Nano-Bio Interaction between Blood Plasma Proteins and Water-Soluble Silicon Quantum Dots with Enabled Cellular Uptake and Minimal Cytotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
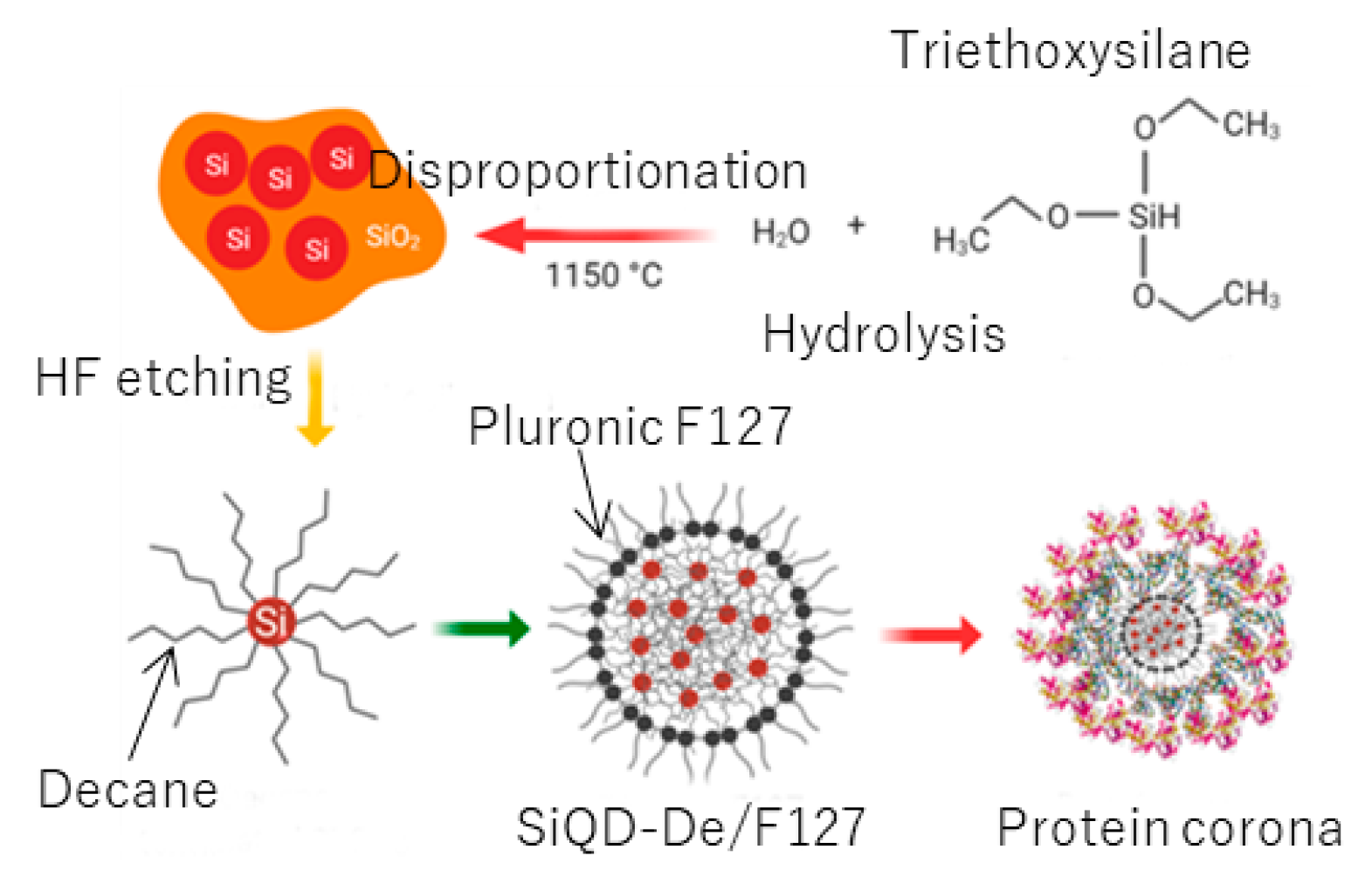
2.2. Synthesis of SiO2-Embedded SiQD Powder
2.3. Synthesis of a Free-Standing, Decane-Terminated SiQD
2.4. Preparation of Pluronic-F127 Coated SiQDs-De
2.5. Characterization
2.6. Asymmetric-Flow Field-Flow Fractionation (AF4) Instrumentation and Separation
2.7. Cell Culture
2.8. Cytotoxicity Assay
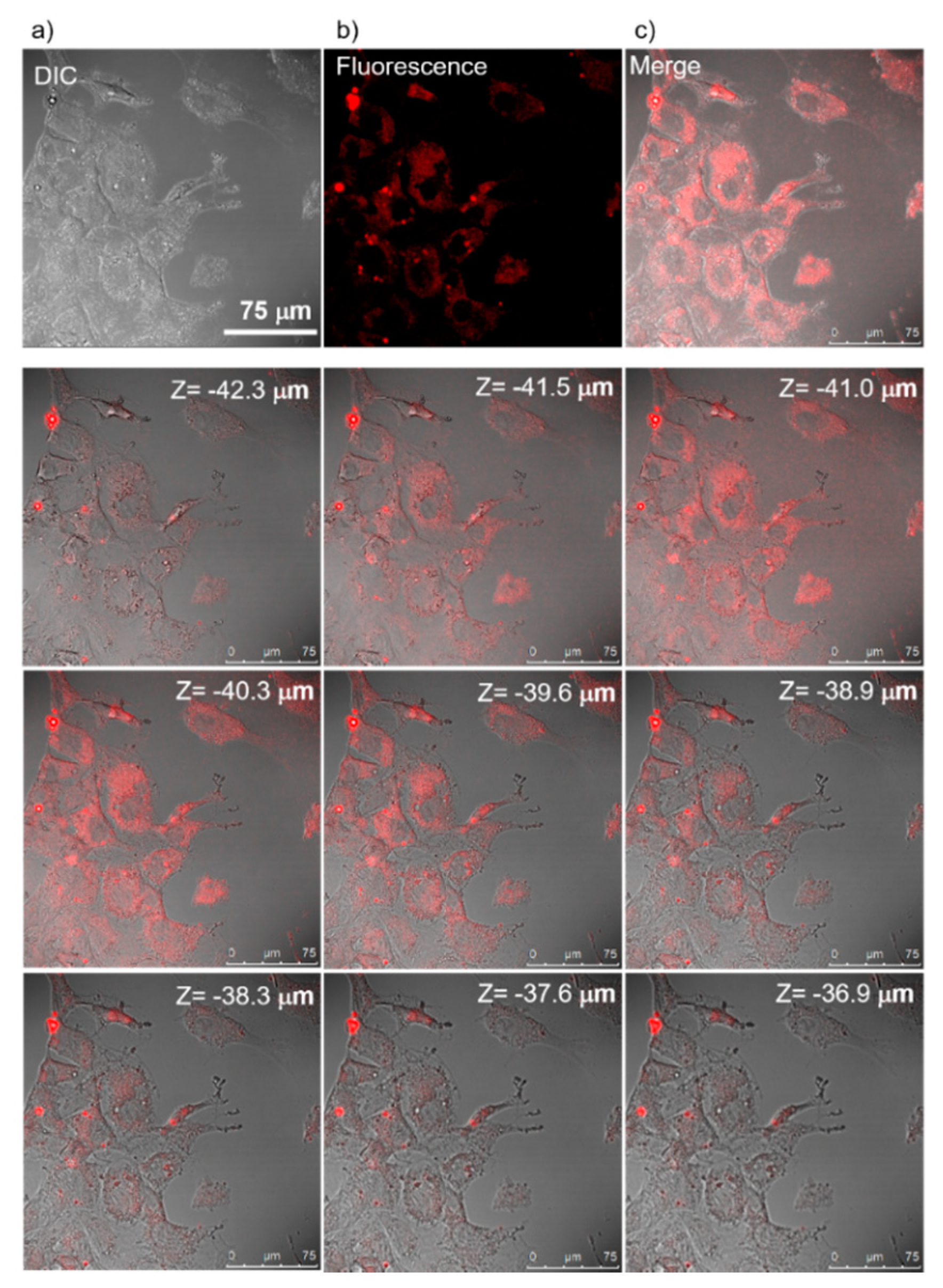
2.9. Cellular Uptake and Confocal Microscopy
3. Results
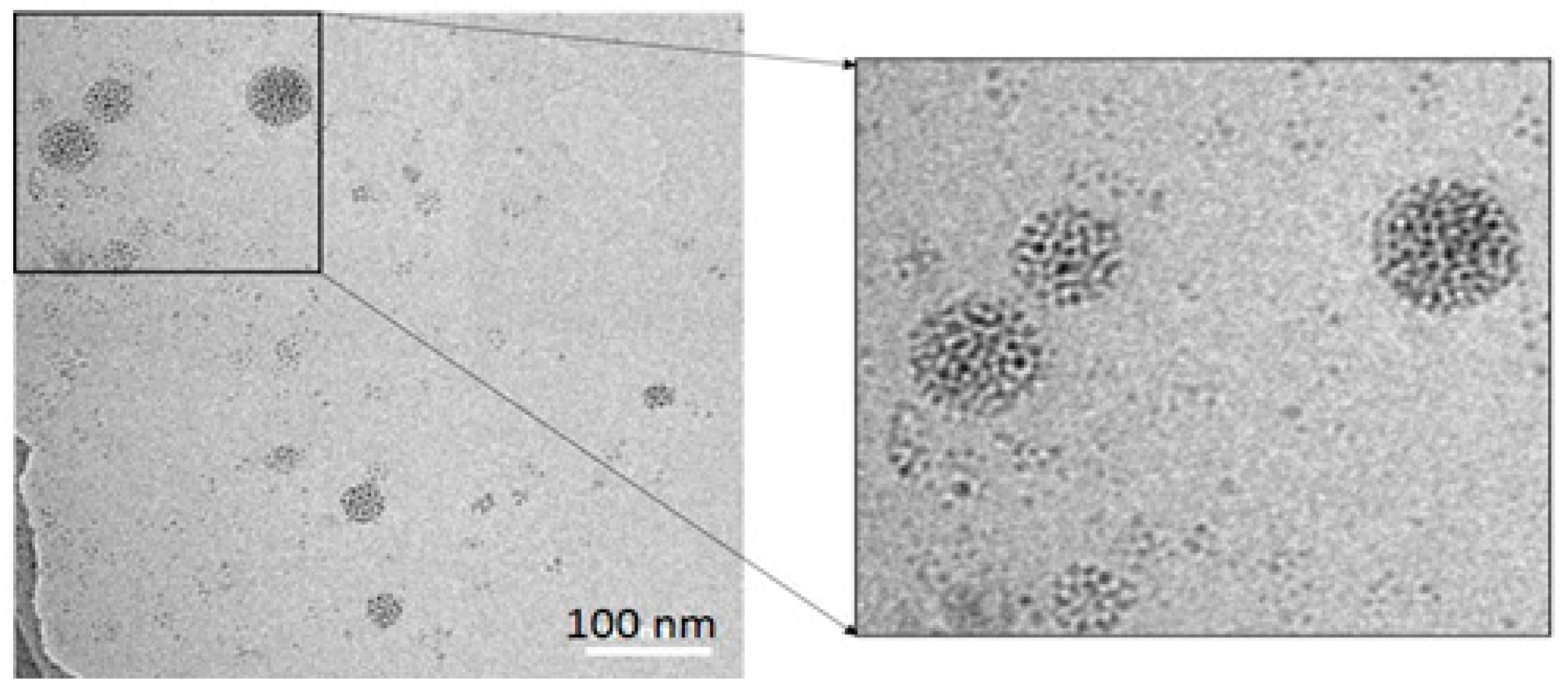
3.1. Synthesis of a Water-Soluble Nanoparticle of SiQDs
3.2. Interaction Between Nanoparticles and Plasma Proteins—Steady-State Fluorescence
3.3. Interaction between Nanoparticles and Plasma Proteins—Excited State Fluorescence
3.4. Protein Secondary Structure Alteration/Recovery upon Interaction with Nanoparticles
3.5. Cellular Uptake and Cytotoxicity of Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv. Mater. 2019, 31, 1900321. [Google Scholar] [CrossRef]
- Martinić, I.; Eliseeva, S.V.; Nguyen, T.N.; Pecoraro, V.L.; Petoud, S. Near-Infrared Optical Imaging of Necrotic Cells by Photostable Lanthanide-Based Metallacrowns. J. Am. Chem. Soc. 2017, 139, 8388–8391. [Google Scholar]
- Dinarvand, M.; Neubert, E.; Meyer, D.; Selvaggio, G.; Mann, F.A.; Erpenbeck, L.; Kruss, S. Near-Infrared Imaging of Serotonin Release from Cells with Fluorescent Nanosensors. Nano Lett. 2019, 19, 6604–6611. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, H.; Geng, X.; Yang, R.; Qu, L.; Kani, A.N.; Li, Z. Rational Design of Far-Red to Near-Infrared Emitting Carbon Dots for Ultrafast Lysosomal Polarity Imaging. ACS Appl. Mater. Interfaces 2020, 12, 31738–31744. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Owens, E.A.; Henary, M.; Fakhri, G.E.; Choi, H.S. Tissue-Specific Near-Infrared Fluorescence Imaging. Acc. Chem. Res. 2016, 49, 1731–1740. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef]
- Genger, U.R.; Grabolle, M.; Jaricot, S.C.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mukherjee, T.K. Spectroscopic investigation of interaction between bovine serum albumin and amine-functionalized silicon quantum dots. Phys. Chem. Chem. Phys. 2014, 16, 8400–8408. [Google Scholar] [CrossRef]
- Pathak, J.; Rawat, K.; Sanwlani, S.; Bohidar, H.B. Interaction of Globular Plasma Proteins with Water-Soluble CdSe Quantum Dots. Chem. Phys. Chem. 2015, 16, 1777–1786. [Google Scholar] [CrossRef]
- Yan, R.; Yu, B.-Q.; Yin, M.-M.; Zhou, Z.Q.; Xiang, X.; Han, X.-L.; Liu, Y.; Jiang, F.-L. The interactions of CdTe quantum dots with serum albumin and subsequent cytotoxicity: The influence of homologous ligands. Toxicol. Res. 2018, 7, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Erogbogbo, F.; Yong, K.-T.; Ye, L.; Liu, J.; Hu, R.; Chen, H.; Hu, Y.; Yang, Y.; Yang, J.; et al. In vivo targeted cancer imaging, sentinel lymph node mapping and multi-channel imaging with biocompatible silicon nanocrystals. ACS Nano 2013, 7, 7303–7310. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Hill, S.K.E.; Zhi, B.; Hudson-Smith, N.V.; Wu, J.J.; White, J.N.; McIntire, E.A.; Santosh, V.S.; Kondeti, K.; Lee, A.L.; et al. Comparative toxicity assessment of novel Si quantum dots and their traditional Cd-based counterparts using bacteria models shewanella oneidensis and bacillus subtilis. Environ. Sci. Nano 2018, 5, 1890–1901. [Google Scholar] [CrossRef]
- Pan, G.-H.; Barras, A.; Boussekey, L.; Boukherroub, R. Silica cross-linked micelles loading with silicon nanoparticles: Preparation and characterization. ACS Appl. Mater. Interfaces 2013, 5, 7042–7049. [Google Scholar] [CrossRef] [PubMed]
- Ruizendaal, L.S.; Bhattacharjee, K.; Ournazari, M.; Rosso-Vasic, L.; De Haan, H.J.; Alink, G.M.; Marcelis, A.T.M.; Zuilhof, H. Synthesis and cytotoxicity of silicon nanoparticles with covalently attached organic monolayers. Nanotoxicology 2009, 3, 339. [Google Scholar] [CrossRef]
- Ravotto, L.; Chen, Q.; Ma, Y.; Vinogradov, S.A.; Locritani, M.; Bergamini, G.; Negri, F.; Yu, Y.; Korgel, B.A.; Ceroni, P. Bright long-lived luminescence of silicon nanocrystals sensitized by two-photon absorbing antenna. Chem 2017, 2, 550–560. [Google Scholar] [CrossRef]
- Pi, X.; Yu, T.; Yang, D. Silicon quantum dots: Water-dispersible silicon-quantum-dot-containing micelles self-assembled from an amphiphilic polymer. Part. Part. Syst. Charact. 2014, 31, 751–756. [Google Scholar] [CrossRef]
- Yong, K.; Law, W.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity assessment of quantum dots: From cellular to primate studies. Chem. Soc. Rev. 2013, 42, 1236–1250. [Google Scholar] [CrossRef]
- Dasog, M.; Kehrle, J.; Rieger, B.; Veinot, J.G.C. Silicon nanocrystals and silicon-polymer hybrids: Synthesis, surface engineering, and applications. Angew. Chem. Int. Ed. 2016, 55, 2322–2339. [Google Scholar] [CrossRef]
- Tu, C.; Ma, X.; House, A.; Kauzlarich, S.M.; Louie, A.Y. PET Imaging and Biodistribution of Silicon Quantum Dots in Mice. ACS Med. Chem. Lett. 2011, 285–288. [Google Scholar] [CrossRef]
- Paik, Y.K.; Overall, C.M.; Corrales, F.; Deutsch, E.W.; Gilbert, L.L.; Omenn, S. Toward Completion of the Human Proteome Parts List: Progress Uncovering Proteins That Are Missing or Have Unknown Function and Developing Analytical Methods. J. Proteome Res. 2018, 17, 4023–4030. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, S.; Velmurugan, D.; Hanagata, N.; Aruna, P.; Ganesan, S. Investigations on the interactions of 5-fluorouracil with bovine serum albumin: Optical spectroscopic and molecular modeling studies. J. Lumin. 2014, 151, 1–10. [Google Scholar] [CrossRef]
- Chinnathambi, C.; Karthikeyan, S.; Rajendiran, M.; Udayakumar, K.; Manoharan, A.; Kandasamy, S.; Hanagata, N. 4-Hydroxycoumarin Derivative: N-(diphenylmethyl)-2-[(2-oxo-2H-chromen-4-yl)oxy]acetamide Interaction with Human Serum Albumin. J. Spectrosc. 2018, 2018, 3480384. [Google Scholar] [CrossRef]
- Matsuda, M.; Sugo, T.; Yoshida, N.; Terukina, S.; Yamazumi, K.; Niwa, K.; Maekawa, H. Structure and function of fibrinogen: Insights from dysfibrinogens. Thromb. Haemost. 1999, 82, 283–290. [Google Scholar]
- Chung, M.C.M. Structure and function of transferrin. Biochem. Educ. 1984, 12, 146–154. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Hadjidemetriou, M.; Kostarelos, K. Nanomedicine: Evolution of the nanoparticle corona. Nat. Nanotechnol. 2017, 12, 288–290. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Song, F.; Liu, R.; Guo, H.; Olsen, D.W.; Cohen, Y.; Emili, A.; Chan, W.C. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 2014, 8, 2439–2455. [Google Scholar] [CrossRef]
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bai, J.; Jiang, X. Monitoring of the enzymatic degradation of protein corona and evaluating the accompanying cytotoxicity of nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 17614–17622. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, R.; Nienhaus, K.; Nienhaus, G.U. Formation of a monolayer protein corona around polystyrene nanoparticles and implications for nanoparticle agglomeration. Small 2019, 15, 1900974. [Google Scholar] [CrossRef] [PubMed]
- Hadjidemetriou, M.; McAdam, S.; Garner, G.; Thackeray, C.; Knight, D.; Smith, D.; Al-Ahmady, Z.; Mazza, M.; Rogan, J.; Clamp, A.; et al. The Human In vivo biomolecule corona onto PEGylated liposomes: A proof-of-concept clinical study. Adv. Mater. 2019, 31, 1803335. [Google Scholar] [CrossRef] [PubMed]
- Vu, V.P.; Gifford, G.B.; Chen, F.; Benasutti, H.; Wang, G.; Groman, E.V.; Scheinman, R.; Saba, L.; Moghimi, S.M.; Simberg, D. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat. Nanotechnol. 2019, 14, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S.; Hadjidemetriou, M.; Gubbins, J.; Kostarelos, K. Formation of protein corona in vivo affects drug release from temperature-sensitive liposomes. J. Control. Release 2018, 28, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Ghosh, B.; Beaune, G.; Nagarajan, U.; Yasui, T.; Nakamura, J.; Tsuruoka, T.; Baba, Y.; Shirahata, N.; Winnik, F.M. Functional double-shelled silicon nanocrystals for two-photon fluorescence cell imaging: Spectral evolution and tuning. Nanoscale 2016, 8, 9009–9019. [Google Scholar] [CrossRef]
- Chandra, S.; Masuda, Y.; Shirahata, N.; Winnik, F.M. Transition-metal-doped NIR-emitting silicon nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 6157–6160. [Google Scholar] [CrossRef]
- Shirahata, N.; Nakamura, J.; Inoue, J.; Ghosh, B.; Nemoto, K.; Nemoto, Y.; Takeguchi, M.; Masuda, Y.; Tanaka, M.; Ozin, G.A. Emerging atomic energy levels in zero-dimensional silicon quantum dots. Nano Lett. 2020, 20, 1491–1498. [Google Scholar] [CrossRef]
- Ghosh, B.; Hamaoka, T.; Nemoto, Y.; Takeguchi, M.; Shirahata, N. Impact of anchoring monolayers on the enhancement of radiative recombination in light-emitting diodes based on silicon nanocrystals. J. Phys. Chem. C 2018, 122, 6422–6430. [Google Scholar] [CrossRef]
- Dohnalová, K.; Poddubny, A.N.; Prokofiev, A.A.; de Boer, W.D.; Umesh, C.P.; Paulusse, J.M.J.; Zuilhof, H.; Gregorkiewicz, T. Surface brightens up Si quantum dots: Direct bandgap-like size-tunable emission. Light Sci. Appl. 2013, 2, e47. [Google Scholar] [CrossRef]
- Kortshagen, U.R.; Sankaran, R.M.; Pereira, R.N.; Girshick, S.L.; Wu, J.J.; Aydil, E.S. Nonthermal plasma synthesis of nanocrystals: Fundamental principles, materials, and applications. Chem. Rev. 2016, 116, 11061–11127. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.K.; Bhattacharjee, K.; Bose, S.; Guchhait, N. A spectroscopic investigation on the interaction of a magnetic ferrofluid with a model plasma protein: Effect on the conformation and activity of the protein. Phys. Chem. Chem. Phys. 2012, 14, 15482–15493. [Google Scholar] [CrossRef] [PubMed]
- Santra, M.K.; Banerjee, A.; Rahaman, O.; Panda, D. Unfolding pathways of human serum albumin: Evidence for sequential unfolding and folding of its three domains. Int. J. Biol. Macromol. 2005, 37, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kamal, J.K.A.; Behera, D.V. Thermal and conformational stability of seed coat soybean peroxidase. Biochemistry 2002, 41, 9034–9042. [Google Scholar] [CrossRef]
- Sahin, Z.; Demir, Y.K.; Kayser, V. Global kinetic analysis of seeded BSA aggregation. Eur. J. Pharm. Sci. 2016, 86, 115–124. [Google Scholar] [CrossRef]
- Konev, S.V. Fluorescence and Phosphorescence of Proteins and Nucleic Acids; Plenum Press: New York, NY, USA, 1967; p. 21. [Google Scholar]
- Ma, C.Y.; Harwalkar, V.R. Study of thermal denaturation of oat globulin by ultraviolet and fluorescence spectrophotometry. J. Agricul. Food Chem. 1998, 36, 155–160. [Google Scholar] [CrossRef]
- Paul, B.K.; Samanta, A.; Guchhait, N.J. Exploring hydrophobic subdomain IIA of the protein bovine serum albumin in the native, intermediate, unfolded, and refolded states by a small fluorescence molecular reporter. Phys. Chem. B 2010, 114, 6183–6196. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed; Plenum: New York, NY, USA, 2006. [Google Scholar]
- Paul, B.K.; Guchhait, N. A spectral deciphering of the binding interaction of an intramolecular charge transfer fluorescence probe with a cationic protein: Thermodynamic analysis of the binding phenomenon combined with blind docking study. Photochem. Photobiol. Sci. 2011, 10, 980–991. [Google Scholar] [CrossRef]
- Kenry, K.; Loh, K.P.; Lim, C.T. Molecular interactions of graphene oxide with human blood plasma proteins. Nanoscale 2016, 8, 9425–9441. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Abu, N.; Hanagata, N. HE CdSe/ZnS quantum dot micelles for long-term cell imaging without alteration to the native structure of the blood plasma protein human serum albumin. RSC Adv. 2017, 7, 2392–2402. [Google Scholar] [CrossRef]
- Jameson, D.M. Introduction to Fluorescence, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 135–138. [Google Scholar]
- Ghosh, B.; Takeguchi, M.; Nakamura, J.; Nemoto, Y.; Hamaoka, T.; Chandra, S.; Shirahata, N. Origin of the photoluminescence quantum yields enhanced by alkane-termination of freestanding silicon nanocrystals: Temperature-dependence of optical properties. Sci. Rep. 2016, 6, 36951. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Sun, F.; Qiao, Z.; Li, J.; Shang, L. In situ investigation on the protein corona formation of quantum dots by using fluorescence resonance energy transfer. Small 2020, 16, 1907633. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Bulyáki, É.; Kun, J.; Moussong, É.; Lee, Y.H.; Goto, Y.; Réfrégiers, M.; Kardos, J. BeStSel: A web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res. 2018, 46, W315–W322. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.C.; Liou, X.Y.; Ye, L.P.; Liang, H.; Wang, Z.Y. Spectroscopic studies on the interaction between human hemoglobin and CdS quantum dots. J. Colloid Interface Sci. 2007, 311, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.X.; Gao, T.; Yang, M.; Jiang, P.; Jiang, F.J.; Liu, Y. Thermodynamics of the Interaction Between Graphene Quantum Dots with Human Serum Albumin and γ-Globulins. J. Solut. Chem. 2020, 49, 100–116. [Google Scholar] [CrossRef]
- Hua, Y.; Li, H.; Meng, P.; Li, K.; Xiong, Y.; Zhang, S.; Yang, Y.; Yin, A.; Huan, P. Interactions between CdTe quantum dots and plasma proteins: Kinetics, thermodynamics and molecular structure changes. Colloids Surf. B Biointerfaces 2020, 189, 110881. [Google Scholar] [CrossRef]
- Seo, J.-H.; Matsuno, R.; Lee, Y.; Takai, M.; Ishihara, K. Conformational recovery and preservation of protein nature from heat-induced denaturation by water-soluble phospholipid polymer conjugation. Biomaterials 2009, 30, 4859–4867. [Google Scholar] [CrossRef]
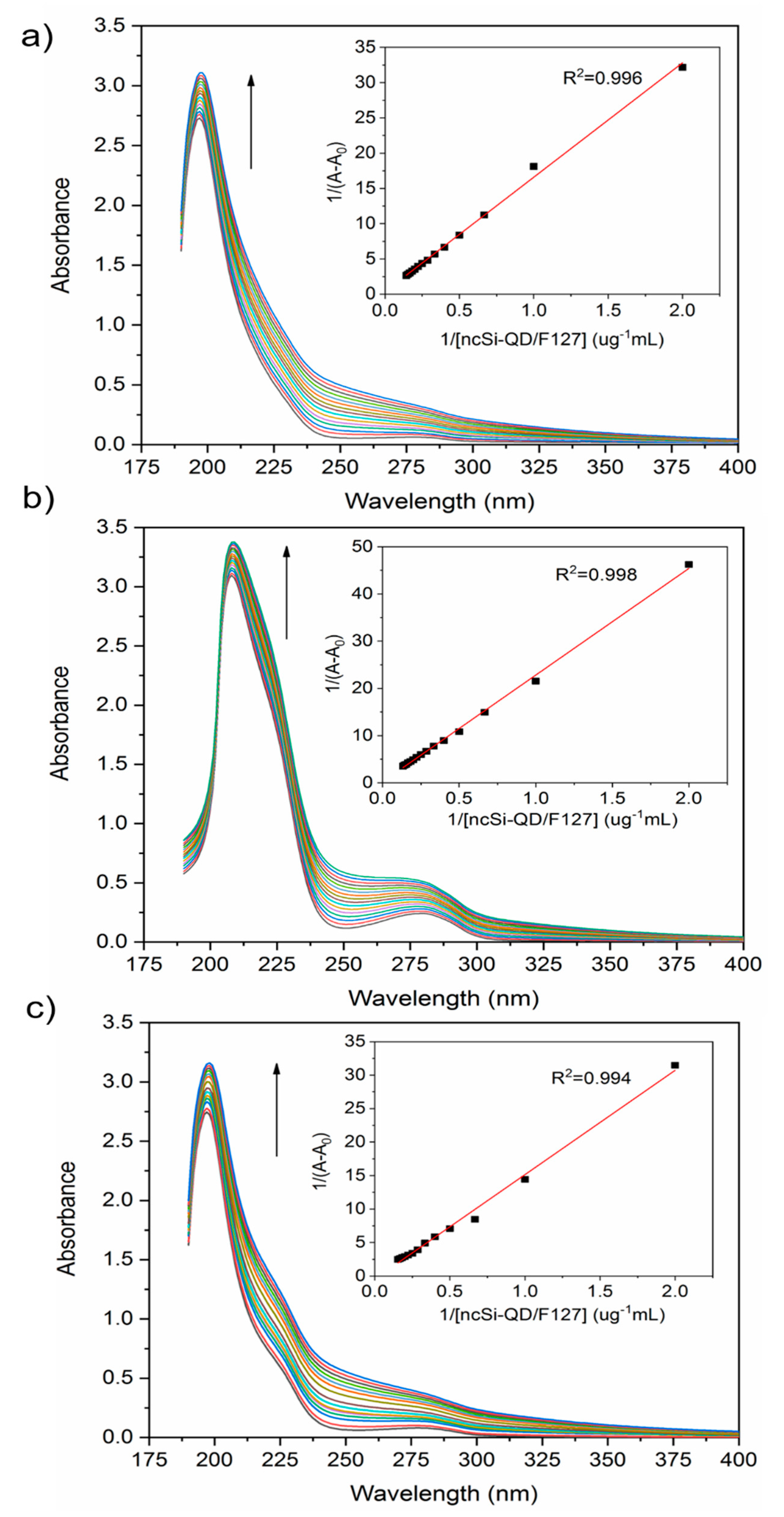
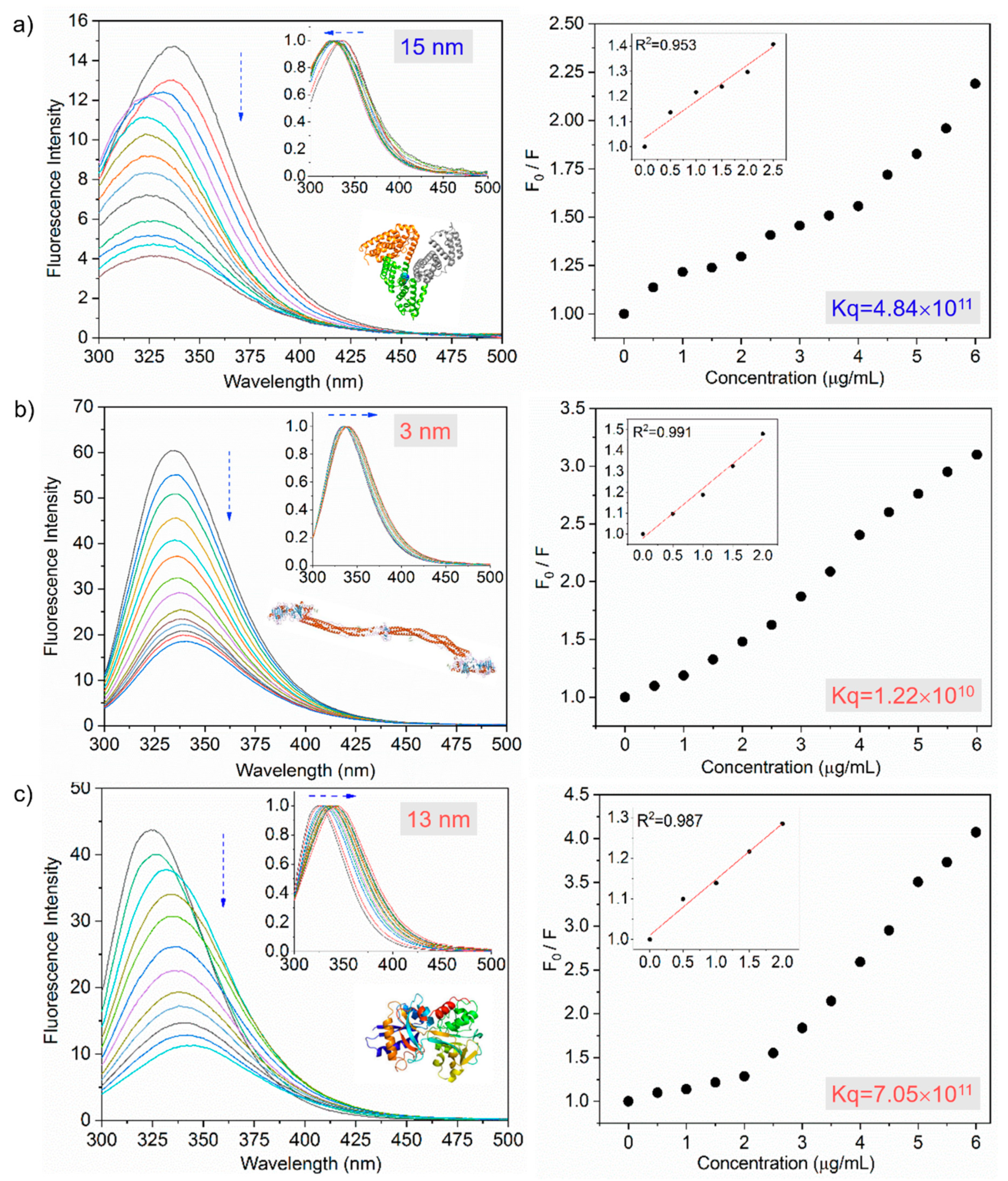

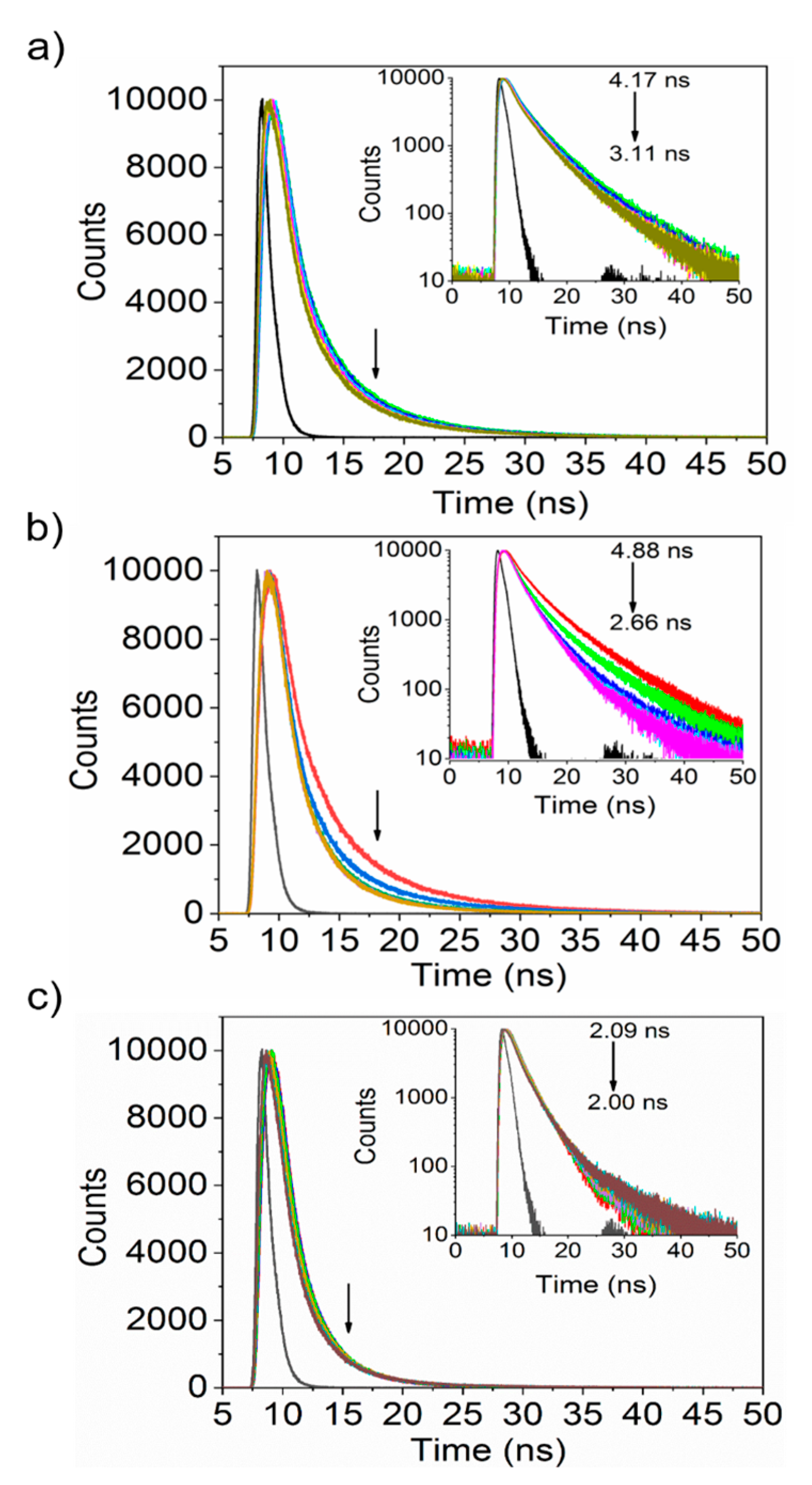


| Protein | Ksv (ug−1 mL) | Kq (ug−1 mLs−1) | τ0 (ns) | n | R2 |
|---|---|---|---|---|---|
| Albumin | 0.20 ± 0.06 | 4.84 × 1011 | 4.17 | 1.03 | 0.95 |
| Fibrinogen | 0.59 ± 0.09 | 1.22 × 1010 | 4.88 | 0.99 | 0.99 |
| Transferrin | 0.15 ± 0.08 | 7.05 × 1011 | 2.09 | 0.95 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnathambi, S.; Hanagata, N.; Yamazaki, T.; Shirahata, N. Nano-Bio Interaction between Blood Plasma Proteins and Water-Soluble Silicon Quantum Dots with Enabled Cellular Uptake and Minimal Cytotoxicity. Nanomaterials 2020, 10, 2250. https://doi.org/10.3390/nano10112250
Chinnathambi S, Hanagata N, Yamazaki T, Shirahata N. Nano-Bio Interaction between Blood Plasma Proteins and Water-Soluble Silicon Quantum Dots with Enabled Cellular Uptake and Minimal Cytotoxicity. Nanomaterials. 2020; 10(11):2250. https://doi.org/10.3390/nano10112250
Chicago/Turabian StyleChinnathambi, Shanmugavel, Nobutaka Hanagata, Tomohiko Yamazaki, and Naoto Shirahata. 2020. "Nano-Bio Interaction between Blood Plasma Proteins and Water-Soluble Silicon Quantum Dots with Enabled Cellular Uptake and Minimal Cytotoxicity" Nanomaterials 10, no. 11: 2250. https://doi.org/10.3390/nano10112250
APA StyleChinnathambi, S., Hanagata, N., Yamazaki, T., & Shirahata, N. (2020). Nano-Bio Interaction between Blood Plasma Proteins and Water-Soluble Silicon Quantum Dots with Enabled Cellular Uptake and Minimal Cytotoxicity. Nanomaterials, 10(11), 2250. https://doi.org/10.3390/nano10112250








