Triplet Energy Transfer Mechanism of Ternary Organic Hybrid Thin Films of PFO/MEH-PPV/CsPbBr3 Perovskite Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Characterizations
3. Results
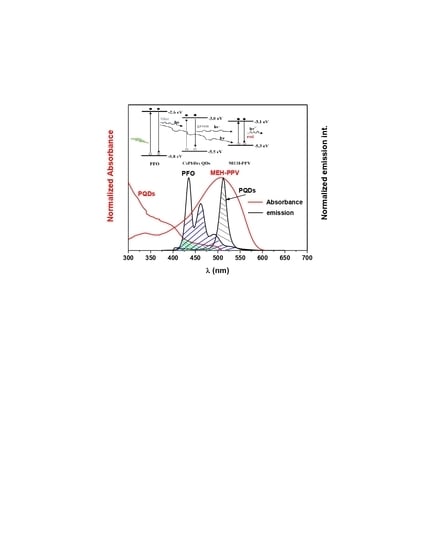
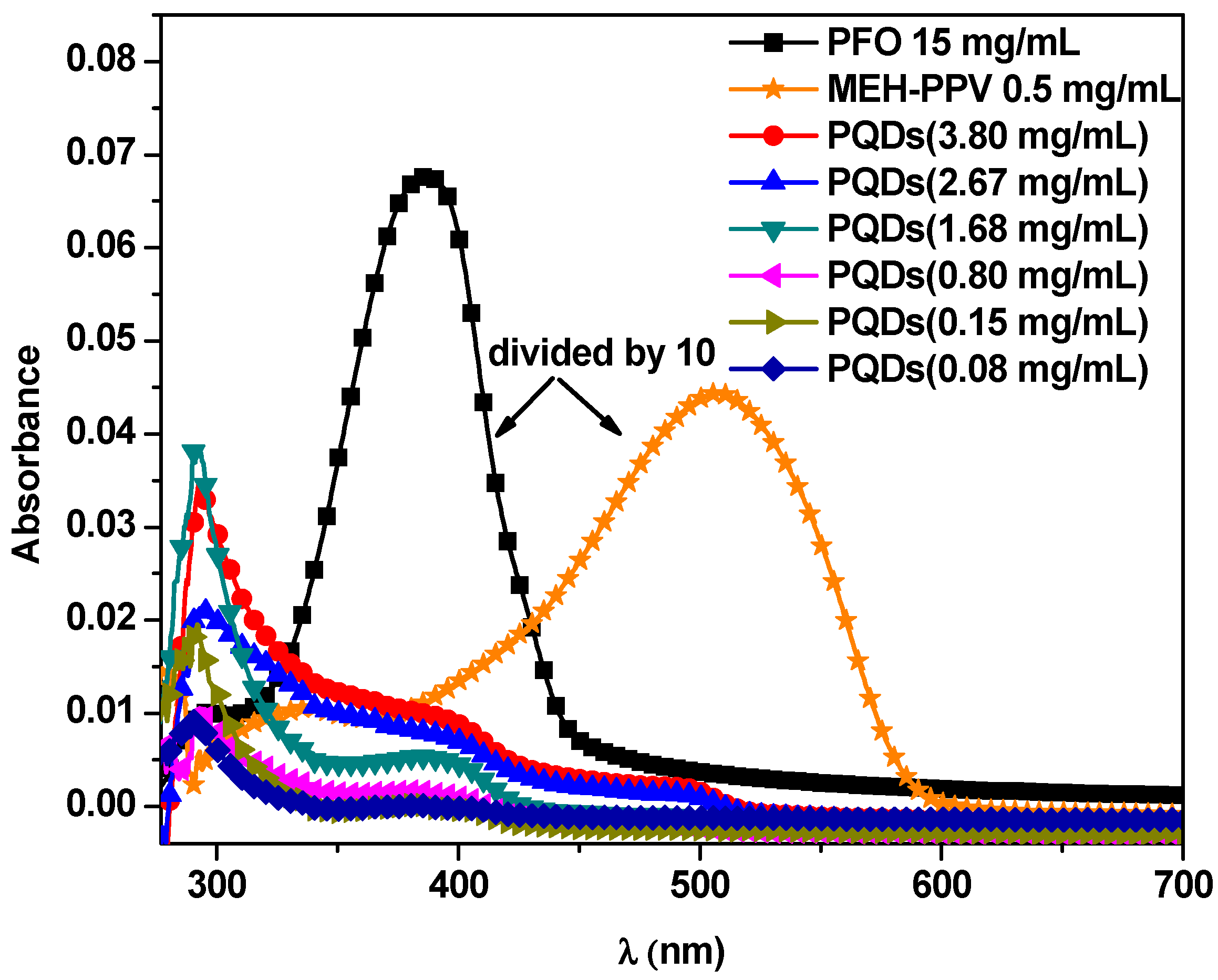
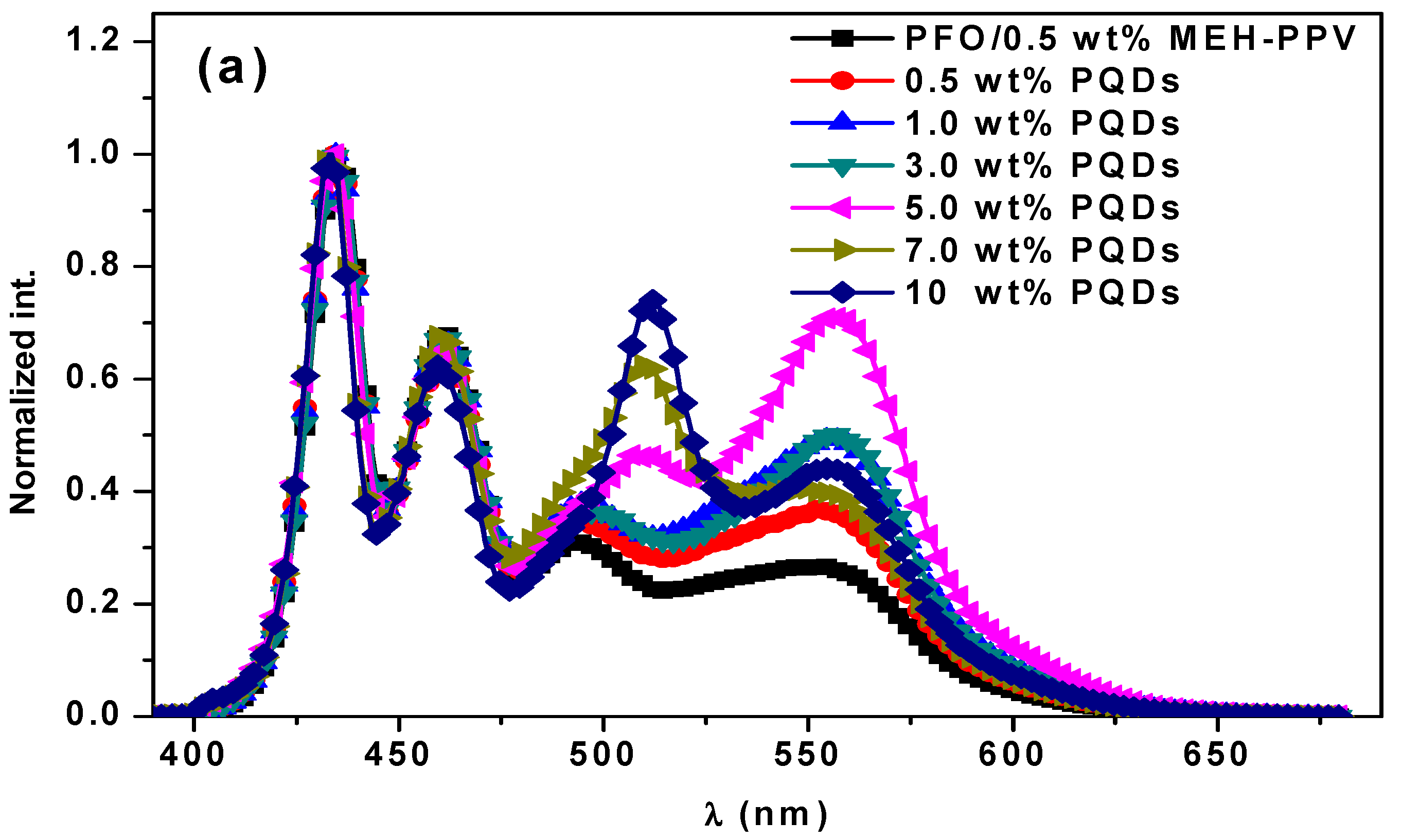
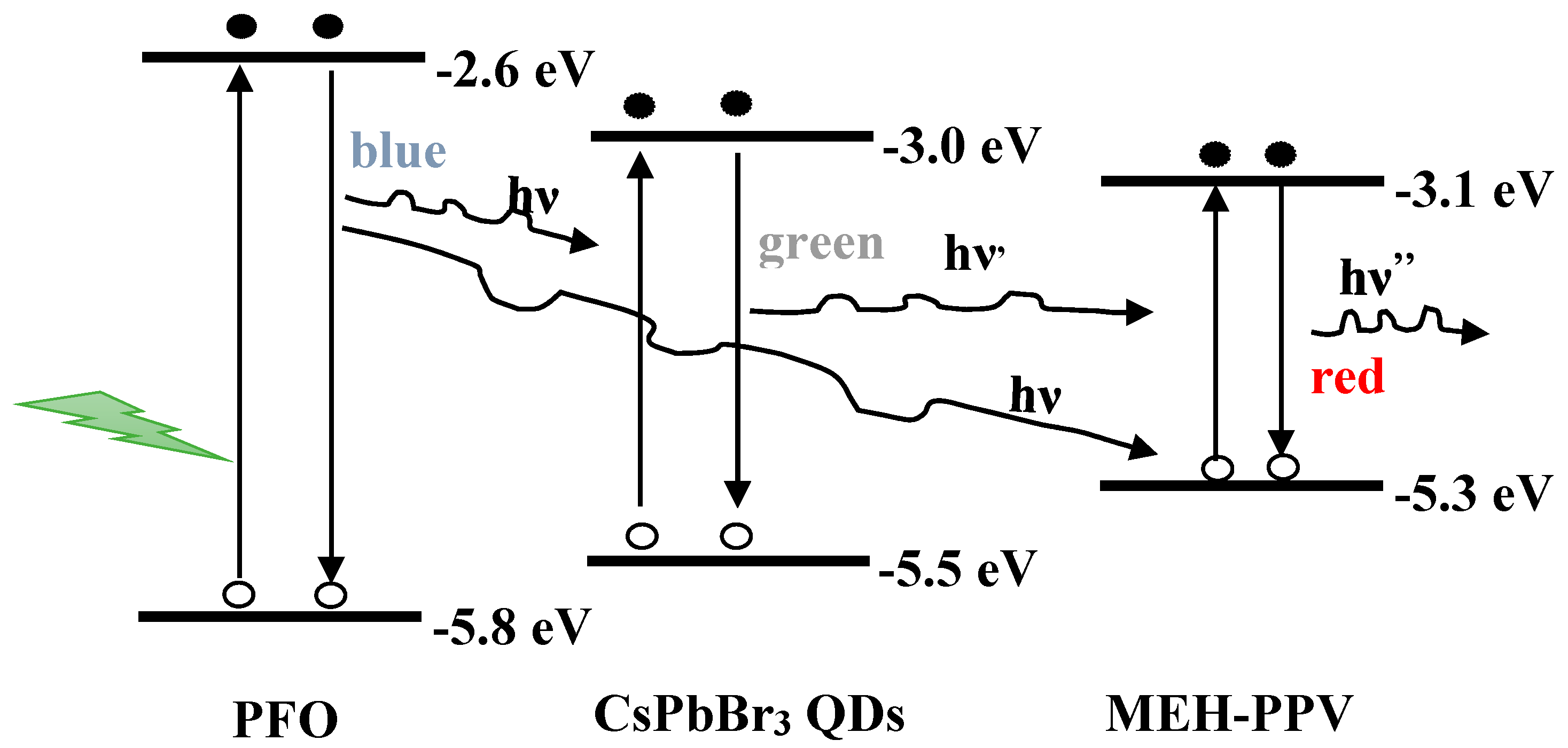
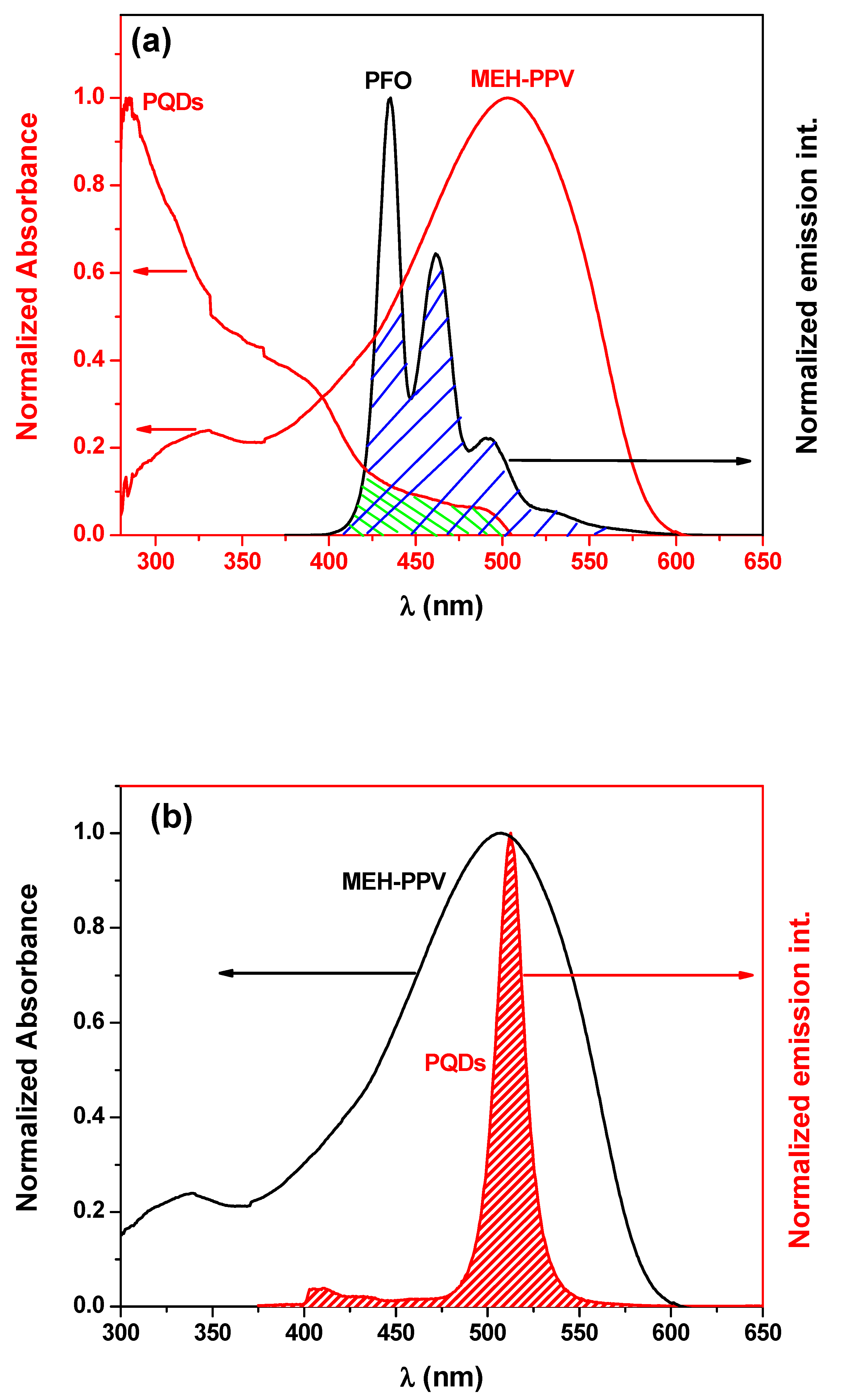
3.1. Optical Properties
3.2. FRET Parameters of the Ternary Hybrid Thin Films
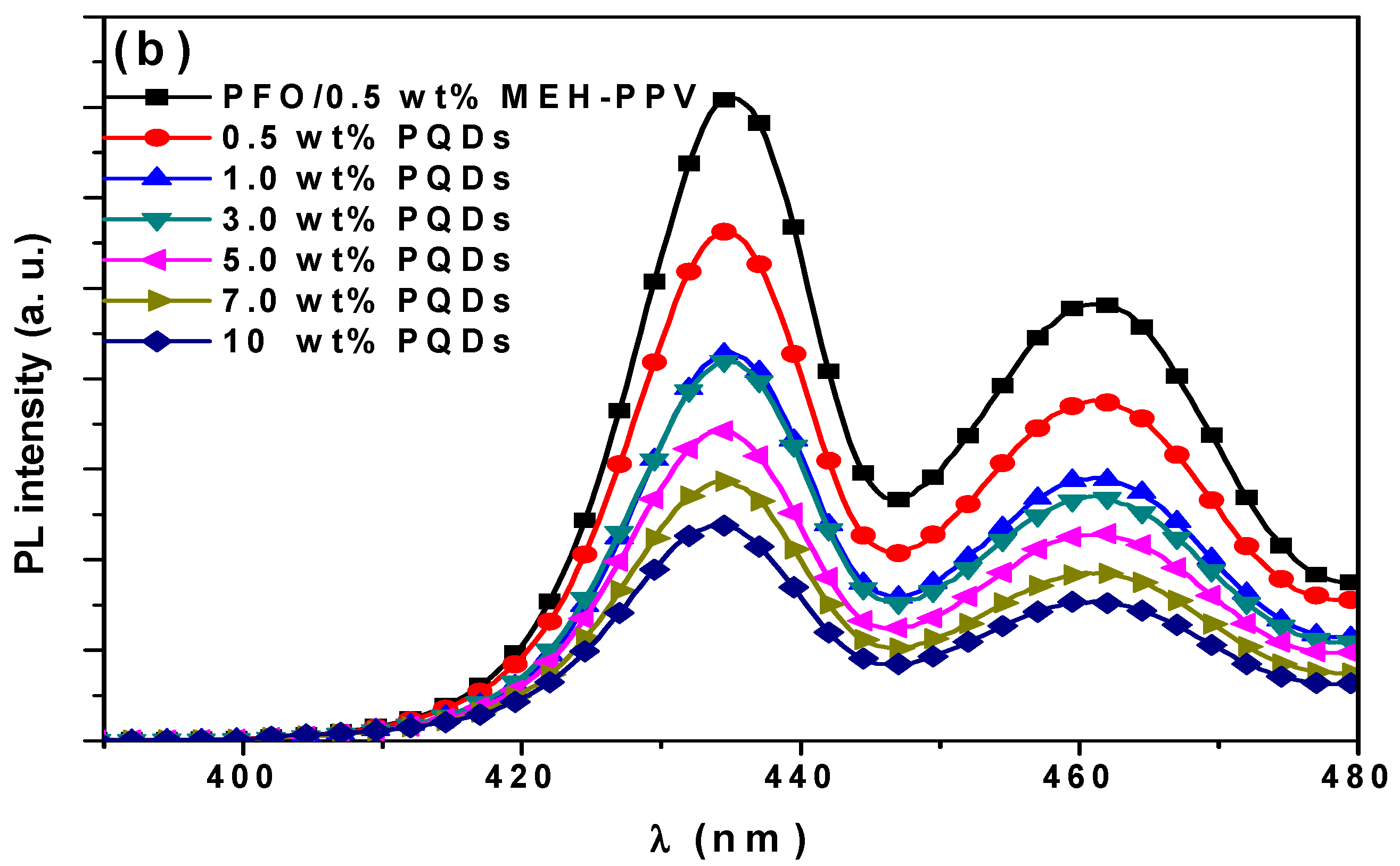
3.2.1. Quantum Yield (φDA) and Lifetime (τDA) of the Donor in the Ternary Hybrid Thin Films
3.2.2. Stern–Volmer (kSV) and the Quenching Rate (kq) Constants
3.2.3. Energy Transfer Distance and Lifetime
3.2.4. Critical Concentration of the Acceptor (Ao) and Conjugation Length (Aπ)
4. Discussion and Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Xu, X.; Li, Z.; Wang, J.; Lin, B.; Ma, W.; Xia, Y.; Andersson, M.R.; Janssen, R.A.J.; Wang, E. High-performance all-polymer solar cells based on fluorinated naphthalene diimide acceptor polymers with fine-tuned crystallinity and enhanced dielectric constants. Nano Energy 2018, 45, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-M.; Su, Y.-W.; Jiang, J.-M.; Chen, H.-C.; Lin, S.-W.; Su, C.-J.; Jeng, U.-S.; Wei, K.-H. Complementary solvent additives tune the orientation of polymer lamellae, reduce the sizes of aggregated fullerene domains, and enhance the performance of bulk heterojunction solar cells. J. Mater. Chem. A 2014, 2, 20760–20769. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-R.; Phan, H.; Luo, C.; Wang, M.; Perez, L.A.; Patel, S.N.; Ying, L.; Kramer, E.J.; Nguyen, T.Q.; Bazan, G.C.; et al. High-Mobility Field-Effect Transistors Fabricated with Macroscopic Aligned Semiconducting Polymers. Adv. Mater. 2014, 26, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J. Currents and photocurrents in organic materials determined by the interface phenomena. Adv. Colloid Interface Sci. 2005, 116, 227–243. [Google Scholar] [CrossRef]
- Lu, J.; Tao, Y.; D’Iorio, M.; Li, Y.; Ding, J.; Day, M. Pure Deep Blue Light-Emitting Diodes from Alternating Fluorene/Carbazole Copolymers by Using Suitable Hole-Blocking Materials. Macromolecules 2004, 37, 2442–2449. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Jumali, M.H.H.; AlSalhi, M.S. Enhanced Optoelectronic Properties of PFO/Fluorol 7GA Hybrid Light Emitting Diodes via Additions of TiO2 Nanoparticles. Polymers 2016, 8, 334. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Energy transfer mechanism and optoelectronic properties of (PFO/TiO2)/Fluorol 7GA nanocomposite thin films. Opt. Mater. 2017, 72, 644–649. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, M.; Zhang, Y.; Bin, Z.; Zhang, D.; Duan, L. Simultaneous Enhancement of Efficiency and Stability of Phosphorescent OLEDs Based on Efficient Forster Energy Transfer from Interface Exciplex. ACS Appl. Mater. Interfaces 2016, 8, 3825–3832. [Google Scholar] [CrossRef]
- Pramanik, A.; Biswas, S.; Tiwary, C.S.; Kumbhakar, P.; Sarkar, R.; Kumbhakar, P. Forster resonance energy transfer assisted white light generation and luminescence tuning in a colloidal graphene quantum dot-dye system. J. Colloid Interface Sci. 2020, 565, 326–336. [Google Scholar] [CrossRef]
- Trindade, F.D.J.; Triboni, E.R.; Castanheira, B.; Brochsztain, S. Color-Tunable Fluorescence and White Light Emission from Mesoporous Organosilicas Based on Energy Transfer from 1,8-Naphthalimide Hosts to Perylenediimide Guests. J. Phys. Chem. C 2015, 119, 26989–26998. [Google Scholar] [CrossRef] [Green Version]
- Al-Asbahi, B.A.; Qaid, S.M.; Jumali, M.H.H.; AlSalhi, M.S.; Aldwayyan, A.S. Long-range dipole–dipole energy transfer enhancement via addition of SiO2/TiO2 nanocomposite in PFO/MEH-PPV hybrid thin films. J. Appl. Polym. Sci. 2019, 136, 47845. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Qaid, S.M.H.; Al Dwayyan, A.S.A. Effect of Donor-Acceptor Concentration Ratios on Non-Radiative Energy Transfer in Zero-Dimensional Cs4PbBr6 Perovskite/MEH-PPV Nanocomposite Thin Films. Polymers 2020, 12, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaid, S.M.H.; Al-Asbahi, B.A.; Ghaithan, H.M.; AlSalhi, M.S.; Al Dwayyan, A.S. Optical and structural properties of CsPbBr3 perovskite quantum dots/PFO polymer composite thin films. J. Colloid Interface Sci. 2020, 563, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Meyns, M.; Perálvarez, M.; Heuer-Jungemann, A.; Hertog, W.; Ibáñez, M.; Nafria, R.; Genç, A.; Arbiol, J.; Kovalenko, M.V.; Carreras, J.; et al. Polymer-Enhanced Stability of Inorganic Perovskite Nanocrystals and Their Application in Color Conversion LEDs. ACS Appl. Mater. Interfaces 2016, 8, 19579–19586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef]
- Rainò, G.; Nedelcu, G.; Protesescu, L.; Bodnarchuk, M.I.; Kovalenko, M.V.; Mahrt, R.F.; Stöferle, T. Single Cesium Lead Halide Perovskite Nanocrystals at Low Temperature: Fast Single-Photon Emission, Reduced Blinking, and Exciton Fine Structure. ACS Nano 2016, 10, 2485–2490. [Google Scholar] [CrossRef] [Green Version]
- Bouzayen, N.; Zaidi, B.; Mabrouk, A.; Chemek, M.; Alimi, K. Density functional theory studies of new bipolar carbazole–benzothiazole: Electronic and vibrational properties. Comput. Theor. Chem. 2012, 984, 1–8. [Google Scholar] [CrossRef]
- Jungsuttiwong, S.; Tarsang, R.; Sudyoadsuk, T.; Promarak, V.; Khongpracha, P.; Namuangruk, S. Theoretical study on novel double donor-based dyes used in high efficient dye-sensitized solar cells: The application of TDDFT study to the electron injection process. Org. Electron. 2013, 14, 711–722. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Jumali, M.H.H.; Yap, C.C.; Salleh, M.M. Influence of TiO2 Nanoparticles on Enhancement of Optoelectronic Properties of PFO-Based Light Emitting Diode. J. Nanomater. 2013, 2013, 561534. [Google Scholar] [CrossRef]
- List, E.J.W.; Guentner, R.; De Freitas, P.S.; Scherf, U. The Effect of Keto Defect Sites on the Emission Properties of Polyfluorene-Type Materials. Adv. Mater. 2002, 14, 374–378. [Google Scholar] [CrossRef]
- Sahare, P.D.; Sharma, V.K.; Mohan, D.; Rupasov, A.A. Energy transfer studies in binary dye solution mixtures: Acriflavine + Rhodamine 6G and Acriflavine + Rhodamine B. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Al-Asbahi, B.A.; Jumali, M.H.H.; Yap, C.C.; Flaifel, M.H.; Salleh, M.M. Photophysical properties and energy transfer mechanism of PFO/Fluorol 7GA hybrid thin films. J. Lumin. 2013, 142, 57–65. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Gilbert, A.; Baggott, J.E. Essentials of Molecular Photochemistry; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 34, 1685–1757. [Google Scholar] [CrossRef]
- Wu, P.; Brand, L. Resonance Energy Transfer: Methods and Applications. Anal. Biochem. 1994, 218, 1–13. [Google Scholar] [CrossRef]
- Mote, U.S.; Patil, S.R.; Bhosale, S.H.; Han, S.H.; Kolekar, G.B. Fluorescence resonance energy transfer from tryptophan to folic acid in micellar media and deionised water. J. Photochem. Photobiol. B Biol. 2011, 103, 16–21. [Google Scholar] [CrossRef]




| PQDs (wt.%) | φDA | τDA (ps) | knr (ns)−1 | ksv (µM)−1 | kq × 1015 (M.S)−1 | Aπ (Å) | A0 (mM) | A1/2 (µM) |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.64 | 308 | 1.17 | 0.067 | 0.192 | 0.576 | 0.57 | 15.0 |
| 0.5 | 0.51 | 245 | 2.00 | 0.220 | 0.635 | 0.039 | 0.52 | 4.55 |
| 1.0 | 0.39 | 186 | 3.29 | 0.459 | 1.326 | −0.456 | 0.48 | 2.18 |
| 3.0 | 0.38 | 180 | 3.48 | 0.492 | 1.423 | −0.514 | 0.45 | 2.03 |
| 5.0 | 0.32 | 149 | 4.62 | 0.704 | 2.034 | −0.798 | 0.40 | 1.42 |
| 7.0 | 0.26 | 125 | 5.94 | 0.948 | 2.739 | −1.049 | 0.42 | 1.06 |
| 10 | 0.22 | 104 | 7.56 | 1.246 | 3.601 | −1.290 | 0.39 | 0.80 |
| PQDs (wt.%) | J(λ) × 1016 (M−1cm−1nm4) | R0 (Å) | RDA (Å) | τET (ps) |
|---|---|---|---|---|
| 0 | 7.32 | 92.3 | 130.6 | 2775 |
| 0.5 | 8.64 | 94.9 | 110.0 | 839 |
| 1.0 | 9.96 | 97.2 | 99.7 | 402 |
| 3.0 | 11.4 | 99.4 | 100.7 | 375 |
| 5.0 | 14.6 | 103.6 | 98.9 | 262 |
| 7.0 | 13.4 | 102.1 | 92.8 | 195 |
| 10 | 15.6 | 104.7 | 90.9 | 148 |
| PQDs (wt.%) | J(λ) × 1016 (M−1cm−1nm4) | R0 (Å) | RDA (Å) | τET (ps) | A0 (mM) |
|---|---|---|---|---|---|
| 0.5 | 1.17 | 68.0 | 78.8 | 7810 | 1.42 |
| 1.0 | 0.68 | 62.2 | 63.8 | 4170 | 1.86 |
| 3.0 | 0.16 | 48.6 | 49.2 | 781 | 3.89 |
| 5.0 | 0.094 | 44.7 | 42.7 | 372 | 5.00 |
| 7.0 | 0.055 | 40.8 | 37.1 | 234 | 6.58 |
| 10 | 0.045 | 39.5 | 34.3 | 164 | 7.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asbahi, B.A.; Qaid, S.M.H.; Ghaithan, H.M.; Aldwayyan, A.S. Triplet Energy Transfer Mechanism of Ternary Organic Hybrid Thin Films of PFO/MEH-PPV/CsPbBr3 Perovskite Quantum Dots. Nanomaterials 2020, 10, 2094. https://doi.org/10.3390/nano10112094
Al-Asbahi BA, Qaid SMH, Ghaithan HM, Aldwayyan AS. Triplet Energy Transfer Mechanism of Ternary Organic Hybrid Thin Films of PFO/MEH-PPV/CsPbBr3 Perovskite Quantum Dots. Nanomaterials. 2020; 10(11):2094. https://doi.org/10.3390/nano10112094
Chicago/Turabian StyleAl-Asbahi, Bandar Ali, Saif M. H. Qaid, Hamid M. Ghaithan, and Abdullah S. Aldwayyan. 2020. "Triplet Energy Transfer Mechanism of Ternary Organic Hybrid Thin Films of PFO/MEH-PPV/CsPbBr3 Perovskite Quantum Dots" Nanomaterials 10, no. 11: 2094. https://doi.org/10.3390/nano10112094
APA StyleAl-Asbahi, B. A., Qaid, S. M. H., Ghaithan, H. M., & Aldwayyan, A. S. (2020). Triplet Energy Transfer Mechanism of Ternary Organic Hybrid Thin Films of PFO/MEH-PPV/CsPbBr3 Perovskite Quantum Dots. Nanomaterials, 10(11), 2094. https://doi.org/10.3390/nano10112094