on the functions of freshwater ecosystems: Insight into nanoZnO-polluted stream. Chemosphere 2019, 214, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Mantzavinos, D.; Venieri, D. Current trends in the application of nanomaterials for the removal of emerging micropollutants and pathogens from water. Molecules 2020, 25, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chaüque, E.F.C.; Zvimba, J.N.; Ngila, J.C.; Musee, N. Fate, behaviour, and implications of ZnO nanoparticles in a simulated wastewater treatment plant. Water SA 2016, 42, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour. Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [Green Version]
- Faisal, M.; Saquib, Q.; Alatar, A.A.; Al-Khedhairy, A.A. Phytotoxicity of Nanoparticles; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-662-44607-2. [Google Scholar]
- Vargas-Estrada, L.; Torres-Arellano, S.; Longoria, A.; Arias, D.M.; Okoye, P.U.; Sebastian, P.J. Role of nanoparticles on microalgal cultivation: A review. Fuel 2020, 280. [Google Scholar] [CrossRef]
- Aravantinou, A.F.; Tsarpali, V.; Dailianis, S.; Manariotis, I.D. Effect of cultivation media on the toxicity of ZnO nanoparticles to freshwater and marine microalgae. Ecotoxicol. Environ. Saf. 2015, 114, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T. Commercial scale production of inorganic nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Aravantinou, A.F.; Andreou, F.; Manariotis, I.D. Long-term toxicity of ZnO nanoparticles to Scenedesmus rubescens cultivated in different media. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hund-Rinke, K.; Sinram, T.; Schlich, K.; Nickel, C.; Dickehut, H.P.; Schmidt, M.; Kühnel, D. Attachment efficiency of nanomaterials to algae as an important criterion for ecotoxicity and grouping. Nanomaterials 2020, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Danabas, D.; Ates, M.; Ertit Tastan, B.; Cicek Cimen, I.C.; Unal, I.; Aksu, O.; Kutlu, B. Effects of Zn and ZnO Nanoparticles on Artemia salina and Daphnia magna organisms: Toxicity, accumulation and elimination. Sci. Total Environ. 2020, 711, 134869. [Google Scholar] [CrossRef]
- Aravantinou, A.F.; Theodorakopoulos, M.A.; Manariotis, I.D. Selection of microalgae for wastewater treatment and potential lipids production. Bioresour. Technol. 2013, 147, 130–134. [Google Scholar] [CrossRef]
- Tsavatopoulou, V.D.; Vakros, J.; Manariotis, I.D. Lipid conversion of Scenedesmus rubescens biomass into biodiesel using biochar catalysts from malt spent rootlets. J. Chem. Technol. Biotechnol. 2019, 95, 2421–2429. [Google Scholar] [CrossRef]
- Lv, J.; Guo, B.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Integration of wastewater treatment and flocculation for harvesting biomass for lipid production by a newly isolated self-flocculating microalga Scenedesmus rubescens SX. J. Clean. Prod. 2019, 240, 118211. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Pérez, S.; Blasco, J. Toxicity of silver and gold nanoparticles on marine microalgae. Mar. Environ. Res. 2015, 111, 60–73. [Google Scholar] [CrossRef]
- Deniel, M.; Errien, N.; Daniel, P.; Caruso, A.; Lagarde, F. Current methods to monitor microalgae-nanoparticle interaction and associated effects. Aquat. Toxicol. 2019, 217, 105311. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.Y.; Lee, Y.C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201. [Google Scholar] [CrossRef]
- Ren, H.Y.; Dai, Y.Q.; Kong, F.; Xing, D.; Zhao, L.; Ren, N.Q.; Ma, J.; Liu, B.F. Enhanced microalgal growth and lipid accumulation by addition of different nanoparticles under xenon lamp illumination. Bioresour. Technol. 2020, 297, 122409. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Freshwater alga and cyanobacteria, growth inhibition test. In OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar]
- Miao, A.J.; Zhang, X.Y.; Luo, Z.; Chen, C.S.; Chin, W.C.; Santschi, P.H.; Quigg, A. Zinc oxide-engineered nanoparticles: Dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010, 29, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, V.; Phan, D.; Pasha, A.B.M.T. Effects of metal oxide nanoparticles on nitrification in wastewater treatment systems: A systematic review. J. Environ. Sci. Health Part. A Toxic Hazard. Subst. Environ. Eng. 2018, 53, 659–668. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef]
- Vallee, B.L.; Auld, D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhou, S.; Zhao, F.J.; Liu, Y.; Fan, P.P.; Wang, G.C. Physiological responses of Porphyra haitanesis to different copper and zinc concentrations. Braz. J. Oceanogr. 2010, 58, 261–267. [Google Scholar] [CrossRef]
- Ali, A.; Phull, A.R.; Zia, M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol. Rev. 2018, 7, 413–441. [Google Scholar] [CrossRef]
- Starodub, M.E.; Wong, P.T.S.; Mayfield, C.I. Short term and long term studies on individual and combined toxicities of copper, zinc and lead to Scenedesmus quadricauda. Sci. Total Environ. 1987, 63, 101–110. [Google Scholar] [CrossRef]
- Shehata, S.A.; Badr, S.A. Growth response of Scenedesmus to different concentrations of copper, cadmium, nickel, zinc and lead. Environ. Int. 1980, 4, 431–434. [Google Scholar] [CrossRef]
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.; Ma, H.; Ma, A.; Terry, N. Effects of nanoparticles on algae: Adsorption, distribution, ecotoxicity and fate. Appl. Sci. 2019, 9, 1534. [Google Scholar] [CrossRef] [Green Version]
- Azov, Y. Effect of pH on inorganic carbon uptake in algal cultures. Appl. Environ. Microbiol. 1982, 43, 1300–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, A.; Deicher, M.; Herman, P.M.J.; Wollenzien, U.I.A. Nitrate and phosphate affect cultivability of cyanobacteria from environments with low nutrient levels. Appl. Environ. Microbiol. 2005, 71, 3379–3383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, H.; Galyean, A.; Leopold, M. Evaluating engineered nanoparticles in natural waters. TrAC Trends Anal. Chem. 2011, 30, 72–83. [Google Scholar] [CrossRef]
- Peng, X.; Palma, S.; Fisher, N.S.; Wong, S.S. Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat. Toxicol. 2011, 102, 186–196. [Google Scholar] [CrossRef]
- Leung, Y.H.; Chan, C.M.N.; Ng, A.M.C.; Chan, H.T.; Chiang, M.W.L.; Djurišić, A.B.; Ng, Y.H.; Jim, W.Y.; Guo, M.Y.; Leung, F.C.C.; et al. Antibacterial activity of ZnO nanoparticles with a modified surface under ambient illumination. Nanotechnology 2012, 23, 1–12. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Shanmugam, V.K. Functionalization of zinc oxide nanoparticles using Mucuna pruriens and its antibacterial activity. Surf. Interfaces 2020, 19, 100521. [Google Scholar] [CrossRef]
- Simon, D.F.; Domingos, R.F.; Hauser, C.; Hutchins, C.M.; Zerges, W.; Wilkinson, K.J. Transcriptome sequencing (RNA-seq) analysis of the effects of metal nanoparticle exposure on the transcriptome of Chlamydomonas reinhardtii. Appl. Environ. Microbiol. 2013, 79, 4774–4785. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aravantinou, A.F.; Andreou, F.; Manariotis, I.D. Long-Term Toxicity of ZnO Nanoparticles on Scenedesmus rubescens Cultivated in Semi-Batch Mode. Nanomaterials 2020, 10, 2262. https://doi.org/10.3390/nano10112262
Aravantinou AF, Andreou F, Manariotis ID. Long-Term Toxicity of ZnO Nanoparticles on Scenedesmus rubescens Cultivated in Semi-Batch Mode. Nanomaterials. 2020; 10(11):2262. https://doi.org/10.3390/nano10112262
Chicago/Turabian StyleAravantinou, Andriana F., Fytoula Andreou, and Ioannis D. Manariotis. 2020. "Long-Term Toxicity of ZnO Nanoparticles on Scenedesmus rubescens Cultivated in Semi-Batch Mode" Nanomaterials 10, no. 11: 2262. https://doi.org/10.3390/nano10112262
APA StyleAravantinou, A. F., Andreou, F., & Manariotis, I. D. (2020). Long-Term Toxicity of ZnO Nanoparticles on Scenedesmus rubescens Cultivated in Semi-Batch Mode. Nanomaterials, 10(11), 2262. https://doi.org/10.3390/nano10112262





