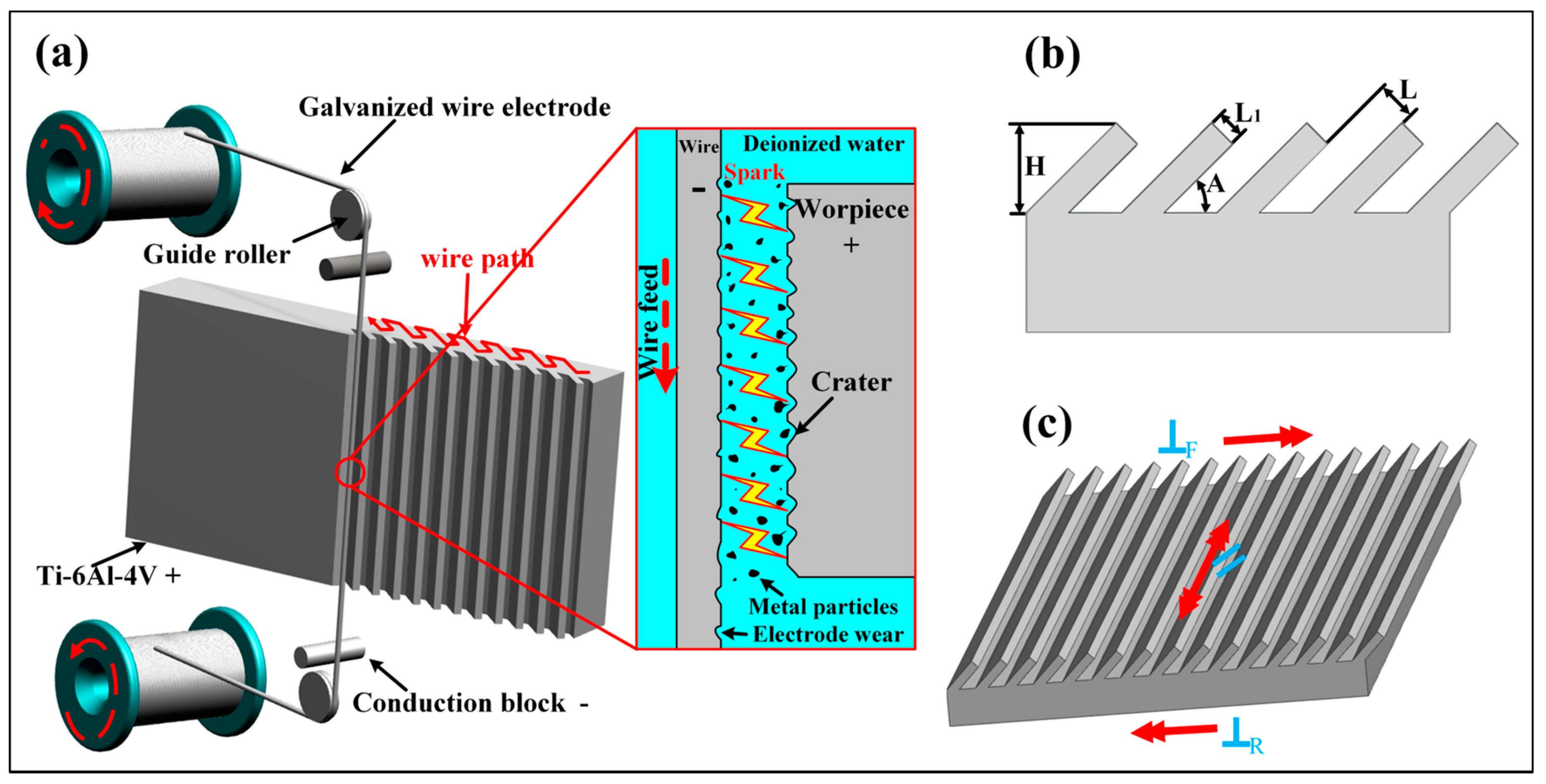
3.1. Relationship between Microstructure and Surface Wettability
A three-level combined bionic groove structure was prepared by using WEDM combined with AH reaction, and the surface morphology of the structure is shown in
Figure 2.
Figure 2a–e is the SEM photographs of the WEDM processed Ti-6Al-4V surface with different inclination angles (
L is 300 μm, and
A is 90°, 75°, 60°, 45°, and 30°), and
Figure 2f is the image of grooves with an
A of 30° and
L of 200 μm. Thus, the precise construction of periodic groove structure with different inclinations and spacing values has been realized by WEDM. By further amplifying the WEDM processed surface, as shown in
Figure 2g, a 10–20 μm sized tiny crater was observed on the groove surface due to local melting and vaporization of Ti-6Al-4V matrix resulting from discharge during the WEDM process while a lot of random-distributed mastoids were found around the crater structure, displaying micron-grade pits and bumps that fairly uniform in size on the processed surface. As shown in
Figure 2g, the micron-grade concave bump structure was relatively smooth without noticeable nanostructures. The WEDM-processed samples were further processed by AH, and the resulting surface morphology is shown in
Figure 2h. It can be observed that the pits and bulges on the surface of Ti-6Al-4V were covered with a layer of the fluff-like structure. After further magnifying, as shown in
Figure 2i, polygonal micropores and nanoscale pore wall structures were observed on the surface. It can be concluded that by combining WEDM and AH, a three-level microstructure with a combination of submillimeter grooves, micrometer-level pits, and bumps, nano-level grids were fabricated on the Ti-6Al-4V surface. Large-scale construction of bionic dual-scale and multi-scale microstructures was realized.
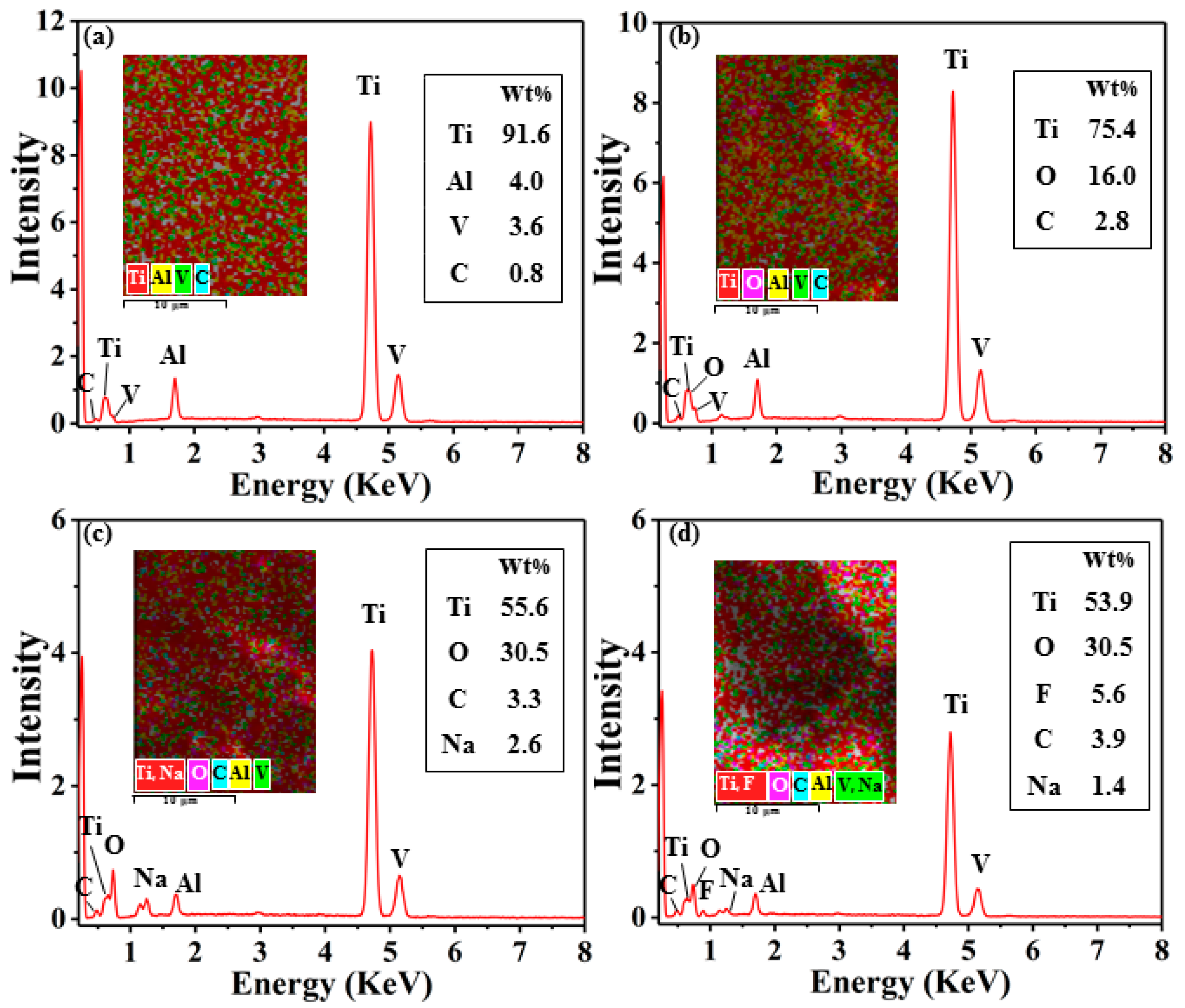
EDS was used to analyze the chemical composition of the sample surfaces treated by different methods, as shown in
Figure 3. As can be seen in
Figure 3a, the C content on the surface increased because certain organic matters present in the air attached on the surface of the sample during polishing the Ti-6Al-4V surface. The content of C and O elements on the surface of Ti-6Al-4V increased significantly after electrical processing, as shown in
Figure 3b. The presence of the O element can be explained that the material removal occurred in the aqueous medium, and the high temperature generated during discharge caused the surface to oxidize resulting in a sharp increase in O element. An increase of C element can be understood that a little organic matter in the aqueous medium decomposed at high temperature and produced a large amount of free C. As the material resolidified during the electrical machining process, free C attached to the surface, making the C content increase. On the other hand, the content of O on the surface of Ti-6Al-4V increased sharply after AH reaction, accompanied by the appearance of Na element, which increased the element O/Na ratio, as shown in
Figure 3c. This is the case where the sample surface is covered with a layer of sodium titanate in a high-temperature alkaline solution environment [
44]. Moreover, the content of C increased after AH reaction because a large amount of organic matter presents in the air deposited on the sample surface during the drying process. In addition, after the WEDM and AH reaction, F was observed on the surface because the samples were modified with fluorosilane. Besides, the carbon content further increased, which caused the lower surface functional groups sequentially to assemble on the surface, reducing the surface energy of the sample surface.
In order to study the influence of surface microstructure characteristics on the wetting property of the Ti-6Al-4V surface, the wettability of different microstructure surfaces was compared and analyzed.
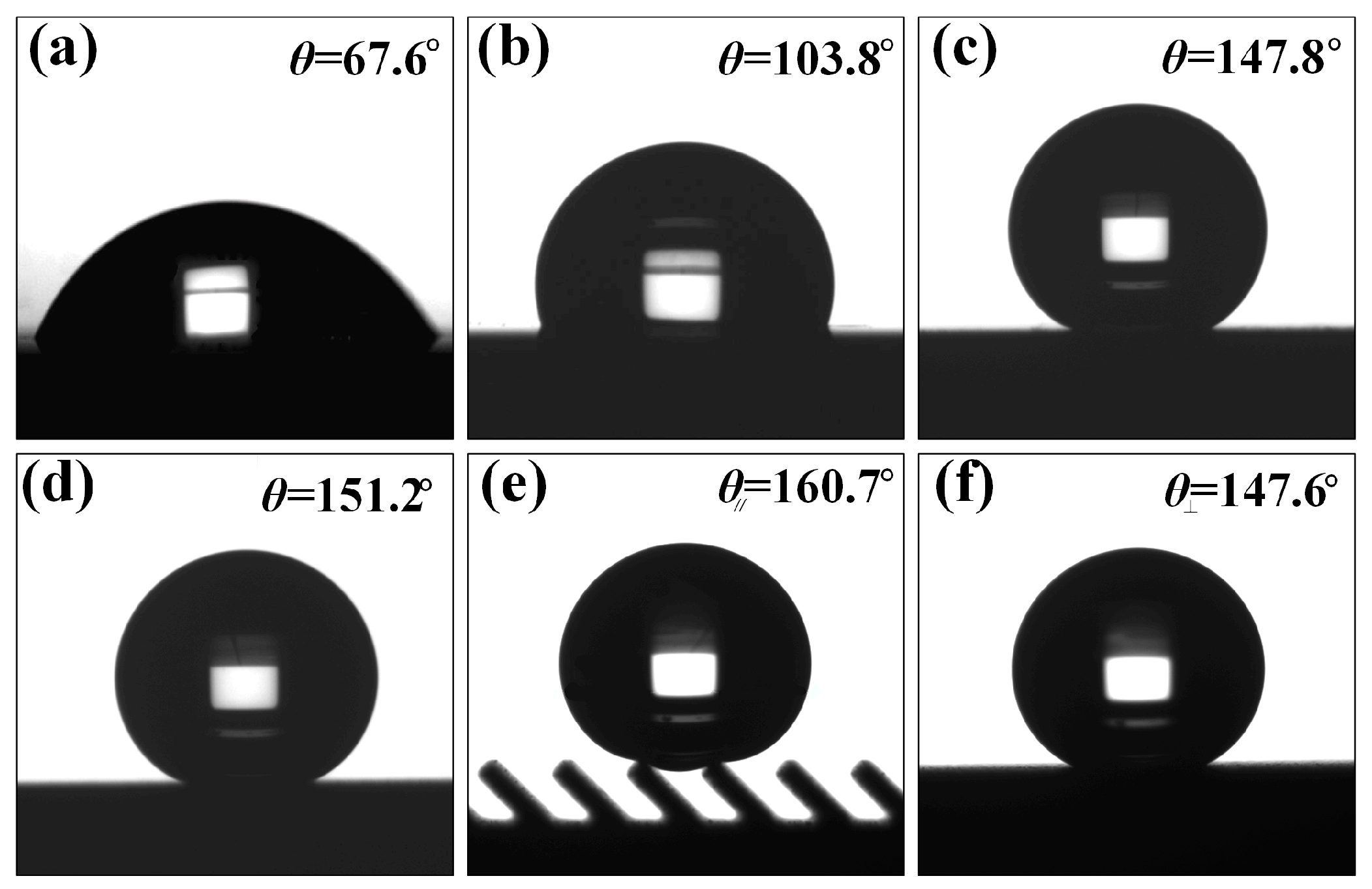
Figure 4 shows the contact angles of water on the Ti-6Al-4V surface with different reaction methods. It can be observed in
Figure 4a that the polished Ti-6Al-4V demonstrated high surface energy and strong hydrophilicity with static contact angle of only 67.6°, as shown in
Figure 4a. After the polished surface was modified with fluorosilane, the surface still failed to achieve the superhydrophobic behavior although the surface contact angle increased to 103.8.
Figure 4b,c shows the measurement results of the contact angle of water droplets on the WEDM processed and fluorosilane modified surface without groove structure. The contact angle of water droplets on the surface with micron-level concave bump structure reached 147.8°, exhibiting similar superhydrophobic properties. According to the Wenzel wetting theory, if the intrinsic contact angle of the modified smooth surface is greater than 90°, the contact angle of the prepared surface will increase with an increase in surface roughness. Therefore, during the WEDM process, micro-pits and bumps structure spontaneously formed during electrical processing effectively increased the surface roughness, thereby improving the hydrophobic properties of the surface. On this basis, after AH reaction, the contact angle of water droplets on the sample surface increased to 151.2°, as shown in
Figure 4d. As the construction of the composite micro-nano structure produced an increased surface roughness, the water droplets were seen to form a discrete contact with the solid surface, and hence the contact angle of the water droplets increased, forming a superhydrophobic surface.
Figure 4e,f shows optical photographs of contact angles in parallel and perpendicular directions with submillimeter inclined grooves (
L is 300 μm,
A is 45°) and composite micro-nano structures superimposed on the surface. The appearance of the structure caused the surface of the sample to exhibit anisotropic wettability. In the direction parallel to the groove, the contact angle of the water droplet reached 160.7°, while in the direction perpendicular to the groove, the contact angle of the water droplet was 147.6°, evidencing a difference between the contact angles in both directions to reach 13.1°. From the above analysis and comparison, it can be seen that the combination of multi-level microstructures makes the Ti-6Al-4V surface exhibit good superhydrophobicity, and the submillimeter-level inclined groove structure is a key factor generating anisotropic wettability. Therefore, the three-level combined bionic structure surface prepared by the combination of WEDM and AH is one of the ideal processing methods to form an anisotropic superhydrophobic surface.
To further investigate the influence of the submillimeter scale groove structure on the wettability of water droplets, the contact angle of the surface of the inclined groove structure was measured and analyzed.
Figure 5 shows the static contact angles in parallel and perpendicular directions at the inclination angle
A of the water droplet on the groove of 45° and spacing
L of 200 μm, 250 μm, 300 μm, and 350 μm, respectively. As shown in
Figure 5b, when
L was 250 μm in the direction parallel to the grooves, the contact angle of water droplets on its surface was 148.4°, showing a similar superhydrophobic effect. If the spacing was 300 μm, the contact angle of the water droplets on its surface reached 160.7°, exhibiting the superhydrophobic property, as shown in
Figure 5c. Therefore, it can be seen that groove spacing is one of the main factors affecting the value of the contact angle of the water droplet. As the contact angle of the solid surface is related to the length of the three-phase contact line (TPCL), the contact angle can be expressed as [
45]
where
h is the height of the water drop and
l is the length of the TPCL. From the formula, it can be known that when the height of the water drop is almost the same, the shorter the length of the TPCL, the greater the contact angle. It can be seen in
Figure 5b,c that when
L was 300 μm, the number of grooves in contact with water droplets was reduced to one. After measurement, the length of TPCL was about 1.27 mm when
L was 250 μm, and the length of TPCL was about 0.91 mm when
L was 300 μm. It can be concluded that the shorter the TPCL, the greater the contact angle. However, by comparing panels (a) and (b), and (c) and (d) in
Figure 5, it can be found that when the number of water droplets supported by the groove was the same, the contact angle of the water droplet decreased as
L increased; at the same time, as can be seen in
Figure 5, the contact angle in the direction perpendicular to the groove was significantly smaller than that in the parallel direction, and the contact angle in the perpendicular direction reduced stepwise with increasing
L.
Figure 6 shows the static contact angles in the directions parallel and perpendicular to the groove with a groove
L of 300 μm and an inclination angle
A of 90°, 75°, 60°, 45°, and 30°, respectively. Under the same groove
L, a decrease in
A caused the equivalent TPCL of the parallel contact between the water droplet and the groove end surface to increase. Therefore, the contact angle of the groove in both directions has the same change rule as the groove
A decreases and
L increases.
To further explore the relationship between the contact angles in two directions and the sub-millimeter groove structures
L and
A, the contact angles of water droplets on the groove surface in parallel and perpendicular directions with different structural parameters were measured. It can be seen in
Figure 7 that the contact angles in the parallel direction of the micro-groove structure surface were larger than those in the perpendicular direction and the water droplets had anisotropic wetting property on the surface. The mechanism of anisotropic wetting is shown in Figure 9f. When the water droplet diffused along the direction perpendicular to the groove, it would be affected by the gap of the groove and the groove produced a peg effect to pin the three-phase contact line of the water droplet, hindering further wetting; while the water droplet spread parallel along the groove, the end surface of the groove made continuous contact with the water droplet, and under the action of the solid surface adhesion and friction resistance, it diffused smoothly in the groove until it reached equilibrium, making the length of TPCL in the parallel direction of the groove smaller than that perpendicular to the groove. As a result, the contact angle of the water droplets in the parallel direction of the groove was larger than that perpendicular to the groove, creating heterogeneous wettability. By comparing the changing trend of the contact angle of the water droplet on each inclined groove sample with groove
L, it can be clearly seen in
Figure 7 that the contact angles of the groove in both directions decreased with increasing
L and decreasing
A except that the contact angle in the parallel direction of the groove showed a significant change with the groove
A of 45°. This is because an increase in
L caused the number of water droplets immersed in the groove to increase gradually, resulting in an increased TPCL, and therefore the contact angles in both directions decreased with increasing
L; when
L was constant, the parallel distance of the end surface of the groove supporting the water droplets gradually increased as the inclination angle
A of groove decreased, and thus the contact angles in perpendicular directions decreased stepwise, showing the same trend with the situation where
L was increased. For the anomalous CAs values at 45° and 30° presented in
Figure 7, the reason is that the number of grooves supporting water droplets in the parallel direction is reduced in the grooves (
Figure 5b,c and
Figure 6c,d), resulting in a sharp decrease in TPCL, the groove surface shows a larger contact angle. Therefore, it can be concluded that when the number of grooves supporting water droplets is the same, as
L increases, the water droplet contact angle in the parallel direction decreases as
L increases, as shown in
Figure 7.
3.2. Anisotropic Sliding Performance of Water Droplets
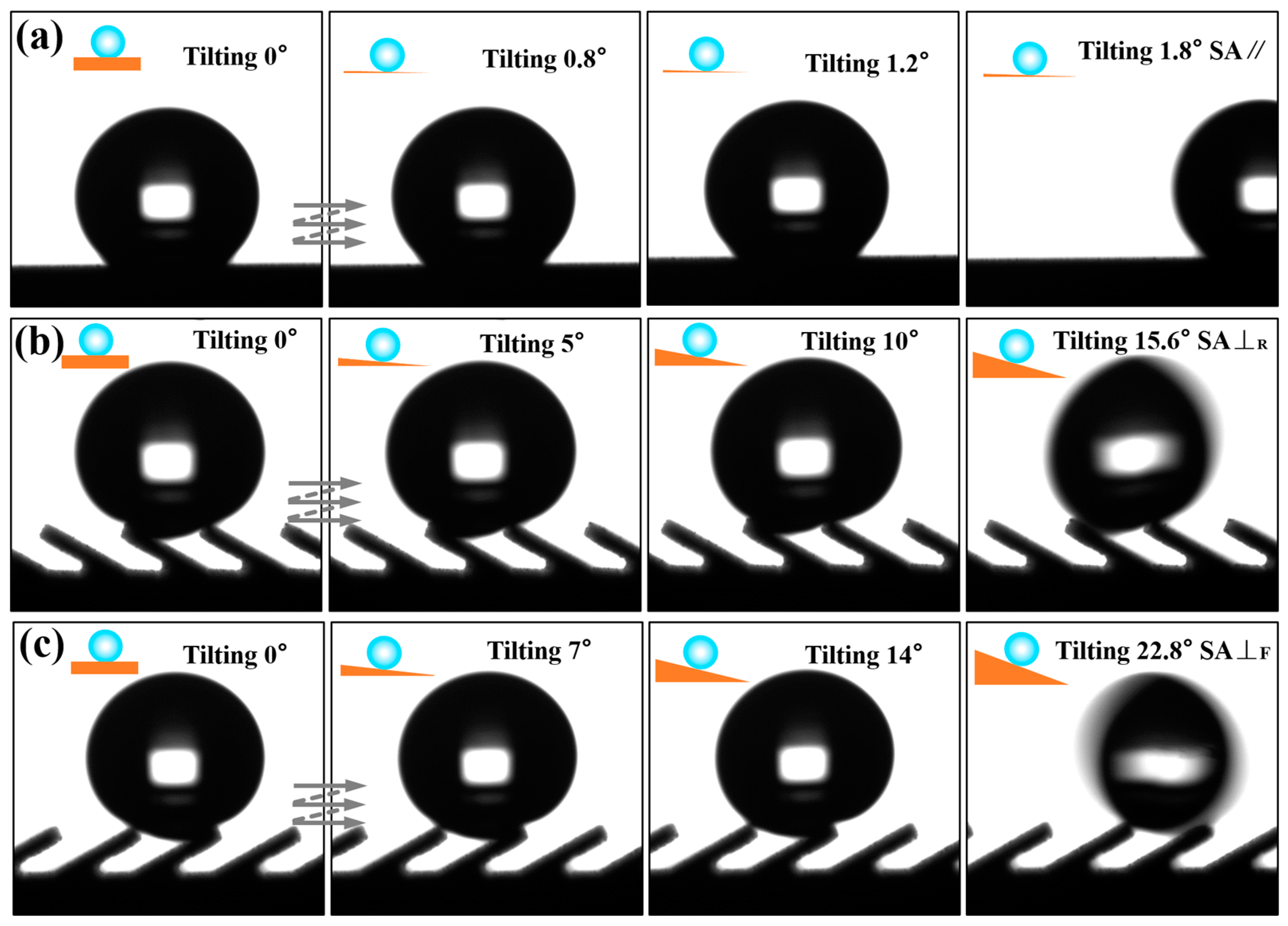
The three-level combined and inclined groove structure prepared by the method of WEDM and AH realizes the three-dimensional anisotropic sliding of water droplets. As shown in
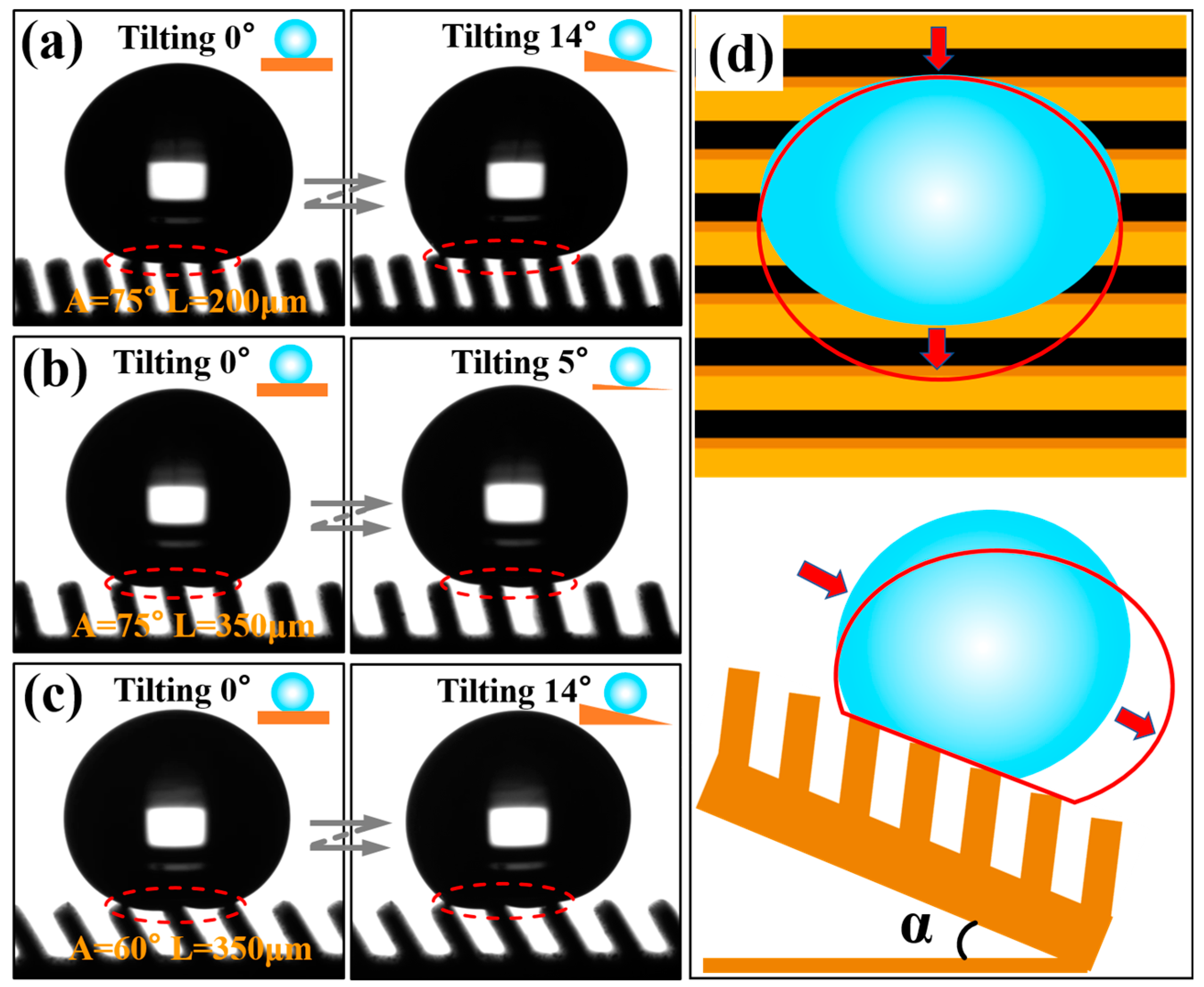
Figure 8, 7 μL water droplets sliding on the prepared Ti-6Al-4V inclined groove structure surface (
L = 300μm,
A = 30°) were photographed in three directions. It is clear that the water droplet had the smallest sliding angle in the direction parallel to the inclined groove. As shown in
Figure 8b, in the direction perpendicular to the inclined groove, the maximum SA⊥
F was 22.8° when sliding perpendicular to the inclined groove due to the influence of the asymmetric heterostructure;
Figure 8c indicates that the SA⊥
R was 15.6°when sliding perpendicular to the inclined groove reversely. It can be seen that the Ti-6Al-4V surface of the three-level combined and inclined groove structure displayed obvious tridirectionally anisotropic sliding characteristics. In order to further explore the influence of the structural parameters of the inclined groove on the droplet sliding performance, we further measured the sliding angles of the groove surface under different structural parameters.
Figure 9 illustrates the images of water droplets sliding on the groove structures with different inclination angles changing with the groove
L. At a 90° angle, the upper end of the groove was parallel to the parallel plane. The water droplets were subject to the same resistance when sliding in both directions of the perpendicular groove, exhibiting only one kind of sliding characteristics. As can be seen in
Figure 9a, the groove surface only showed the anisotropy in two directions at a 90° angle. However, when the groove was inclined, the upper end of the groove was at a corresponding angle to the parallel plane, and the surface formed an asymmetrical-shaped structure. The resistance of the water droplet was different when sliding in two directions perpendicular to the groove, showing two sliding characteristics. At the same time, the influence of different forms of solid–liquid contact lines in the perpendicular and parallel directions made the inclined groove surface exhibit tridirectionally anisotropic sliding characteristics, as shown in
Figure 9b–e. When the inclination angle
A was 75°, 60° and 45°, the sliding angles in three directions on the surface can be expressed as SA⊥
R > SA⊥
F > SA//; if
A was 30° and
L was greater than 200 μm, the sliding angles in three directions on the groove structure surface can be described as SA⊥
F > SA⊥
R > SA//. Due to the influence of the groove pitch
L and the inclination angle
A on the sliding process of water droplets, when the inclination angle
A of the groove was 90° and 75°, respectively, the sliding angles in the directions parallel and perpendicular to the groove surface decreased with increasing
L, as shown in
Figure 9a,b, while the inclination angle
A was 60°, 45°, and 30°, respectively, the tridirectionally anisotropic sliding angles increased as
L increased, as shown in
Figure 9c–e.
3.3. Mechanism of Anisotropic Sliding Behavior of Water Droplets
This section studied and analyzed the anisotropic sliding behavior of water droplets on the surface of the prepared three-level inclination groove structure so as to understand the influence of groove structure parameters on the anisotropic sliding performance of water droplets. As a matter of fact, there are several factors affecting the sliding angle when water droplets slide on a solid surface. According to the theory of Furmidge and Frenkel, the sliding angle α of the solid plane can be described as follows [
7],
where
θA is the advancing angle,
θR is the receding angle, m is the mass of the droplet,
R is the contact radius of the droplet and the solid surface, and
γLG is the interfacial tension between the liquid and the gas. The left part of the equation is the driving force of the water droplet on the surface under the influence of gravity, and the right part is the sliding resistance caused by the surface adhesion. It can be seen from the formula that when the water droplet quality was constant, the smaller the adhesion resistance of the water droplet, the smaller the sliding angle of the surface. The adhesion of sliding water droplets was closely related to the morphology of the surface microstructure. A decrease in
R caused the contact area between the water droplets and the solid surface to decrease, leading to a smaller adhesion resistance of the water droplets on the surface.
R is mainly affected by large-scale grooves. Specifically, the diffusion degree of water droplets on the surface was different with a change of
L and
A of the groove, resulting in varied
R. On the other hand, for the surface with microstructures, the combination of micro- and nanostructures established discrete contact between the solid and the liquid to avoid the intrusion of external water droplets, which effectively reduced the contact area between the liquid and the solid, thereby effectively reducing the adhesion resistance of the surface [
6]. The combination of the two methods will effectively reduce the adhesion resistance of the solid surface, resulting in a very low sliding angle. Therefore, a bionic groove structure with a three-level combination was prepared by a process method combining WEDM and AH reaction, so that the sliding of water droplets in the direction parallel to the groove was kept within 10°, and the minimum sliding angle reached 1.8° at the suitable submillimeter groove structure parameters.
Due to the influence of the large-scale groove structure, the water droplets spread differently along with the parallel and perpendicular directions of the groove. The diameters of the TPCL in the two directions are shown in
Figure 9f. When sliding in the parallel direction, the diameter of the solid-liquid junction was short, and the adhesion resistance was relatively small; the diameter of the solid-liquid connection was long when sliding in the perpendicular direction showing a discontinuity. Not only did the water droplet experience a large adhesion resistance when sliding, but also the water droplet needed to overcome a larger energy barrier during sliding [
19,
20] due to the discontinuous radius of the solid–liquid connection, which can explain why the sliding angle in the direction parallel to the groove smaller than that in the perpendicular direction.
In addition, when the groove changed from a right angle to an inclined angle, the upper-end surface of the inclined groove formed an asymmetric inclined surface. When the water droplet slid in the perpendicular direction, it presented two scenarios. The water droplet sliding forward along the direction of the inclined groove appeared as a release effect while sliding reversely along the direction of the inclined grooves exhibited a pinning effect. The sliding resistance of the water droplets under different sliding modes of action was different, demonstrating bidirectionally anisotropic sliding characteristics in the perpendicular direction [
46]. Therefore, the surface of the inclined groove exhibited tridirectionally anisotropic sliding characteristics. As shown in
Figure 10a, when the inclination angle
A of inclined groove was 90°, the upper-end surface of the groove was parallel to the parallel plane, and the entire groove surface only exhibited bidirectionally anisotropic sliding property. However, as the inclination angle
A decreased, the end face perpendicular to the groove direction produced an asymmetrical shaped structure, as shown in
Figure 10b,c. The water droplets demonstrated tridirectionally anisotropic sliding characteristics on the entire inclined groove surface.
Anisotropic sliding was greatly affected by the submillimeter inclined groove structure size, as shown in
Figure 10. When the inclination angle
A of the groove was 75° and 60°, respectively, it could effectively avoid the intrusion of external water droplet due to the small horizontal distance between the grooves, and the water droplets were always in a non-wetting state on the inclined groove surface. Under this circumstance, when the water droplet slid forward along the direction perpendicular to the inclined groove, they were subjected to the energy barrier generated by the discontinuous TPCL and the adhesion force of the inclined end face of the groove, while water droplets sliding reversely along the inclined groove in the perpendicular direction were supported by the inclined end surface of the groove, so that the resistance of the water droplets sliding reversely along the inclined groove direction was greater than that sliding forward along the inclined groove direction, as shown in
Figure 10b and
Figure 11c. It can be concluded that when the angle
A of the inclined groove was 75°, 60°, and 45°, respectively, the sliding angles in the tridirectionally appeared as SA⊥
R>SA⊥
F>SA//. In addition, the energy barrier generated by the discontinuous TPCL in the unwetted state and the resistance produced by the tilted end face of the groove were relatively low, the difference between the sliding angles in each direction was also very limited. However, at a 30° angle, the droplet began to be supported by one inclined groove, and the external water droplet were immersed in the groove and contact the trench wall because the horizontal distance between the inclined grooves was enlarged as the inclination angle became smaller, as shown in
Figure 10c. Water droplet sliding along the direction perpendicular to the groove were subjected to greater resistance, which increased the sliding angle and broadened the difference between the sliding angles in each direction, as shown in
Figure 9e. When
L was greater than 200 μm, the sliding angles in three directions showed the change rule of SA⊥
F>SA⊥
R>SA//. This is because at a 30° degree, the contact area between the water droplet immersed in the groove and the two side walls of the groove was asymmetric, as shown in
Figure 8b and
Figure 10c. SA⊥
R was relatively small because the wall adhesion force worked against the gravity of the water droplet when the water droplet slid reversely in the direction perpendicular to the inclined groove. On the other hand, if the water droplet slide forward along the direction perpendicular to the inclined groove, in addition to overcoming the adhesive force on the inclined groove wall, the gravity of the water droplet accumulated directly above the inclined surface of the groove wall with increasing sliding angle, which caused greater adhesive force on the groove wall, thereby making SA⊥
F increase.
As shown in
Figure 9a,b, when the inclination angle of the inclined groove was at 90° and 75°, the sliding angle in each direction decreased as
L increased. By analyzing the sliding behavior of the water droplets in the dynamic video, it can be observed that if the inclination angle of the groove end surface and
L was small, water droplets were deformed under the combined action of the adhesion force of the groove end surface and their own gravity during the sliding process. As the tilt angle of the sample increased, the number of grooves supporting water droplets increased since the deformed water droplet surface contacted the end surface of the groove, as shown in
Figure 11a, which in turn led to an increased solid–liquid contact radius and increased surface adhesion force as well as increased sliding angle. The change process of TPCL during the sliding process is shown in
Figure 11d. However, an increase in
L caused the solid–liquid contact area between the water droplet and the groove surface to decrease. Moreover, the water droplet, driven by its own gravity and insufficient deformation degree, rolled off the groove surface during the sliding process, as shown in
Figure 11b. When the inclination angle of the groove reduced, the inclination of the end face of the groove increased, and therefore the pinning effect became enhanced when the water droplets slide. Meanwhile, the equivalent solid-liquid contact distance in the parallel direction of a single groove increased with decreasing inclination angle and increasing
L. Consequently, the number of water droplets immersed in the groove increased as
L increased, as a result, the pinning effect of water droplets on the inclined end face of the groove was further increased [
7,
31], as shown in
Figure 11c. Therefore, when the inclination angle
A of the groove was 60°, 45°, and 30°, the sliding angles in the three directions increased with increasing
L.