Preparations of NiFe2O4 Yolk-Shell@C Nanospheres and Their Performances as Anode Materials for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
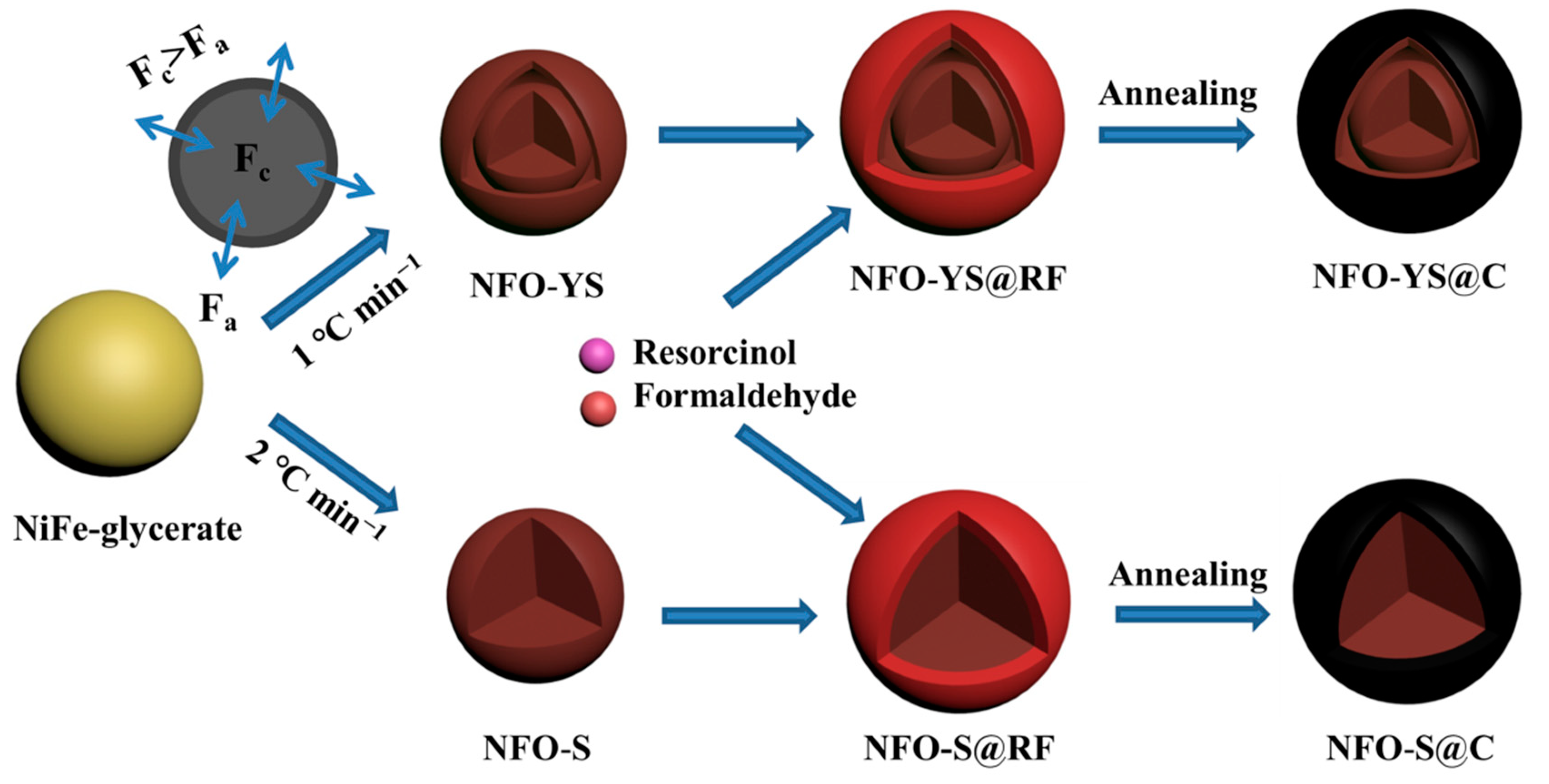
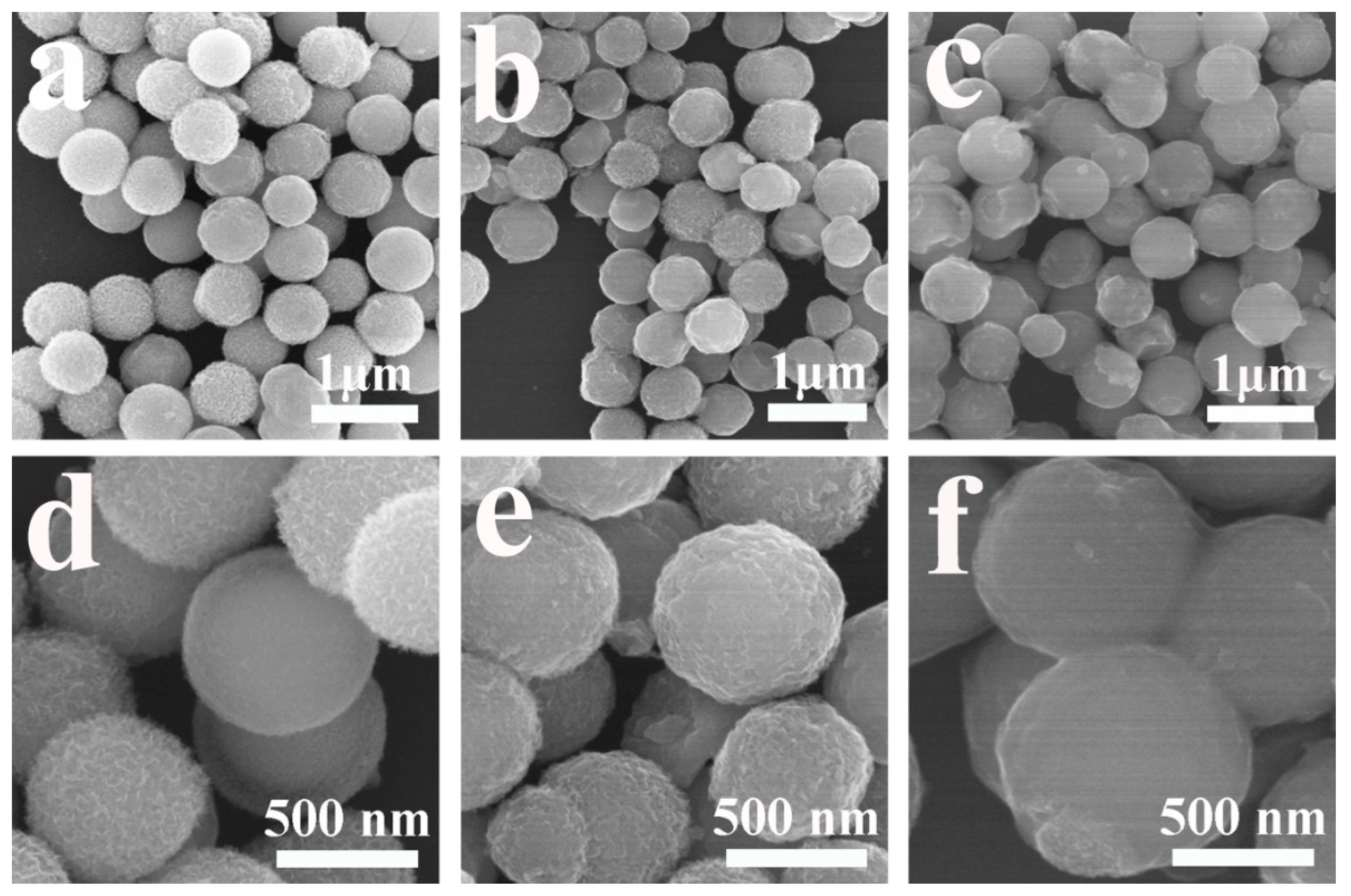
2.2. Synthesis of NiFe2O4 Yolk-Shell Nanospheres
2.3. Synthesis of NiFe2O4@C Nanospheres
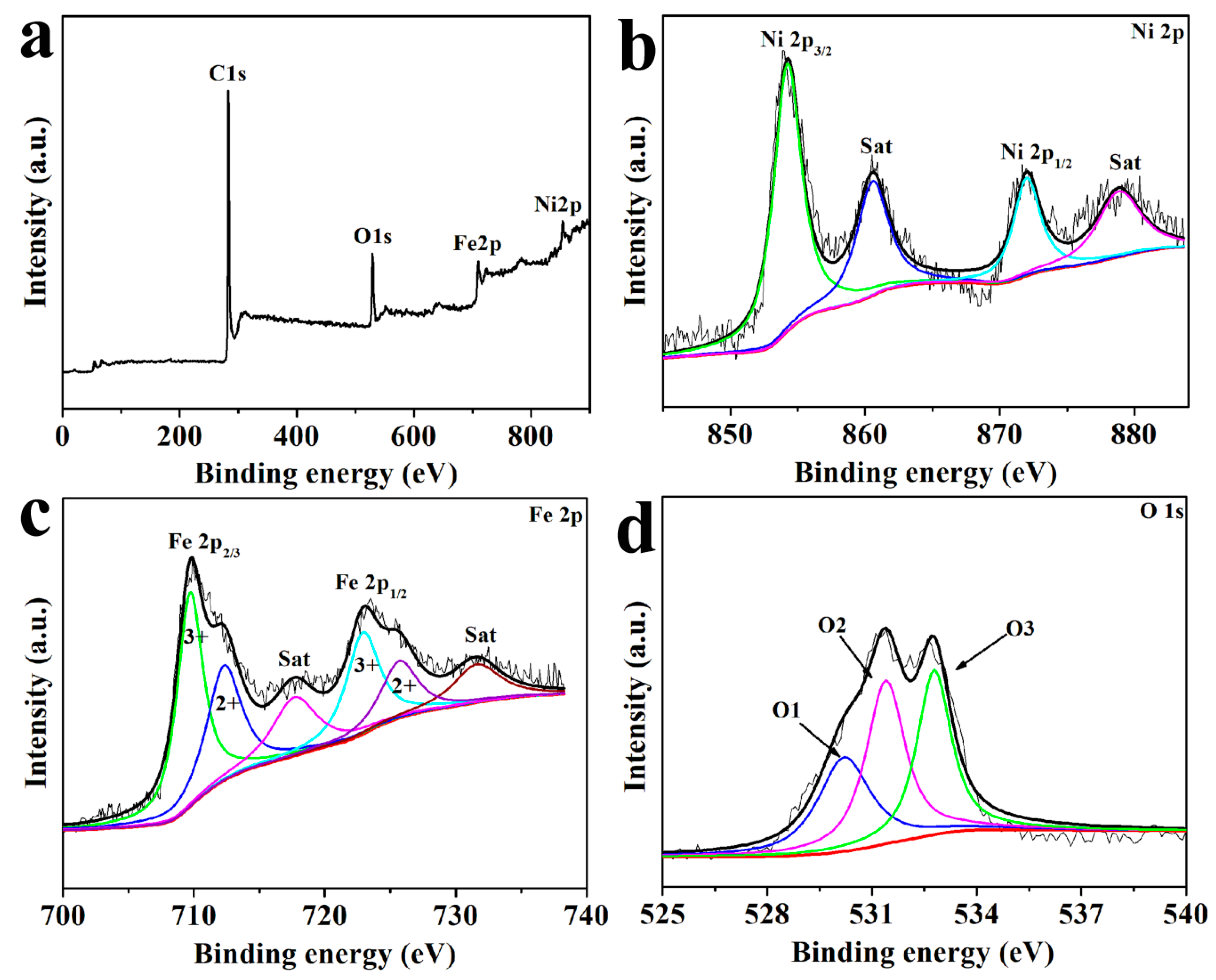
2.4. Characterization
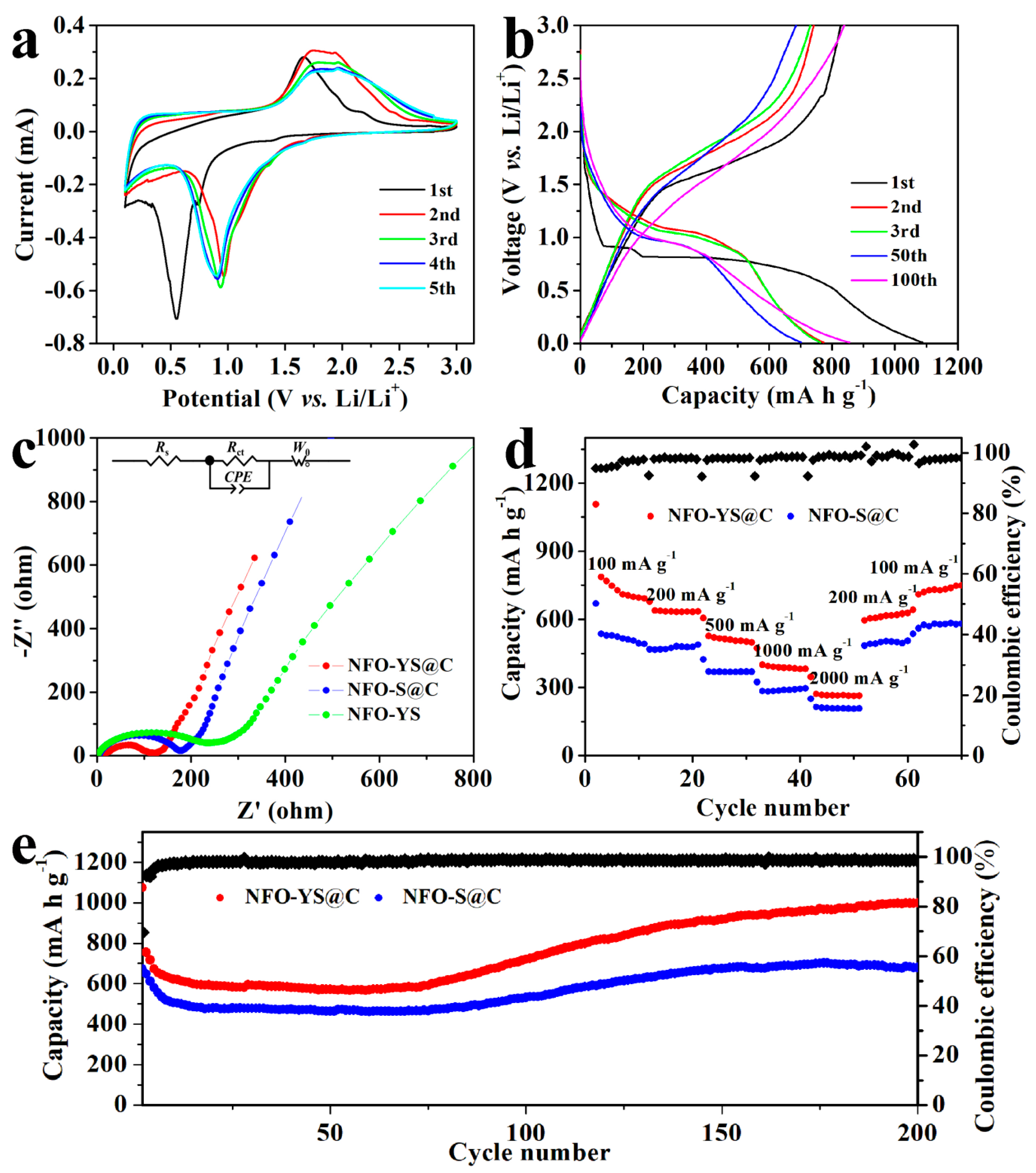
2.5. Electrochemical Measurements
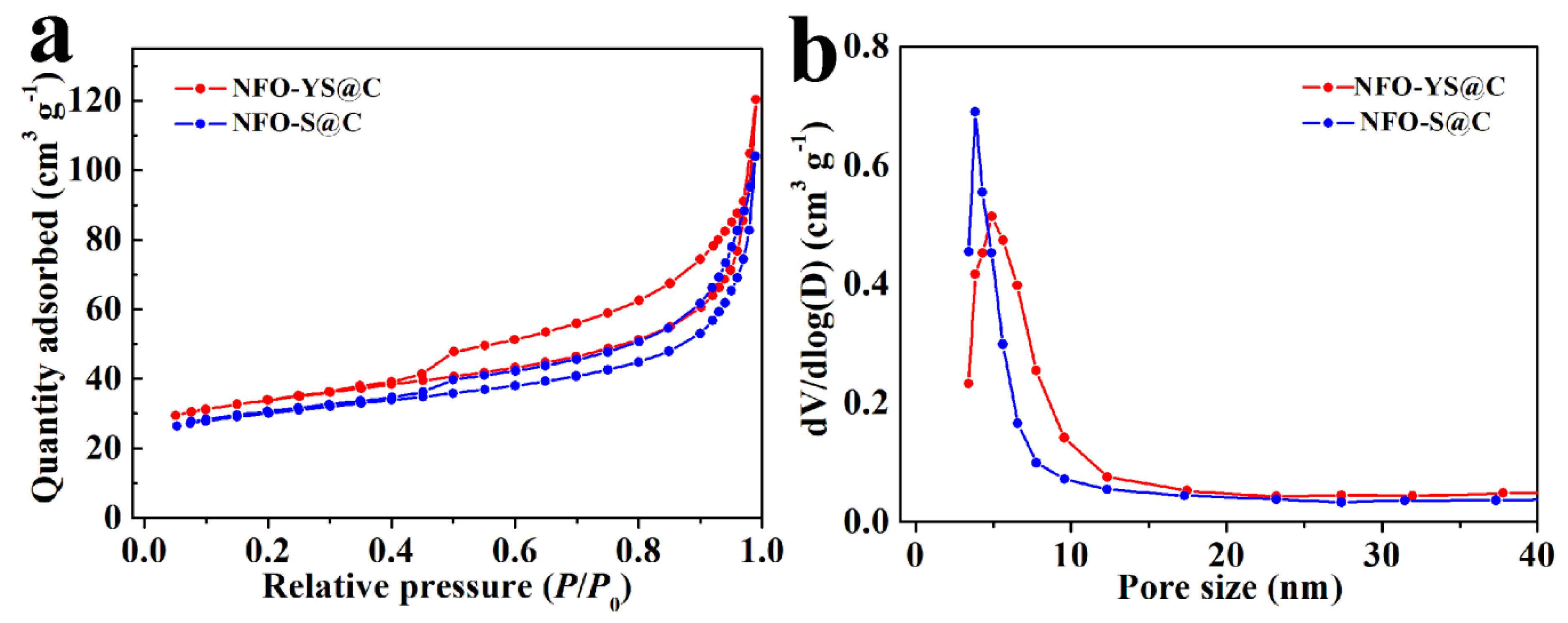
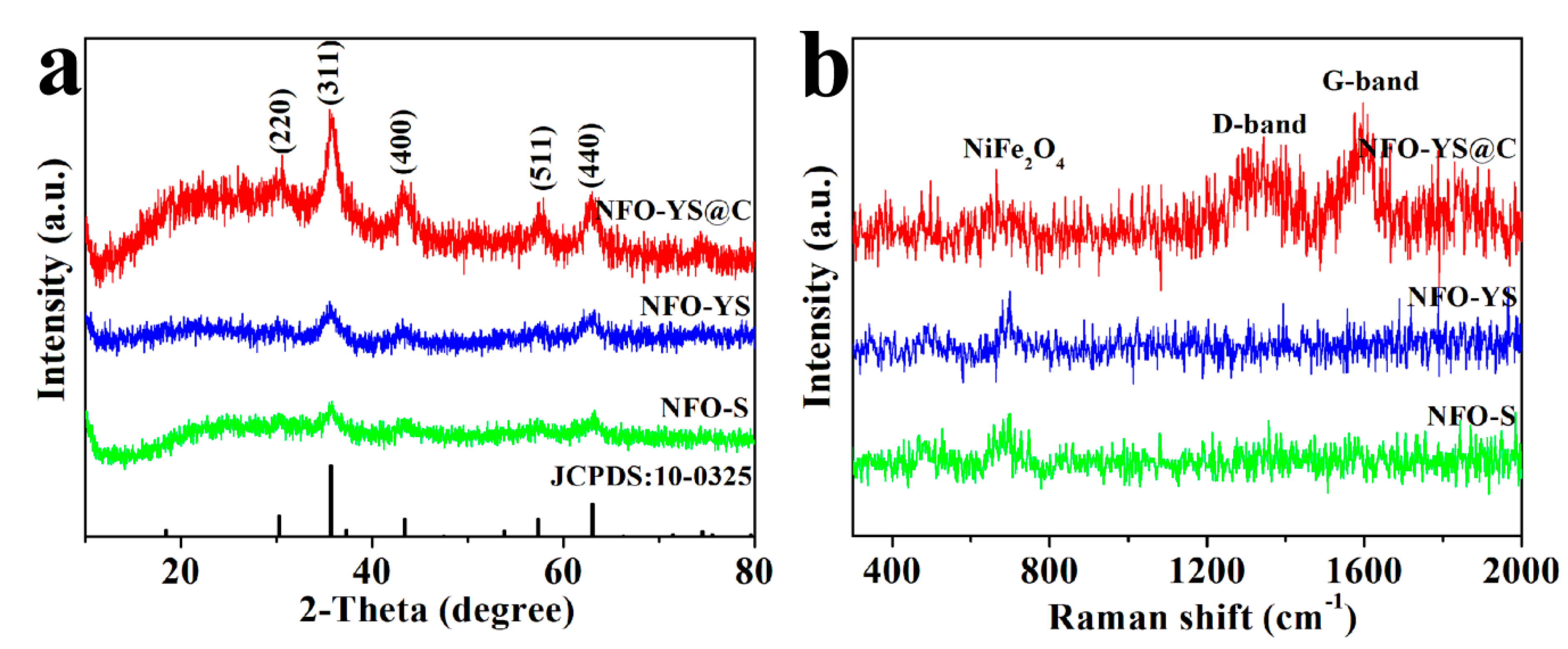
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, B.; Pan, Z.F.; Su, X.Y.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Zhong, S.Y.; Liu, H.Z.; Wei, D.H.; Hu, J.; Zhang, H.; Hou, H.S.; Peng, M.X.; Zhang, G.H.; Duan, H.G. Long-aspect-ratio N-rich carbon nanotubes as anode material for sodium and lithium ion batteries. Chem. Eng. J. 2020, 395, 125054. [Google Scholar] [CrossRef]
- Sun, F.; Wang, K.F.; Wang, L.J.; Pei, T.; Gao, J.H.; Zhao, G.B.; Lu, Y.F. Hierarchical porous carbon sheets with compressed framework and optimized pore configuration for high-rate and long-term sodium and lithium ions storage. Carbon 2019, 155, 166–175. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition metal oxide anodes for electrochemical energy storage in lithium-and sodium-ion batteries. Adv. Energy Mater. 2019, 10, 1902485. [Google Scholar] [CrossRef]
- Zheng, M.B.; Tang, H.; Li, L.L.; Hu, Q.; Zhang, L.; Xue, H.G.; Pang, H. Hierarchically nanostructured transition metal oxides for lithium-ion batteries. Adv. Sci. 2018, 5, 1700592. [Google Scholar] [CrossRef]
- Aminu, I.S.; Geaney, H.; Imtiaz, S.; Adegoke, T.E.; Kapuria, N.; Collins, G.A.; Ryan, K.M. A copper silicide nanofoam current collector for directly grown Si nanowire networks and their application as lithium-ion anodes. Adv. Funct. Mater. 2020, 30, 2003278. [Google Scholar] [CrossRef]
- Huang, G.; Han, J.H.; Lu, Z.; Wei, D.X.; Kashani, H.; Watanabe, K.; Chen, M.W. Ultrastable silicon anode by three-dimensional nanoarchitecture design. ACS Nano 2020, 14, 4374–4382. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Kim, I.T. W2C/WS2 alloy nanoflowers as anode materials for Lithium-ion storage. Nanomaterials 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.T.; Wu, X.; Wen, J.W.; Li, J.; Zeng, M. A novel core-shell structure NiFe2O4@SiO2 as high-performance anode materials for Lithium-ion batteries. Chem. Electro. Chem. 2019, 6, 911–916. [Google Scholar]
- Weng, C.Z.; Sun, X.; Han, B.; Ye, X.C.; Zhong, Z.Q.; Li, W.; Liu, W.Z.; Deng, H.; Lin, Z. Targeted conversion of Ni in electroplating sludge to nickel ferrite nanomaterial with stable lithium storage performance. J. Hazard. Mater. 2020, 393, 122296. [Google Scholar] [CrossRef]
- Li, J.; Meng, Q.P.; Zhang, Y.M.; Peng, L.L.; Yu, G.H.; Marschilok, A.C.; Wu, L.J.; Su, D.; Takeuchi, K.J.; Takeuchi, E.S.; et al. Size-dependent kinetics during non-equilibrium lithiation of nano-sized zinc ferrite. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, L.; Li, X.W.; Zhang, Q.Y.; Zhang, Y.X.; Gu, J.L.; Wang, K.; Zhang, Y.H. CuCo2S4–rGO microflowers: First-principle calculation and application in energy storage. Small 2020, 16, 2001468. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.T.; Zhang, C.Q.; Luo, Z.H.; Zhang, T.; Liu, J.F.; Li, J.S.; Zuo, Y.; Biendicho, J.J.; Llorca, J.; Arbiol, J.; et al. A low temperature solid state reaction to produce hollow MnxFe3-xO4 nanoparticles as anode for lithium-ion batteries. Nano Energy 2019, 66, 104199. [Google Scholar] [CrossRef]
- Li, Y.Z.; Meng, Y.S.; Liu, X.L.; Xiao, M.J.; Hu, Q.R.; Li, R.N.; Ke, X.Y.; Ren, G.F.; Zhu, F.L. Double-protected zinc ferrite nanospheres as high rate and stable anode materials for lithium ion batteries. J. Power Sources 2019, 442, 227256. [Google Scholar] [CrossRef]
- Sharma, M.; Sundriyal, S.; Panwar, A.K.; Gaur, A. Facile synthesis and electrochemical performance of Mg-substituted Ni1−xMgxCo2O4 mesoporous nanoflakes for energy storage applications. Electrochim. Acta 2019, 294, 53–59. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.L.; Chu, R.X.; Jiang, H.; Zeng, Y.B.; Zhang, Y.; Huang, N.M.; Guo, H. Pseudocapacitive P-doped NiCo2O4 microspheres as stable anode for lithium ion batteries. J. Alloy Compd. 2019, 787, 1051–1062. [Google Scholar] [CrossRef]
- Ma, Y.; Tai, C.; Younesi, R.; Gustafsson, T.; Lee, J.Y.; Edström, K. Iron doping in spinel NiMn2O4: Stabilization of the mesoporous cubic phase and kinetics activation toward highly reversible Li+ storage. Chem. Mater. 2015, 27, 7698–7709. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Wang, C.; Wang, Y.; Han, X. Morphology controllable synthesis of NiO/NiFe2O4 hetero-structures for ultrafast Lithium-ion battery. Front. Chem. 2018, 6, 654. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Liang, X.L.; Li, J.F.; Qian, J.M.; Liu, Y.G.; Yang, S.L.; Wang, Y.; Gao, D.Q.; Xue, D.S. Interfacial engineering of NiO/NiCo2O4 porous nanofibers as efficient bifunctional catalysts for rechargeable zinc–air batteries. ACS Appl. Mater. Inter. 2020, 12, 21661–21669. [Google Scholar] [CrossRef]
- Luo, L.; Chen, Z.; Ke, H.Z.; Sha, S.; Cai, G.G.; Li, D.W.; Yang, H.G.; Yang, X.W.; Zhang, R.Q.; Li, J.Q.; et al. Facile synthesis of three-dimensional MgFe2O4/graphene aerogel composites for high lithium storage performance and its application in full cell. Mater. Design 2019, 182, 108043. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Fu, Y.S.; Liu, W.W.; Lim, L.; Wang, X.; Yu, A.P. A general approach for fabricating 3D MFe2O4 (M=Mn, Ni, Cu, Co)/graphitic carbon nitride covalently functionalized nitrogen-doped graphene nanocomposites as advanced anodes for lithium-ion batteries. Nano Energy 2019, 57, 48–56. [Google Scholar] [CrossRef]
- Yang, C.Y.; Sun, M.Q.; Zhang, L.; Liu, P.Y.; Wang, P.; Lu, H.B. ZnFe2O4@carbon core-shell nanoparticles encapsulated in reduced graphene oxide for high-performance Li-ion hybrid supercapacitors. ACS Appl. Mater. Inter. 2019, 11, 14713–14721. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Qiu, Z.Z.; Chen, W.; Lin, Y.M.; Xin, H.L.; Yang, B.; Fan, H.S.; Zhu, C.Z.; Xu, J. NiFe2O4 porous nanorods/graphene composites as high-performance anode materials for lithium-ion batteries. Electrochim. Acta 2017, 248, 292–298. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Zhu, X.X.; Liu, Q.Y. One-step preparation of one dimensional nickel ferrites/graphene composites for supercapacitor electrode with excellent cycling stability. J. Power Sources 2018, 396, 41–48. [Google Scholar] [CrossRef]
- Möller, L.; Thauer, E.; Ottmann, A.; Deeg, L.; Ghunaim, R.; Hampel, S.; Klingeler, R. CoFe2O4-filled carbon nanotubes as anode material for lithium-ion batteries. J. Alloy Compd. 2020, 834, 155018. [Google Scholar] [CrossRef]
- Yang, T.B.; Zhang, W.X.; Li, L.L.; Jin, B.; Jin, E.M.; Jeong, S.; Jiang, Q. In-situ synthesized ZnFe2O4 firmly anchored to the surface of MWCNTs as a long-life anode material with high lithium storage performance. Appl. Surf. Sci. 2017, 425, 978–987. [Google Scholar] [CrossRef]
- Mujahid, M.; Ullah Khan, R.; Mumtaz, M.; Soomro, S.A.; Ullah, S. NiFe2O4 nanoparticles/MWCNTs nanohybrid as anode material for lithium-ion battery. Ceram. Int. 2019, 45, 8486–8493. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hu, X.; Wang, L.N.; Jin, B.J.; Zou, G.J.; Huang, Z.W.; Liu, Q.; Hu, G.Z.; Zhang, K.; Park, J.H. Rationally designed hybrids of NiCo2O4 and polymeric carbon nitride as faradaic electrodes with enhanced electrochemical performance. Electrochim. Acta 2019, 299, 717–726. [Google Scholar] [CrossRef]
- Zhang, L.H.; Wei, T.; Jiang, Z.M.; Liu, C.Q.; Jiang, H.; Chang, J.; Sheng, L.Z.; Zhou, Q.H.; Yuan, L.B.; Fan, Z.J. Electrostatic interaction in electrospun nanofibers: Double-layer carbon protection of CoFe2O4 nanosheets enabling ultralong-life and ultrahigh-rate lithium ion storage. Nano Energy 2018, 48, 238–247. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.F.; Wu, Q.H.; Li, W.L.; Shen, C.; Ni, L.B.; Yan, H.; Diao, G.W.; Chen, M. Ultrathin WS2 nanosheets vertically embedded in hollow mesoporous carbon framework a triple-shelled structure with enhanced lithium storage and electrocatalytic properties. J. Mater. Chem. A 2018, 6, 19004–19012. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Lu, X.; Lu, J.; Rabia, K.; Chen, H.; Hu, R.; Tang, H.; Zhang, Q.; Li, Z. Core-shell ZnO@C: N hybrids derived from MOFs as long-cycling anodes for lithium ion batteries. Chem. Commun. 2020, 56, 1980–1983. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Cui, Y.; Wang, D. Design of hollow nanostructures for energy storage, conversion and production. Adv. Mater. 2018, 31, 1801993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhuang, Z.C.; Zhao, H.H.; Lin, M.T.; Zhao, D.Y.; Mai, L.Q. Intricate hollow structures: Controlled synthesis and applications in energy storage and conversion. Adv. Mater. 2017, 29, 1602914. [Google Scholar] [CrossRef]
- Gong, Q.H.; Gao, T.T.; Hu, T.T.; Zhou, G.W. Synthesis and electrochemical energy storage applications of micro/nanostructured spherical materials. Nanomaterials 2019, 9, 1207. [Google Scholar] [CrossRef]
- Kundu, M.; Karunakaran, G.; Kolesnikov, E.; Sergeevna, V.E.; Kumari, S.; Gorshenkov, M.V.; Kuznetsov, D. Hollow NiCo2O4 nano-spheres obtained by ultrasonic spray pyrolysis method with superior electrochemical performance for lithium-ion batteries and supercapacitors. J. Ind. Eng. Chem. 2018, 59, 90–98. [Google Scholar] [CrossRef]
- Yu, M.; Huang, Y.; Wang, K.; Han, X.P.; Wang, M.Y.; Zhu, Y.D.; Liu, L. Complete hollow ZnFe2O4 nanospheres with huge internal space synthesized by a simple solvothermal method as anode for lithium ion batteries. Appl. Surf. Sci. 2018, 462, 955–962. [Google Scholar] [CrossRef]
- Deng, J.J.; Yu, X.L.; Qin, X.Y.; Liu, B.; He, Y.; Li, B.H.; Kang, F.Y. Controlled synthesis of anisotropic hollow ZnCo2O4 octahedrons for high-performance lithium storage. Energy Storage Mater. 2018, 11, 184–190. [Google Scholar] [CrossRef]
- Park, S.; Yang, S.H.; Kang, Y.C. Rational design of metal-organic framework-templated hollow NiCo2O4 polyhedrons decorated on macroporous CNT microspheres for improved lithium-ion storage properties. Chem. Eng. J. 2018, 349, 214–222. [Google Scholar] [CrossRef]
- Yu, L.; Hu, H.; Wu, H.B.; Lou, X.W. Complex hollow nanostructures: Synthesis and energy-related applications. Adv. Mater. 2017, 29, 1604563. [Google Scholar] [CrossRef]
- Qu, L.N.; Hou, X.H.; Huang, X.Y.; Liang, Q.; Ru, Q.; Wu, B.; Lam, K.H. Self-assembled porous NiFe2O4 floral microspheres inlaid on ultrathin flake graphite as anode materials for lithium ion batteries. Chemelectrochem 2017, 4, 3148–3155. [Google Scholar] [CrossRef]
- Tong, X.B.; Zhao, L.Z.; Lin, X.P.; Pan, X.; Zhang, J.M.; Duan, X.C.; Li, Q.H. High-index faceted nickel ferrite nanocrystals encapsulated by graphene with high performance for lithium-ion batteries. Electrochim. Acta 2017, 257, 99–108. [Google Scholar] [CrossRef]
- Islam, M.; Ali, G.; Jeong, M.; Choi, W.; Chung, K.Y.; Jung, H. Study on the electrochemical reaction mechanism of NiFe2O4 as a high-performance anode for Li-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 14833–14843. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yu, L.; Wu, H.B.; Yu, X.Y.; Zhang, X.; Lou, X.W. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 2015, 6, 6694. [Google Scholar] [CrossRef] [PubMed]
- Kamari Kaverlavani, S.; Moosavifard, S.E.; Bakouei, A. Self-templated synthesis of uniform nanoporous CuCo2O4 double-shelled hollow microspheres for high-performance asymmetric supercapacitors. Chem. Commun. 2017, 53, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, B.Q.; Zhang, L.Y.; Huo, Y.Q.; Li, L.; Xie, H.M.; Wang, C.G.; Su, Z.M. One-pot controllable synthesis of CoFe2O4 solid, hollow and multi-shell hollow nanospheres as superior anode materials for lithium ion batteries. J. Mater. Chem. A 2017, 5, 21994–22003. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.P.; Luo, B.; Zhu, H.C.; Chu, W.; Huang, K. Microwave-assisted synthesis of NiCo2O4 double-shelled hollow spheres for high-performance sodium ion batteries. Nano Micro Lett. 2018, 10, 13–20. [Google Scholar] [CrossRef]
- Liu, X.P.; Deng, S.F.; Liu, P.F.; Liang, J.N.; Gong, M.X.; Lai, C.L.; Lu, Y.; Zhao, T.H.; Wang, D.L. Facile self-template fabrication of hierarchical nickel-cobalt phosphide hollow nanoflowers with enhanced hydrogen generation performance. Sci. Bull. 2019, 64, 1675–1684. [Google Scholar] [CrossRef]
- Xia, D.C.; Quan, J.P.; Wu, G.D.; Liu, X.L.; Zhang, Z.T.; Ji, H.P.; Chen, D.L.; Zhang, L.Y.; Wang, Y.; Yi, S.S.; et al. Linear-polyethyleneimine-templated synthesis of N-doped carbon nanonet flakes for high-performance supercapacitor electrodes. Nanomaterials 2019, 9, 1225. [Google Scholar] [CrossRef]
- Dos, R.G.; Larsson, S.H.; de Oliveira, H.P.; Thyrel, M.; Claudio Lima, E. Sustainable biomass activated carbons as electrodes for battery and supercapacitors—A mini-review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, S.; Peng, J.; Zuo, Y.T.; Wang, G.; Guo, X.H.; Zhao, N.Q.; Ma, Y.Q.; Ma, L. Synthesis of micro—and meso-porous carbon derived from cellulose as an electrode material for supercapacitors. Electrochim. Acta 2017, 241, 170–178. [Google Scholar] [CrossRef]
- Wu, X.Y.; Niu, H.L.; Fu, S.S.; Song, J.M.; Mao, C.J.; Zhang, S.Y.; Zhang, D.W.; Chen, C.L. Core-shell CeO2@C nanospheres as enhanced anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 6790–6795. [Google Scholar] [CrossRef]
- Choi, W.; Choi, M.; Choi, J.; Lee, S.; Jung, J.; Jung, Y.; Park, J.; Park, S.; Won, H.; Moon, J.; et al. The high capacity and cycle stability of NiFe2O4 thin film prepared by E-beam evaporation method for lithium ion batteries. J. Alloys Compd. 2017, 729, 802–808. [Google Scholar] [CrossRef]
- Pan, Y.M.; Zhang, J.J.; Lu, H.B. Uniform Yolk-shell MoS2@carbon microsphere anodes for high-performance lithium-ion batteries. Chemistry 2017, 23, 9937–9945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.Y.; Chen, Y.N.; Wang, X.G.; Xie, Z.J.; Zhou, Z. Binder-free NiFe2O4/C nanofibers as air cathodes for Li-O2 batteries. J. Power Sources 2018, 377, 136–141. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.L.; Liu, H.; Wang, J.X.; Dong, X.T.; Liu, G.X.; Yu, W.S.; Yang, Y.; Zhang, H.B. Facile synthesis of Fe3O4/NiFe2O4 nanosheets with enhanced Lithium-ion storage by one-step chemical dealloying. J. Mater. Sci. 2018, 53, 15631–15642. [Google Scholar] [CrossRef]
- Gong, Q.H.; Li, Y.L.; Huang, H.; Zhang, J.; Gao, T.T.; Zhou, G.W. Shape-controlled synthesis of Ni-CeO2@PANI nanocomposites and their synergetic effects on supercapacitors. Chem. Eng. J. 2018, 344, 290–298. [Google Scholar] [CrossRef]
- Liu, Y.S.; Liu, H.T.; Huang, W.L.; Yu, Y.; Dai, X.Q.; Shan, Z.Q. Self-assembly of hierarchical microsized hard carbon-supported Si encapsulated in nitrogen-doped carbon as anode for lithium-ion batteries. J. Mater. Sci. 2020, 55, 12373–12384. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Liu, X.; Zhang, W.; Zhang, Y.; Li, Y.; Qin, C.; Zhao, W.; Bakenov, Z. Dual-network nanoporous NiFe2O4/NiO composites for high performance Li-ion battery anodes. Chem. Eng. J. 2020, 388, 124207. [Google Scholar] [CrossRef]
- Gao, X.J.; Wang, J.W.; Zhang, D.; Nie, K.Q.; Ma, Y.Y.; Zhong, J.; Sun, X.H. Hollow NiFe2O4 nanospheres on carbon nanorods as a highly efficient anode material for lithium ion batteries. J. Mater. Chem. A 2017, 5, 5007–5012. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.F.; Shao, H.X. One-pot synthesis of NiFe2O4/C composite as an anode material for lithium-ion batteries. J. Power Sources 2013, 244, 610–613. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Zai, J.T.; Tian, B.B.; Qian, X.F. Formation of NiFe2O4/expanded graphite nanocomposites with superior lithium storage properties. Nano Micro Lett. 2017, 9, 123–130. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Cao, W.Q.; Cai, Y.Z.; Shu, J.C.; Cao, M.S. Rational design of NiFe2O4-rGO by tuning the compositional chemistry and its enhanced performance for a Li-ion battery anode. Inorg. Chem. Front. 2019, 6, 961–968. [Google Scholar] [CrossRef]
- Zou, Y.L.; Li, Z.Y.; Liu, Y.L.; Duan, J.L.; Long, B. Coaxial structure of NiFe2O4/CNTs composites as anodes for enhanced lithium ion batteries. J. Alloys Compd. 2020, 820, 153085. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, K.; Zhou, Y.L.; Xia, Y.B.; Yu, N.F.; Wu, G.L.; Zhu, Y.S.; Wu, Y.P.; Huang, H.B. A facile, one-step synthesis of silicon/silicon carbide/carbon nanotube nanocomposite as a cycling-stable anode for lithium ion batteries. Nanomaterials 2019, 9, 1624. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Y.; Shua, F.F.; Guo, X.W.; Xue, D.F. High performance porous MnO@C composite anode materials for lithium-ion batteries. Electrochim. Acta 2016, 188, 793–800. [Google Scholar] [CrossRef]
- Hong, Y.J.; Kang, Y.C. One-pot synthesis of core-shell-structured tin oxide-carbon composite powders by spray pyrolysis for use as anode materials in Li-ion batteries. Carbon 2015, 88, 262–269. [Google Scholar] [CrossRef]
- Maharajan, S.; Kwon, N.H.; Brodard, P.; Fromm, K.M. A nano-rattle SnO2@carbon composite anode material for high-energy Li-ion batteries by melt diffusion impregnation. Nanomaterials 2020, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Yang, G.R.; Wang, L.; Yan, W. Synthesis of one-dimensional NiFe2O4 nanostructures: Tunable morphology and high-performance anode materials for Li ion batteries. J. Mater. Chem. A 2016, 4, 8620–8629. [Google Scholar] [CrossRef]
- Sun, H.T.; Xin, G.Q.; Hu, T.; Yu, M.P.; Shao, D.L.; Sun, X.; Lian, J. High-rate lithiation-induced reactivation of mesoporous hollow spheres for long-lived lithium-ion batteries. Nat. Commun. 2014, 5, 4526–4533. [Google Scholar] [CrossRef]
- He, Y.Y.; Xu, L.Q.; Li, C.C.; Chen, X.X.; Xu, G.; Jiao, X.Y. Mesoporous Mn-Sn bimetallic oxide nanocubes as long cycle life anodes for Li-ion half/full cells and sulfur hosts for Li-S batteries. Nano Res. 2018, 11, 3555–3566. [Google Scholar] [CrossRef]
- Bao, S.S.; Xiao, Y.F.; Li, J.F.; Yue, B.; Li, Y.J.; Sun, W.X.; Liu, L.; Huang, Y.; Wang, L.; Zhang, P.C.; et al. Coral-like NiFe2O4/C composite as the high-performance anode material for lithium-ion batteries. Electron. Mater. Lett. 2020, 16, 207–215. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, W.S.; Hou, X.H.; Wei, C.Y.; Qin, H.Q.; Chen, F.M. Nano silicon embedded porous NiFe2O4 floral microspheres with the improved performance of lithium storage. Mater. Lett. 2019, 238, 70–73. [Google Scholar] [CrossRef]
- Preetham, P.; Mohapatra, S.; Nair, S.V.; Santhanagopalan, D.; Rai, A.K. Ultrafast pyro-synthesis of NiFe2O4 nanoparticles within a full carbon network as a high-rate and cycle-stable anode material for lithium ion batteries. RSC Adv. 2016, 6, 38064–38070. [Google Scholar]

| Materials | Current Densies (mA g−1) | Cycle Numbers | Reversible Capacities (mA h g−1) | Capacity Retention (%) | Refs |
|---|---|---|---|---|---|
| NiFe2O4/C | 100 | 50 | 892.4 | 69.8 | [71] |
| NiFe2O4/Si | 100 | 100 | 906 | 57.5 | [72] |
| NiFe2O4 | 500 | 100 | 786 | 85.2 | [42] |
| NiFe2O4/graphene | 100 | 50 | 805 | 71.4 | [23] |
| NiFe2O4/CNTs | 100 | 100 | 624.6 | 46.3 | [63] |
| NiFe2O4/expanded graphite | 100 | 120 | 800 | 67.7 | [61] |
| NiFe2O4/C | 915 | 100 | 381.8 | 29.3 | [73] |
| NiFe2O4/graphite | 200 | 300 | 963.4 | 86.3 | [40] |
| NFO-YS/C | 200 | 200 | 1074.5 | 95.3 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Gong, Q.; Cao, P.; Sun, X.; Ren, J.; Gu, S.; Zhou, G. Preparations of NiFe2O4 Yolk-Shell@C Nanospheres and Their Performances as Anode Materials for Lithium-Ion Batteries. Nanomaterials 2020, 10, 1994. https://doi.org/10.3390/nano10101994
Liu T, Gong Q, Cao P, Sun X, Ren J, Gu S, Zhou G. Preparations of NiFe2O4 Yolk-Shell@C Nanospheres and Their Performances as Anode Materials for Lithium-Ion Batteries. Nanomaterials. 2020; 10(10):1994. https://doi.org/10.3390/nano10101994
Chicago/Turabian StyleLiu, Tianli, Qinghua Gong, Pei Cao, Xuefeng Sun, Jing Ren, Shaonan Gu, and Guowei Zhou. 2020. "Preparations of NiFe2O4 Yolk-Shell@C Nanospheres and Their Performances as Anode Materials for Lithium-Ion Batteries" Nanomaterials 10, no. 10: 1994. https://doi.org/10.3390/nano10101994
APA StyleLiu, T., Gong, Q., Cao, P., Sun, X., Ren, J., Gu, S., & Zhou, G. (2020). Preparations of NiFe2O4 Yolk-Shell@C Nanospheres and Their Performances as Anode Materials for Lithium-Ion Batteries. Nanomaterials, 10(10), 1994. https://doi.org/10.3390/nano10101994








